Abstract
Hippocampal-dependent cognitive functions rely on production of new neurons and maintenance of dendritic structures to provide the synaptic plasticity needed for learning and formation of new memories. Hippocampal formation is exquisitely sensitive to patho-physiological changes, and reduced antioxidant capacity and exposure to low dose irradiation can significantly impede hippocampal-dependent functions of learning and memory by reducing the production of new neurons and alter dendritic structures in the hippocampus. Although the mechanism leading to impaired cognitive functions is complex, persistent oxidative stress likely plays an important role in the SOD-deficient and radiation-exposed hippocampal environment. Aging is associated with increased production of pro-oxidants and accumulation of oxidative end products. Similar to the hippocampal defects observed in SOD-deficient mice and mice exposed to low dose irradiation, reduced capacity in learning and memory, diminishing hippocampal neurogenesis, and altered dendritic network are universal in the aging brains. Given the similarities in cellular and structural changes in the aged, SOD-deficient, and radiation-exposed hippocampal environment and the corresponding changes in cognitive decline, understanding the shared underlying mechanism will provide more flexible and efficient use of SOD deficiency or irradiation to model age-related changes in cognitive functions and identify potential therapeutic or intervention methods.
Hippocampal-dependent learning and memory
Hippocampus is critical for the acquisition (learning), consolidation and retrieval of declarative memories (reviewed in (1)). It is also important for the formation of spatial memory (2, 3). The hippocampus is located in the medial temporal lobe of the brain and is composed of two separate structures: the dentate gyrus (DG) and the Cornu Ammonis areas (CA1 and CA3). The entorhinal cortex serves as a connection between hippocampus and other parts of the cerebral cortex. Information from the cerebral cortex is relayed to the granule cells of hippocampal dentate gyrus by axonal projection from the entorhinal cortex. Axons form the dentate granule cell (called mossy fibers) then relay the information to the CA3 pyramidal cells whose axons project to the CA1 pyramidal cells, which then send the information back to the deeper layers of entorhinal cortex to complete the neural circuit in the hippocampal formation (4–6). Severe damage to the hippocampus often leads to failure in forming new memories (7, 8).
In mammals, adult neurogenesis – the process of production and maturation of new borne neurons beyond the early postnatal stage – occurs primarily in two areas of the central nervous system (9). These areas are subventricular zone (SVZ) of the lateral ventricles, which supplies new interneurons to the olfactory bulb; and the subgranular zone (SGZ) of dentate gyrus, which generates granule cells to the dentate gyrus of hippocampus. More recently, olfactory neuroepithelium has been shown to continue production of sensory neurons into old age in mice (10). Results from experimental animals show that the production of new neurons in these areas is important for synaptic plasticity (11, 12). However, the process of neurogenesis can be impeded by various physiological changes. Hippocampal neurogenesis in particular, is exquisitely sensitive to changes in its microenvironment, often leading to reduced production of new neurons.
In the hippocampal dentate SGZ, neural progenitor cells go through asymmetrical replication and generate neuroblasts, which then differentiate into immature neurons. In the next three to four weeks, newborn immature neurons put out axons and complex dendritic trees as they migrate further into the granule cell layer and finally establish synaptic integration into the existing neuronal network in hippocampal formation. It is estimated that roughly 10% immature neurons survive the maturation process and develop into fully functional mature neurons (13).
The SGZ of hippocampal dentate gyrus is an active site of neurogenesis throughout life in humans and other mammals (14, 15), and recent data show that the newly born neurons are functionally integrated into the hippocampal circuitry (16–20). In laboratory mice, the rate of adult hippocampal neurogenesis decreases exponentially from 1 month of age at the rate of 30–40% per month (21). At the same time, the rate of apoptotic cell death in the granule cell layer peaks at 1 month and gradually decreases with age. In balance, the number of granule cells in the hippocampus stays relatively constant (21), and the majority of granule cells will probably last for the entire lifespan of the animal. Similar observation has also been made in other species.
The potential importance of neurogenesis, as it relates to the hippocampal functions of learning and memory, is highlighted in normal animals where a positive correlation has been established between dentate neurogenesis and behavioral performance (18, 22–26). Environmental enrichment (e.g. increased environmental complexity, toys, running wheels, etc.) increases the numbers of new hippocampal neurons (27, 28), suggesting that the new cells may be important in learning and forming new memories. Recent studies show that these new cells become functionally integrated into the dentate gyrus and have passive membrane properties, action potentials and functional synaptic inputs similar to those found in mature dentate granule cells (29). Most importantly, the new neurons play a significant role in synaptic plasticity, which can be considered a cellular substrate for learning. A recent study provides strong evidence that these are not just correlative observations. Cell lineage tracking and expression of learning-related immediate early genes show that adult-generated granule cells in the SGZ are preferentially incorporated into the spatial memory networks in the dentate gyrus of the hippocampus (30).
In addition to production of new neurons in the hippocampus, maintenance of axons and dendrites – the structures that project from the cell body of neurons and form synapses with axons and dendrites from other neurons for transmission of electrochemical signals – is equally important for learning and memory. Consequently, reductions in hippocampal neurogenesis are not always consistent with defects in hippocampal functions of learning and memory (31–33), and loss of dendritic spines – the post synaptic element of excitatory synapses -- is an early event in mouse models of Alzheimer disease, and it correlates with synaptic loss and deficits in learning and memory (34, 35).
Redox potential and cell fate decision
Changes in intracellular redox potential and the extracellular microenvironment can impact cell fate decisions, including entering or exiting cell cycle, proliferation or differentiation, and survival or cell death. Redox couples, such as GSH/GSSG and NADPH/NADP+ are presence in high abundance in cells and the status of these redox couples can serve as important indicators for the reduction potential of intracellular environment, which then influence the activity of redox-sensitive proteins involved in cell fate decisions (36). Within the normal cellular environment, a more reduced redox environment usually favors proliferation, whereas a more oxidized environment favors differentiation. In a pathogenic environment, moderately increased oxidative stress usually leads to a more controlled apoptotic cell death, whereas very high level of oxidative stress would lead to a more sweeping necrotic cell death (36). The redox potential also changes as cells progress through the cell cycle with the level of superoxide radicals increasing as cells progress from G1 to M phase (37, 38), and the balance between hydrogen peroxide and superoxide concentration influences the decision to enter or exit the cell cycle (39).
At the center of redox control is the superoxide-metabolizing enzymes, superoxide dismutases (SODs) (40, 41), and the H2O2/peroxide-metabolizing enzymes, catalase, glutathione peroxidases (Gpxs), peroxiredoxins (Prdxs), and glutaredoxins (Grxs). In the mammalian system, there are three SODs that are encoded by different genes and the proteins are located in different subcellular compartments. The cytoplasmic Cu and Zn-containing SOD (CuZnSOD) is a dimeric enzyme and is present in high abundance in most tissues. In addition to the cytosol, CuZnSOD is also present in the intermembrane space of mitochondria and in the nucleus (42). Mutant mice deficient in CuZnSOD (Sod1−/− mice) are viable, but have increased incidence of liver cancer as they age and have a reduced life span compared to wild type controls (43). Sod1−/− mice also have defects in the neuromuscular junctions, resulting in skeletal muscle atrophies (44). Although Sod1−/− mice are viable, primary cells derived from Sod1−/− mice are not able to survive in the conventional tissue culture system due to toxicity from the ambient oxygen (45).
The mitochondrial Mn-containing SOD (MnSOD) is a tetrameric enzyme and is present in the matrix of mitochondria. The protein is present at low levels in most tissues, but the production MnSOD is highly inducible under conditions of oxidative stress. Tissues that rely heavily on oxidative phosphorylation for energy production maintain a higher level of MnSOD. Mutant mice deficient in MnSOD (Sod2−/− mice) suffer from severe mitochondrial defects and, depending on the genetic background, die prenatally or peri-natally with dilated cardiomyopathy, metabolic acidosis, and vacuolar degeneration in the brain (46–48). MnSOD has been shown to be important for cell cycle progression by controlling the balance between hydrogen peroxide and superoxide as cells progress from G1 to M phase (39, 49). Marked reduction in MnSOD levels is commonly observed in transformed cells, and reconstitution of MnSOD expression in transformed cells can increase cell doubling time and reduce tumorigenicity (50). Consistent with the in vitro observation, mutant mice with 50% reduction in MnSOD (Sod2−/+) develop significantly higher incidence of lymphoma as they age (51), whereas increased MnSOD in normal tissues is shown to delay entry into the S phase in a partial hepatectomy experimental model with transgenic mice expressing elevated levels of MnSOD in the liver (52). Reduction of MnSOD in transformed cells is usually due to deletion or epigenetic modification of Sod2 (53–56). The role of MnSOD in cancer initiation, progression, and metastasis is more complex than cell cycle control. Multiple studies have shown elevated levels of MnSOD in the invading front of metastatic tumors, and molecular and biochemical studies suggest a role of MnSOD in enhancing the invasive and migratory activity of tumor cells by controlling the signaling events that drive tumor metastasis (57–59).
The extracellular Cu and Zn-containing SOD (EC-SOD) is a tetrameric enzyme and is mainly present on cell surfaces by attachment to the extracellular matrix via its heparin binding domain at the C-terminus (60). The heparin binding domain is cleaved in a small percentage of EC-SOD, allowing the enzyme to diffuse further away from the cell surface and, in the case of endothelial cell-derived EC-SOD, enter the circulation (61). Compared to CuZnSOD and MnSOD, EC-SOD is produced at very low level in most tissues. Mutant mice deficient in EC-SOD (Sod3−/− mice) are viable and with no overt abnormalities when maintained under normal laboratory conditions (62). The life span of Sod3−/− mice is comparable to that of wild type controls (63). All three SODs function similarly in their ability to enzymatically convert superoxide radicals into hydrogen peroxide. The resulting hydrogen peroxide is then converted into water by peroxidases. Because superoxide radicals are highly reactive and have a very short half life, they need to be removed at the site of their generation. Therefore, it is possible to make compartment-specific changes in redox potentials by changing the level of a specific SOD.
In addition to SODs, compartment-specific peroxidases, including peroxiredoxins (Prdxs), glutaredoxins (Grxs), and glutathione peroxidases (Gpxs), are important in controlling the cellular thiol pools and thereby, controlling redox-sensitive proteins that are important in various aspects of cellular functions. Of particular interest is the role of Prdxs in stem cell self renewal and differentiation. In the mammalian system, there are 6 Prdxs (Prdx I – VI) (64). Prdx I, II, and VI are located in the cytosol, Prdx III is located in the mitochondria, Prdx IV is located in the endoplastic reticulum, and Prdx V is found in multiple subcellular locations including cytosol, mitochondria, and peroxisome. Whereas Prdx I – IV are the typical 2-Cys Prdxs and form inter-molecular disulfide bonds, Prdx V is classified as atypical Prdx and form intra-molecular disulfide bonds upon oxidation by H2O2. Oxidized Prdx I–V can be returned to the reduced state by thioredoxins (Trx), which is then regenerated by thioredoxin reductase (TrxR) and NADPH (64). Prdx I and Prdx II were shown to play an important role in maintaining the stemness of mouse embryonic stem cells (ESC) and differentiation towards the neuronal lineage, respectively (65). Prdx I had also been shown to play an important role in spinal motor neuron differentiation by controlling redox-activation of the glycerophosphodiester phosphodiesterase 2 (GDE2) via reduction of an intramolecular disulfide bond between the N- and C-terminal domain of GDE2 (66).
Redox balance and hippocampal neurogenesis
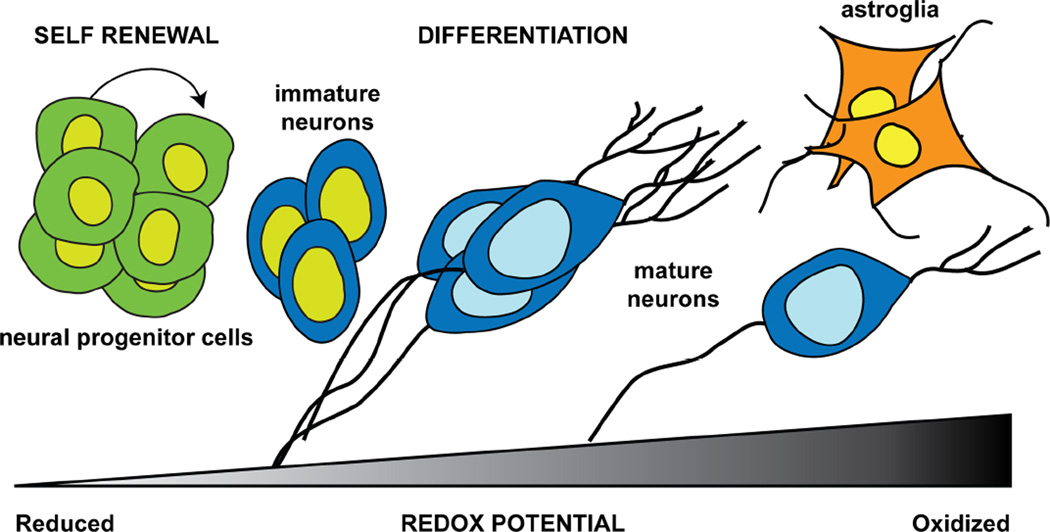
Stem cells usually reside in an environment with low oxygen tension, and recent studies suggest that activation of hypoxia-inducible factor 1 alpha (HIF-1α) in low oxygen environment facilitates signaling pathways that favors self renewal and inhibits pathways that promote differentiation (67). In additional to the external environment, intracellular ROS production increases as cells proceed through the differentiation process. Within the hippocampal redox environment that favors differentiation, a more reduced environment favors differentiation of progenitor cells into the neural lineage, whereas a more oxidized environment favors astroglial lineage (Figure 1) (68). Consistent with this finding, production of newborn cells in the SGZ of hippocampal dentate gyrus was shifted towards a lowered percentage of cells bearing neuronal markers and an increased percentage bearing astroglial markers in mice deficient in SOD (Figure 2A and B) (69–72). Interestingly, this shift was seen in all SOD deficient mice (Sod1−/+, Sod2−/+, and Sod3−/− mice), suggesting the importance of maintaining a normal redox environment in all subcellular compartments. The largest shift to astroglial lineage was observed in CuZnSOD deficient mice (Sod1−/+) even though the mice were only missing 50% CuZnSOD, and the smallest shift was seen in EC-SOD null mice (Sod3−/−) (70, 72).
Figure 1. Redox potential and cell fate decision.
Neural progenitor cells reside in a relatively hypoxic environment that favors self renewal (left panel). As the environment becomes more oxidized, signaling pathways that promote differentiation are activated, leading to the generation of new neurons (middle panel). The differentiation process also leads to increased production of intracellular oxygen free radicals. Higher number of astroglial cells are generated in an environment where the redox potential is more oxidized than the normal condition for neuronal differentiation (right panel), resulting in reduced production of new neurons.
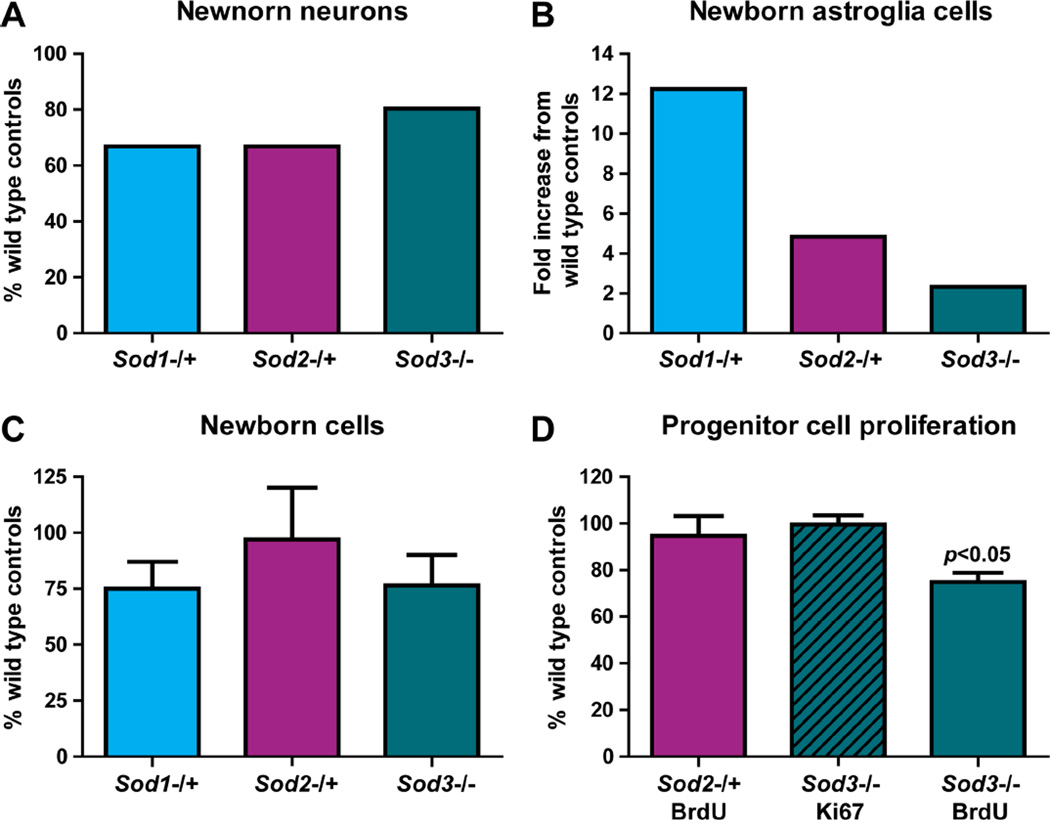
Figure 2. In vivo lineage selection during neural progenitor cell differentiation was affected by SOD deficiency.
The percentage of newborn neurons (A) and astroglia (B) in the SGZ of hippocampal dentate gyrus from each SOD deficient mouse strain was normalized to its wild type controls with the wild type value set as 100% (A) or 1 (B). Deviations from the wild type levels suggested changes in the lineage selection during progenitor cell differentiation in the SOD-deficient environment. Similarly, total number of newborn cells (C) or proliferating cells (D) in the SGZ of each SOD deficient mouse strain was normalized to its wild type controls. Mice were injected with 7 doses (C) (1× per day for 7 days) or 2 doses (D) (2× per day, 8 hours apart) of BrdU at 3 months of age and tissues collected for stereological cell counting 3 weeks after the 7th BrdU injection (for total number of newborn cells and differentiation study) or 16 hours after the second BrdU injection (for proliferating cells), respectively. Discrepancy in Ki67 and BrdU detection of proliferating cells in Sod2−/+ may be due to the presence of Ki67 in all phases of the cell cycle, while BrdU can only be incorporated by cells in the S phase of cell cycle. Data derived from Fishman et al (Sod1−/+ and Sod2−/+ (70)) and Rola et al (Sod3−/− (71)). Progenitor cell proliferation was significantly reduced in Sod3−/− mice when measured by BrdU incorporation (p<0.05, Bonferroni post test). All the other comparisons in C and D did not reach significant level.
Successful neurogenesis relies heavily on long-term survival of newborn neurons. Therefore, it was not surprising to find reduced number of newborn granule cells, which represented long-term survival of newborn neurons, in the SGZ of all three strains of SOD deficient mice (Figure 2C) (69–72). The reduced number of newborn granule cells was not due to reduced proliferation of progenitor cells in the SGZ of Sod2−/+ mice as the number of proliferating cells, measured by BrdU incorporation, was similar between Sod2+/+ and Sod2−/+ mice (Figure 2D) (69). Proliferation of progenitor cells in the SGZ of Sod3−/− mice, on the other hand was either reduced when measured by BrdU incorporation (72), or not changed when measured by the expression of Ki67 (Figure 2D) (71). Proliferation of progenitor cells in the SGZ was not determined in Sod1−/+ mice.
Redox balance and dendritic structure
Hippocampal-dependent learning is mainly mediated by excitatory neurons with granule cells in the dentate gyrus and pyramidal cells in the CA areas. Granule cells and pyramidal cells put out extensive dendrites that synapse with axons and dendrites from other neurons for proper control of synaptic transmissions. Dendritic spines are small protrusions on dendrites that receive input from synapses on axons. They constitute the post synaptic element of excitatory synapses and mediate the majority of excitatory connections in the brain. The structure and density of dendritic spines are important factors in synaptic functions, and dynamic changes in the shapes and sizes of dendritic spines can affect spine stability and synaptic strength. Deficiency in MnSOD (Sod2−/+ mice) or EC-SOD (Sod3−/− mice) did not lead to a significant change in spine density in dentate granule cells (69, 72). However, a single dose of cranial irradiation, which was known to cause persistent increase in oxidative stress and neuroinflammation, produced a 20% reduction in spine density in dentate granule cells at 2 months post-irradiation (69, 72). More detailed analyses of spine morphology showed a significant increase in thin spines and a significant decrease in mushroom spines in neurons in the dentate gyrus and CA1 (73), suggesting a potential change in synaptic activities following cranial irradiation.
Dendritic arbors are also sensitive to changes in redox balance. Dendritic extension in immature neurons (Dcx+ cells) becomes more elaborate when Dcx+ cells reach the post-mitotic stage (categories E and F Dcx+ cells) (74). Studies with mice with altered levels of EC-SOD suggested that higher levels of EC-SOD promoted dendritic arborization in Dcx+ cells and EC-SOD transgenic mice had proportionally higher percentage of categories E and F Dcx+ cells in the dentate gyrus (72). In mature granule cells, on the other hand, higher levels of EC-SOD did not affect the overall branching and the length of dendrites. However, deficiency in EC-SOD had a devastating effect on dendritic arborization in granule cells with marked reductions in the complexity of dendritic arbors in Sod3−/− mice (72). Reduced dendritic complexity has also been reported in hippocampal granule cells and pyramidal cells following irradiation (75). Taken together, maintenance of dendritic structures, including dendritic arbors and dendritic spines, is sensitive to perturbation of redox balance, and increased oxidative stress in the hippocampal formation can negatively affect learning and memory via changes in dendritic structures.
Redox balance and hippocampal-dependent learning and memory
Several behavioral studies are designed based on the natural curiosity of rodents to explore novel objects and locations or their natural instincts to freeze when frightened to examine hippocampal-dependent and independent cognitive functions. In the novel object recognition paradigm, mice have the natural tendency to spend relatively more time exploring a novel object when it is presented with a familiar object at the same time. Such recognition memory depends on multiple brain areas, but most critically involving prefrontal cortex and the hippocampus (76). In contrast, the novel location recognition test relies on spatial memory, and a mouse would spend relatively more time exploring a familiar object when it is moved to a new location than a familiar object that remains in its original location (i.e. familiar or old location). Spatial memory relies heavily on hippocampal functions (77, 78). The combination of novel object and novel location recognition test helps to decipher the finer differences in hippocampal- and non-hippocampal-dependent functions. Given the changes observed in hippocampal neurogenesis, dendritic arborization, and spine density in mice deficient in EC-SOD (Sod3−/−), it was important to confirm that the cellular and structural defects were associated with functional defects in behavioral output. The cognitive tests showed that Sod3−/− mice were not able to clearly distinguish between a novel object and a familiar object; however, the same group of mice had no problem recognizing novel placement of a familiar object (72).
Morris water maze and its related mazes where experimental animals rely on visual cues in the testing environment to find a hidden escape platform from the water are usually used as more stringent tests for spatial memory. In the traditional Morris water maze test with probe trials at the end of each training day, EC-SOD deficient (Sod3−/−) mice did not spend significantly more time exploring the area housing the hidden escape platform until completing three days of training (79). Wild type control mice, on the other hand, were able to pass the test with only two days of training. In a radial arm water maze test, which is a modified water maze that includes a 6-arm or 8-arm radial arm in the water tank with a hidden escape platform located at the end of one arm, the number of entries into the arms without the hidden escape platform can be counted and scored as errors in the search for the hidden escape platform. Consistent with the Morris water maze results, EC-SOD deficient mice failed to significantly reduce the number of errors after 30 rounds of training (72). Wild type controls, on the other hand, were able to efficiently reduce the number of errors with training (72). Similar to this finding, mutant mice deficient in Prdx II (Prdx II−/−) showed an accelerated age-related decline in long-term potentiation and learning in Morris water maze (80). Mutant mice deficient in MnSOD (Sod2−/+ mice) on the other hand, did not show defects in novel object recognition, novel location recognition, or learning in radial arm water maze (69). Given that changes in hippocampal neurogenesis and spine density in Sod2−/+ mice were not as extensive as that in Sod3−/− mice (Figure 2 and (69)), it was possible that the cellular defects in Sod2−/+ mice were not sufficient to negatively affect cognitive function at the age (4 months) the mice were tested. Mice deficient in CuZnSOD have not been tested for cognitive functions.
Radiation, oxidative stress, and hippocampal functions
The brain is exposed to ionizing radiation in a number of clinical situations, predominantly in those involving cancer treatments. Radiation brain injury could involve macroscopic tissue destruction after relatively high doses of irradiation (81). Less severe morphologic injury also occurs after radiotherapy and this injury can result in variable degrees of cognitive dysfunction (82, 83). Such cognitive changes can occur in both pediatric and adult patients, and are often manifested as deficits in hippocampal-dependent functions of learning, memory, and spatial information processing (82–84). The generation of reactive oxygen species (ROS) is considered a main cause of radiation-mediated tissue damages. Ionizing radiation not only results in the acute generation of short-lived ROS (85), it also results in a persistent oxidative stress that extends up to several months after irradiation (71, 79, 86). Therefore, exposure to low level irradiation can be used to model the effects of persistent oxidative stress on neurocognitive functions. Hippocampal neurogenesis is exquisitely sensitive to irradiation, and irradiation in the CNS leads to a marked dose-dependent reduction in proliferating cells and immature neurons in the SGZ of hippocampal dentate gyrus within the first 48 hours after irradiation (71). Beyond the period immediately after radiation exposure, the effects of irradiation continue to manifest as suppression of hippocampal neurogenesis and reduction in dendritic arborization and spine densities (71, 72). Because of the persistent perturbation in redox balance following radiation exposure, changes in the hippocampal formation and the associated cognitive defects are similar to that observed in SOD-deficient mice (69, 72, 79). Consequently, enhanced antioxidant capacity has been shown to minimize irradiation-induced hippocampal defects (72, 87).
Oxidative stress and age-related cognitive decline
Tissue levels of protein oxidation, lipid peroxidation, and DNA/RNA oxidation all go up with age (88–91), and this is impart due to increased production of reactive oxygen species (ROS) and in part, due to decreased repair. Age-related increase of superoxide radicals in the brain can be visually illustrated by in vivo conversion of dihydroethidium (DHE) into its oxidized products, 2-hydroethidium and ethidium, which are DNA and RNA interchelating fluorescent dyes (92). Whereas 20-month old C57BL/6 mice showed a marked increase in fluorescent signals from oxidation of DHE in pyramidal and granule cells in the hippocampal formation, age-matched transgenic mice with higher levels of extracellular superoxide dismutase (EC-SOD) only showed a modest increase of DHE oxidation compared to 3-month old controls (93). Parallel to age-related increase in superoxide radicals in the hippocampus, 20-month old C57BL/6 mice showed impairment in long-term potentiation, which is an in vitro model for learning, and defects in spatial learning. In contrast, higher levels of EC-SOD in aged transgenic mice resulted a relatively normal hippocampal LTP and alleviated age-related decline in spatial learning (93).
Age-related reduction in hippocampal neurogenesis is well documented in rodents (94), whether this is due to depletion of progenitor cells and/or failure of progenitor cells to enter cell cycle is not entirely clear. Aging is commonly associated with memory decline (95–97), which is often attributed to reduced hippocampal neurogenesis (98, 99). This conclusion is supported mainly by studies that show a cause-effect relationship between improved cognitive functions and increased hippocampal neurogenesis in aged rodents (100, 101). However, not all study results support the correlation between cognitive deficits and reduced hippocampal neurogenesis in older rodents (94, 102), and the discrepancy cannot be simply attributed to the sensitivity of tests used to assess cognitive functions. Moreover, improved cognitive functions do not always correlate with increased hippocampal neurogenesis (33, 103). In addition to hindrance of new neuron production, the pro-oxidant environment in aged brain can affect lineage commitment of newborn cells towards astroglial lineage. It can also affect the dendritic structure, leading to reduced complexity in dendritic arborization and reduced spine density (104–106). Therefore, it is quite possible that aging-related cognitive decline is caused not only by reduced hippocampal neurogenesis, but also by defects in the dendritic structures as well as changes in the rest of the neuronal network (106).
In summary, data from experimental animals showed (1) a persistent increase in oxidative stress, (2) a marked reduction in the production of newborn neurons in the hippocampus, due to reduced proliferation of progenitor cells, reduced long-term survival of newborn neurons, and reduced differentiation towards the neuronal lineage, (3) alterations in dendritic arborization and spine densities in newborn and existing hippocampal neurons, and (4) reduced hippocampal-dependent functions of learning and memory in aged mice, SOD-deficient mice, and mice exposed to low dose radiation. Given the similarity in the hippocampal environment and the outcome in hippocampal neurogenesis, dendritic structures, and cognitive performance in these experimental systems, it would be important to determine if they also share a common underlying mechanism. In addition, the cellular and molecular mechanisms underlying SODs-mediated determination of cell fate decision and changes in cognitive function could be an important direction of future study. The cooperation of hippocampus and other areas of the brain in cognitive function under conditions of oxidative stress would be another interesting area of study since memory encoding, retention, and retrieval rely on concerted efforts from multiple brain regions (107, 108). SOD-deficient mice and radiation-exposed mice manifest defects at young age and can be a significant time saver if they can be used to model the aging brain, even if just for a limited aspect of the age-related declines, for experimental treatments or intervention of oxidative stress induced functional defects.
Highlights.
Hippocampus is central to learning and memory, but is sensitive to oxidative stress
Reduced hippocampal neurogenesis and dendritic complexity affect memory formation
Aging, SOD deficiency and radiation exposure cause oxidative stress in hippocampus
Redox imbalance alters hippocampal neurogenesis, dendritic complexity, and learning
Redox control may play important roles in hippocampal-dependent cognitive functions
Acknowledgement
This work was supported by VA Merit Review, Geriatric Research, Education, and clinical Center (GRECC), and the use of facility and resources at the VA Palo Alto Health Care System.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Eichenbaum H. The hippocampus and declarative memory: cognitive mechanisms and neural codes. Behav Brain Res. 2001;127(1–2):199–207. doi: 10.1016/s0166-4328(01)00365-5. [DOI] [PubMed] [Google Scholar]
- 2.Budson AE. Understanding memory dysfunction. The neurologist. 2009;15(2):71–79. doi: 10.1097/NRL.0b013e318188040d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Papp G, Witter MP, Treves A. The CA3 network as a memory store for spatial representations. Learn Mem. 2007;14(11):732–744. doi: 10.1101/lm.687407. [DOI] [PubMed] [Google Scholar]
- 4.Amaral DG, Witter MP. The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience. 1989;31(3):571–591. doi: 10.1016/0306-4522(89)90424-7. [DOI] [PubMed] [Google Scholar]
- 5.Zola-Morgan S, Squire LR. Neuroanatomy of memory. Annu Rev Neurosci. 1993;16:547–563. doi: 10.1146/annurev.ne.16.030193.002555. [DOI] [PubMed] [Google Scholar]
- 6.Amaral DG, Scharfman HE, Lavenex P. The dentate gyrus: fundamental neuroanatomical organization (dentate gyrus for dummies) Progress in brain research. 2007;163:3–22. doi: 10.1016/S0079-6123(07)63001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Squire LR, Zola SM. Structure and function of declarative and nondeclarative memory systems. Proc Natl Acad Sci U S A. 1996;93(24):13515–13522. doi: 10.1073/pnas.93.24.13515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacKay DG, Johnson LW. Errors, error detection, error correction and hippocampalregion damage: data and theories. Neuropsychologia. 2013;51(13):2633–2650. doi: 10.1016/j.neuropsychologia.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 9.Braun SM, Jessberger S. Adult neurogenesis: mechanisms and functional significance. Development. 2014;141(10):1983–1986. doi: 10.1242/dev.104596. [DOI] [PubMed] [Google Scholar]
- 10.Margolis FL, Verhaagen J, Biffo S, Huang FL, Grillo M. Regulation of gene expression in the olfactory neuroepithelium: a neurogenetic matrix. Progress in brain research. 1991;89:97–122. doi: 10.1016/s0079-6123(08)61718-5. [DOI] [PubMed] [Google Scholar]
- 11.Stuchlik A. Dynamic learning and memory, synaptic plasticity and neurogenesis: an update. Frontiers in behavioral neuroscience. 2014;8:106. doi: 10.3389/fnbeh.2014.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gu Y, Janoschka S, Ge S. Neurogenesis and hippocampal plasticity in adult brain. Current topics in behavioral neurosciences. 2013;15:31–48. doi: 10.1007/7854_2012_217. [DOI] [PubMed] [Google Scholar]
- 13.Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- 14.Eriksson PS, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4(11):1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 15.Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J. Neurosci. 1996;16(6):2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Markakis EA, Gage FH. Adult-generated neurons in the dentate gyrus send axonal projections to field CA3 and are surrounded by synaptic vesicles. J Comp Neurol. 1999;406(4):449–460. [PubMed] [Google Scholar]
- 17.Ramirez-Amaya V, Marrone DF, Gage FH, Worley PF, Barnes CA. Integration of new neurons into functional neural networks. J Neurosci. 2006;26(47):12237–12241. doi: 10.1523/JNEUROSCI.2195-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96(23):13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nature Neurosci. 1999;2(3):266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 20.Zhao C, Teng EM, Summers RG, Jr, Ming GL, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci. 2006;26(1):3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ben Abdallah NM, Slomianka L, Vyssotski AL, Lipp HP. Early age-related changes in adult hippocampal neurogenesis in C57 mice. Neurobiol Aging. 2010;31(1):151–161. doi: 10.1016/j.neurobiolaging.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 23.Kempermann G. Why new neurons? Possible functions for adult hippocampal neurogenesis. J Neurosci. 2002;22(3):635–638. doi: 10.1523/JNEUROSCI.22-03-00635.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kempermann G, Brandon EP, Gage FH. Environmental stimulation of 129/SvJ mice causes increased cell proliferation and neurogenesis in the adult dentate gyrus. Curr Biol. 1998;8(16):939–942. doi: 10.1016/s0960-9822(07)00377-6. [DOI] [PubMed] [Google Scholar]
- 25.Kempermann G, Kuhn HG, Gage FH. Experience-induced neurogenesis in the senescent dentate gyrus. J Neurosci. 1998;18(9):3206–3212. doi: 10.1523/JNEUROSCI.18-09-03206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prickaerts J, Koopmans G, Blokland A, Scheepens A. Learning and adult neurogenesis: survival with or without proliferation? Neurobiol Learn Mem. 2004;81(1):1–11. doi: 10.1016/j.nlm.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 27.Nithianantharajah J, Hannan AJ. Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat Rev Neurosci. 2006;7(9):697–709. doi: 10.1038/nrn1970. [DOI] [PubMed] [Google Scholar]
- 28.Huang FL, Huang KP, Wu J, Boucheron C. Environmental enrichment enhances neurogranin expression and hippocampal learning and memory but fails to rescue the impairments of neurogranin null mutant mice. J Neurosci. 2006;26(23):6230–6237. doi: 10.1523/JNEUROSCI.1182-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Praag H, et al. Functional neurogenesis in the adult hippocampus. Nature. 2002;415(6875):1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kee N, Teixeira CM, Wang AH, Frankland PW. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat Neurosci. 2007;10(3):355–362. doi: 10.1038/nn1847. [DOI] [PubMed] [Google Scholar]
- 31.Saxe MD, et al. Paradoxical influence of hippocampal neurogenesis on working memory. Proc Natl Acad Sci U S A. 2007;104(11):4642–4646. doi: 10.1073/pnas.0611718104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jaholkowski P, et al. New hippocampal neurons are not obligatory for memory formation; cyclin D2 knockout mice with no adult brain neurogenesis show learning. Learn Mem. 2009;16(7):439–451. doi: 10.1101/lm.1459709. [DOI] [PubMed] [Google Scholar]
- 33.Rosi S, et al. The polyamine inhibitor alpha-difluoromethylornithine modulates hippocampus-dependent function after single and combined injuries. PLoS One. 2012;7(1):e31094. doi: 10.1371/journal.pone.0031094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perez-Cruz C, et al. Reduced spine density in specific regions of CA1 pyramidal neurons in two transgenic mouse models of Alzheimer's disease. J Neurosci. 2011;31(10):3926–3934. doi: 10.1523/JNEUROSCI.6142-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Middei S, et al. Learning discloses abnormal structural and functional plasticity at hippocampal synapses in the APP23 mouse model of Alzheimer's disease. Learn Mem. 2010;17(5):236–240. doi: 10.1101/lm.1748310. [DOI] [PubMed] [Google Scholar]
- 36.Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30(11):1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 37.Menon SG, Goswami PC. A redox cycle within the cell cycle: ring in the old with the new. Oncogene. 2007;26(8):1101–1109. doi: 10.1038/sj.onc.1209895. [DOI] [PubMed] [Google Scholar]
- 38.Sarsour EH, Kumar MG, Chaudhuri L, Kalen AL, Goswami PC. Redox control of the cell cycle in health and disease. Antioxid Redox Signal. 2009;11(12):2985–3011. doi: 10.1089/ars.2009.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Menon SG, et al. Redox regulation of the G1 to S phase transition in the mouse embryo fibroblast cell cycle. Cancer Res. 2003;63(9):2109–2117. [PubMed] [Google Scholar]
- 40.Huang TT, Zou Y, Corniola R. Oxidative stress and adult neurogenesis--effects of radiation and superoxide dismutase deficiency. Semin Cell Dev Biol. 2012;23(7):738–744. doi: 10.1016/j.semcdb.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang TT. Redox balance- and radiation-mediated alteration in hippocampal neurogenesis. Free radical research. 2012;46(8):951–958. doi: 10.3109/10715762.2012.664770. [DOI] [PubMed] [Google Scholar]
- 42.Sturtz LA, Diekert K, Jensen LT, Lill R, Culotta VC. A fraction of yeast Cu,Zn-superoxide dismutase and its metallochaperone, CCS, localize to the intermembrane space of mitochondria. A physiological role for SOD1 in guarding against mitochondrial oxidative damage. J Biol Chem. 2001;276(41):38084–38089. doi: 10.1074/jbc.M105296200. [DOI] [PubMed] [Google Scholar]
- 43.Elchuri S, et al. CuZnSOD deficiency leads to persistent and widespread oxidative damage and hepatocarcinogenesis later in life. Oncogene. 2005;24(3):367–380. doi: 10.1038/sj.onc.1208207. [DOI] [PubMed] [Google Scholar]
- 44.Jang YC, et al. Increased superoxide in vivo accelerates age-associated muscle atrophy through mitochondrial dysfunction and neuromuscular junction degeneration. FASEB J. 2010;24(5):1376–1390. doi: 10.1096/fj.09-146308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang TT, et al. Superoxide-mediated cytotoxicity in superoxide dismutase-deficient fetal fibroblasts. Arch Biochem Biophys. 1997;344(2):424–432. doi: 10.1006/abbi.1997.0237. [DOI] [PubMed] [Google Scholar]
- 46.Huang TT, et al. Genetic modification of prenatal lethality and dilated cardiomyopathy in Mn superoxide dismutase mutant mice. Free Radic Biol Med. 2001;31(9):1101–1110. doi: 10.1016/s0891-5849(01)00694-3. [DOI] [PubMed] [Google Scholar]
- 47.Huang TT, et al. Genetic modifiers of the phenotype of mice deficient in mitochondrial superoxide dismutase. Hum Mol Genet. 2006;15(7):1187–1194. doi: 10.1093/hmg/ddl034. [DOI] [PubMed] [Google Scholar]
- 48.Li Y, et al. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat Genet. 1995;11(4):376–381. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- 49.Sarsour EH, Agarwal M, Pandita TK, Oberley LW, Goswami PC. Manganese superoxide dismutase protects the proliferative capacity of confluent normal human fibroblasts. J Biol Chem. 2005;280(18):18033–18041. doi: 10.1074/jbc.M501939200. [DOI] [PubMed] [Google Scholar]
- 50.Dhar SK, St Clair DK. Manganese superoxide dismutase regulation and cancer. Free Radic Biol Med. 2012;52(11–12):2209–2222. doi: 10.1016/j.freeradbiomed.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 51.Van Remmen H, et al. Life-long reduction in MnSOD activity results in increased DNA damage and higher incidence of cancer but does not accelerate aging. Physiol Genomics. 2003;16(1):29–37. doi: 10.1152/physiolgenomics.00122.2003. [DOI] [PubMed] [Google Scholar]
- 52.Kim A, et al. Enhanced expression of mitochondrial superoxide dismutase leads to prolonged in vivo cell cycle progression and up-regulation of mitochondrial thioredoxin. Free Radic Biol Med. 2010;48(11):1501–1512. doi: 10.1016/j.freeradbiomed.2010.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cyr AR, Hitchler MJ, Domann FE. Regulation of SOD2 in cancer by histone modifications and CpG methylation: closing the loop between redox biology and epigenetics. Antioxid Redox Signal. 2013;18(15):1946–1955. doi: 10.1089/ars.2012.4850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hitchler MJ, Oberley LW, Domann FE. Epigenetic silencing of SOD2 by histone modifications in human breast cancer cells. Free Radic Biol Med. 2008;45(11):1573–1580. doi: 10.1016/j.freeradbiomed.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hitchler MJ, et al. Epigenetic regulation of manganese superoxide dismutase expression in human breast cancer cells. Epigenetics : official journal of the DNA Methylation Society. 2006;1(4):163–171. doi: 10.4161/epi.1.4.3401. [DOI] [PubMed] [Google Scholar]
- 56.Huang Y, He T, Domann FE. Decreased expression of manganese superoxide dismutase in transformed cells is associated with increased cytosine methylation of the SOD2 gene. DNA and cell biology. 1999;18(8):643–652. doi: 10.1089/104454999315051. [DOI] [PubMed] [Google Scholar]
- 57.Hempel N, Carrico PM, Melendez JA. Manganese superoxide dismutase (Sod2) and redox-control of signaling events that drive metastasis. Anti-cancer agents in medicinal chemistry. 2011;11(2):191–201. doi: 10.2174/187152011795255911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Connor KM, et al. Manganese superoxide dismutase enhances the invasive and migratory activity of tumor cells. Cancer Res. 2007;67(21):10260–10267. doi: 10.1158/0008-5472.CAN-07-1204. [DOI] [PubMed] [Google Scholar]
- 59.Ranganathan AC, et al. Manganese superoxide dismutase signals matrix metalloproteinase expression via H2O2-dependent ERK1/2 activation. J Biol Chem. 2001;276(17):14264–14270. doi: 10.1074/jbc.M100199200. [DOI] [PubMed] [Google Scholar]
- 60.Karlsson K, Marklund SL. Heparin-induced release of extracellular superoxide dismutase to human blood plasma. Biochem J. 1987;242(1):55–59. doi: 10.1042/bj2420055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bowler RP, et al. Furin proteolytically processes the heparin-binding region of extracellular superoxide dismutase. J Biol Chem. 2002;277(19):16505–16511. doi: 10.1074/jbc.M105409200. [DOI] [PubMed] [Google Scholar]
- 62.Carlsson LM, Jonsson J, Edlund T, Marklund SL. Mice lacking extracellular superoxide dismutase are more sensitive to hyperoxia. Proc Natl Acad Sci U S A. 1995;92(14):6264–6268. doi: 10.1073/pnas.92.14.6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sentman ML, et al. Phenotypes of mice lacking extracellular superoxide dismutase and copper- and zinc-containing superoxide dismutase. J Biol Chem. 2006;281(11):6904–6909. doi: 10.1074/jbc.M510764200. [DOI] [PubMed] [Google Scholar]
- 64.Rhee SG, Woo HA, Kil IS, Bae SH. Peroxiredoxin functions as a peroxidase and a regulator and sensor of local peroxides. J Biol Chem. 2012;287(7):4403–4410. doi: 10.1074/jbc.R111.283432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim SU, et al. Dominant role of peroxiredoxin/JNK axis in stemness regulation during neurogenesis from embryonic stem cells. Stem Cells. 2014;32(4):998–1011. doi: 10.1002/stem.1593. [DOI] [PubMed] [Google Scholar]
- 66.Yan Y, Sabharwal P, Rao M, Sockanathan S. The antioxidant enzyme Prdx1 controls neuronal differentiation by thiol-redox-dependent activation of GDE2. Cell. 2009;138(6):1209–1221. doi: 10.1016/j.cell.2009.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Panchision DM. The role of oxygen in regulating neural stem cells in development and disease. J Cell Physiol. 2009;220(3):562–568. doi: 10.1002/jcp.21812. [DOI] [PubMed] [Google Scholar]
- 68.Prozorovski T, et al. Sirt1 contributes critically to the redox-dependent fate of neural progenitors. Nat Cell Biol. 2008;10(4):385–394. doi: 10.1038/ncb1700. [DOI] [PubMed] [Google Scholar]
- 69.Corniola R, Zou Y, Leu D, Fike JR, Huang TT. Paradoxical Relationship between Mn Superoxide Dismutase Deficiency and Radiation-Induced Cognitive Defects. PLoS One. 2012;7(11):e49367. doi: 10.1371/journal.pone.0049367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fishman K, et al. Radiation-induced reductions in neurogenesis are ameliorated in mice deficient in CuZnSOD or MnSOD. Free Radic Biol Med. 2009;47(10):1459–1467. doi: 10.1016/j.freeradbiomed.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rola R, et al. Lack of extracellular superoxide dismutase (EC-SOD) in the microenvironment impacts radiation-induced changes in neurogenesis. Free Radic Biol Med. 2007;42(8):1133–1145. doi: 10.1016/j.freeradbiomed.2007.01.020. discussion 1131–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zou Y, et al. Extracellular superoxide dismutase is important for hippocampal neurogenesis and preservation of cognitive functions after irradiation. Proc Natl Acad Sci U S A. 2012;109(52):21522–21527. doi: 10.1073/pnas.1216913110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chakraborti A, Allen A, Allen B, Rosi S, Fike JR. Cranial irradiation alters dendritic spine density and morphology in the hippocampus. PLoS One. 2012;7(7):e40844. doi: 10.1371/journal.pone.0040844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Plumpe T, et al. Variability of doublecortin-associated dendrite maturation in adult hippocampal neurogenesis is independent of the regulation of precursor cell proliferation. BMC Neurosci. 2006;7:77. doi: 10.1186/1471-2202-7-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Parihar VK, Limoli CL. Cranial irradiation compromises neuronal architecture in the hippocampus. Proc Natl Acad Sci U S A. 2013;110(31):12822–12827. doi: 10.1073/pnas.1307301110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Barker GR, Warburton EC. When is the hippocampus involved in recognition memory? J Neurosci. 2011;31(29):10721–10731. doi: 10.1523/JNEUROSCI.6413-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Manns JR, Eichenbaum H. A cognitive map for object memory in the hippocampus. Learn Mem. 2009;16(10):616–624. doi: 10.1101/lm.1484509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bachevalier J, Nemanic S. Memory for spatial location and object-place associations are differently processed by the hippocampal formation, parahippocampal areas TH/TF and perirhinal cortex. Hippocampus. 2008;18(1):64–80. doi: 10.1002/hipo.20369. [DOI] [PubMed] [Google Scholar]
- 79.Raber J, et al. Irradiation enhances hippocampus-dependent cognition in mice deficient in extracellular superoxide dismutase. Hippocampus. 2011;21(1):72–80. doi: 10.1002/hipo.20724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim SU, et al. Peroxiredoxin II preserves cognitive function against age-linked hippocampal oxidative damage. Neurobiol Aging. 2011;32(6):1054–1068. doi: 10.1016/j.neurobiolaging.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 81.Sheline GE, Wara WM, Smith V. Therapeutic irradiation and brain injury. Int J Radiat Oncol Biol Phys. 1980;6(9):1215–1228. doi: 10.1016/0360-3016(80)90175-3. [DOI] [PubMed] [Google Scholar]
- 82.Grill J, et al. Long-term intellectual outcome in children with posterior fossa tumors according to radiation doses and volumes. Int J Radiat Oncol Biol Phys. 1999;45(1):137–145. doi: 10.1016/s0360-3016(99)00177-7. [DOI] [PubMed] [Google Scholar]
- 83.Meyers CA, Geara F, Wong PF, Morrison WH. Neurocognitive effects of therapeutic irradiation for base of skull tumors. Int J Radiat Oncol Biol Phys. 2000;46(1):51–55. doi: 10.1016/s0360-3016(99)00376-4. [DOI] [PubMed] [Google Scholar]
- 84.Surma-aho O, et al. Adverse long-term effects of brain radiotherapy in adult low-grade glioma patients. Neurology. 2001;56(10):1285–1290. doi: 10.1212/wnl.56.10.1285. [DOI] [PubMed] [Google Scholar]
- 85.Riley PA. Free radicals in biology: oxidative stress and the effects of ionizing radiation. Int J Radiat Biol. 1994;65(1):27–33. doi: 10.1080/09553009414550041. [DOI] [PubMed] [Google Scholar]
- 86.Limoli CL, Giedzinski E, Baure J, Rola R, Fike JR. Redox changes induced in hippocampal precursor cells by heavy ion irradiation. Radiat Environ Biophys. 2007;46(2):167–172. doi: 10.1007/s00411-006-0077-9. [DOI] [PubMed] [Google Scholar]
- 87.Motomura K, Ogura M, Natsume A, Yokoyama H, Wakabayashi T. A free-radical scavenger protects the neural progenitor cells in the dentate subgranular zone of the hippocampus from cell death after X-irradiation. Neurosci Lett. 2010;485(1):65–70. doi: 10.1016/j.neulet.2010.08.065. [DOI] [PubMed] [Google Scholar]
- 88.Levine RL, Stadtman ER. Oxidative modification of proteins during aging. Exp Gerontol. 2001;36(9):1495–1502. doi: 10.1016/s0531-5565(01)00135-8. [DOI] [PubMed] [Google Scholar]
- 89.Stadtman ER. Protein oxidation and aging. Free radical research. 2006;40(12):1250–1258. doi: 10.1080/10715760600918142. [DOI] [PubMed] [Google Scholar]
- 90.Pamplona R. Membrane phospholipids, lipoxidative damage and molecular integrity: a causal role in aging and longevity. Biochim Biophys Acta. 2008;1777(10):1249–1262. doi: 10.1016/j.bbabio.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 91.Santos RX, et al. Mitochondrial DNA oxidative damage and repair in aging and Alzheimer's disease. Antioxid Redox Signal. 2013;18(18):2444–2457. doi: 10.1089/ars.2012.5039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ali SS, et al. Gender differences in free radical homeostasis during aging: shorter-lived female C57BL6 mice have increased oxidative stress. Aging Cell. 2006;5(6):565–574. doi: 10.1111/j.1474-9726.2006.00252.x. [DOI] [PubMed] [Google Scholar]
- 93.Hu D, Serrano F, Oury TD, Klann E. Aging-dependent alterations in synaptic plasticity and memory in mice that overexpress extracellular superoxide dismutase. J Neurosci. 2006;26(15):3933–3941. doi: 10.1523/JNEUROSCI.5566-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bizon JL, Gallagher M. Production of new cells in the rat dentate gyrus over the lifespan: relation to cognitive decline. Eur J Neurosci. 2003;18(1):215–219. doi: 10.1046/j.1460-9568.2003.02733.x. [DOI] [PubMed] [Google Scholar]
- 95.Meyer RC, Spangler EL, Kametani H, Ingram DK. Age-associated memory impairment. Assessing the role of nitric oxide. Ann N Y Acad Sci. 1998;854:307–317. doi: 10.1111/j.1749-6632.1998.tb09911.x. [DOI] [PubMed] [Google Scholar]
- 96.Flood JF, Morley JE. Learning and memory in the SAMP8 mouse. Neurosci Biobehav Rev. 1998;22(1):1–20. doi: 10.1016/s0149-7634(96)00063-2. [DOI] [PubMed] [Google Scholar]
- 97.Gallagher M, Pelleymounter MA. Spatial learning deficits in old rats: a model for memory decline in the aged. Neurobiol Aging. 1988;9(5–6):549–556. doi: 10.1016/s0197-4580(88)80112-x. [DOI] [PubMed] [Google Scholar]
- 98.Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16(6):2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jinno S. Decline in adult neurogenesis during aging follows a topographic pattern in the mouse hippocampus. J Comp Neurol. 2011;519(3):451–466. doi: 10.1002/cne.22527. [DOI] [PubMed] [Google Scholar]
- 100.van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25(38):8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Villeda SA, et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477(7362):90–94. doi: 10.1038/nature10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Martinez-Canabal A, Akers KG, Josselyn SA, Frankland PW. Age-dependent effects of hippocampal neurogenesis suppression on spatial learning. Hippocampus. 2012 doi: 10.1002/hipo.22054. [DOI] [PubMed] [Google Scholar]
- 103.Kamsler A, Avital A, Greenberger V, Segal M. Aged SOD overexpressing mice exhibit enhanced spatial memory while lacking hippocampal neurogenesis. Antioxid Redox Signal. 2007;9(2):181–189. doi: 10.1089/ars.2007.9.181. [DOI] [PubMed] [Google Scholar]
- 104.Zeng Y, Liu Y, Wu M, Liu J, Hu Q. Activation of TrkB by 7,8-dihydroxyflavone prevents fear memory defects and facilitates amygdalar synaptic plasticity in aging. J Alzheimers Dis. 2012;31(4):765–778. doi: 10.3233/JAD-2012-120886. [DOI] [PubMed] [Google Scholar]
- 105.Villeda SA, et al. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat Med. 2014;20(6):659–663. doi: 10.1038/nm.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Petralia RS, Mattson MP, Yao PJ. Communication breakdown: the impact of ageing on synapse structure. Ageing research reviews. 2014;14:31–42. doi: 10.1016/j.arr.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.de Vanssay-Maigne A, et al. Modulation of encoding and retrieval by recollection and familiarity: mapping the medial temporal lobe networks. NeuroImage. 2011;58(4):1131–1138. doi: 10.1016/j.neuroimage.2011.06.086. [DOI] [PubMed] [Google Scholar]
- 108.Opitz B. Memory function and the hippocampus. Frontiers of neurology and neuroscience. 2014;34:51–59. doi: 10.1159/000356422. [DOI] [PubMed] [Google Scholar]