Abstract
Mast cells are critical components of the innate immune system and important for host defense, allergy, autoimmunity, tissue regeneration, and tumor progression. Dysregulated mast cell development leads to systemic mastocytosis, a clinically variable but often devastating family of hematologic disorders. Here we report that induced expression of Lin28, a heterochronic gene and pluripotency factor implicated in driving a fetal hematopoietic program, caused mast cell accumulation in adult mice in target organs such as the skin and peritoneal cavity. In vitro assays revealed a skewing of myeloid commitment in LIN28B-expressing hematopoietic progenitors, with increased levels of LIN28B in common myeloid and basophil-mast cell progenitors altering gene expression patterns to favor cell fate choices that enhanced mast cell specification. In addition, LIN28B-induced mast cells appeared phenotypically and functionally immature, and in vitro assays suggested a slowing of mast cell terminal differentiation in the context of LIN28B upregulation. Finally, interrogation of human mast cell leukemia samples revealed upregulation of LIN28B in abnormal mast cells from patients with systemic mastocytosis (SM). This work identifies Lin28 as a novel regulator of innate immune function and a new protein of interest in mast cell disease.
Introduction
Mast cells (MCs) are key effectors in allergic responses, expressing (along with basophils) the high-affinity receptor for IgE (FcεRI). Crosslinking FcεRI on tissue MCs initiates the immediate hypersensitivity reaction, with local release of histamine and inflammatory cytokines. This supports innate immune defense against infections and plays an important role in autoimmunity (1–4). Aside from their central role in allergy and inflammation, it is increasingly clear that MCs play a pivotal role in tissue regeneration and tumor remodeling (5–9).
Dysregulated MC development and activation leads to mastocytosis, a poorly-understood group of myeloproliferative neoplasms characterized by abnormal growth and activation of immature MCs and their precursors. The WHO recently classified mastocytosis into seven variants (1–4,10), ranging from cutaneous mastocytosis to mast cell leukemia (MCL). These are highly clinically variable, with median survival rates of 2 months for MCL (11,12) but virtually no mortality for mild forms. Mastocytosis is characterized by upregulated c-Kit signaling (13) and the vast majority of systemic mastocytoses harbor an imatinib-insensitive activating c-KIT mutation (usually D816V) (14–17), but this cannot explain the wide clinical variability. Understanding normal MC development and its dysregulation in SM is of central importance to developing new therapies for these disorders.
In contrast to other myeloid lineages, relatively little is known about MC development, in part because MCs are rare and difficult to isolate. Developing mast cell progenitors (MCPs) circulate through the bloodstream and only complete differentiation after migrating into skin, heart, lung, and other target organs (18–20). MCPs arise from lineage-negative (Lin−) c-kit+Sca-1−myeloid progenitors (MPs) in the bone marrow, although there is controversy regarding their specific lineal relationship with other myeloid precursors (18,21,22). MC development and differentiation is influenced by the balance between core myeloid transcription factors such as C/EBPα, MITF, GATA-1, PU.1, and GATA-2, and responsive to signals elaborated by PLA2G4 and PI-3K (19,23–26). During maturation, MCs upregulate c-kit and FcεRIαand induce expression of neutral granule components such as carboxypeptidase A3, chymase, cathepsin G, granzyme B, and the tryptases (2).
The heterochronic RNA-binding factor Lin28 is highly expressed in embryonal tissues (27–29) and, along with Oct4, Sox2, and Nanog, reprograms somatic fibroblasts into pluripotent stem cells (30). Lin28 has been heavily studied in tumorigenesis (28,29,31–34), and has been implicated in obesity (35), metabolism (36), and tissue regeneration (37). Mammals express two isoforms of Lin28 (a and b). Both proteins can enforce proliferative programs and oppose cellular differentiation, and can have similar physiological functions, although it is clear that each protein has unique properties as well (reviewed in (27)). Although the canonical downstream effect of both isoforms is to inhibit biogenesis of the let-7 family of microRNAs, other functions have also been ascribed to these proteins (38,39). Recent studies have begun to examine the role of LIN28B, in particular, in hematopoiesis (40–43), and indicate that reactivation of LIN28B in adult blood cells can revert their phenotypes to an immature stage and upregulate a fetal hematopoietic program resulting in fetal globin expression and increased production of “primitive” γδ T and B-1 B cells. A physiologic role for LIN28B in hematopoietic development remains uncertain; knockout model development is challenged by redundancies in the Lin28 isoforms and the essential role of these genes in embryonal development.
The role of Lin28 in solid tumors is well documented (28,29,32–34), but its association with hematologic malignancy is largely undefined. Some reports suggest that LIN28B overexpression can result in lymphoid malignancy (44,45), whereas other studies have not supported a role for Lin28 in hematologic malignancy (43,46). We show here that expression of LIN28B in adult mice drives accumulation of immature MCs, resulting in an overabundance of immature MCs that are hypofunctional upon antigen challenge in vivo. We further demonstrate that LIN28B acts at multiple stages of MC development, both to favor MC fate choice and to impair terminal differentiation. Finally, we find that LIN28B is highly overexpressed in abnormal MCs from patients with ASM; this and the proliferation of immature MCs characteristic of SM mirror our animal model findings. Together, these data implicate aberrant Lin28 expression in mast cell disease and dysfunction.
Methods
iLIN28B and ilet7 mice
iLIN28B and ilet7 mice have been described (36) and were maintained on a C57BL/6 background. LIN28B or let-7 transgenes were induced with 1mg/mL of doxycycline in drinking water for 2 weeks. Unless otherwise stated, mice were 6–12 weeks old at induction. Bone marrow transplant recipients were induced for 2 weeks after 16–20 weeks of post-transplant recovery. All control mice were age- and gender-matched.
Flow cytometric analysis
Mononuclear cells harvested from hematopoietic tissues, peritoneal lavage, in vitro cell culture, or methylcellulose were stained with directly-conjugated antibodies or with biotinylated antibodies followed by fluorophore-conjugated streptavidin, then analyzed by flow cytometry on a BD LSRII or BD AriaII (Becton Dickinson, East Rutherford NJ). Data were analyzed using FlowJo (Treestar Industries, Ashland OR). Antibodies are listed in Supplemental Methods.
In vitro methylcellulose cultures
FACSorted progenitors were plated in Methocult m3434 (Stem Cell Technologies, Vancouver BC) at 100–1000 cells/1.5mL/3cm dish and cultured for 10 days. Colonies were counted and morphologically categorized as CFU-G, CFU-M, CFU-GM, CFU-GEMM, or CFU-E/BFU-E before harvest for further analysis.
Bone marrow-derived mast cell cultures
Bone marrow was isolated from uninduced C57BL/6, iLIN28B, and ilet-7 mice and cultured in IMDM supplemented with 10% FCS, 50ng/mL rmSCF, and 10ng/mL rmIL-3 (R&D Systems, Minneapolis MN). Cultures were induced with 1µg/mL doxycycline. After 5 weeks, >95% of suspended cells from wild-type mice displayed MC morphology and granulation and were toluidine-blue positive and c-kithiFcεRIαhi by flow cytometry.
Histologic evaluation
Cytospins were prepared using mononuclear cell suspensions derived from hematopoietic tissues or in vitro cell culture on a Shandon Cytospin 2 (Thermo Fisher Scientific, Waltham MA) and stained with Wright-Giemsa (Sigma-Aldrich, St. Louis MO). Peripheral blood smears were hand-drawn on glass slides and stained with Wright-Giemsa. MCs were stained with acid toluidine blue solution after fixation in Mota’s solution. Tissues were fixed in neutral-buffered formalin and processed by the DF/HCC Specialized Histopathology facility.
Electron Microscopy (EM)
Cell pellets were fixed in 2.5% glutaraldehyde, 1.25% paraformaldehyde, and 0.03% picric acid in 0.1M sodium cacodylate (pH 7.4). Subsequent preparation and EM were performed at the EM Core Facility at Harvard Medical School.
Bone marrow transplantation
Mononuclear cells were harvested from the bone marrow of age-and-gender matched male or female mice and either directly retroorbitally injected(1×106 cells/recipient) or stained using SLAM markers, FACSorted for LT-HSCs (Lin−Sca-1+c-Kit+CD150+CD48−), and then injected into anesthetized, lethally-irradiated (950 rads) congenic (CD45.1+)recipients, either with competitorCD45.1+BM cells or with 3×105CD45.1+helper cells to avoid radiation-induced hematologic failure.
Passive cutaneous anaphylaxis (PCA) assay
PCA assays were performed according to standard protocols (47). Briefly, control and iLIN28B mice were induced for 2 weeks and the near pinnae were injected with PBS (control) or anti-dinitrophenol (DNP) IgE. 24h later, mice were tail-vein injected with DNP-human serum albumin (HSA) in a solution of Evans blue dye; histamine release resulted in increased vascular permeability and dye extravasation into the ear pinna. Pinnae were removed, macerated, and incubated overnight in acetone or formamide. After incubation, 570nm absorbance of the supernatant was spectrophotometrically measured. Positive control pinnae were injected directly with histamine followed by DNP-HSA in Evans blue dye.
RT-PCR
RNA extraction from Trizol and RT-PCR were carried out according to manufacturer’s instructions (Life Technologies, Carlsbad CA). Banked patient samples were obtained through Dana-Farber Cancer Institute (DFCI) IRB protocol 01–206 (R. Soiffer, PI). Bone marrow aspirates were centrifuged for buffy coat isolation, then subjected to dextran erythrocyte sedimentation followed by lysis in ammonium-chloride-potassium buffer. Subsequently, bone marrow mononuclear cells were stained with antibodies to c-Kit, CD25, CD2, and CD45; abnormal MCs (as defined in the patients’ medical records) and control cells were FACSorted into Trizol for further analysis. Primer sequences are provided in Supplemental Methods.
Results
Lin28b is normally downregulated upon mast cell differentiation in adult mice
Recent analyses have implicated Lin28b in the specification of primitive hematopoietic lineages during fetal development, and shown that it is highly expressed in mouse fetal liver but that expression declines during postnatal life ((42); https://gexc.stanford.edu/search). To evaluate the possible involvement of Lin28b in post-natal mast cell development, we first examined Lin28b expression during MC differentiation in bone marrow-derived mast cell cultures (BMMCs). MC development is poorly understood, and its kinetics are difficult to study in vivo; the BMMC system represents a very useful and well-accepted model system for the analysis of MC development and function (48).In addition, BMMCs permit the synchronous generation of large numbers of MCs, enabling kinetic evaluation of MC development. Lin28b transcription decreased over time in BMMC culture (Fig 1), indicating that changes in Lin28 levels may be important for MC maturation.
Figure 1. Lin28 plays a role in mast cell development.
Bone marrow mast cell (BMMC) cultures are a well-accepted model system of mast cell development. Examination of the expression of Lin28b in BMMC cultures showed that Lin28b is downregulated as mast cells mature. Values are expressed as fold-change over expression in fetal liver, which has been shown to express high levels of Lin28b. p=0.0005 by one-way ANOVA in both cases.
Expression of LIN28 Bleads to increased numbers of mast cells and their progenitors
To study further the role of Lin28b in MC development, we used inducible transgenic mice that express LIN28B upon doxycycline treatment (iLIN28B mice (36)). After a two-week induction (Fig. 2A), hematopoietic cells were harvested from spleen, bone marrow, peritoneal cavity, and peripheral blood and analyzed by histology and flow cytometry. LIN28B-expressing mice harbored greater numbers of peritoneal MCs, identified as c-kit+FcεRIα+,than age- and gender-matched controls (5.22±0.287% of live peritoneal cavity cells in iLIN28B mice (n=12) vs. 2.98±0.202% in control C57BL/6 mice (n=7), Fig 2B). Sectioning and staining of ear pinnae revealed a similar (~1.5-fold) increase in MCs in the skin of induced iLIN28B mice as assessed by toluidine blue staining (Fig 2C, arrowheads). iLIN28B mice also had a 3.6-fold increase in splenic mast cell precursors (MCPs)(Fig 2D, 0.055±0.006%of live cells in iLIN28B (n=12) vs. 0.015±0.002% in controls (n=7)) and twice as many bone marrow MCPs (Fig 2E, 0.122±0.009% of live cells in iLIN28B mice vs. 0.064±0.005% in controls, n=9 of each genotype) as controls. MCPs were defined as lin−c-Kit+FcγRII/III+intβ7+ in the spleen and lin−Sca −1−c-Kit+CD150− Flk2− intβ7+CD27dim in the bone marrow (18,20).
Figure 2. Induced expression of LIN28B leads to the accumulation of mast cells and mast cell progenitors.
(A) iLIN28B mice, which express LIN28B under the control of a tet-on promoter, were induced with doxycycline-containing drinking water for 2 weeks and then sacrificed. (B) Induction of LIN28B leads to a 1.75-fold increase in peritoneal mast cells. Peritoneal lavage mononuclear cells were stained with antibodies to c-Kit and FcεRIα and analyzed by flow cytometry (left) or cytospun and stained with toluidine blue (right). Images were obtained on an Olympus BX60 light microscope equipped with an Olympus DP70 camera using Olympus DP Controller software; original magnification x600 for all images. (C) iLIN28B mice have higher numbers of skin-resident mast cells than controls after induction. Ear sections were obtained and stained with toluidine blue (left), and toluidine blue (+) mast cells per high-powered field (hpf) were enumerated (right). Images were obtained on an Olympus BX60 light microscope equipped with an Olympus DP70 camera using Olympus DP Controller software; original magnification x600 for all images. (D,E) LIN28B expression results in increases in splenic (D) and bone marrow (E) mast cell progenitors (MCPs). Spleens from induced iLIN28B (circles) and control mice (squares) were harvested, processed, and stained for lineage markers, c-Kit, integrinβ7, and FcγRII/III; splenic mast cell precursors are lin−c-Kit+FcγRII/III+intβ7+. Bone marrow mononuclear cells were stained for lineage markers, Sca-1, c-Kit, CD150, Flk2, integrinβ7, and CD27. BMMCPs are lin−Sca-1−c-Kit+CD150−Flk2−intβ7+CD27+.
Lin28 expression promotes mast cell progenitor accumulation
To understand how Lin28 expression augments MC numbers, we next examined MC developmental stages in iLIN28B mice. MC provenance is not completely understood, and MCs appear capable of developing from several different progenitors (18–21,49). However, the most exhaustive studies indicate that MCs develop predominantly from the megakaryocyte-erythroid progenitor (MEP) pool of myeloid progenitors (18,20). Interestingly, induction of LIN28B caused skewing of the myeloid progenitor compartment in the marrow of iLIN28B mice, with significantly higher percentages of MEPs than in controls (Fig 3). This increase in MEPs was accompanied by decreases in the proportions of myeloid progenitors (MPs) that were granulocyte-macrophage precursors (GMPs, Fig 3), although the percentage of live cells that were GMPs was less affected. There were no statistically significant differences in the proportions of cells in the common myeloid progenitor (CMP) pool, suggesting that Lin28 may act at the level of CMP fate choice. In keeping with these findings, mice induced to express let-7 for two weeks were found to have fewer numbers of bone marrow MCPs compared to controls (Fig S1, 0.01±0.003% vs. 0.048±0.007%, p=0.007). Because the lifespan of fully differentiated MCs exceeds the 2 week induction period used (50,51), we did not expect to see differences in the frequencies of peritoneal MCs. However, a trend towards reduced peritoneal MC numbers was observed (1.88±0.3% vs. 2.39±0.02% of live cells; p=0.16) (Fig S1). Taken together, these data indicate that Lin28b expression acts to promote MC lineage commitment at the CMP→MEP transition.
Figure 3. LIN28B induction favors the differentiation of CMPs into MEPs rather than GMPs.
Bone marrow from induced iLIN28B mice or control animals was harvested and mononuclear cells were stained for lineage markers, c-Kit, Sca-1, FcγRII/III, and CD34. Within the lin−c-Kit+Sca-1− myeloid progenitor (MP) pool, common myeloid progenitors (CMPs) are FcγRII/III−CD34+ (green box) and differentiate into FcγRII/III+CD34+ granulocyte-monocyte precursors (GMPs, blue box) and FcγRII/III−CD34− megakaryocyte-erythrocyte precursors (MEPs, red box). Overexpression of LIN28B resulted in increased percentages of MEPs and decreased percentages of GMPs (expressed as a percentage of myeloid progenitors on the left and as a percentage of all live bone marrow mononuclear cells on the right). Total numbers of bone marrow cells were not significantly different.
The mast cell phenotype in iLIN28B mice is intrinsic to hematopoietic cells
To determine whether the effects of LIN28B expression on MC development in iLIN28B mice were driven by LIN28B expression in MC precursors themselves, we sorted Lin−Sca-1+c-kit+(LSK) progenitor cells from induced iLIN28B bone marrow and cultured them in methylcellulose media containing doxycycline and cytokines that support unbiased myeloid differentiation (m3434, Stem Cell Technologies, Vancouver BC). After 10 days, LSK cells from iLIN28B mice that differentiated in culture generated predominantly c-Kit+FcεRIloMC progenitors (Fig S2), whereas control LSKs produced a variety of different myeloid cell types. This result indicates that the MC-promoting effects ofLIN28B induction in vivo are maintained ex vivo in differentiating hematopoietic progenitors and suggests that LIN28B induction may delay or impair MC terminal differentiation in a cell autonomous manner. However, because the cells assayed in these cultures were harvested from mice in which LIN28B had already been induced in vivo, it remained possible that durable cell-extrinsic effects of prior induction were carried forward into the in vitro setting. Thus, we performed a further, definitive in vivo experiment by analyzing iLIN28B hematopoietic chimeras generated by transplantation of bone marrow from uninduced LIN28B-transgenic mice into lethally-irradiated CD45.1 congenic recipients. After full hematopoietic reconstitution (16–24 weeks), recipient mice were induced to express LIN28B for 2 weeks and analyzed for MC phenotypes. We again found increased numbers of MCPs in the spleen (0.05±0.005% of live splenocytes in iLIN28B chimeras vs. 0.03±0.005% in controls, n= 10 in each group) and bone marrow of iLIN28B chimeric mice (0.15±0.008% of live marrow mononuclear cells in iLIN28B chimeras vs. 0.07±0.007% in controls, n=10 in each group; Fig 4, panels B and C). These results mirror those obtained in induced, transgenici LIN28B mice (Fig 2, panels D and E).
Figure 4. The effects of LIN28B overexpression on mast cell development are cell-intrinsic.
(A) LT-HSCs (Lin−Sca-1+c-Kit+CD150+CD48−) were sorted from uninduced iLIN28B (circles) and control mice (squares) and transplanted into lethally-irradiated congenic (CD45.1) recipients. After long-term hematopoietic reconstitution, recipients were induced for 2 weeks with 1g/L doxycycline drinking water and then sacrificed. (B,C) Restriction of LIN28B overexpression to the hematopoietic compartment results in an accumulation of bone marrow and splenic mast cell progenitors very similar to that seen in induced iLIN28B mice. As in Figure 2, splenic mast cell precursors are lin−c-Kit+FcγRII/III+intβ7+. BMMCPs are lin−Sca-1−c-Kit+CD150−Flk2−intβ7+CD27+. Because peritoneal mast cells are long-lived, the majority of MC recovered from the peritoneum were of recipient origin (i.e. CD45.1+). Thus, the transplant experiment was repeated (D) and distilled water was intraperitoneally injected at the time of induction to lyse resident recipient mast cells. After 2 weeks of induction, iLIN28B overexpression resulted in an increase in peritoneal mast percentages.
MCs are long-lived (50,51), and recipient peritoneal MCs persisted in situ in hematopoietic chimeras, preventing donor reconstitution in the peritoneum. Therefore, another transplantation experiment was performed using recipients that were injected intraperitoneally with distilled water at the time of LIN28B induction to lyse resident recipient MCs (50). Increased numbers of donor MCs were found in the peritoneal lavage of recipients receiving iLIN28B bone marrow as compared to control marrow (4.94±0.66% of live peritoneal mononuclear cells in iLIN28B chimeras vs. 2.49±0.48% in controls, n=5 for each group, Fig 4D). These findings, taken together, indicate that the effects of LIN28B in promoting MC fate choice are intrinsic to the hematopoietic compartment.
Expression of LIN28B impedes terminal mast cell differentiation
To investigate further the finding that LIN28B-induced LSK cells are impaired in their ability to differentiate fully into MCs in methylcellulose culture, we studied the kinetics of c-kit and FcεRIαreceptor expression, as well as transcriptional and ultrastructural features of developing MCs in iLIN28B and control BMMC cultures. These experiments corroborated our methylcellulose culture studies and demonstrated that, in vitro, LIN28B-expressing BMMCs matured with delayed kinetics relative to control BMMCs (Fig 5A). Concordantly, acquisition of toluidine blue staining in cultured cells (Fig 5B) was delayed. Furthermore, electron microscopy demonstrated a decrease in the number of mature, electron-dense granules in LIN28B-expressing BMMCs (Fig 5C and D, 7.8±1.06 v. 16.83±1.62 per cell at d32 of culture, n=25 cells, representative of BMMCs from 3 different mice per condition), reminiscent of features reported in fetal liver-derived MCs and in human MC disease (52,53).Thus, these data suggest that induced LIN28B expression prevents MCs from fully maturing.
Figure 5. iLIN28B induction results in delayed maturation of mast cells in vitro.
(A) Induction of LIN28B slows BMMC differentiation and results in an accumulation of immature c-Kit+FcεRIα− cells. Cultures were initiated from uninduced control or iLIN28B mice, as described, with or without subsequent induction, and samples were taken at defined time points for flow cytometric analysis. Plots represent a time course of maturation; each condition was performed in biological triplicate. These data are summarized in graphical format, showing a clear shift to the right of the immunophenotypic maturation curve of induced iLIN28B BMMC (p=0.005 by one-way ANOVA). (B) LIN28B overexpression slows acquisition of toluidine blue-positive mast cell granules. Cells were taken from BMMC cultures at the indicated time points and cytospun, then stained with Wright-Giemsa preparation (left) or toluidine blue (right). Although iLIN28B cultures did acquire toluidine blue positivity (far right), it occurred with kinetics similar to those seen in (A). Images were obtained on an Olympus BX60 light microscope equipped with an Olympus DP70 camera using Olympus DP Controller software; original magnification x600 for all images. (C) Electron microscopy demonstrates that iLIN28B BMMCs have fewer dense granules than controls. BMMCs at the specified time points were visualized by transmission EM and their electron-dense granules enumerated by visual inspection. Differences in total granule number did not reach statistical significance whereas differences in dense granule number were highly statistically significant (D), whether expressed as number of dense granules per cell or percentage of granules that were dense per cell (not shown). Images were acquired on a Philips/FEI Tecnai 12 Biotwin/Spirit TEM equipped with an AMT XR60 camera; original magnification ×2000 for all images.
iLIN28B mast cells are hypofunctional
Delayed kinetics of LIN28B-expressing mast cell development were not apparent in vivo, although increased numbers of mast cell progenitors are consistent with slowed maturation. In vivo development is likely too asynchronous to permit kinetic analyses, particularly as in vitro iLIN28B BMMCs ultimately attained the same immunophenotypic endpoint as controls (Fig 5A). To evaluate mast cell differentiation in vivo, we hypothesized that differences in developmental kinetics might have functional manifestations. To test this, we examined MC histamine release in end organs by performing passive cutaneous anaphylaxis (PCA) assays. Recipient tissue MCs persist through bone marrow transplant, precluding performance of these assays in transplant recipients. Therefore, we analyzed MC function in iLIN28B transgenic mice. The histamine response to a mast cell-specific IgE-mediated stimulus was attenuated in iLIN28B mice relative to controls (Fig 6A). Histamine treatment per se caused equivalent increases in vascular permeability in control and iLIN28B mice (Fig S3), indicating that the diminished extravasation seen in response to MC-specific stimuli was not attributable to an inability of iLIN28B vasculature to respond to histamine. Thus, LIN28B-expressing MCs exhibit an impaired response to IgE signaling in vivo, consistent with a failure to mature fully. To test this further, we interrogated MC cytokine responses to IgE-mediated receptor stimulation in BMMC cultures from control and iLIN28B mice that were induced to express LIN28B in vitro at the initiation of the cultures, but not before. iLIN28B BMMCs produced less TNFα and IL-6 in response to FcεRIα stimulation than controls (Fig 6B), confirming that iLIN28B MCs are intrinsically hypofunctional.
Figure 6. Mast cells from iLIN28B mice are hypofunctional.
(A) iLIN28B mice have impaired IgE-mediated histamine responses. Control and iLIN28B mice were induced for two weeks with doxycycline. Their pinnae were then injected subcutaneously with PBS or anti-DNP IgE. 24 hours later, mice were intravenously injected with DNP-HSA in a solution of Evans’ blue dye; local histamine release resulted in increased vascular permeability and extravasation of the dye into the ear pinna. iLIN28B mice were observed to have decreased extravasation (right panel, bottom) as compared to control mice (right panel, top) indicating that they released less histamine in response to IgE signaling. Pinnae were subsequently removed and macerated to release the dye, and absorbance was quantitated (left panel). (B) iLIN28B mast cells have impaired cytokine production in response to IgE stimulation. Bone marrow mast cell cultures from iLIN28B and control mice were challenged with anti-DNP IgE and DNP-HSA at varying concentrations. Intracellular TNFα expression was measured by flow cytomery (left panels) and IL-6 secretion was evaluated by serum ELISA (right panel).
LIN28B downregulates C/ebpa expression
To investigate the transcriptional program underlying the production of immature MCs in LIN28B-expressing mast cell precursors, we performed RT-PCR analysis of RNA isolated from BMMCs of induced or uninduced iLIN28B mice to evaluate genes important in MC development and function. We confirmed that iLIN28B BMMCs expressed high levels of LIN28B and low levels of let-7 species (Fig 7A), consistent with increased LIN28B activity. Transcription of the let-7 target Hmga2 was not significantly elevated (Fig 7B), which may explain why induced iLIN28B animals do not develop mast cell malignancies (see below). The let-7 target Igfbp2 was not expressed in BMMCs, with or without LIN28B induction (not shown). However, iLIN28B BMMCs expressed less Fcer1a as determined by flow cytometry (Fig 7B). Additionally, although expression of MC genes such as Kit and Gata-1 was not substantially altered, LIN28B-expressing BMMC cultures had significantly decreased expression of C/ebpa (0.23±0.07 relative to controls; Fig 7B). C/ebpα is a key regulator of granulocyte differentiation (54,55), and especially of basophil differentiation (25). Previous studies have demonstrated that fate specification at the level of the basophil-MCP is dictated by the balance between C/ebpα and MITF, with C/ebpαspecifying basophil fate(25). Thus, downregulation of C/ebpαin iLIN28B BMMCs would be expected to favor MC fate choice. Expression of Mitf was also decreased (0.63±0.07 fold relative to controls; Fig 7B), although to a lesser extent than the decrease in C/ebpa. Thus, expression of LIN28B results in downregulation of genes critical for terminal differentiation of basophils and mast cells, with preferential downregulation of the basophil-specification branch of that program. These molecular alterations likely underlie the accumulation of immature mast cells seen in iLIN28B mice.
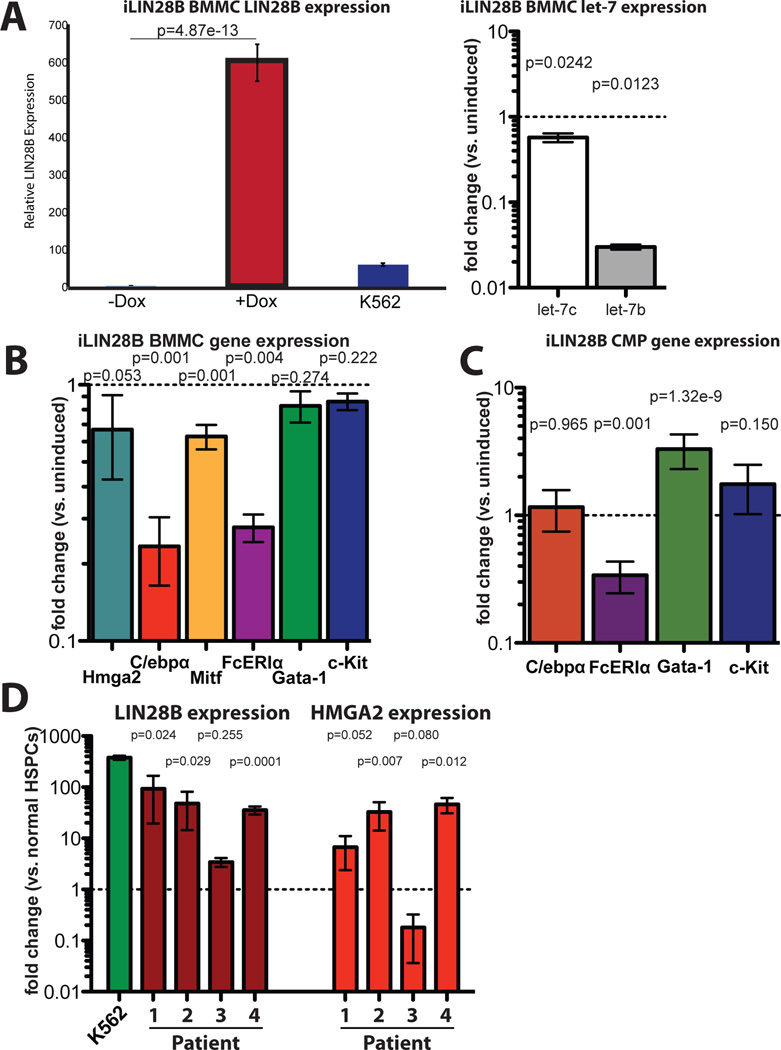
Figure 7. Induction of LIN28B favors mast cell fate choice but enforces an immature mast cell phenotype, and LIN28 is upregulated in SM and MCL.
(A) (Left) RNA was extracted from uninduced (left column) or induced (center column) iLIN28B BMMCs and qPCR was performed on LIN28B. As expected, induction of iLIN28B BMMCs resulted in significant expression of LIN28B. Values are expressed as fold-change over control BMMC expression. K562 cells were used as a control (right column); fold-change is displayed on a linear y-axis. (Right) qPCR was performed for let-7c (left) and let-7b (right), and markedly decreased expression of these species was observed. Values are expressed as fold change over expression in control cultures and are displayed on a logarithmic y-axis. (B) iLIN28B BMMCs downregulate C/ebpa and Mitf expression, indicating that Lin28 plays a role in basophil-mast cell fate choice. However, C/ebpa expression is significantly more downregulated than Mitf, such that the balance of these transcription factors skews towards the mast cell fate. Expression of mast cell genes Gata-1 and Kit is not significantly different (p=0.274 and 0.222 respectively).Expression of let-7 target Hmga2 is not significantly upregulated, indicating that let-7 downregulation is insufficient for Hmga2 upregulation. Values are normalized to control BMMC expression levels. Results are compiled from 2 separate experiments, each of which comprised 3–4 technical replicates of 3 biological replicates for each condition (n=6 mice total for each condition). Data are expressed as mean of means ±SEM, and displayed on a logarithmic y-axis. (C) As in BMMCs, CMPs from induced iLIN28B mice downregulate Fcer1a expression. Additionally, they upregulate Gata-1 expression, suggesting a mechanism whereby CMPs favor an MEP over GMP fate choice. RNA was isolated from flow sorted CMPs (lin−c-Kit+Sca-1−FcγRII/III−CD34+) from induced iLIN28B mice. Each column represents the aggregate of 4 technical replicates each from 3 biological replicates, and data are represented in terms of fold change over CMPs from control mice (±SD). Values are displayed on a logarithmic y-axis.(D) Abnormal mast cells from patients with ASM express high levels of LIN28B and HMGA2. Abnormal (+) and control (−) cells were sorted from bone marrow aspirates from patients with ASM followed at the Dana-Farber Cancer Institute according to clinically-reported cell surface markers (Patient 1 and 4: abnormal: c-KithiCD25+; NL: c-Kit+CD25−. Pt 2: abnormal: c-KithiCD25+CD2+; NL:c-Kit+CD25−. Pt 3: abnormal: c-Kit+; NL: c-Kit−). Pt 3 had mast cell leukemia that had transformed from prior cutaneous mastocytosis and was refractory to therapy, and had higher expression of both LIN28B and HMGA2 in control cells relative to other patients. K562 cells were used as a positive control for LIN28B. Values are expressed as fold change versus each patient’s HSPCs, and displayed on a logarithmic y-axis. P values obtained by t-test.
We also performed transcriptional analysis on myeloid progenitors from iLIN28B mice. Adult myeloid progenitors represent an earlier stage of development than the cultured BMMCs, and do not express Lin28b at appreciable levels (not shown, but concordant with published datasets at https://gexc.stanford.edu/search and http://www.immgen.org/databrowser/index.html). Enforced expression of LIN28B in CMPs resulted intranscriptional changes distinct from those seen in BMMCs. Although Fcer1a expression was low in iLIN28B CMPs just as in iLIN28B BMMCs, C/ebpa levels in CMPs were comparable to controls. Furthermore, Gata-1 expression was higher in iLIN28B CMPs than in controls (Fig 7C). Gata-1, a master regulator of erythropoiesis, is also critical for differentiation of the megakaryocyte, eosinophil, and mast cell lineages (24), and ectopic expression of Gata-1 converts lymphoid and myelomonocytic precursors into megakaryocyte/erythroid precursors (56).These findings indicate that induced LIN28B in mast cell progenitors augments mast cell production through distinct mechanisms acting at different stages of development:first, it promotes CMP differentiation to form MEP over GMP by upregulating Gata-1, and second, it slows terminal differentiation while favoring MC fate choice at the basophil-MCP level by downregulating C/ebpa more than Mitf.
Human systemic mastocytosis overexpresses LIN28
Lin28 has been implicated in tumorigenesis (28,29,31–33), and the accumulation of immature MCs in animals induced to express LIN28B suggested a potential role for this protein in MC disease. We therefore analyzed dysplastic MCs from patients with SM/MCL treated at the DFCI. SM is rare, and patients often have a low disease burden in comparison to other hematologic malignancies (10,15). Additionally, dysplastic MCs are difficult to dislodge from the bone marrow, such that aspirates frequently contain very few (<10%) abnormal MCs (57). Between 2010 and 2014, 19 patients with SM/MCL were enrolled in the DFCI hematologic malignancy registry. We were able to obtain primary samples from four of these patients, two of whom were treated with midostaurin, a tyrosine kinase inhibitor with multiple targets including c-Kit (Table I)(58,59). Bone marrow mononuclear cells from these patients were stained with antibodies for abnormal mast cell markers previously identified by clinical flow cytometry, including c-Kit, CD45, CD25, and CD2. Cells were sorted and analyzed by RT-PCR for LIN28B expression. Interestingly, and in contrast to the iLIN28B animal model, LIN28B and HMGA2 were both found to be highly expressed (Fig. 7D) in abnormal human MCs as compared to their corresponding nondysplastic bone marrow cells, regardless of clinical subtype or c-Kit mutational status. These results implicate LIN28 as an important cofactor in the pathogenesis of human SM/MCL.
Table I.
Patients With Mast Cell Disorders
| Patient | Age | Gender/race | Diagnosis | c-kit | Treatment | Outcome |
|---|---|---|---|---|---|---|
| 1 | 73 | M/Af-American | ASM | Wildtype | Midostaurin (PKC412) |
Major Incomplete Response ; alive |
| 2 | 86 | F/Caucasian | ISM | D816V | Supp care H1/H2 antihistamines Prednisone |
Alive |
| 3 | 77 | F/Caucasian | MCL | D816V | Midostaurin (PKC412) |
Major Incomplete Response ; dead |
| 4 | 61 | F/Caucasian | ASM | D816V | Cladribine | PR; alive |
- Major Response: complete resolution of at least one C-Finding and no progression of other C-Findings. C-Findings (Clinical findings) are cytopenias, osteolysis with pathologic fractures, hepatosplenomegaly and/or impaired liver function and/or ascites, and malabsorption.
- Complete response – complete disappearance of mast cell infiltrates in affected organs, decrease of tryptase levels to below 20 ng/mL, and disappearance of SM-related organomegaly
- Incomplete response- less than 50% decrease of mast cell infiltrates in affected organs, and/or 50% decrease of tryptase levels, and /or 50% visible regression of SM-related organomegaly
- Pure Clinical Response – without decrease in mast call infiltrates, without decrease in tryptase levels, and without regression of organomelgaly.
- Partial Response: Incomplete regression of one or more C-Finding without complete regression and without progression in other C-Findings
- Good partial response – more than 50% regression
- Minor response – equal to or less than 50 % regression
- No Response: C –Findings persistent or progressive
- Stable disease – C-Findings parameters show constant range
- Progressive Disease – one or more C-Findings show progression
Discussion
Growing evidence highlights the central role of mast cells in innate immunity. MCs mediate inflammatory responses in multiple contexts and also coordinate the responses of other immune cells (1–3). In rare instances, MC development is subverted to cause mastocytosis or MCL, usually as a result of a somatically-acquired c-Kit mutation(14,58).However, a subset of pediatric and aggressive MC dyscrasias do not have c-Kit mutations, and MCL often loses c-Kit mutations(15,60). Here, we ascribe an important function to the RNA binding protein Lin28 in MC development and associate upregulation of this protein with aggressive mast cell malignancy.
Lin28 has been extensively studied as a pluripotency and proliferation factor that impairs cellular differentiation(28,30). We demonstrate here that enforced expression of LIN28B causes accumulation of MCPs in bone marrow as well as increased MC numbers in end organs. However, the MCs found in iLIN28 tissues are immature in both immunophenotype (FcεRIαlo, c-Kithi) and function. Our in vitro studies confirm that Lin28 is normally downregulated during MC development, and reveal that LIN28B induction impedes MC development and results in diminished responses to prototypical MC stimuli. iLIN28B mice also exhibit alterations in cell fate decisions at the CMP and basophil-MCP stage, associated with deregulated expression of transcription factors involved in MC specification and differentiation. It is likely that the accumulation of MCPs in vivo in iLIN28B mice reflects both a slowing interminal differentiation of these cells and a skewing of cell fate decisions by progenitor cells in the myeloid lineage. It is also possible that LIN28B expression affects mast cell progenitor compartmentalization or recruitment and/or mature mast cell proliferation, and that these mechanisms augment the developmental effects of LIN28B to cause accumulation of MCPs in vivo.
Lin28 activity impacts a number of biological processes. The best-characterized of these is its inhibition of the biogenesis of the microRNA let-7, although let-7 independent functions of Lin28 have been described(38,39). In MC development, enforced expression of let-7 yields an opposite phenotype to that of Lin28 expression, suggesting that the effects of LIN28B in this system may be mediated in part through let-7 (Fig. S2). However, although expression of LIN28B results in marked suppression of let-7 (Fig 7A), canonical downstream targets such as Hmga2 are not uniformly upregulated. This finding suggests a context-dependency to Lin28b expression, as well as let-7 independent mechanisms of Lin28B action.
Our investigation into the role of LIN28B in MC development agrees with previous work implicating Lin28 as a factor that favors more primitive cellular phenotypes. The phenotype of MCs in adult mice induced to express LIN28B resembles the published phenotype of fetal liver-derived MCs (52). In our analyses, hematopoiesis in LIN28B-induced fetal liver showed the same bias toward MEPs at the expense of GMPs (data not shown), indicating that upregulation of LIN28B impacts myelopoiesis even when Lin28b expression is already high.
Previous studies have reported differences in the ability of ectopic Lin28 to induce hematologic malignancy(43,44,46). In our system, we did not observe malignancy in transplant recipients of mouse iLIN28B bone marrow, even after months ofLIN28B induction. Our finding that Hmga2 expression was not increased in mouse BMMCs induced to express LIN28B may explain these discrepancies, suggesting that mechanisms downstream of let-7 can abrogate the oncogenic effects of Lin28b on Hmga2 in some settings. Nonetheless, LIN28B upregulation has been described in human hematologic malignancies such as blast crisis CML(29), and we describe here the marked upregulation of both LIN28B and HMGA2 in abnormal MCs from patients with SM and MCL. It is interesting to note that this upregulation occurred regardless of clinical subtype or of c-kit mutation status, suggesting that LIN28B may complement c-kit in the pathogenesis of mast cell disease. The induction of LIN28B expression in SM is intriguing and warrants further investigation, ideally on untreated primary patient samples, to determine the role of LIN28 in mast cell disorders and its interaction with the c-Kit mutation. Thus, our findings implicating Lin28b as a novel regulator of mast cell fate and function highlight the need for more extensive study of this protein in human MC disease.
Supplementary Material
Acknowledgements
The authors are grateful to Cem Akin, Alberto Orfao, Ann Dvorak, David Weinstock, Susan Buchanan, Girijesh Buruzula, Joyce LaVecchio, Atsuya Wakabayashi, Jacobo Ramirez, Elsa Lindhe, Louise Trakim as and Marie Ericsson of the HMS EM core facility, and the DF/HCCS pecialized Histopathology facility for their assistance. This work was supported by a Damon Runyon-Sohn Foundation Cancer Research Fellowship (DSRG 02-2) and a St. Baldrick’s Foundation-PALS Scholar Award (243625)(L.D.W.), a Helen Hay Whitney Cancer Fellowship (S.D.), a Burroughs-Wellcome Fund Career Award (H.Z.), and grants from the NIH(R01-HL088582 and P30DK036836)(A.J.W.),(R01-GM107536)(G.Q.D.), (K08-CA157727)(H.Z.), and the Ellison Medical Foundation (G.Q.D.).
Footnotes
Conflict-of-interest
The authors declare no competing financial interests.
Authorship
L.D.W., T.N.R., D.P., R.G.R., G.Q.D., and A.J.W. designed experiments and interpreted data. L.D.W., T.N.R., P.T.N., D.P., J.S., and R.G.R. performed experiments. S.D., H.Z., and G.Q.D. developed and characterized transgenic mice. R.C.L. and D.D. provided samples through DFCI Protocol 01-206. L.D.W. wrote the manuscript; L.D.W.,R.G.R., T.N.R., D.D, D.S.P., G.Q.D., and A.J.W edited it.
Supplementary information is available at Leukemia’s website.
References
- 1.Caughey GH. Mast cell tryptases and chymases in inflammation and host defense. Immunol Rev. 2007 Jun;217:141–154. doi: 10.1111/j.1600-065X.2007.00509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galli SJ, Tsai M. Mast cells in allergy and infection: Versatile effector and regulatory cells in innate and adaptive immunity. Eur J Immunol. 2010 Jun 25;40(7):1843–1851. doi: 10.1002/eji.201040559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Filippo K, Dudeck A, Hasenberg M, Nye E, van Rooijen N, Hartmann K, et al. Mast cell and macrophage chemokines CXCL1/CXCL2 control the early stage of neutrophil recruitment during tissue inflammation. Blood. 2013 Jun 13;121(24):4930–4937. doi: 10.1182/blood-2013-02-486217. [DOI] [PubMed] [Google Scholar]
- 4.Sismanopoulos N, Delivanis DA, Mavrommati D, Hatziagelaki E, Conti P, Theoharides TC. Do mast cells link obesity and asthma? Allergy. 2012 Oct 16;68(1):8–15. doi: 10.1111/all.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jung M, Lord MS, Cheng B, Lyons JG, Alkhouri H, Hughes JM, et al. Mast Cells Produce Novel Shorter Forms of Perlecan That Contain Functional Endorepellin: A ROLE IN ANGIOGENESIS AND WOUND HEALING. Journal of Biological Chemistry. 2013 Feb 1;288(5):3289–3304. doi: 10.1074/jbc.M112.387811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Younan GJ, Heit YI, Dastouri P, Kekhia H, Xing W, Gurish MF, et al. Mast Cells Are Required in the Proliferation and Remodeling Phases of Micro deformational Wound Therapy. Plastic and Reconstructive Surgery. 2011 Dec;128(6):649e–658e. doi: 10.1097/PRS.0b013e318230c55d. [DOI] [PubMed] [Google Scholar]
- 7.Glimelius I, Edström A, Fischer M, Nilsson G, Sundström C, Molin D, et al. Angiogenesis and mast cells in Hodgkin lymphoma. Leukemia. 2005 Oct 13;19(12):2360–2362. doi: 10.1038/sj.leu.2403992. [DOI] [PubMed] [Google Scholar]
- 8.Mizuno H, Nakayama T, Miyata Y, Saito S, Nishiwaki S, Nakao N, et al. Mast cells promote the growth of Hodgkin’s lymphoma cell tumor by modifying the tumor microenvironment that can be perturbed by bortezomib. Leukemia. 2012 Oct;26(10):2269–2276. doi: 10.1038/leu.2012.81. [DOI] [PubMed] [Google Scholar]
- 9.Ribatti D, Molica S, Vacca A, Nico B, Crivellato E, Roccaro AM, et al. Tryptase-positive mast cells correlate positively with bone marrow angiogenesis in B-cell chronic lymphocytic leukemia. Leukemia. 2003 Jul;17(7):1428–1430. doi: 10.1038/sj.leu.2402970. [DOI] [PubMed] [Google Scholar]
- 10.Gotlib J, Pardanani A, Akin C, Reiter A, George T, Hermine O, et al. International Working Group-Myeloproliferative Neoplasms Research and Treatment (IWG-MRT) & European Competence Network on Mastocytosis (ECNM) consensus response criteria in advanced systemic mastocytosis. Blood. 2013 Mar 28;121(13):2393–2401. doi: 10.1182/blood-2012-09-458521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pardanani A. Systemic mastocytosis in adults: 2013 update on diagnosis, risk stratification, and management. Am J Hematol. 2013 Jul;88(7):612–624. doi: 10.1002/ajh.23459. [DOI] [PubMed] [Google Scholar]
- 12.Pardanani A, Tefferi A. Systemic mastocytosis in adults: a review on prognosis and treatment based on 342 Mayo Clinic patients and current literature. Current Opinion in Hematology. 2010 Mar;17(2):125–132. doi: 10.1097/MOH.0b013e3283366c59. [DOI] [PubMed] [Google Scholar]
- 13.Nagata H, Worobec AS, Semere T, Metcalfe DD. Elevated expression of the proto-oncogene c-kit in patients with mastocytosis. Leukemia. 1998 Feb;12(2):175–181. doi: 10.1038/sj.leu.2400906. [DOI] [PubMed] [Google Scholar]
- 14.Valent P, Sperr WR, Akin C. How I treat patients with advanced systemic mastocytosis. Blood. 2010 Dec 23;116(26):5812–5817. doi: 10.1182/blood-2010-08-292144. [DOI] [PubMed] [Google Scholar]
- 15.Georgin-Lavialle S, Lhermitte L, Dubreuil P, Chandesris MO, Hermine O, Damaj G. Mast cell leukemia. Blood. 2013 Feb 21;121(8):1285–1295. doi: 10.1182/blood-2012-07-442400. [DOI] [PubMed] [Google Scholar]
- 16.Lasho T, Tefferi A, Pardanani A. Inhibition of JAK-STAT signaling by TG101348: a novel mechanism for inhibition of KITD816V-dependent growth in mast cell leukemia cells. Leukemia. 2010 Jul;24(7):1378–1380. doi: 10.1038/leu.2010.109. [DOI] [PubMed] [Google Scholar]
- 17.Bubnoff von N, Gorantla SHP, Kancha RK, Lordick F, Peschel C, Duyster J. The systemic mastocytosis-specific activating cKit mutation D816V can be inhibited by the tyrosine kinase inhibitor AMN107. Leukemia. 2005 Jul 14;19(9):1670–1671. doi: 10.1038/sj.leu.2403887. [DOI] [PubMed] [Google Scholar]
- 18.Chen C-C, Grimbaldeston MA, Tsai M, Weissman IL, Galli SJ. Identification of mast cell progenitors in adult mice. Proc Natl Acad Sci USA. 2005 Aug 9;102(32):11408–11413. doi: 10.1073/pnas.0504197102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arinobu Y, Iwasaki H, Gurish MF, Mizuno S-I, Shigematsu H, Ozawa H, et al. Developmental checkpoints of the basophil/mast cell lineages in adult murine hematopoiesis. Proc Natl Acad Sci USA. 2005 Dec 13;102(50):18105–18110. doi: 10.1073/pnas.0509148102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franco CB, Chen C-C, Drukker M, Weissman IL, Galli SJ. Distinguishing Mast Cell and Granulocyte Differentiation at the Single-Cell Level. Cell Stem Cell. 2010 Apr;6(4):361–368. doi: 10.1016/j.stem.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salmon JM, Slater NJ, Hall MA, McCormack MP, Nutt SL, Jane SM, et al. Aberrant mast-cell differentiation in mice lacking the stem-cell leukemia gene. Blood. 2007 Nov 15;110(10):3573–3581. doi: 10.1182/blood-2006-10-053124. [DOI] [PubMed] [Google Scholar]
- 22.Motakis E, Guhl S, Ishizu Y, Itoh M, Kawaji H, de Hoon M, et al. Redefinition of the human mast cell transcriptome by deep-CAGE sequencing. Blood. 2014 Apr 24;123(17):e58–e67. doi: 10.1182/blood-2013-02-483792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma P, Mali RS, Munugalavadla V, Krishnan S, Ramdas B, Sims E, et al. The PI3K pathway drives the maturation of mast cells via microphthalmia transcription factor. Blood. 2011 Sep 29;118(13):3459–3469. doi: 10.1182/blood-2011-04-351809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Migliaccio AR, Rana RA, Sanchez M, Lorenzini R, Centurione L, Bianchi L, et al. GATA-1 as a Regulator of Mast Cell Differentiation Revealed by the Phenotype of the GATA-1low Mouse Mutant. Journal of Experimental Medicine. 2003 Feb 3;197(3):281–296. doi: 10.1084/jem.20021149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qi X, Hong J, Chaves L, Zhuang Y, Chen Y, Wang D, et al. Immunity. 1. Vol. 39. Elsevier Inc; 2013. Jul 25, Antagonistic Regulation by the Transcription Factors C/EBPa and MITF Specifies Basophil and Mast Cell Fates; pp. 97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walsh JC, DeKoter RP, Lee HJ, Smith ED, Lancki DW, Gurish MF, et al. Cooperative and antagonistic interplay between PU. 1 and GATA-2 in the specification of myeloid cell fates. Immunity. 2002 Nov;17(5):665–676. doi: 10.1016/s1074-7613(02)00452-1. [DOI] [PubMed] [Google Scholar]
- 27.Shyh-Chang N, Daley GQ. Lin28: Primal Regulator of Growth and Metabolism in Stem Cells. Cell Stem Cell. 2013 Apr;12(4):395–406. doi: 10.1016/j.stem.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.West JA, Viswanathan SR, Yabuuchi A, Cunniff K, Takeuchi A, Park I-H, et al. A role for Lin28 in primordial germ-cell development and germ-cell malignancy. Nature. 2009 Jul 5; doi: 10.1038/nature08210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Viswanathan SR, Powers JT, Einhorn W, Hoshida Y, Ng TL, Toffanin S, et al. Lin28 promotes transformation and is associated with advanced human malignancies. Nat Genet. 2009 May 31;41(7):843–848. doi: 10.1038/ng.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, et al. Induced Pluripotent Stem Cell Lines Derived from Human Somatic Cells. science. 2007 Dec 21;318(5858):1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 31.Iliopoulos D, Hirsch HA, Struhl K. An Epigenetic Switch Involving NF-κB, Lin28, Let-7 MicroRNA, and IL6 Links Inflammation to Cell Transformation. Cell. 2009 Nov;139(4):693–706. doi: 10.1016/j.cell.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Molenaar JJ, Domingo-Fernández R, Ebus ME, Lindner S, Koster J, Drabek K, et al. LIN28B induces neuroblastoma and enhances MYCN levels via let-7 suppression. Nat Genet. 2012 Oct 7;44(11):1199–1206. doi: 10.1038/ng.2436. [DOI] [PubMed] [Google Scholar]
- 33.King CE, Cuatrecasas M, Castells A, Sepulveda AR, Lee JS, Rustgi AK. LIN28B Promotes Colon Cancer Progression and Metastasis. Cancer Research. 2011 Jun 14;71(12):4260–4268. doi: 10.1158/0008-5472.CAN-10-4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Urbach A, Yermalovich A, Zhang J, Spina CS, Zhu H, Perez-Atayde AR, et al. Lin28 sustains early renal progenitors and induces Wilms tumor. Genes & Development. 2014 Apr 14; doi: 10.1101/gad.237149.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu H, Shah S, Shyh-Chang N, Shinoda G, Einhorn WS, Viswanathan SR, et al. Lin28a transgenic mice manifest size and puberty phenotypes identified in human genetic association studies. Nat Genet. 2010 May 30;42(7):626–30. doi: 10.1038/ng.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu H, Shyh-Chang N, Segrè AV, Shinoda G, Shah SP, Einhorn WS, et al. Cell. 1. Vol. 147. Elsevier Inc; 2011. Sep 30, The Lin28/let-7 Axis Regulates Glucose Metabolism; pp. 81–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shyh-Chang N, Zhu H, de Soysa TY, Shinoda G, Seligson MT, Tsanov KM, et al. Cell. 4. Vol. 155. Elsevier Inc; 2013. Nov 7, Lin28 Enhances Tissue Repair by Reprogramming Cellular Metabolism; pp. 778–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilbert ML, Huelga SC, Kapeli K, Stark TJ, Liang TY, Chen SX, et al. LIN28 Binds Messenger RNAs at GGAGA Motifs and Regulates Splicing Factor Abundance. Molecular Cell. 2012 Oct;48(2):195–206. doi: 10.1016/j.molcel.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cho J, Chang H, Kwon SC, Kim B, Kim Y, Choe J, et al. LIN28A Is a Suppressor of ER-Associated Translation in Embryonic Stem Cells. Cell. 2012 Nov;151(4):765–777. doi: 10.1016/j.cell.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 40.Yuan J, Nguyen CK, Liu X, Kanellopoulou C, Muljo SA. Lin28b Reprograms Adult Bone Marrow Hematopoietic Progenitors to Mediate Fetal-Like Lymphopoiesis. science. 2012 Mar 8;335(6073):1195–1200. doi: 10.1126/science.1216557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee YT, de Vasconcellos JF, Yuan J, Byrnes C, Noh SJ, Meier ER, et al. LIN28B-mediated expression of fetal hemoglobin and production of fetal-like erythrocytes from adult human erythroblasts ex vivo. Blood. 2013 Jun 24; doi: 10.1182/blood-2012-12-472308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Copley MR, Babovic S, Benz C, Knapp DJHF, Beer PA, Kent DG, et al. The Lin28b-let-7-Hmga2 axis determines the higher self-renewal potential of fetal haematopoietic stem cells. Nature Cell Biology. 2013 Jun 30; doi: 10.1038/ncb2783. [DOI] [PubMed] [Google Scholar]
- 43.Chaudhuri AA, So AYL, Mehta A, Minisandram A, Sinha N, Jonsson VD, et al. Oncomir miR-125b regulates hematopoiesis by targeting the gene Lin28A. Proceedings of the National Academy of Sciences. 2012 Feb 24; doi: 10.1073/pnas.1200677109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beachy SH, Onozawa M, Chung YJ, Slape C, Bilke S, Francis P, et al. Enforced expression of Lin28b leads to impaired T-cell development, release of inflammatory cytokines, and peripheral T-cell lymphoma. Blood. 2012 Aug 2;120(5):1048–1059. doi: 10.1182/blood-2012-01-401760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rao S, Lee SY, Gutierrez A, Perrigoue J, Thapa RJ, Tu Z, et al. Inactivation of ribosomal protein L22 promotes transformation by induction of the stemness factor, Lin28B. Blood. 2012 Nov 1;120(18):3764–3773. doi: 10.1182/blood-2012-03-415349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yuan J, Muljo SA. Exploring the RNA world in hematopoietic cells through the lens of RNA-binding proteins. Immunol Rev. 2013 May;253(1):290–303. doi: 10.1111/imr.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mican JA, Arora N, Burd PR, Metcalfe DD. Passive cutaneous anaphylaxis in mouse skin is associated with local accumulation of interleukin-6 mRNA and immunoreactive interleukin-6 protein. J Allergy Clin Immunol. 1992 Nov;90(5):815–824. doi: 10.1016/0091-6749(92)90107-d. [DOI] [PubMed] [Google Scholar]
- 48.Arock M, Le Nours A, Malbec O, Daëron M. Ex vivo and in vitro primary mast cells. Methods in molecular biology (Clifton, NJ) 2008;415:241–254. doi: 10.1007/978-1-59745-570-1_14. [DOI] [PubMed] [Google Scholar]
- 49.Hallgren J, Gurish MF. Mast cell progenitor trafficking and maturation. Adv Exp Med Biol. 2011;716:14–28. doi: 10.1007/978-1-4419-9533-9_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jamur MC, Moreno AN, Mello LF, Souza Júnior DA, Campos MRC, Pastor MVD, et al. Mast cell repopulation of the peritoneal cavity: contribution of mast cell progenitors versus bone marrow derived committed mast cell precursors. BMC Immunol. 2010;11:32. doi: 10.1186/1471-2172-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ekoff M, Nilsson G. linkspringercomezp-prod1hulharvardedu. Springer US: Boston, MA; 2011. Mast Cell Apoptosis and Survival; pp. 47–60. [Google Scholar]
- 52.Fukuishi N, Igawa Y, Kunimi T, Hamano H, Toyota M, Takahashi H, et al. Mezey E, editor. Generation of Mast Cells from Mouse Fetus: Analysis of Differentiation and Functionality, and Transcriptome Profiling Using Next Generation Sequencer. PLoS ONE. 2013 Apr 3;8(4):e60837. doi: 10.1371/journal.pone.0060837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dvorak AM. Ultrastructural Studies of Human Basophils and Mast Cells. Journal of Histochemistry and Cytochemistry. 2005 May 27;53(9):1043–1070. doi: 10.1369/jhc.5R6647.2005. [DOI] [PubMed] [Google Scholar]
- 54.Tenen DG. Abnormalities of the CEBP alpha transcription factor: a major target in acute myeloid leukemia. Leukemia. 2001 Apr;15(4):688–689. doi: 10.1038/sj.leu.2402088. [DOI] [PubMed] [Google Scholar]
- 55.Radomska HS, Huettner CS, Zhang P, Cheng T, Scadden DT, Tenen DG. CCAAT/enhancer binding protein alpha is a regulatory switch sufficient for induction of granulocytic development from bipotential myeloid progenitors. Molecular and Cellular Biology. 1998 Jul;18(7):4301–4314. doi: 10.1128/mcb.18.7.4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Iwasaki H, Mizuno S-I, Wells RA, Cantor AB, Watanabe S, Akashi K. GATA-1 converts lymphoid and myelomonocytic progenitors into the megakaryocyte/erythrocyte lineages. Immunity. 2003 Sep;19(3):451–462. doi: 10.1016/s1074-7613(03)00242-5. [DOI] [PubMed] [Google Scholar]
- 57.Sánchez-Muñoz L, Teodósio C, Morgado JM, Escribano L. Methods in Cell Biology. Elsevier Inc; 2011. Immunophenotypic Characterization of Bone Marrow Mast Cells in Mastocytosis and Other Mast Cell Disorders; p. 27. [DOI] [PubMed] [Google Scholar]
- 58.Verstovsek S. Advanced systemic mastocytosis: the impact of KIT mutations in diagnosis, treatment, and progression. Eur J Haematol. 2013 Jan 22;90(2):89–98. doi: 10.1111/ejh.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gotlib J, DeAngelo DJ, George TI, Corless CL, Linder A, Langford C, et al. KIT inhibitor midostaurin exhibits a high rate of clincally meaningful and durable responses in advanced systemic mastocytosis: report of a full accrued phase II trial. Blood. 2010 Jan 14;116(21):316. [Google Scholar]
- 60.Elliott MA, Pardanani A, Li CY, Tefferi A. Immunophenotypic normalization of aberrant mast cells accompanies histological remission in imatinib-treated patients with eosinophilia-associated mastocytosis. Leukemia. 2004 Mar 11;18(5):1027–1029. doi: 10.1038/sj.leu.2403329. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.