Abstract
This analysis examined the influence of quantifiable parameters of daily sleep continuity, primarily sleep duration and sleep fragmentation, on daily pain in adults with Sickle Cell Disease (SCD). Seventy-five adults with SCD completed baseline psychosocial measures and daily morning (sleep) and evening (pain) diaries over a three-month period. Mixed-effect modeling was used to examine daily between- and within-subjects effects of sleep continuity parameters on pain, as well as the synergistic effect of sleep fragmentation and sleep duration on pain. Results revealed nights of shorter sleep duration and time in bed, increased fragmentation, and less efficient sleep (relative to one’s own mean) were followed by days of greater pain severity. Further, the analgesic benefit of longer sleep duration was attenuated when sleep fragmentation was elevated. These results suggest that both the separate and combined effects of sleep duration and fragmentation should be considered in evaluating pain in adults with SCD.
Keywords: daily diaries, pain, Sickle Cell Disease, sleep fragmentation, sleep continuity
Introduction
Sleep is disrupted among individuals with chronic pain conditions, with daily diary studies finding abnormal sleep onset (> 30 minutes), fragmented sleep (1–2 awakenings during the night and for > 30 minutes), and inefficient sleep (80–85% sleep efficiency).10,20–21,35
A growing body of evidence suggesting that poor sleep prospectively predicts increases in clinical and experimental pain.8 Specifically, a limited number of studies have demonstrated that indices of sleep continuity, such as decreased sleep duration,17,35 delayed sleep onset latency (SOL; > 30 minutes),17,35 and increased sleep fragmentation (i.e. wake after sleep onset; WASO) predict increased pain severity.17 Investigations of sleep continuity are important because they provide an estimate of the association of sleep and pain that is less susceptible to retrospective heuristic biases than ratings of sleep quality, which may be influenced by feeling states present at the time of reporting.15,27
A few studies have identified sleep fragmentation (e.g., high WASO) as a particularly harmful characteristic of sleep continuity. Smith et al.28 demonstrated that experimentally disrupting sleep continuity (i.e. increasing WASO) significantly decreased endogenous pain-inhibition and increased spontaneous pain in healthy participants. In two observational studies, increased sleep fragmentation significantly predicted higher next-day pain among adolescents and adults with chronic pain.1,18 Although evidence generally supports the notion that sleep fragmentation increases vulnerability to pain, it is not known if sleep fragmentation interacts with other aspects of sleep continuity in predicting pain. For example, it would be important to know if the effects of sleep duration on pain are more or less pronounced in the context of fragmented versus non-fragmented sleep, thereby elucidating the value of acquiring both longer and consolidated sleep.
We elected to study sleep continuity and pain in adults with Sickle Cell Disease (SCD) for several reasons. First, pain is the most commonly reported symptom of SCD and exhibits day to day variability.19,31 Second, up to 70% of patients with SCD report sleep disturbances including difficulty initiating and maintaining sleep.14,34 Sleep continuity has not been quantified among non-sleep clinic adults with SCD, however sleep-disordered breathing (e.g. obstructive sleep apnea)6,9 and pain12–13,33 are common etiologies. Although disturbed sleep correlates with greater SCD pain,12–13,33 and sleep is impaired during an vaso-occlusive crisis,12–13 little is known about day-to-day variations in sleep, as well as the extent to which disrupted sleep continuity influences daily SCD pain.
Given the limitations of previous investigations, the goals of the present analyses were to examine the direct effect of parameters of sleep continuity during the night ( sleep duration, latency, and fragmentation) on SCD pain experienced between waking and going to bed the following day (i.e. next-day pain). We also examined the interacting, or synergistic effect of sleep fragmentation and sleep duration on next-day pain in the course of daily life in adults with SCD. For the second goal, we hypothesized that the synergistic effect of decreased sleep duration and greater sleep fragmentation would be associated with the highest next-day pain severity.
Methods
Participants
Participants in these analyses are from a larger NIH-funded study investigating dimensions of pain among individuals with SCD. Participants for the parent study were recruited through SCD clinics as well as posted flyers and advertisements. Individuals with SCD were eligible to participate in the parent study if they were 1) 18 years of age or older, 2) diagnosed with a SCD hemoglobinopathy genotype (HbSS, HbSC, HbS/β-thalassemia), 3) on a stable dose of NSAIDs, acetaminophen, or opioids (i.e. no change in pain management regimen made by a clinician during the weeks prior to enrollment), 5) without a vaso-occlusive crisis within the past three weeks, and 5) willing to provide informed consent. Individuals with SCD were excluded from participation if they 1) were an active substance abuser, 2) had a significant cognitive impairment or mental disorder, 3) current infection, 4) diagnosis of an autoimmune disorder, 5) HIV infection with a neuropathy, or 6) were currently pregnant, lactating, or planned to become pregnant in the subsequent 6 months of the study. A total of 236 individuals with SCD were screened by phone for participation in the parent study, 84 were eligible after the phone screen, and 84 provided study consent.
Only participants with SCD from the parent study who completed the electronic daily diary assessment portion of the study (n = 78) were used in the present analyses. The diary assessment period was intended to be approximately three months, however the total number of diary days varied between participants. We excluded participants (n = 3) who completed less than one week (< 7 days) of diary entries and/or had a diary completion ratio (an index of diary adherence calculated as number of diaries completed out of the total number of days the participant carried the PDA) of less than or equal to 25%. At least one-week of diaries is recommended for examining variations in sleep.4 Additionally, large intervals between reporting days would decrease our ability to examine day-to-day variations in sleep. In total, 75 adults (96% of all participants with SCD who completed the electronic diary portion of the study protocol) were included in the subsequent analyses.
Procedure
An Institutional Review Board at the study site approved all study procedures. Written informed consent was obtained from each study participant at the baseline study session. During the baseline session, psychosocial measures (e.g. Centers for Epidemiological Studies Depression (CES-D) and Pain Catastrophizing Scale (PCS)), demographic questions, and a medical and psychiatric history were collected.
At the conclusion of the baseline session, participants were provided with and trained on the use of an electronic handheld diary. The electronic diary included two reporting segments (morning for sleep and evening for daily pain) and was used to record daily experiences over the subsequent three month period.
Measures
Daily Measures
Electronic morning (sleep) and evening (pain) diaries were completed on a personal digital assistant (PDA; Palm® personal electronic organizer) using a customized application. Responses to each sleep and pain item were logged into customized menus and data entry screens with the use of a stylus pen. Participants did not receive daily reminders to complete entries; however participants were scheduled for a one-week and as-needed follow-up to answer any questions about the diary and to identify any technical issues regarding its use in order to enhance compliance with data entry procedures. Participants received financial incentives based on the number of diaries completed.
During data cleaning, entries that were not completed within 12 hours of waking or bedtime were removed to decrease recall bias. Out of 5,839 days in which a PDA was carried across participants (average of 78 days per participant), 4,411 morning diaries (average of 59 entries per participant) and 4,549 evening diaries (average of 61 entries per participant) were included in our analysis.
Sleep Diary
Immediately upon waking, participants were instructed to record what time they went to bed that night, their final awakening time, and what time they got out of bed that morning. Participants were also asked to estimate how long it took them to fall asleep (i.e. sleep onset latency, SOL) and the total amount of time they spent awake during the night (i.e. WASO). These variables were used to calculate sleep duration (i.e. total sleep time, TST), time in bed (TIB), and sleep efficiency for each participant. Sleep efficiency (SE), an index of the proportionate time in bed spent asleep, was calculated as a percentage of TST to TIB.
The reliability and validity of sleep diaries to measure parameters of sleep continuity, given the potential for recall bias, has been assessed. Overall, self-reported sleep parameters are used frequently and have been validated against objective measures.5 Furthermore, while both individual and systematic variability in measurement exists, an assessment period of at least 3 weeks or longer for sleep diaries is sufficient to achieve accurate and stable estimates in both insomniacs and normal sleepers.36 As noted above, each participant in the present analysis contributed an average of 59 morning (sleep) diaries, which supports the adequacy of using sleep diaries to measure parameters of sleep continuity.
Pain Diary
Just before bed, participants were instructed to record their average pain level for the day. Participants manipulated an electronic slider on the screen between zero (“no pain”) and 100 (“pain as bad as you can imagine”). Participants were also instructed to self-report the degree to which pain interfered with daytime activities and “Are you in a sickle cell [vaso-occlusive] crisis today?” As has been done in prior work, a proportion of diary days in pain (end of day pain rating greater than 0 out of 100, indicating some pain that day)30 and proportion of diary days for which a vaso-occlusive crisis was reported30 were calculated for each participant as a proxy of sickle cell disease severity.
Covariates
Demographic information including age, gender, education level, and marital status, as well as information about pain medication use, were collected at the baseline. Additionally, participants completed questionnaires on pain catastrophizing and depressive symptom severity at baseline. Both measures were included as covariates in the present analyses since both are highly associated with clinical pain severity and sleep continuity.3,23
Depressive Symptoms
The Centers for Epidemiological Studies Depression (CESD) is a self-report instrument that assesses depressive symptom severity.24 Responses to each item are reported on a 4-point Likert scale from zero (“rarely” or “none of the time”) to three (“most” or “all of the time”). A summary total score is calculated (scores of 16 or greater suggest clinically relevant depressive symptoms).24 The reliability of the CESD in our sample was good (Cronbach’s alpha = 0.78) and is similar to estimates found in another study of adults with SCD.16
Pain Catastrophizing
The Pain Catastrophizing Scale (PCS) is a 13-item scale used to measure negative cognitive-emotional appraisals of pain.32 The PCS, which was initially validated among undergraduate students, has been subsequently validated among a sample of community-dwelling adults.22 Each item is reported on a 5-point Likert scale from zero (“not at all”) to four (“all of the time”) where scores of 30 or greater indicate clinically relevant catastrophizing.32 The PCS scale had excellent reliability in our sample (Cronbach’s alpha = 0.91) and was similar to the reliability found in other community-dwelling adults.22
Data Analytic Strategy
Multilevel modeling (MLM) was used to evaluate daily between- and within-subjects effects of sleep continuity characteristics on pain and the potential moderating effect of WASO on the relationship between sleep duration and clinical pain. Multilevel models can account for dependencies created by repeated measurements over time on independent units (i.e. the participant). The MLM approach can also distinguish between changes over time in the dependent variable both within an individual (i.e. Level 1) and between individuals (i.e. Level 2).25
In our first set of MLMs we tested separate within-subjects (Level 1) models specifying an independent fixed effect of sleep duration, time in bed, SOL, WASO, and sleep efficiency during the night on our dependent variable next-day pain, which was the severity of pain reported between waking and going to bed the following day. In our second set of MLMs we tested an expanded model that included WASO, TST, and their interaction term as independent variables predicting next-day pain severity. We allowed the intercept to vary randomly across participants for all MLM models and controlled for autoregressive correlation between observations by modeling the variance/covariance matrix using an autoregressive 1 function. Age, gender, depressive symptoms, pain catastrophizing, and opioid medication use (yes or no) were tested as between-subjects (Level 2) covariates. Covariates that were not significant (p > 0.20) were removed in order to improve model fit and to achieve the most parsimonious model. All Level 1 independent variables were centered at each participant’s mean whereas Level 2 covariates were centered at the mean values across all participants, as recommended by Enders & Tofighi (2007).7
Descriptive statistics, Spearman’s rho correlations, and multilevel models were all computed and performed in Statistical Package of Social Sciences (SPSS), Version 21.0.11
Results
Descriptive Statistics
Seventy-five adults with SCD completed more than one week (> 7 days) and at least 25% of their daily morning (sleep) and evening (pain) diaries over the study period. Descriptive characteristics of the study participants are displayed in Table 1. Overall, participants ranged from 19 to 64 years of age (mean 38.5 years). Most of the sample was female, African-American, single, and had at least a college or technical degree. The mean CESD and PCS scores in our sample were 14.6 and 13, respectively.
Table 1.
Sample demographic, psychosocial, and pain characteristics (n = 75)
| Characteristic | Mean (SD) | n (%) |
|---|---|---|
| Demographic | ||
| Age, years | 38.5 (11.8) | |
| Female | 54 (72.0) | |
| African-American | 75 (100.0) | |
| Marital Status | ||
| Married/Cohabitating | 23 (30.6) | |
| Single | 42 (56.0) | |
| Separated/Divorced | 10 (13.3) | |
| Education | ||
| High School or less | 11 (14.7) | |
| Some College | 28 (37.3) | |
| Tech School/College Grad | 28 (37.3) | |
| Master/Doctoral Degree | 8 (10.7) | |
| Pain | ||
| Percentage of diary days with pain† | 63% (1%–100%) | |
| ≤ 5% | 8 (10.7) | |
| 6%–49% | 22 (29.3) | |
| 50%–95% | 19 (25.3) | |
| > 95% | 26 (34.7) | |
| Percentage of diary days with VOC† | 6% (0%–88%) | |
| 0% | 22 (29.3) | |
| 1%–49% | 47 (62.6) | |
| ≥50% | 6 (8.0) | |
| Opioid Use | 43 (57.3) | |
| NSAID Use | 22 (29.3) | |
| Psychosocial | ||
| CESD‡ | 14.6 (10.9) | |
| PCS‡ | 13 (9.3) | |
CESD = Centers for Epidemiological Studies Depression, PCS = Pain Catastrophizing Scale, VOC = Vaso-occlusive Crisis
Across participants over the study period
n < 75 due to missing value
Average pain severity on pain days (without crisis) and pain days (with crisis) over the study period were 32.4 and 50.7 out of 100, respectively, and are consistent with pain severity reported in previous studies.30 Approximately 35% of participants reported pain almost every day (> 95%) while 11% of the sample rarely reported pain (≤ 5%). In addition, only 8% reported a vaso-occlusive (VOC) on more than 50% of their diary day while almost a third of the sample (29.3%) did not report any VOC during the daily diary period. Approximately 57% of the sample reported taking some type of opioid (short and/or long acting), while 29% reported taking and NSAID during the study period (Table 1).
Table 2 displays the sleep continuity characteristics of the sample. Compared to recommendations and abnormal sleep indicators for adults,2,26 on average our sample of adults with SCD reported normal sleep duration (average of 7 hours), but abnormal sleep latency (> 30 minutes), WASO (> 30 minutes), and sleep efficiency (<85%). Correlations between sleep continuity indices and diary pain severity are provided in Table 3. Overall, mean pain severity correlated significantly with all indices of sleep continuity in the hypothesized direction.
Table 2.
Sleep continuity characteristics of the sample (n = 75)
| Sleep Parameter | M (SD)* | Range* |
|---|---|---|
| Total Sleep Time (TST), hr | 7.0 (2.2) | 1.9 – 19.5 |
| Time in Bed (TIB), hr | 8.6 (2.0) | 5.8 – 20.8 |
| Sleep Onset Latency (SOL), min | 35.5 (35.4) | 1.60 – 190.9 |
| Wake After Sleep Onset (WASO), min | 32.2 (35.8) | 0.1 – 242.0 |
| Sleep Efficiency (SE), % | 81.4 (14.2) | 24.0 – 97.0 |
M = mean; Min = Minutes; SD = Standard Deviation
Computed from participant mean sleep continuity characteristics
Table 3.
Correlations of primary study variables
| Variables
|
||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| 1. Mean TST | 1 | .56** | −.47** | −.50** | .59** | −.31** |
| 2. Mean TIB | 1 | .01 | .21 | −.14 | .24* | |
| 3. Mean SOL | 1 | .63** | −.70** | .41** | ||
| 4. Mean WASO | 1 | −.76** | .66** | |||
| 5. Mean SE | 1 | −.54** | ||||
| 6. Mean Pain Severity | 1 | |||||
SE = Sleep efficiency; SOL = Sleep onset latency; TIB = Time in bed; TST = Total sleep time; WASO = Wake after sleep onset
p < 0.05
p < 0.01
Main Effect Models
Results of our main effect multilevel models revealed, relative to the within-person mean across days, lower TST (β = −0.01 [SE = 0.001], p < 0.001), lower sleep efficiency (β = −0.03 [SE = 0.01], p = 0. 02), lower TIB (β = −0.004 [SE = 0.002], p = 0. 01), and higher WASO (β = 0.02 [SE = 0.004], p < 0.001) predicted higher next-day pain. Sleep onset latency did not significantly predict next-day pain severity (p = 0.39). These models suggest a 30-minute increase in total sleep time or 5% increase in sleep efficiency (above an individual’s mean) is associated with lower pain severity the next day (a decrease of 0.30 and 0.15 points on a 0–100 point scale respectively). Similarly, for every 30-minute decrease in WASO, next-day pain severity is estimated to be lower by 0.60 points.
Interaction Model
We tested separate multilevel models that included WASO and TST, as an interaction term to investigate the combined effect of sleep duration and fragmentation on pain severity (Table 4). In Model 1, the interaction term was significant (p = 0.04) and suggested next-day pain severity increases as both sleep fragmentation and sleep duration increase above an individual’s norm. The small effect from the MLM model may be related to variation within and between-subjects. In post-hoc testing, we used linear regression to estimate a mean between-subjects interaction effect as a test of the reliability of the daily interaction effect. In this model, the interaction effect was still significant (β = 0.001 [SE = 0.00001], p = 0.04) suggesting reliability of the daily interaction of WASO and TST. However, as displayed in Figure 1, the effect of TST on next-day pain is attenuated by high WASO. The daily interaction effect accounted for approximately 7% of the within–subject variance in clinical pain severity (Pseudo R2 = 0.067). We examined whether the daily interaction effect of sleep duration and sleep fragmentation remained significant and whether model fit improved after removing covariates that were not significant (Model 2). We also examined whether model fit would change if depressive symptoms were removed, since this variable decreased our sample size by eight participants (Model 3). We found no change in the magnitude, direction, or significance of the fixed effects. We also found a negligible change in model fit with the removal of non-significant covariates (χ2 (3) = 4, p = .26), but model fit was worse with the removal of depressive symptoms (χ2 (1) = 3,092, p < .001). Therefore, depressive symptoms were retained as a covariate in the final model (Model 2) suggesting that, even after accounting for depressive symptoms, there is a benefit to obtaining longer and consolidated sleep.
Table 4.
Multilevel Model of Total Sleep Time and Wake After Sleep Onset Predicting Daytime Clinical Pain Severity
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
|
| |||
| Fixed effects | β (SE) | β (SE) | β (SE) |
| Intercept | 31.7(3.0)*** | 20.7 (4.0)*** | 33.1(2.7)*** |
| WASO | .02(.01)*** | .02 (.01)*** | .02(.01)*** |
| TST | −.005(.002)** | −.005 (.002) ** | −.004(.002)* |
| TST × WASO | 2.8E−5(1.4E−5)* | 2.8E−5(1.4E−5)* | 2.8E−5(1.4E−5)* |
| Opioid nonuse† | −20.9(4.2)*** | −20.5 (4.1)*** | −23.3(4.2) *** |
| CESD | .55(.21)** | .6(.2)** | |
| PCS | .23(.24) | ||
| Age | .06(.17) | ||
| Male†† | −4.6(4.7) | ||
| Random Effects | |||
| Intercept | 247.1(46.5)*** | 241.9 (44.4)*** | 306.0(52.7) *** |
| Deviance | 27,048.6 | 27,052.3 | 30,144.3 |
| AIC | 27,054.6 | 27,058.3 | 30,150.3 |
| BIC | 27,072.9 | 27,076.7 | 30,169.0 |
Note: CESD = Centers for Epidemiological Studies Depression (grand-mean centered), PCS = Pain Catastrophizing Scale (grand-mean centered); TST = Total Sleep Time (person-centered), TST*WASO = Total Sleep Time × Wake after Sleep Onset (person-centered), WASO = Wake after Sleep Onset (person-centered)
Relative to opioid use.
Relative to Females
p < 0.2.
p < 0.05.
p < 0.01.
p < 0.001
Figure 1.
Moderation of the within subjects effect of sleep duration on clinical pain by sleep fragmentation (Adjusted)
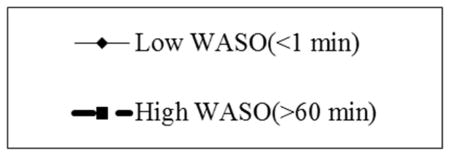
WASO = Wake after Sleep Onset
Discussion
The present study sought to examine the relationship between indicators of sleep continuity and clinical pain in a population of adults with SCD. Through the use of electronic daily sleep and pain diaries during 3 months of observation in patients’ natural environment, we found nights characterized by shorter sleep duration, more sleep fragmentation, and less efficient sleep than typical were followed by days of higher than typical clinical pain severity. Latency of sleep had no effect on next-day pain severity. We also found a small yet significant interaction effect between sleep fragmentation and sleep duration, which supported our hypothesis that the effects of sleep duration on pain is different on fragmented versus non-fragmented days. In general, as sleep duration increases, next-day clinical pain severity decreases; however, this benefit occurs only with low levels of sleep fragmentation.
This paper adds to the growing research literature highlighting sleep continuity, specifically sleep fragmentation (WASO) and not delayed sleep onset, as a predictor of next-day pain severity. Similar to prior studies, we demonstrated that daily changes in sleep fragmentation and total sleep time predict variation in next-day clinical pain severity.1,18,28 We also show, however, that the effects of sleep continuity should not be considered in isolation; the benefits of longer sleep may only be realized in the absence of sleep fragmentation.
Several clinical and research implications can be drawn from our results. First, to our knowledge this is the first study to characterize the sleep continuity characteristics of adults with SCD using electronic daily diaries. Our findings suggest that it may be important for clinicians working with adults with SCD to routinely include assessments of sleep continuity through daily diaries and discussions with patients about their sleep habits, particularly when pain is poorly controlled. These discussions should include an assessment of total sleep duration, sleep latency, sleep fragmentation (i.e., minutes awake after sleep onset), and sleep efficiency (ratio of total sleep time to total time in bed). Furthermore, although the sleep duration and sleep fragmentation interaction effect was small, we find that the combination of increasing sleep duration and reducing sleep fragmentation may be most beneficial to pain reduction, which may occur through changes in central pain processing. Sleep fragmentation has already been shown to reduce endogenous opioid pain modulation in healthy adults28 and may provide a mechanism underlying the effects of daily sleep fragmentation and sleep duration on next day clinical pain in patients with SCD. In addition, increase sleep fragmentation and shorter sleep duration may promote hyperalgesia through increased inflammatory activity.29 Nevertheless, the small effects observed in the present study should be considered with the qualification that they represent self-report responses observed from day-to-day; these small daily effects, however, may accumulate over years of coping with SCD and thus integratively represent a contributor to disease burden and poor quality of life in these patients. Future research and treatment studies should consider the interacting effects of sleep fragmentation and sleep duration and seek to determine if specifically altering sleep continuity in patients with SCD yields clinically meaningful changes in SCD pain. In addition, the interaction between sleep continuity and opioid use on clinical pain should be explored given the known effect of opioids on sleep continuity.
There are a few notable limitations of our analyses. First, all data were self-report; no objective measures of sleep were obtained. However, self-reported sleep parameters are used frequently and have been validated against more objective measures.5 Nonetheless, future work may integrate objective sleep measurement in the home with intensive longitudinal self-report data. Second, the exclusive focus on SCD pain in our analysis limits the generalizability of our findings to other pain populations. Third, nearly 25% of both morning (sleep) and evening (pain) diaries were missing. The adherence rate of approximately 75% is consistent with earlier work and is reasonable considering the number of participants and length of the assessment period. Furthermore, our analytic method (MLM) is fairly robust to missing data.
Finally, the parent study sampled adults with SCD who were on a stable pain management regimen, free of infection, and relatively stable in terms of the management of their sickle cell disease. Thus, it is possible that our sample is healthier than the population norm for SCD. However, we found approximately a third of the sample (35%) reported pain almost every day during the study period (similar to Smith et al.30). This finding supports the notion that SCD pain in the general public and in our population is common.30–31
Despite these limitations, our investigation has a number of strengths. A major strength is the emphasis on daily reports of sleep and pain using electronic diaries, which have become the gold standard for measuring time-varying fluctuations in daily life. Additionally, we followed participants for up to three months, which provided an extensive and thorough assessment period through which the relationship between sleep and pain may be reliably estimated. Finally, we had a sufficiently large sample size of participants with SCD (n = 75) who collectively provided over 4,000 records of daily sleep and pain diaries during the assessment period.
Conclusion
Overall our results suggest that parameters of sleep continuity (i.e. sleep duration, sleep fragmentation, and sleep efficiency) are predictors of clinical pain severity in individuals with SCD. Furthermore, sleep duration should not be considered in isolation and that its daily association with SCD pain may be qualified by the amount of sleep fragmentation present. Therefore, clinicians and researchers working with pain populations should include assessments and interventions targeting sleep continuity, with a focus on both sleep duration and sleep fragmentation, when addressing pain in SCD.
Highlights.
Used daily diaries to examine sleep continuity and pain in adults with SCD.
Subjective parameters of sleep continuity predicted clinical pain severity.
Analgesic benefits of sleep duration may be qualified by sleep fragmentation.
Perspective.
Subjective parameters of sleep continuity (e.g. sleep duration, fragmentation, and efficiency) predict clinical pain in individuals with SCD. Additionally, sleep duration should not be considered in isolation and its association with pain may be qualified by sleep fragmentation. Research and practice should include assessments of both when addressing pain severity.
Footnotes
Conflicts of Interest/Disclosures: This study was supported by grants from the National Institutes of Health: National Heart, Lung, and Blood Institute Investigator Grant 5R01HL098110 (Haythornthwaite) and National Institute of Nursing Research Predoctoral Training Fellowships T32NR012704 and F31NR014598 (Moscou-Jackson). All authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alsaadi SM, McAuley JH, Hush JM, Lo S, Bartlett DJ, Grunstein RR, Maher CG. The Bidirectional Relationship Between Pain Intensity and Sleep Disturbance/Quality in Patients with Low Back Pain. Clin J Pain. 2014;30(9):755–765. doi: 10.1097/AJP.0000000000000055. [DOI] [PubMed] [Google Scholar]
- 2.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5. Arlington, VA: American Psychiatric Association; 2013. [Accessed May 9, 2014]. Available at: dsm.psychiatryonline.org. [Google Scholar]
- 3.Buenaver LF, Quartana PJ, Grace EG, Sarlani E, Simango M, Edwards RR, Haythronthwaite JA, Smith MT. Evidence for indirect effects of pain catastrophizing on clinical pain among myofascial temporomandibular disorder participants: the mediating role of sleep disturbance. Pain. 2012;153(6):1159–1166. doi: 10.1016/j.pain.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 4.Buysse DJ, Ancoli-Israel S, Edinger JD, Lichstein KL, Morin CM. Recommendations for a standard research assessment of insomnia. Sleep. 2006;29(9):1155–73. doi: 10.1093/sleep/29.9.1155. [DOI] [PubMed] [Google Scholar]
- 5.Buysse DJ, Hall ML, Strollo PJ, Kamarch TW, Owens J, Lee L, Reis SE, Matthews KA. Relationships between the Pittsburgh Sleep Quality Index (PSQI), Epworth Sleepiness Scale (ESS), and clinical/polysomnographic measures in a community sample. J Clin Sleep Med. 2008;4(6):563–571. [PMC free article] [PubMed] [Google Scholar]
- 6.Daniel LC, Grant M, Kothare SV, Dampier C, Barakat LP. Sleep patterns in pediatric sickle cell disease. Pediatr Blood Cancer. 2010;55(3):501–507. doi: 10.1002/pbc.22564. [DOI] [PubMed] [Google Scholar]
- 7.Enders CK, Tofighi D. Centering predictor variables in cross-sectional multilevel models: a new look at an old issue. Psychol Methods. 2007;12(2):121–138. doi: 10.1037/1082-989X.12.2.121. [DOI] [PubMed] [Google Scholar]
- 8.Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. J Pain. 2013;14(12):1539–1552. doi: 10.1016/j.jpain.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hargrave DR, Wade A, Evans JPM, Hewes DKM, Kirkham FJ. Nocturnal oxygen saturation and painful sickle cell crises in children. Blood. 2003;101(3):846–848. doi: 10.1182/blood-2002-05-1392. [DOI] [PubMed] [Google Scholar]
- 10.Haythornthwaite JA, Hegel MT, Kerns RD. Development of a sleep diary for chronic pain patients. J Pain Symptom Manage. 1991;6(2):65–72. doi: 10.1016/0885-3924(91)90520-e. [DOI] [PubMed] [Google Scholar]
- 11.IBM Corp. IBM SPSS Statistics for Windows. 2012. [Google Scholar]
- 12.Jacob E. The pain experience of patients with sickle cell anemia. Pain Manag Nurs. 2001;2(3):74–83. doi: 10.1053/jpmn.2001.26119. [DOI] [PubMed] [Google Scholar]
- 13.Jacob E, Miaskowski C, Savedra M, Beyer JE, Treadwell M, Styles L. Changes in Sleep, Food Intake, and Activity Levels During Acute Painful Episodes in Children with Sickle Cell Disease. J Pediatr Nurs. 2006;21(1):23–34. doi: 10.1016/j.pedn.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Jacob E, Stinson J, Duran J, Gupta A, Gerla M, Ann Lewis M, Zeltzer L. Usability testing of a Smartphone for accessing a web-based e-diary for self-monitoring of pain and symptoms in sickle cell disease. J Pediatr Hematol Oncol. 2012;34(5):326–335. doi: 10.1097/MPH.0b013e318257a13c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kahneman D, Tversky A. Prospect Theory: An Analysis of Decision under Risk. [Accessed January 21, 2015];Econometrica. 1979 47(2):263–292. Retrieved from http://links.jstor.org/sici?sici=0012-9682%28197903%2947%3A2%3C263%3APTAAOD%3E2.0.CO%3B2-3. [Google Scholar]
- 16.Laurence B, George D, Woods D. Association between elevated depressive symptoms and clinical disease severity in African-American adults with sickle cell disease. J Natl Med Assoc. 2006;98(3):365–369. [PMC free article] [PubMed] [Google Scholar]
- 17.Lavigne G, Smith MT, Denis R, Zucconi M. Pain and sleep. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 5. St. Louis: Elsevier Saunders; 2011. [Accessed December 27, 2013]. pp. 1442–1451. Available at: https://www.clinicalkey.com/ [Google Scholar]
- 18.Lewandowski AS, Palermo TM, De la Motte S, Fu R. Temporal daily associations between pain and sleep in adolescents with chronic pain versus healthy adolescents. Pain. 2010;151(1):220–225. doi: 10.1016/j.pain.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCavit TL. Sickle cell disease. Pediatr Rev. 2012;33(5):195–204. doi: 10.1542/pir.33-5-195. quiz 205–6. [DOI] [PubMed] [Google Scholar]
- 20.O’Brien EM, Waxenberg LB, Atchison JW, Gremillion HA, Staud RM, McCrae CS, Robinson ME. Intraindividual variability in daily sleep and pain ratings among chronic pain patients: bidirectional association and the role of negative mood. Clin J Pain. 2011;27(5):425–33. doi: 10.1097/AJP.0b013e318208c8e4. [DOI] [PubMed] [Google Scholar]
- 21.O’Donoghue GM, Fox N, Heneghan C, Hurley DA. Objective and subjective assessment of sleep in chronic low back pain patients compared with healthy age and gender matched controls: a pilot study. BMC Musculoskelet Disord. 2009;10:122. doi: 10.1186/1471-2474-10-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Osman A, Barrios FX, Gutierrez PM, Kopper BA, Merrifield T, Grittmann L. The Pain Catastrophizing Scale: further psychometric evaluation with adult samples. J Behav Med. 2000;23(4):351–365. doi: 10.1023/a:1005548801037. [DOI] [PubMed] [Google Scholar]
- 23.Parmelee PA, Tighe CA, Dautovich ND. Sleep disturbance in Osteoarthritis: Linkages with pain, disability and depressive symptoms. Arthritis Care Res (Hoboken) 2014 doi: 10.1002/acr.22459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Appl Psychol Meas. 1977;1(3):385–401. doi: 10.1177/014662167700100306. [DOI] [Google Scholar]
- 25.Raudenbush SW, Bryk AS. Hierarchical Linear Models: Applications and Data Analysis Methods. 2. Thousand Oaks: SAGE Publications; 2002. [Google Scholar]
- 26.Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4(5):487–504.43. [PMC free article] [PubMed] [Google Scholar]
- 27.Shiffman S, Stone AA, Tukkan JS, Bachrach CA, Jobe JB, Kurtzman HS, Cain VS. The Science of Self-report: Implications for Research and Practice. Mawah: Lawrence Erlbaum Associates; Real-time self report of momentary states in the natural environment: computerized momentary ecological assessment; pp. 277–296. [Google Scholar]
- 28.Smith MT, Edwards RR, McCann UD, Haythornthwaite JA. The effects of sleep deprivation on pain inhibition and spontaneous pain in women. Sleep. 2007;30(4):494–505. doi: 10.1093/sleep/30.4.494. [DOI] [PubMed] [Google Scholar]
- 29.Smith MT, Quartana PJ, Okonkwo RM, Nasir A. Mechanisms by which sleep disturbance contributes to osteoarthritis pain: a conceptual model. Curr Pain Headache Rep. 2009;13(6):447–54. doi: 10.1007/s11916-009-0073-2. [DOI] [PubMed] [Google Scholar]
- 30.Smith WR, Penberthy LT, Bovbjerg VE, McClish DK, Roberts JD, Dahman B, Aisiku IP, Levenson JL, Roseff SD. Daily assessment of pain in adults with sickle cell disease. Ann Intern Med. 2008;148(2):94–101. doi: 10.7326/0003-4819-148-2-200801150-00004. [DOI] [PubMed] [Google Scholar]
- 31.Smith WR, Scherer M. Sickle-cell pain: advances in epidemiology and etiology. Hematology Am Soc Hematol Educ Program. 2010;2010(1):409–415. doi: 10.1182/asheducation-2010.1.409. [DOI] [PubMed] [Google Scholar]
- 32.Sullivan MJL. The pain catastrophizing scale: User manual. Montreal: 2009. [Google Scholar]
- 33.Valrie CR, Gil KM, Redding-Lallinger R, Daeschner C. Brief Report: Sleep in Children with Sickle Cell Disease: An Analysis of Daily Diaries Utilizing Multilevel Models. J Pediatr Psychol. 2007;32(7):857–861. doi: 10.1093/jpepsy/jsm016. [DOI] [PubMed] [Google Scholar]
- 34.Wallen GR, Minniti CP, Krumlauf M, Eckes E, Allen D, Oguhebe A, Seamon C, Darbari DS, Hildesheim M, Yang L, Schulden JD, Kato GJ, Taylor JG., 6th Sleep disturbance, depression and pain in adults with sickle cell disease. BMC Psychiatry. 2014;14:207. doi: 10.1186/1471-244X-14-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilson KG, Watson ST, Currie SR. Daily diary and ambulatory activity monitoring of sleep in patients with insomnia associated with chronic musculoskeletal pain. Pain. 1998;75(1):75–84. doi: 10.1016/S0304-3959(97)00207-8. [DOI] [PubMed] [Google Scholar]
- 36.Wohlgemuth WK, Edinger JD, Fins AI, Sullivan RJJ. How many nights are enough? The short-term stability of sleep parameters in elderly insomniacs and normal sleepers. Psychophysiology. 1999;36:233–244. [PubMed] [Google Scholar]