Abstract
Respiratory syncytial virus (RSV) is a leading cause of pneumonia and bronchiolitis, but despite decades of research a safe and effective vaccine has remained elusive. The viral fusion glycoprotein (RSV F) plays an obligatory role in the entry process and is the major target of neutralizing antibodies, making it an attractive target for vaccine development. This review will summarize the recently determined structures of RSV F in the pre- and postfusion conformations and describe the location and properties of neutralizing epitopes on RSV F, including the newly identified prefusion-specific epitopes. The influence of these findings on vaccine development will also be discussed, with a focus on the rational design and optimization of vaccine antigens.
Introduction
Respiratory syncytial virus (RSV) is a leading cause of lower respiratory tract diseases such as pneumonia and bronchiolitis [1]. It is an extremely infectious paramyxovirus, infecting almost all children within their first two years of life [2]. The very young are most susceptible to severe disease, and in the U.S. the peak age of hospitalization is 1-2 months of age [3,4]. In a recent analysis of global mortality, it was estimated that RSV is responsible for 6.7% of all deaths for children between 1 month and 1 year of age [5]. RSV infection elicits neutralizing antibodies and an effective T cell response, but immunity to the virus wanes over time, leading to reinfections throughout life [6]. Although disease severity is mild for older children and healthy adults, RSV causes substantial morbidity and mortality in elderly people [7,8].
An effective RSV vaccine is urgently needed, and over 40 are currently in development covering a broad range of modalities, including live-attenuated and protein subunit vaccines [9,10]. Of the three proteins on the virion surface, only the fusion glycoprotein (RSV F) is absolutely required for infection, and it is the main target of neutralizing antibodies in human sera [11**,12]. RSV F is also the target of palivizumab, a monoclonal antibody that passively protects infants from severe disease [13]. Therefore, the elicitation of potent, neutralizing antibodies against RSV F is a major goal for most vaccine developers [14].
In the last few years, there have been new developments in RSV research that have significantly advanced our understanding of the structure, antigenicity, and immunogenicity of RSV F. These include the elucidation of the X-ray crystal structures of RSV F in the pre and postfusion conformations, the isolation of extremely potent neutralizing antibodies that target novel epitopes, and the rational design of vaccine antigens. The goal of this review is to summarize these recent advances and highlight how this knowledge is being used to inform vaccine development.
RSV F Structure
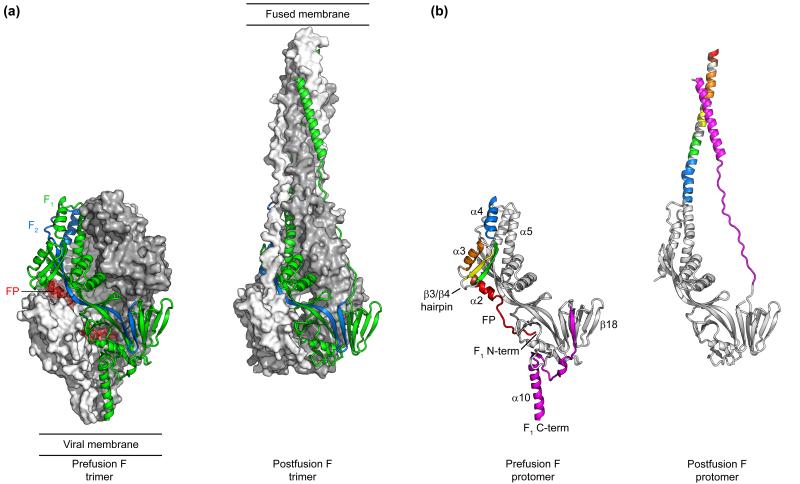
RSV F is a class I fusion glycoprotein that, like influenza HA and HIV-1 env, is synthesized as a precursor (F0) that requires proteolytic cleavage for activation [15]. The mature protein contains three copies of two polypeptides (F2 and F1) held together by two disulfide bonds. After initially folding into a metastable prefusion conformation, RSV F undergoes a dramatic rearrangement that results in a stable postfusion conformation, leading to fusion of the viral and cellular membranes.
Recently, cryoelectron tomography of cell culture-grown RSV has revealed that both the preand postfusion conformations are present on the virion surface [16*]. This provides additional evidence that the prefusion conformation is metastable, and converts to the postfusion conformation at a basal rate that has yet to be determined. Both high temperature [17] and low osmolality [18] have been shown to trigger the conversion, but the nature of the physiological trigger remains unknown. One possibility is that direct interaction with a host-cell receptor, such as the recently identified nucleolin [19], could initiate the fusion process at the correct time and place.
We, as well as a group at Novartis, were initially able to engineer a soluble form of postfusion RSV F and determine its structure by X-ray crystallography [20,21]. The structure revealed that F2 and F1 are deeply intertwined and combine to form several beta sheets (Figure 1a). The overall structure resembles a golf tee and is consistent with previous negative-stain electron microscopy images [22]. Interestingly, the structures revealed that all of the previously identified antigenic sites are present on the postfusion conformation.
Figure 1.
Conformations of the RSV F ectodomain. (a) Structures of trimeric RSV F in the prefusion (left) and postfusion (right) conformations. A molecular surface is shown for two of the protomers, colored gray and white, whereas the third protomer is displayed as a ribbon with F2 colored blue and F1 colored green. The fusion peptide (FP) is colored red and is shown with a transparent molecular surface. (b) Structures of RSV F protomers in the prefusion (left) and postfusion (right) conformations. Secondary structure elements that exist in substantially different orientations, conformations, or both are uniquely colored. The first 10 amino acids of the fusion peptide are not present in the postfusion structure.
To stabilize a soluble form of the prefusion conformation, we appended a heterologous trimerization motif to the C-terminus of F1 and co-expressed the soluble protein in the presence of a monoclonal antibody that specifically binds the prefusion conformation [23**]. The X-ray crystal structure revealed a compact, oval-shaped structure with a large cavity in the interior, partially occupied by the hydrophobic fusion peptide (Figure 1a). Of the ~470 amino acids in the RSV F ectodomain, approximately 300 are in similar positions in both the pre- and postfusion states. The remaining residues, mostly localized to the N- and C-terminus of F1, exist in substantially different positions between the two states (Figure 1b). The N-terminus of F1 undergoes the most dramatic rearrangement, with five secondary structure elements (α2, α3, α4, β3 and β4) refolding into an extended alpha helix in the postfusion state (Figure 1b). Many of the antibodies that are specific for the prefusion conformation interact with regions at the N- or C-terminus of the F1 subunit, whereas antibodies that bind both conformations recognize the structurally conserved central region (Figure 2).
Figure 2.
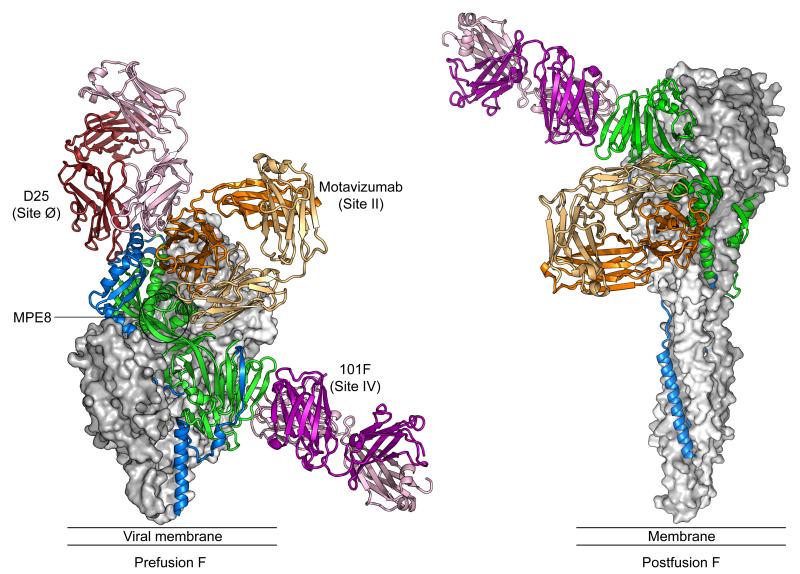
Neutralizing epitopes on RSV F. For the pre- and postfusion RSV F trimers, a molecular surface is shown for two of the protomers, colored gray and white, whereas the third protomer is displayed as a green ribbon with residues that are displaced by more than 5 Å between the structures colored blue. Antigen-binding fragments (Fabs) for antibodies D25, motavizumab and 101F are shown as ribbons, with the heavy chains colored darker than the light chains.
Epitopes Shared on Pre- and Postfusion F
There are two major neutralizing epitopes, antigenic sites II and IV, found on both the preand postfusion conformations of RSV F (Figure 2). Antibodies against both sites fail to block virus attachment but they neutralize infection by blocking fusion of the viral and cellular membranes [24].
Antibodies that recognize antigenic site II include murine 47F and 1129 [25,26], with humanization of the latter resulting in the licensed monoclonal antibody palivizumab [27] and its more potent derivative motavizumab [28]. Escape mutations for these antibodies include F1 residues Asn262, Asn268 and Lys272, and some antibodies bind peptides spanning residues 255-275. The X-ray crystal structure of motavizumab in complex with an RSV F-derived peptide spanning residues 254-277 revealed that the epitope forms a helix-loop-helix motif, with motavizumab recognizing both helices [29]. Although the epitope consists of discontinuous residues, they all reside within a 20 amino acid linear peptide, making this epitope an attractive target for immunization and vaccine development.
Immunization with the epitope peptide fails to elicit neutralizing antibodies though [30], and the structure suggests that maintaining the helix-turn-helix motif is critical for antibody recognition and elicitation. To stabilize this conformation, we attempted to transplant the epitope onto heterologous scaffold proteins that contained two anti-parallel alpha helices [31]. Although the epitope-scaffolds bind motavizumab, their affinity is much weaker than the interaction between motavizumab and RSV F, and these epitope-scaffolds do not elicit significant neutralizing titers when injected into mice. Schief and colleagues recently succeeded in eliciting neutralizing antibodies in macaques by creating superior motavizumab epitope-scaffolds through a combination of computational design and display on multivalent nanoparticles [32**]. These new scaffolds have picomolar affinity for motavizumab and elicit motavizumab-like monoclonal antibodies. Although the elicited neutralizing titers are low, such epitope-specific vaccine antigens represent a promising approach for precisely focusing the immune response.
The other major antigenic site shared on both the pre- and postfusion conformations is site IV, which is the target of antibodies such as 101F and MAb19 [26,33]. Escape mutations include F1 residues Arg429, Ile432, Lys433 and Val447, and due to variation in antibody recognition there were originally thought to be three distinct antigenic sites in this region (sites IV, V, VI) [34]. The X-ray crystal structure of 101F bound to an RSV F-derived peptide spanning residues 422-438 revealed that the peptide adopts a mostly linear conformation [35]. On the pre- and postfusion RSV F structures, this peptide corresponds to the outer most beta-strand (β18) of a 7-stranded beta-sandwich in the cysteine-rich region. Modeling of 101F binding to the RSV F ectodomain suggests that antigenic site IV is much larger and more complex than the linear peptide, and includes additional elements of the beta-sandwich (Figure 2). This is consistent with the ~10,000-fold lower affinity of 101F for the peptide than for RSV F [35].
Recently, a human monoclonal antibody called 54G10 was isolated that neutralizes all four subtypes of human metapneumovirus (hMPV), which like RSV is a paramyxovirus that causes acute respiratory tract infections [36]. This antibody also neutralizes RSV, and based on resistance mutations, 54G10 is predicted to recognize antigenic site IV on RSV F. Thus, site IV is a target of broadly neutralizing antibodies, which may be desirable for passive prophylaxis.
The presence of both site II and site IV on postfusion RSV F provides a rationale for the use of this protein as a subunit vaccine antigen. In addition to being extremely stable, postfusion RSV F elicits site II-directed antibodies in cotton rats [37]. Of course, non-neutralizing postfusion-specific antibodies may also be elicited, such as those directed against the six-helix bundle [38].
Epitopes Unique to Prefusion F
By passing human sera over immobilized postfusion RSV F, Melero and colleagues found that the majority of the neutralizing activity was not retained on the column. This demonstrated not only that prefusion-specific epitopes exist, but also that they are the main target of neutralizing antibodies elicited by natural infection in humans [11**].
Antibodies D25, AM22 and 5C4 were the first antibodies shown to be prefusion-specific. D25 and AM22 were isolated by AIMM Therapeutics from immortalized B cells derived from a human donor [39*], and we isolated 5C4 from mouse hybridomas through a collaborative effort with Professor Ningshao Xia at Xiamen University [23**]. All three antibodies have extremely potent neutralizing activity and have no detectable binding to furin-cleaved postfusion RSV F. Using a combination of X-ray crystallography and electron microscopy, we determined that all three antibodies bind to the membrane-distal apex of the prefusion RSV F trimer, which we named antigenic site Ø (zero). The site Ø epitope includes an unstructured region in F2 (residues 62-69) as well as the α4 helix in F1 (residues 196-209). Both regions are displaced by more than 5 Å in the postfusion conformation, with the α4 helix inverting ~180° (Figure 1b). This large change in conformation explains the prefusion-specificity of these antibodies, as well as their mechanism of fusion-inhibition. Interestingly, D25 binds monomeric RSV F [40], suggesting that monomers have prefusion-like structures, or that D25 is able to bind to a range of RSV F conformations, with the exception of the fully cleaved postfusion state.
Additional prefusion-specific antibodies have recently been isolated, such as the human antibody MPE8, which cross-competes with palivizumab for binding to prefusion RSV F. Mutagenesis indicates that its epitope is likely to be focused on the residues just below the helix-loop-helix motif of antigenic site II and also include residues in F2 [41*]. Interestingly, residues in this region are in similar conformations in the pre- and postfusion states, suggesting that the prefusion-specificity of MPE8 is due to more subtle conformational changes than those observed in antigenic site Ø. The MPE8 epitope appears to be particularly well-conserved amongst pneumoviruses, since MPE8 can neutralize not only human and bovine RSV, but also hMPV and pneumonia virus of mice (PVM). Other antibodies, such as AM14 and RSD5, appear to recognize unique prefusion-specific epitopes, but like MPE8, precise definition via structural studies are required.
The isolation of prefusion-specific antibodies is expected to have a major impact on the prevention of RSV infection and disease. Since many of these antibodies are 10-fold more potent than palivizumab, they represent new opportunities for passive prophylaxis. Indeed, a variant of D25, called MEDI8897, is currently being tested in clinical trials. In addition, prefusion-specific antibodies have been instrumental in the development of RSV F vaccine antigens stabilized in the prefusion conformation. For example, we used the D25-bound structure of RSV F to rationally design mutations that stabilize RSV F in the prefusion conformation, and then used binding of D25, AM22 and 5C4 to evaluate the conformation and stability of these variants [42**]. Our most stable variant, called DS-Cav1, elicits very high titers of RSV neutralizing activity in mice and rhesus macaques, and these titers are many fold higher than those elicited by postfusion RSV F. A benefit of prefusion RSV F as a vaccine antigen is that it contains all of the known neutralizing epitopes. As a result, prefusion RSV F, as well as other antigens bearing prefusion-specific epitopes [40], are now being developed by multiple companies as subunit vaccine antigens.
Conclusions
RSV causes substantial morbidity and mortality in infants and the elderly, and an effective vaccine has yet to be developed. Recent advances in our understanding of the structure, function and antigenicity of RSV F, as well as the isolation and characterization of novel RSV F-directed antibodies, have created new opportunities for passive prophylaxis and vaccine development. Antibodies that specifically recognize the prefusion conformation of RSV F are especially potent, and elicitation of such antibodies is now a major goal of vaccine developers. Importantly, the structural definition of neutralizing epitopes and the pre- and postfusion conformations of RSV F has allowed for the rational design of vaccine antigens that elicit high titers of neutralizing antibodies.
Despite the recent progress, there remain many outstanding questions regarding the antigenicity and immunogenicity of RSV F, including (i) Are there discrete antigenic sites (and if so, how many), or is there a continuum of sites covering the protein surface? (ii) Is antibody binding to prefusion RSV F sufficient for neutralization, or do antibodies have to target select regions in specific ways to effect neutralization? and (iii) What are the characteristics of antibodies (sequence, potency, specificity) elicited by natural infection in each of the target vaccine populations? Answers to these questions will allow for the optimization and refinement of vaccine antigens that elicit the most potent antibodies that each target population is capable of producing while avoiding the elicitation of non-neutralizing or potentially deleterious antibodies.
Conflict of interest
Dr. Jason McLellan is an inventor on several patent applications related to the use of motavizumab-epitope scaffolds, antibody 5C4 and prefusion RSV F.
Highlights.
X-ray crystal structures of pre- and postfusion RSV F have been determined
Antibodies specific for the prefusion conformation are extremely potent
Select antibodies neutralize both RSV and human metapneumovirus
Structure-based vaccine design has produced promising immunogens
Acknowledgements
The author would like to thank José Melero and members of the McLellan laboratory for helpful comments on the manuscript. Dr. McLellan was supported in part by NIAID grant 1R43AI112124.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Hall CB, Simoes EA, Anderson LJ. Clinical and epidemiologic features of respiratory syncytial virus. Curr Top Microbiol Immunol. 2013;372:39–57. doi: 10.1007/978-3-642-38919-1_2. [DOI] [PubMed] [Google Scholar]
- 2.Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child. 1986;140:543–546. doi: 10.1001/archpedi.1986.02140200053026. [DOI] [PubMed] [Google Scholar]
- 3.Hall CB. The burgeoning burden of respiratory syncytial virus among children. Infect Disord Drug Targets. 2012;12:92–97. doi: 10.2174/187152612800100099. [DOI] [PubMed] [Google Scholar]
- 4.Hall CB, Weinberg GA, Blumkin AK, Edwards KM, Staat MA, Schultz AF, Poehling KA, Szilagyi PG, Griffin MR, Williams JV, et al. Respiratory syncytial virus-associated hospitalizations among children less than 24 months of age. Pediatrics. 2013;132:e341–348. doi: 10.1542/peds.2013-0303. [DOI] [PubMed] [Google Scholar]
- 5.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2013;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Varga SM, Braciale TJ. The adaptive immune response to respiratory syncytial virus. Curr Top Microbiol Immunol. 2013;372:155–171. doi: 10.1007/978-3-642-38919-1_8. [DOI] [PubMed] [Google Scholar]
- 7.Widmer K, Zhu Y, Williams JV, Griffin MR, Edwards KM, Talbot HK. Rates of hospitalizations for respiratory syncytial virus, human metapneumovirus, and influenza virus in older adults. J Infect Dis. 2012;206:56–62. doi: 10.1093/infdis/jis309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walsh EE, Falsey AR. Respiratory syncytial virus infection in adult populations. Infect Disord Drug Targets. 2012;12:98–102. doi: 10.2174/187152612800100116. [DOI] [PubMed] [Google Scholar]
- 9.Anderson LJ. Respiratory syncytial virus vaccine development. Semin Immunol. 2013;25:160–171. doi: 10.1016/j.smim.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 10.Rudraraju R, Jones BG, Sealy R, Surman SL, Hurwitz JL. Respiratory syncytial virus: current progress in vaccine development. Viruses. 2013;5:577–594. doi: 10.3390/v5020577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11 **.Magro M, Mas V, Chappell K, Vazquez M, Cano O, Luque D, Terron MC, Melero JA, Palomo C. Neutralizing antibodies against the preactive form of respiratory syncytial virus fusion protein offer unique possibilities for clinical intervention. Proc Natl Acad Sci U S A. 2012;109:3089–3094. doi: 10.1073/pnas.1115941109. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors demonstrate the existence of antibodies that are specific for the prefusion conformation of RSV F, and show that these antibodies account for the majority of neutralizing activity in human sera.
- 12.Collins PL, Melero JA. Progress in understanding and controlling respiratory syncytial virus: still crazy after all these years. Virus Res. 2011;162:80–99. doi: 10.1016/j.virusres.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The IMpact-RSV Study Group Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics. 1998;102:531–537. [PubMed] [Google Scholar]
- 14.Anderson LJ, Dormitzer PR, Nokes DJ, Rappuoli R, Roca A, Graham BS. Strategic priorities for respiratory syncytial virus (RSV) vaccine development. Vaccine. 2013;31(Suppl 2):B209–215. doi: 10.1016/j.vaccine.2012.11.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McLellan JS, Ray WC, Peeples ME. Structure and function of respiratory syncytial virus surface glycoproteins. Curr Top Microbiol Immunol. 2013;372:83–104. doi: 10.1007/978-3-642-38919-1_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16 *.Liljeroos L, Krzyzaniak MA, Helenius A, Butcher SJ. Architecture of respiratory syncytial virus revealed by electron cryotomography. Proc Natl Acad Sci U S A. 2013;110:11133–11138. doi: 10.1073/pnas.1309070110. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reveals the presence of pre- and postfusion F on the surface of RSV virions. It also shows that the presence of an assembled layer of matrix protein is associated with ordered rows of prefusion RSV F.
- 17.Yunus AS, Jackson TP, Crisafi K, Burimski I, Kilgore NR, Zoumplis D, Allaway GP, Wild CT, Salzwedel K. Elevated temperature triggers human respiratory syncytial virus F protein six-helix bundle formation. Virology. 2010;396:226–237. doi: 10.1016/j.virol.2009.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chaiwatpongsakorn S, Epand RF, Collins PL, Epand RM, Peeples ME. Soluble respiratory syncytial virus fusion protein in the fully cleaved, pretriggered state is triggered by exposure to low-molarity buffer. J Virol. 2011;85:3968–3977. doi: 10.1128/JVI.01813-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tayyari F, Marchant D, Moraes TJ, Duan W, Mastrangelo P, Hegele RG. Identification of nucleolin as a cellular receptor for human respiratory syncytial virus. Nat Med. 2011;17:1132–1135. doi: 10.1038/nm.2444. [DOI] [PubMed] [Google Scholar]
- 20.McLellan JS, Yang Y, Graham BS, Kwong PD. Structure of respiratory syncytial virus fusion glycoprotein in the postfusion conformation reveals preservation of neutralizing epitopes. J Virol. 2011;85:7788–7796. doi: 10.1128/JVI.00555-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swanson KA, Settembre EC, Shaw CA, Dey AK, Rappuoli R, Mandl CW, Dormitzer PR, Carfi A. Structural basis for immunization with postfusion respiratory syncytial virus fusion F glycoprotein (RSV F) to elicit high neutralizing antibody titers. Proc Natl Acad Sci U S A. 2011;108:9619–9624. doi: 10.1073/pnas.1106536108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calder LJ, Gonzalez-Reyes L, Garcia-Barreno B, Wharton SA, Skehel JJ, Wiley DC, Melero JA. Electron microscopy of the human respiratory syncytial virus fusion protein and complexes that it forms with monoclonal antibodies. Virology. 2000;271:122–131. doi: 10.1006/viro.2000.0279. [DOI] [PubMed] [Google Scholar]
- 23 **.McLellan JS, Chen M, Leung S, Graepel KW, Du X, Yang Y, Zhou T, Baxa U, Yasuda E, Beaumont T, et al. Structure of RSV fusion glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Science. 2013;340:1113–1117. doi: 10.1126/science.1234914. [DOI] [PMC free article] [PubMed] [Google Scholar]; This manuscript reveals the prefusion conformation of RSV F and demonstrates that three newly identified prefusion-specific monoclonal antibodies target a novel antigenic site at the apex of the prefuson conformation.
- 24.Magro M, Andreu D, Gomez-Puertas P, Melero JA, Palomo C. Neutralization of human respiratory syncytial virus infectivity by antibodies and low-molecular-weight compounds targeted against the fusion glycoprotein. J Virol. 2010;84:7970–7982. doi: 10.1128/JVI.00447-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beeler JA, Coelingh KV. Neutralization Epitopes of the F-Glycoprotein of Respiratory Syncytial Virus - Effect of Mutation Upon Fusion Function. J Virol. 1989;63:2941–2950. doi: 10.1128/jvi.63.7.2941-2950.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arbiza J, Taylor G, Lopez JA, Furze J, Wyld S, Whyte P, Stott EJ, Wertz G, Sullender W, Trudel M, et al. Characterization of two antigenic sites recognized by neutralizing monoclonal antibodies directed against the fusion glycoprotein of human respiratory syncytial virus. J Gen Virol. 1992;73(Pt 9):2225–2234. doi: 10.1099/0022-1317-73-9-2225. [DOI] [PubMed] [Google Scholar]
- 27.Johnson S, Oliver C, Prince GA, Hemming VG, Pfarr DS, Wang SC, Dormitzer M, O’Grady J, Koenig S, Tamura JK, et al. Development of a humanized monoclonal antibody (MEDI-493) with potent in vitro and in vivo activity against respiratory syncytial virus. J Infect Dis. 1997;176:1215–1224. doi: 10.1086/514115. [DOI] [PubMed] [Google Scholar]
- 28.Wu H, Pfarr DS, Johnson S, Brewah YA, Woods RM, Patel NK, White WI, Young JF, Kiener PA. Development of motavizumab, an ultra-potent antibody for the prevention of respiratory syncytial virus infection in the upper and lower respiratory tract. J Mol Biol. 2007;368:652–665. doi: 10.1016/j.jmb.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 29.McLellan JS, Chen M, Kim A, Yang Y, Graham BS, Kwong PD. Structural basis of respiratory syncytial virus neutralization by motavizumab. Nat Struct Mol Biol. 2010;17:248–250. doi: 10.1038/nsmb.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lopez JA, Andreu D, Carreno C, Whyte P, Taylor G, Melero JA. Conformational constraints of conserved neutralizing epitopes from a major antigenic area of human respiratory syncytial virus fusion glycoprotein. J Gen Virol. 1993;74(Pt 12):2567–2577. doi: 10.1099/0022-1317-74-12-2567. [DOI] [PubMed] [Google Scholar]
- 31.McLellan JS, Correia BE, Chen M, Yang Y, Graham BS, Schief WR, Kwong PD. Design and characterization of epitope-scaffold immunogens that present the motavizumab epitope from respiratory syncytial virus. J Mol Biol. 2011;409:853–866. doi: 10.1016/j.jmb.2011.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32 **.Correia BE, Bates JT, Loomis RJ, Baneyx G, Carrico C, Jardine JG, Rupert P, Correnti C, alyuzhniy O, Vittal V, et al. Proof of principle for epitope-focused vaccine design. Nature. 2014;507:201–206. doi: 10.1038/nature12966. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this study the authors computationally design an antigen that correctly displays the motavizumab epitope, and demonstrate that this antigen can elicit motavizumab-like antibodies in macaques.
- 33.Wu SJ, Schmidt A, Beil EJ, Day ND, Branigan PJ, Liu C, Gutshall LL, Palomo C, Furze J, Taylor G, et al. Characterization of the epitope for anti-human respiratory syncytial virus F protein monoclonal antibody 101F using synthetic peptides and genetic approaches. J Gen Virol. 2007;88:2719–2723. doi: 10.1099/vir.0.82753-0. [DOI] [PubMed] [Google Scholar]
- 34.Lopez JA, Bustos R, Orvell C, Berois M, Arbiza J, Garcia-Barreno B, Melero JA. Antigenic structure of human respiratory syncytial virus fusion glycoprotein. J Virol. 1998;72:6922–6928. doi: 10.1128/jvi.72.8.6922-6928.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McLellan JS, Chen M, Chang JS, Yang Y, Kim A, Graham BS, Kwong PD. Structure of a major antigenic site on the respiratory syncytial virus fusion glycoprotein in complex with neutralizing antibody 101F. J Virol. 2010;84:12236–12244. doi: 10.1128/JVI.01579-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schuster JE, Cox RG, Hastings AK, Boyd KL, Wadia J, Chen Z, Burton DR, Williamson RA, Williams JV. A broadly neutralizing human monoclonal antibody exhibits in vivo efficacy against both human metapneumovirus and respiratory syncytial virus. J Infect Dis. 2015;211:216–225. doi: 10.1093/infdis/jiu307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raghunandan R, Lu H, Zhou B, Xabier MG, Massare MJ, Flyer DC, Fries LF, Smith GE, Glenn GM. An insect cell derived respiratory syncytial virus (RSV) F nanoparticle vaccine induces antigenic site II antibodies and protects against RSV challenge in cotton rats by active and passive immunization. Vaccine. 2014;32:6485–6492. doi: 10.1016/j.vaccine.2014.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palomo C, Mas V, Vazquez M, Cano O, Luque D, Terron MC, Calder LJ, Melero JA. Polyclonal and monoclonal antibodies specific for the six-helix bundle of the human respiratory syncytial virus fusion glycoprotein as probes of the protein post-fusion conformation. Virology. 2014;460:461–119. doi: 10.1016/j.virol.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 39 *.Kwakkenbos MJ, Diehl SA, Yasuda E, Bakker AQ, van Geelen CM, Lukens MV, van Bleek GM, Widjojoatmodjo MN, Bogers WM, Mei H, et al. Generation of stable monoclonal antibody-producing B cell receptor-positive human memory B cells by genetic programming. Nat Med. 2010;16:123–128. doi: 10.1038/nm.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]; This manuscript describes the isolation of antibody D25 and other potent antibodies from human B cells.
- 40.Swanson KA, Balabanis K, Xie Y, Aggarwal Y, Palomo C, Mas V, Metrick C, Yang H, Shaw CA, Melero JA, et al. A monomeric uncleaved respiratory syncytial virus F antigen retains prefusion-specific neutralizing epitopes. J Virol. 2014;88:11802–11810. doi: 10.1128/JVI.01225-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41 *.Corti D, Bianchi S, Vanzetta F, Minola A, Perez L, Agatic G, Guarino B, Silacci C, Marcandalli J, Marsland BJ, et al. Cross-neutralization of four paramyxoviruses by a human monoclonal antibody. Nature. 2013;501:439–443. doi: 10.1038/nature12442. [DOI] [PubMed] [Google Scholar]; This manuscript describes the isolation and characterization of antibody MPE8, and demonstrates that this antibody neutralizes RSV and hMPV, as well as two animal paramyxoviruses.
- 42 **.McLellan JS, Chen M, Joyce MG, Sastry M, Stewart-Jones GB, Yang Y, Zhang B, Chen L, Srivatsan S, Zheng A, et al. Structure-based design of a fusion glycoprotein vaccine for respiratory syncytial virus. Science. 2013;342:592–598. doi: 10.1126/science.1243283. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors use structure-based design to engineer the first soluble RSV F protein stabilized in the prefusion conformation, and demonstrate that this protein elicits high neutralizing antibody titers in mice and macaques.