Highlights
-
•
Many human pathogens have a narrow host range.
-
•
Engraftment of human tissues into mice can render animals susceptible to infection.
-
•
Genetic humanization has yielded mice with inheritable susceptible to viral infection.
-
•
Additional refinements are needed to improve the utility of humanized mice.
Abstract
Many of the viral pathogens that cause infectious diseases in humans have a highly restricted species tropism, making the study of their pathogenesis and the development of clinical therapies difficult. The improvement of humanized mouse models over the past 30 years has greatly facilitated researchers’ abilities to study host responses to viral infections in a cost effective and ethical manner. From HIV to hepatotropic viruses to Middle East Respiratory Syndrome coronavirus, humanized mice have led to the identification of factors crucial to the viral life cycle, served as an outlet for testing candidate therapies, and improved our abilities to analyze human immune responses to infection. In tackling both new and old viruses as they emerge, humanized mice will continue to be an indispensable tool.
Current Opinion in Virology 2015, 11:14–20
This review comes from a themed issue on Viral pathogenesis
Edited by Luca G Guidotti and Matteo Iannacone
For a complete overview see the Issue and the Editorial
Available online 22nd January 2015
http://dx.doi.org/10.1016/j.coviro.2015.01.002
1879-6257/© 2015 Elsevier B.V. All rights reserved.
Introduction
Viruses make a staggering contribution to morbidity and mortality in the human populations of both industrial and developing countries. At least 500 million people are chronically infected with hepatitis B (HBV) or C viruses (HCV), placing them at risk for developing severe liver disease. 33 million individuals are infected with HIV, leading to 1.7 million AIDS-related deaths every year. Of the approximately 400 million people who contract dengue virus (DENV) annually, almost 100 million present with clinical symptoms. 60–90% of the global population is infected with herpes simplex viruses (HSV), resulting in orolabial and genital lesions. Human cytomegalovirus (HCMV), which persistently infects 40% of the world, can be life-threatening for newborns and immunocompromised individuals.
Many of the viruses causing disease in humans have a narrow host range, often limited to humans and closely related non-human primates (NHPs). This has created challenges in studying the pathogenesis of human-tropic viruses as experiments in NHPs are hampered by logistical, financial, and ethical concerns. This creates a pressing need for more tractable small animal models to study existing and emerging viral diseases. In the last few decades, humanized mice have emerged as a solution to this problem. Humanized mice can be generated by expressing human genes whose products are needed for viral infection (Table 1 ), such as entry factors, or through xenotransplantation of hematopoietic stem cells (creating human immune system mice, known as HIS) and/or other human tissues (Figure 1 ).
Table 1.
Prominent examples of factors allowing or restricting aspects of different viral life cycles
| Pathogen | Disease/symptoms | Host factors needed at different steps of the viral life cycle in humans | Factors restricting infection in mice |
|---|---|---|---|
| HIV (as reviewed in [59]) | Leads to decreased levels of CD4+ T cells, ultimately resulting in AIDS | Entry: CD4, CCR5, CXCR4 (some T-tropic HIV-1 viruses can use the murine ortholog of CXCR4) Post-entry: Cyclin T1 |
Transcription: low Tat activity (needs human cyclinT1 as cofactor for successful binding to trans-activation response element) Post-translation: excessive splicing of HIV-1 RNA Poor particle assembly |
| Polio virus [60] | Poliomyelitis, with paralysis in some individuals due to nerve cell damage | Entry: poliovirus receptor | |
| Measles virus [61] | Measles (also known as rubeola), which leads to respiratory infection | Entry: CD46 | |
| HCV (as reviewed in [42]) | Hepatitis C, which can lead to liver cirrhosis, fibrosis, and hepatocellular carcinoma | Entry: OCLN and CD81 (minimal necessary entry factors) | Replication: Innate immune responses |
| HBV [56••] | Hepatitis B, which has similar effects on liver health as hepatitis C | Entry: NTCP | Post-entry: no cccDNA formation; other post-entry restrictions unknown |
| Ebola virus [62] | Fever, diarrhea, and disrupted liver and kidney function; can lead to internal and external bleeding | Entry: Niemann-Pick C1 | Unknown |
Figure 1.
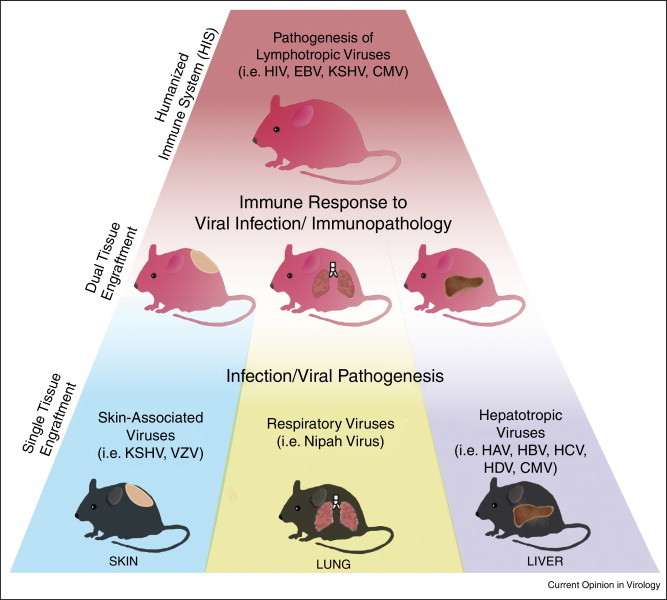
Humanized mice for study of viral pathogenesis. The direct cytopathic effects of a virus on a particular tissue can be studied in mice engrafted with a single human tissue. Skin engraftments have been utilized to study KSHV, where mice exhibit the development of skin lesions and latent infection in cells with altered morphology [43]. Similarly, VZV-infected humanized mice form lesions and have necrosis of multiple skin layers [44]. Respiratory viruses, such as Nipah Virus, can be studied in mice following engraftment of human fetal lung tissue, resulting in viral infection at high titers specifically in the lungs [62]. Following human liver engraftment, mice are susceptible to infection with both HCV [41] and HBV [42]. Lymphotropic viruses, such as HIV-1 and EBV, have been studied in humanized immune system (HIS) mice. However, these HIS mice engrafted with other human tissues have also proven their utility for studying human immune responses exhibited during viral infection.
This paper highlights the recent progress and challenges in studying viral pathogenesis in humanized mice. We will discuss four groups of human-tropic viruses — HIV, DENV, herpesviruses, and hepatitis viruses — as examples of diseases for which specific types of humanized mice were and still are enabling experimental platforms. Using these examples, we will provide a general outlook on how humanized mice can be adapted and refined through genetic host adaptations and/or co-engraftment of multiple tissues to facilitate analysis of other viral infections.
Human immunodeficiency virus (HIV)
In 2013 alone, 1.5 million people worldwide died from AIDS, and 33 million were cited as living with HIV. Besides humans, only chimpanzees are readily susceptible to HIV, but since they usually do not progress to AIDS, they have not gained traction as HIV animal models. In searching for alternatives, it was shown that smaller NHPs, specifically rhesus macaques, were susceptible to simian immunodeficiency virus (SIV), leading to AIDS-like symptoms. To improve the utility of this model, chimeric viruses closely resembling HIV-1, namely simian-human immunodeficiency virus (SHIV) and simian-tropic HIV (stHIV), were generated [1].
Despite intense efforts, it has not yet become possible to genetically overcome species barriers and recapitulate the HIV life-cycle in small animal models. Advances have been made, but they are primarily focused on establishing HIV uptake in mice [2]. Since HIV is a lymphotropic virus primarily infecting CD4 T cells, engraftment of human immune system components proved a viable approach to establish HIV infections in a small animal model. Early models pioneered by McCune and colleagues, based on engrafting xenorecipients with human fetal thymic or lymph node implants, demonstrated that an acute infection of human lymphoid organs with HIV-1 can be followed in humanized mice [3]. With the improvement of xenorecipient strains and humanization protocols (extensively reviewed in [4]), HIS mice have deepened our knowledge about HIV viral transmission, immune responses to HIV and the efficacy of novel therapeutic interventions. The ability of HIV-1-infected cells to form latent reservoirs has been especially challenging in completely curing individuals of the virus [5]. Recently, several groups have shown that HIV-1 latency can be observed in humanized mouse models [6, 7, 8]. These mice have made it possible to model in vivo, for example, how treatment using broadly neutralizing antibodies in combination with inducers can prevent viral rebound following removal from antiretrovirals [9]. In hindering transmission, vectored immunoprophylaxis has shown promise as a way to obstruct intravenous [10] and mucosal transmission of HIV in humanized mice [11]. As the latter is the primary route by which individuals become infected, the in vivo model for mucosal HIV transmission is physiologically relevant and provides a venue for testing anti-viral therapies. Immune responses in HIS mice are suboptimal because of a variety of incompatibilities between the mouse and human immune system. Nonetheless, it was shown that in a particular version of HIS mice, so-called bone-marrow liver thymus (BLT) mice, the dynamic interplay of HIV-specific cellular immunity and viral escape from immune pressure can be accurately modeled [12••].
Dengue virus (DENV)
Dengue is a mosquito-borne disease, caused by DENV, a positive-sense, single-stranded RNA virus belonging to the family Flaviviridae. Four genetically and antigenically distinct serotypes, DENV-1 to DENV-4, have been described, annually causing ∼390 million infections which range in severity from completely asymptomatic to lethal hemorrhagic fever or shock syndrome (DHF and DSS, respectively) [13]. Since a vaccine still does not exist, studying the immune response to DENV is of especial importance, as individuals with previous immunity are more susceptible to developing DHF and DSS [14, 15]. Murine xenorecipient strains expressing HLA-A2 were injected with human blood-forming stem cells and demonstrated improved immune responses to tissue-culture derived DENV, especially in assessing human T-cell response to DENV during and after acute infection [16]. Additionally, it was shown that viremia can be suppressed by administration of direct-acting antivirals (DAAs) to humanized mice that displayed symptoms similar to those in humans following infection with a clinical DENV isolate [17], paving the way for creating and testing DAAs that could be utilized in treating DENV. However, while priming of DENV-specific B and T cell responses occurs at some level, it is not sufficiently robust in existing models. This poses challenges for untangling the mechanisms of why DHF/DSS is so much higher in individuals with secondary heterologous DENV infections. Further light has also been shed on identifying the cells targeted by DENV. Past research in humanized mice concluded that T cells were not infected by DENV [18, 19], but two groups have recently observed evidence to the contrary [17, 20]. Finally, since DENV is mosquito-borne, understanding transmission from host to vector and vice versa is important for examining viral spread in populations and preventing large-scale outbreaks. Thus, the examination for the first time of the human immune response in humanized mice following DENV infection by mosquito bite is an encouraging step [21].
Human herpesviruses (HHVs)
The nine HHVs are prevalent and can establish long-lasting latent infections, leading to skin lesions, epilepsy, cancer, and autoimmune disease (for review see [22]).
Epstein Barr Virus (EBV) is widespread and linked to ∼2% of human tumors originating in lymphocytes and epithelial cells [23]. Only B cell EBV infection can be studied in humanized mice, and different stages of the latent and lytic viral life cycle have been observed in these cells [24, 25]. EBV-associated malignancies have also been studied in HIS mice. A viral mutant lacking EBV latent nuclear antigen 3B (EBNA3B) led to formation of large B cell lymphomas in HIS mice [26•]. Further research on this strain has provided evidence that various EBNA3 antigens regulate expression of the chemokine CXCL10, leading to reduced T cell action [27]. EBV-associated hemophagocytic lymphohistiocytosis, with a pathology highly similar to that seen in humans [28], and erosive arthritis [29] have both been observed in humanized mice. Finally, HIS mice have also been a platform for examining the role of innate immunity in EBV infection (reviewed in [30]).
Human cytomegalovirus (HCMV) is the most common causative agent of congenital viral infection, resulting in children with growth defects or, most detrimental, CNS injury. Additionally, immune-compromised patients, such as individuals living with AIDS or recent organ transplant recipients, are also at risk for HCMV-mediated disease [31]. CMV is found in numerous species, but the determinants of species tropism are not yet defined. While rodents and other animals have been utilized to study congenital CMV infection via their species-specific virus [32, 33], it is still not possible to specifically model congenital HCMV infection. However, progress is being made — immunocompromized mice engrafted with human CD34+ hematopoietic stem cells were able for the first time to establish both systemic HCMV infection and also viral latency and reactivation [34]. Even more recently, HCMV infection was established in the human hepatocytes of a human liver chimeric mouse, building toward a resource for in vivo testing of candidate therapies [35].
Human T cell leukemia virus type 1 (HTLV-1) is strongly linked to the development of adult T cell leukemia/lymphoma (ATL) and the inflammatory disease HTLV-1-associated myelopathy/tropical spastic paraparesis. HIS mice are primarily used to study human T cells during early HTLV-1 infection and initial stages of HTLV-associated diseases. These mice are able to reproduce some aspects of infection in humans, such as CD4+ T cell lymphomas [36] and symptoms associated with changes in human thymopoiesis [37]. Efforts to improve the adaptive immune response in CD133+ mice by injecting human hematopoietic stem cells into the bone marrow of these mice has led to more consistent B-to-T-cell ratios over time and is thus a better approach for studying ATL development [38].
Finally, immunocompromised mice with human skin transplants have been utilized for studying the herpesviruses most highly associated with skin lesions, such as Kaposi's sarcoma-associated herpesviruses [39] and Varicella-zoster virus [40].
Hepatitis B and C Virus (HBV and HCV)
HBV and HCV together infect ∼490 million individuals worldwide, causing liver cirrhosis, fibrosis, and hepatocellular carcinoma if left untreated. These two viruses robustly infect only chimpanzees and humans. In the absence of a permissive mouse model, numerous transgenic strains expressing individual or combinations of HCV gene products were developed to study HBV and HCV-induced liver disease (Table 2 , see [41] for HBV and [42] for HCV for in-depth reviews on existing humanized mouse models for these viruses). HBV transgenic mice have contributed substantially to our understanding of many aspects of HBV biology, immunobiology and pathogenesis (reviewed in [43]). In contrast, reports on the histopathology in HCV protein transgenic mice differ vastly depending on the expressed HCV gene product, mouse background or differences in the promoters used for the expression of viral proteins. Their utility is further lessened as they are not bona fide infection models as any pathological processes develop in the absence of the inflammatory milieu established during chronic infection.
Table 2.
Examples of transgenic mice and their associated phenotypes in studying HBV and HCV
| Pathogen | Component of virus expressed | Resultant phenotype in mouse |
|---|---|---|
| HCV (as reviewed in [42]) | Core | Apoptosis of hepatocytes, lipogenesis |
| NS4B | No liver disease observed | |
| E1-E2-NS2 | Liver injury | |
| Core-E2 | No liver disease observed | |
| HBV (as reviewed in [41]) | HBV surface antigen (HBsAg) and pre-S and X antigens | No viral replication or signs of liver disease |
| X gene | Tumor formation in the liver | |
| Hepatitis B core antigen (HBcAg) | T cell tolerance in response to HBcAg, but no liver disease observed | |
| 1.3 HBV-DNA | High viral particle production | |
To study HBV and HCV infection in mice, humanization of the mouse liver by xenoengraftment of permissive human primary hepatocytes has been explored. Most of the commonly used xenorecipients share common features: they are immunodeficient to prevent xenograft rejection and often suffer from an endogenous liver injury to promote hepatocyte proliferation and provide human donor cells a competitive growth advantage over mouse hepatocytes. In 2001, the development of an Alb-uPA/Rag-2 mouse, which could be engrafted with primary human hepatocytes, was successfully infected with HBV [44] and also HCV [45]. To improve robustness and throughput, several other immunodeficient liver injury models, including fumaryl acetoacetate hydrolase deficient mice (FAH−/− [46]), MUP-uPA [47] and Alb-HSV1-tk [48], have been generated. These mice can be engrafted to very high levels with human hepatocytes and subsequently become susceptible to HBV and HCV infection. Human liver chimeric mice have been critical tools for studying HBV, HCV and HDV infections but also serve as important tools for preclinically assessing the efficacy of novel therapeutics. However, HBV and HCV pathogenesis, human adaptive immune responses and vaccine development can only be studied in a mouse model harboring both a human liver graft and a functional human immune system. Several groups have now reported that dual engraftment of components of a human immune system and a (matching) human liver can be achieved in a single recipient [49, 50]. When infected with HBV [51] or HCV [52], dually engrafted mice indeed mount virus-specific immune responses and develop histopathological features reminiscent of liver disease in humans. However, the difficulties of donor matching for the two tissue compartments, variation in the level of engraftment, the low throughput and the limited functionality of the engrafted human immune system lessen the utility of this model.
An inbred mouse model with inheritable susceptibility to HBV or HCV would overcome the technical difficulties of the xenotransplantation model. The challenge is to systematically identify and overcome any restrictions to viral growth in murine cells. Both the HBV and HCV life-cycles are blocked at numerous steps. For HCV, the minimal set of human specific entry factors, that is human CD81 and occludin (OCLN) have been identified, facilitating HCV uptake into mouse cells in vitro [53] and in vivo [54]. The entire HCV life-cycle can be recapitulated in mice transgenically expressing human CD81 and OCLN with severely impaired antiviral innate immunity [55••]. The recent identification of human taurocholate co-transporting polypeptide (hNTCP) as an HBV receptor [56••] is a promising first step toward creation of a mouse model with inheritable susceptibility to HBV infection. However, it should be noted that there are still numerous blocks to overcome. While HBV assembly and release are supported in mouse hepatocytes, expression of hNTCP does not render mouse cells permissive for HBV uptake, pointing toward post-attachment and post-entry blocks. These include, but are not limited to, the inability of HBV to form covalently closed circular DNA, its main transcriptional template.
Conclusions
The use of humanized mice in infectious disease research provides a forum for studying viruses previously less accessible due to their species tropism. With so many types of humanized mice now available, researchers will continue to improve and expand upon these models. The research discussed here has provided invaluable lessons for handling emerging viral threats, as exemplified by the quick development of a humanized mouse for studying Middle Eastern Respiratory Syndrome (MERS [57•]) and a lung xenotransplantation model for the emerging Nipah Virus [58••]. As viruses continue to evolve and adapt to new hosts, humanized mice will be an indispensable tool for studying pathogenesis and will increase the likelihood of developing more efficacious therapeutics.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
We would like to thank Florian Douam, Benjamin Winer, and Qiang Ding for their helpful discussion and comments on drafts of this paper. Work in the laboratory is in part supported by grants from the National Institutes of Health (2 R01 AI079031-05A1, 1 R01 AI107301-01, 1 R56 AI106005-01), the Walter Reed Army Institute of Research, the Bill and Melinda Gates Foundation and the Grand Challenge Program of Princeton University. JMG is supported by co-funding from NIAID on iNRSA 5T32GM007388. We apologize to all colleagues whose work could not be cited due to space constraints.
References
- 1.Hatziioannou T., Ambrose Z., Chung N.P.Y., Piatak M., Yuan F., Trubey C.M., Coalter V., Kiser R., Schneider D., Smedley J. A macaque model of HIV-1 infection. Proc Natl Acad Sci U S A. 2009;106:4425–4429. doi: 10.1073/pnas.0812587106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pietzsch J., Gruell H., Bournazos S., Donovan B.M., Klein F., Diskin R., Seaman M.S., Bjorkman P.J., Ravetch J.V., Ploss A. A mouse model for HIV-1 entry. Proc Natl Acad Sci U S A. 2012;109:15859–15864. doi: 10.1073/pnas.1213409109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Namikawa R., Kaneshima H., Lieberman M., Weissman I.L., McCune J.M. Infection of the SCID-hu mouse by HIV-1. Science. 1988;242:1684–1686. doi: 10.1126/science.3201256. [DOI] [PubMed] [Google Scholar]
- 4.Shultz L.D., Brehm M.A., Garcia-Martinez J.V., Greiner D.L. Humanized mice for immune system investigation: progress, promise and challenges. Nat Rev Immunol. 2012;12:786–798. doi: 10.1038/nri3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marsden M.D., Zack J.A. HIV/AIDS eradication. Bioorg Med Chem Lett. 2013;23:4003–4010. doi: 10.1016/j.bmcl.2013.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choudhary S.K., Archin N.M., Cheema M., Dahl N.P., Garcia J.V., Margolis D.M. Latent HIV-1 infection of resting CD4+ T cells in the humanized Rag2−/− c−/− mouse. J Virol. 2012;86:114–120. doi: 10.1128/JVI.05590-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denton P.W., Olesen R., Choudhary S.K., Archin N.M., Wahl A., Swanson M.D., Chateau M., Nochi T., Krisko J.F., Spagnuolo R.A. Generation of HIV latency in humanized BLT mice. J Virol. 2012;86:630–634. doi: 10.1128/JVI.06120-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marsden M.D., Kovochich M., Suree N., Shimizu S., Mehta R., Cortado R., Bristol G., An D.S., Zack J.A. HIV latency in the humanized BLT mouse. J Virol. 2012;86:339–347. doi: 10.1128/JVI.06366-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halper-Stromberg A., Lu C.L., Klein F., Horwitz J.A., Bournazos S., Nogueira L., Eisenreich T.R., Liu C., Gazumyan A., Schaefer U. Broadly neutralizing antibodies and viral inducers decrease rebound from HIV-1 latent reservoirs in humanized mice. Cell. 2014 doi: 10.1016/j.cell.2014.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balazs A.B., Chen J., Hong C.M., Rao D.S., Yang L., Baltimore D. Antibody-based protection against HIV infection by vectored immunoprophylaxis. Nature. 2011;481:81–84. doi: 10.1038/nature10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balazs A., Ouyang Y., Hong C., Chen J., Nguyen S., Rao D., An D., Baltimore D. Vectored immunoprophylaxis protects humanized mice from mucosal HIV transmission. Retrovirology. 2014;20:296–300. doi: 10.1038/nm.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12••.Dudek T.E., No D.C., Seung E., Vrbanac V.D., Fadda L., Bhoumik P., Boutwell C.L., Power K.A., Gladden A.D., Battis L. Rapid evolution of HIV-1 to functional CD8+ T cell responses in humanized BLT mice. Sci Transl Med. 2012;4:143ra98. doi: 10.1126/scitranslmed.3003984. [DOI] [PMC free article] [PubMed] [Google Scholar]; Analyzing HIV-1 infection in humanized BLT mice, this study observed CD8+ T cell responses that were highly similar in their specificity, kinetics and magnitude to those observed in humans. The rapid appearance of viral escape mutations from host immune responses, as also seen in humans, further underscored the utility of humanized BLT mice for studying human-specific immunopathology of pathogens.
- 13.Bhatt S., Gething P.W., Brady O.J., Messina J.P., Farlow A.W., Moyes C.L., Drake J.M., Brownstein J.S., Hoen A.G., Sankoh O. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Costa V.V., Fagundes C.T., Souza D.G., Teixeira M.M. Inflammatory and innate immune responses in dengue infection: protection versus disease induction. Am J Pathol. 2013;182:1950–1961. doi: 10.1016/j.ajpath.2013.02.027. [DOI] [PubMed] [Google Scholar]
- 15.Guabiraba R., Ryffel B. Dengue virus infection: current concepts in immune mechanisms and lessons from murine models. Immunology. 2014;141:143–156. doi: 10.1111/imm.12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaiswal S., Pazoles P., Woda M., Shultz L.D., Greiner D.L., Brehm M.A., Mathew A. Enhanced humoral and HLA-A2-restricted dengue virus-specific T-cell responses in humanized BLT NSG mice. Immunology. 2012;136:334–343. doi: 10.1111/j.1365-2567.2012.03585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frias-Staheli N., Dorner M., Marukian S., Billerbeck E., Labitt R.N., Rice C.M., Ploss A. Utility of humanized BLT mice for analysis of dengue virus infection and antiviral drug testing. J Virol. 2014;88:2205–2218. doi: 10.1128/JVI.03085-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blackley S., Kou Z., Chen H., Quinn M., Rose R.C., Schlesinger J.J., Coppage M., Jin X. Primary human splenic macrophages, but not T or B cells, are the principal target cells for dengue virus infection in vitro. J Virol. 2007;81:13325–13334. doi: 10.1128/JVI.01568-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kou Z., Quinn M., Chen H., Rodrigo W.W.S.I., Rose R.C., Schlesinger J.J., Jin X. Monocytes, but not T or B cells, are the principal target cells for dengue virus (DV) infection among human peripheral blood mononuclear cells. J Med Virol. 2008;80:134–146. doi: 10.1002/jmv.21051. [DOI] [PubMed] [Google Scholar]
- 20.Mota J., Rico-Hesse R. Humanized mice show clinical signs of dengue fever according to infecting virus genotype. J Virol. 2009;83:8638–8645. doi: 10.1128/JVI.00581-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cox J., Mota J., Sukupolvi-Petty S., Diamond M.S., Rico-Hesse R. Mosquito bite delivery of dengue virus enhances immunogenicity and pathogenesis in humanized mice. J Virol. 2012;86:7637–7649. doi: 10.1128/JVI.00534-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berges B.K., Tanner A. Modelling of human herpesvirus infections in humanized mice. J Gen Virol. 2014;95:2106–2117. doi: 10.1099/vir.0.067793-0. [DOI] [PubMed] [Google Scholar]
- 23.Cohen J.I., Fauci A.S., Varmus H., Nabel G.J. Epstein-Barr virus: an important vaccine target for cancer prevention. Sci Transl Med. 2011;3:107fs7. doi: 10.1126/scitranslmed.3002878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chijioke O., Azzi T., Nadal D., Münz C. Innate immune responses against Epstein Barr virus infection. J Leukoc Biol. 2013;94:1185–1190. doi: 10.1189/jlb.0313173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heuts F., Rottenberg M.E., Salamon D., Rasul E., Adori M., Klein G., Klein E., Nagy N. T cells modulate Epstein-Barr virus latency phenotypes during infection of humanized mice. J Virol. 2014;88:3235–3245. doi: 10.1128/JVI.02885-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26•.White R.E., Rämer P.C., Naresh K.N., Meixlsperger S., Pinaud L., Rooney C., Savoldo B., Coutinho R., Bödör C., Gribben J. EBNA3B-deficient EBV promotes B cell lymphomagenesis in humanized mice and is found in human tumors. J Clin Invest. 2012;122:1487–1502. doi: 10.1172/JCI58092. [DOI] [PMC free article] [PubMed] [Google Scholar]; Here, huNSG mice infected with an EBNA3B-deficient EBV were found to develop highly aggressive tumors. These findings connected well to clinical samples where aggressive lymphomas were associated with mutations in EBNA3B. This work in humanized mice provided novel evidence that EBNA3B might be the first example of a virus-encoded tumor suppressor.
- 27.Harth-Hertle M.L., Scholz B.A., Erhard F., Glaser L.V., Dölken L., Zimmer R., Kempkes B. Inactivation of intergenic enhancers by EBNA3A initiates and maintains polycomb signatures across a chromatin domain encoding CXCL10 and CXCL9. PLoS Pathog. 2013:9. doi: 10.1371/journal.ppat.1003638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sato K., Misawa N., Nie C., Satou Y., Iwakiri D., Matsuoka M., Takahashi R., Kuzushima K., Ito M., Takada K. A novel animal model of Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis in humanized mice. Blood. 2011;117:5663–5673. doi: 10.1182/blood-2010-09-305979. [DOI] [PubMed] [Google Scholar]
- 29.Kuwana Y., Takei M., Yajima M., Imadome K.I., Inomata H., Shiozaki M., Ikumi N., Nozaki T., Shiraiwa H., Kitamura N. Epstein-Barr virus induces erosive arthritis in humanized mice. PLoS ONE. 2011:6. doi: 10.1371/journal.pone.0026630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chatterjee B., Leung C.S., Münz C. Animal models of Epstein Barr virus infection. J Immunol Methods. 2014 doi: 10.1016/j.jim.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 31.Söderberg-Nauclér C. Does cytomegalovirus play a causative role in the development of various inflammatory diseases and cancer? J Intern Med. 2006;259:219–246. doi: 10.1111/j.1365-2796.2006.01618.x. [DOI] [PubMed] [Google Scholar]
- 32.Slavuljica I., Kveštak D., Huszthy P.C., Britt K.K., Jonjić W.J.S. Immunobiology of congenital cytomegalovirus infection of the central nervous system—the murine cytomegalovirus model. Cell Mol Immunol. 2014 doi: 10.1038/cmi.2014.51. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schleiss M.R. Developing a vaccine against congenital cytomegalovirus (CMV) infection: what have we learned from animal models? Where Should We Go Next? Future Virol. 2013;8:1161–1182. doi: 10.2217/fvl.13.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith M.S., Goldman D.C., Bailey A.S., Pfaffle D.L., Kreklywich C.N., Spencer D.B., Othieno F.A., Streblow D.N., Garcia J.V., Fleming W.H. Granulocyte-colony stimulating factor reactivates human cytomegalovirus in a latently infected humanized mouse model. Cell Host Microbe. 2010;8:284–291. doi: 10.1016/j.chom.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawahara T., Lisboa L.F., Cader S., Douglas D.N., Nourbakhsh M., Pu C.H., Lewis J.T., Churchill T.A., Humar A., Kneteman N.M. Human cytomegalovirus infection in humanized liver chimeric mice. Hepatol Res. 2013;43:679–684. doi: 10.1111/j.1872-034X.2012.01116.x. [DOI] [PubMed] [Google Scholar]
- 36.Banerjee P., Tripp A., Lairmore M.D., Crawford L., Sieburg M., Ramos J.C., Harrington W., Beilke M.A., Feuer G. Adult T-cell leukemia/lymphoma development in HTLV-1-infected humanized SCID mice. Blood. 2010;115:2640–2648. doi: 10.1182/blood-2009-10-246959. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37.Villaudy J., Wencker M., Gadot N., Gillet N.A., Scoazec J.Y., Gazzolo L., Manz M.G., Bangham C.R.M., Dodon M.D. Htlv-1 propels thymic human t cell development in ‘human immune system’ rag2−/− gamma c−/− mice. PLoS Pathog. 2011:7. doi: 10.1371/journal.ppat.1002231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tezuka K., Xun R., Tei M., Ueno T., Tanaka M., Takenouchi N., Fujisawa J.I. An animal model of adult T-cell leukemia: humanized mice with HTLV-1-specific immunity. Blood. 2014;123:346–355. doi: 10.1182/blood-2013-06-508861. [DOI] [PubMed] [Google Scholar]
- 39.Foreman K.E., Friborg J., Chandran B., Katano H., Sata T., Mercader M., Nabel G.J., Nickoloff B.J. Injection of human herpesvirus-8 in human skin engrafted on SCID mice induces Kaposi's sarcoma-like lesions. J Dermatol Sci. 2001;26:182–193. doi: 10.1016/s0923-1811(01)00087-1. [DOI] [PubMed] [Google Scholar]
- 40.Moffat J.F., Zerboni L., Kinchington P.R., Grose C., Kaneshima H., Arvin A.M. Attenuation of the vaccine Oka strain of Varicella-zoster virus and role of glycoprotein C in alphaherpesvirus virulence demonstrated in the SCID-hu mouse. J Virol. 1998;72:965–974. doi: 10.1128/jvi.72.2.965-974.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dandri M., Lütgehetmann M. Mouse models of hepatitis B and delta virus infection. J Immunol Methods. 2014 doi: 10.1016/j.jim.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 42.Billerbeck E., De Jong Y., Dorner M., De La Fuente C., Ploss A. Animal models for hepatitis C. Curr Top Microbiol Immunol. 2013;369:49–86. doi: 10.1007/978-3-642-27340-7_3. [DOI] [PubMed] [Google Scholar]
- 43.Chisari F.V. Hepatitis B virus transgenic mice: models of viral immunobiology and pathogenesis. Curr Top Microbiol Immunol. 1996;206:149–173. doi: 10.1007/978-3-642-85208-4_9. [DOI] [PubMed] [Google Scholar]
- 44.Dandri M., Burda M.R., Torok E., Pollok J.M., Iwanska A., Sommer G., Rogiers X., Rogler C.E., Gupta S., Will H. Repopulation of mouse liver with human hepatocytes and in vivo infection with hepatitis B virus. Hepatology. 2001;33:981–988. doi: 10.1053/jhep.2001.23314. [DOI] [PubMed] [Google Scholar]
- 45.Mercer D.F., Schiller D.E., Elliott J.F., Douglas D.N., Hao C., Rinfret A., Addison W.R., Fischer K.P., Churchill T.A., Lakey J.R. Hepatitis C virus replication in mice with chimeric human livers. Nat Med. 2001;7:927–933. doi: 10.1038/90968. [DOI] [PubMed] [Google Scholar]
- 46.Bissig K.-D., Wieland S.F., Tran P., Isogawa M., Le T.T., Chisari F.V., Verma I.M. Human liver chimeric mice provide a model for hepatitis B and C virus infection and treatment. J Clin Invest. 2010;120:924–930. doi: 10.1172/JCI40094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tesfaye A., Stift J., Maric D., Cui Q., Dienes H.P., Feinstone S.M. Chimeric mouse model for the infection of hepatitis B and C viruses. PLOS ONE. 2013:8. doi: 10.1371/journal.pone.0077298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kosaka K., Hiraga N., Imamura M., Yoshimi S., Murakami E., Nakahara T., Honda Y., Ono A., Kawaoka T., Tsuge M. A novel TK-NOG based humanized mouse model for the study of HBV and HCV infections. Biochem Biophys Res Commun. 2013;441:230–235. doi: 10.1016/j.bbrc.2013.10.040. [DOI] [PubMed] [Google Scholar]
- 49.Gutti T.L., Knibbe J.S., Makarov E., Zhang J., Yannam G.R., Gorantla S., Sun Y., Mercer D.F., Suemizu H., Wisecarver J.L. Human hepatocytes and hematolymphoid dual reconstitution in treosulfan-conditioned uPA-NOG mice. Am J Pathol. 2014;184:101–109. doi: 10.1016/j.ajpath.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilson E., Bial J., Bial G., Jensen B., Greiner D., Brehm M., Grompe M. Extensive double humanization of both liver and hematopoiesis in FRGN mice. Stem Cell Res. 2014;13:404–412. doi: 10.1016/j.scr.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bility M.T., Cheng L., Zhang Z., Luan Y., Li F., Chi L., Zhang L., Tu Z., Gao Y., Fu Y. Hepatitis B virus infection and immunopathogenesis in a humanized mouse model: induction of human-specific liver fibrosis and M2-like macrophages. PLoS Pathog. 2014:10. doi: 10.1371/journal.ppat.1004032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Washburn M.L., Bility M.T., Zhang L., Kovalev G.I., Buntzman A., Frelinger J.A., Barry W., Ploss A., Rice C.M., Su L. A humanized mouse model to study hepatitis C virus infection, immune response, and liver disease. Gastroenterology. 2011;140:1334–1344. doi: 10.1053/j.gastro.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ploss A., Evans M.J., Gaysinskaya V.A., Panis M., You H., de Jong Y.P., Rice C.M. Human occludin is a hepatitis C virus entry factor required for infection of mouse cells. Nature. 2009;457:882–886. doi: 10.1038/nature07684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dorner M., Horwitz J.A., Robbins J.B., Barry W.T., Feng Q., Mu K., Jones C.T., Schoggins J.W., Catanese M.T., Burton D.R. A genetically humanized mouse model for hepatitis C virus infection. Nature. 2011;474:208–211. doi: 10.1038/nature10168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55••.Dorner M., Horwitz J.a, Donovan B.M., Labitt R.N., Budell W.C., Friling T., Vogt A., Catanese M.T., Satoh T., Kawai T. Completion of the entire hepatitis C virus life cycle in genetically humanized mice. Nature. 2013;501:237–241. doi: 10.1038/nature12427. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using mice with diminished antiviral immunity that also stably expressed the minimal human factors, human CD81 and OCLN, for viral uptake in murine cells, this study was the first to reproduce the entire HCV life cycle in mice with inheritable susceptibility to HCV. This is a significant improvement upon previous xenotransplant mouse models, allowing for higher throughput work and decreased variabilities between experiments.
- 56••.Yan H., Zhong G., Xu G., He W., Jing Z., Gao Z., Huang Y., Qi Y., Peng B., Wang H. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife. 2012:2012. doi: 10.7554/eLife.00049. [DOI] [PubMed] [Google Scholar]; This paper identified the first HBV and HDV receptor, sodium taurocholate cotransporting polypeptide (NTCP), using tandem affinity purification and mass spectrometry. This finding greatly enhances researchers’ abilities to create in vitro platforms and small-animal models for studying these two viruses.
- 57•.Zhao J., Li K., Wohlford-Lenane C., Agnihothram S.S., Fett C., Zhao J., Gale M.J., Baric R.S., Enjuanes L., Gallagher T. Rapid generation of a mouse model for Middle East respiratory syndrome. Proc Natl Acad Sci U S A. 2014;111:4970–4975. doi: 10.1073/pnas.1323279111. [DOI] [PMC free article] [PubMed] [Google Scholar]; Following transduction of mice with a recombinant, nonreplicating adenovirus expressing the human receptor for MERS, DPP4, these mice were successfully infected with MERS-CoV, developing pneumonia. The quick timeline of 2–3 weeks to create such a model is a promising approach for rapid study of emerging pathogens.
- 58••.Valbuena G., Halliday H., Borisevich V., Goez Y., Rockx B. A human lung xenograft mouse model of Nipah Virus infection. PLoS Pathog. 2014:10. doi: 10.1371/journal.ppat.1004063. [DOI] [PMC free article] [PubMed] [Google Scholar]; Here, the first human lung xenograft model in mice was made and successfully infected with Nipah Virus, which replicated to high titers in the lungs. This is a promising, more physiologically relevant approach for studying the pathogenesis of other respiratory viruses in the context of the human lung microenvironment.
- 59.Takeuchi H., Matano T. Host factors involved in resistance to retroviral infection. Microbiol Immunol. 2008;52:318–325. doi: 10.1111/j.1348-0421.2008.00040.x. [DOI] [PubMed] [Google Scholar]
- 60.Ren R.B., Costantini F., Gorgacz E.J., Lee J.J., Racaniello V.R. Transgenic mice expressing a human poliovirus receptor: a new model for poliomyelitis. Cell. 1990;63:353–362. doi: 10.1016/0092-8674(90)90168-e. [DOI] [PubMed] [Google Scholar]
- 61.Oldstone M.B., Lewicki H., Thomas D., Tishon A., Dales S., Patterson J., Manchester M., Homann D., Naniche D., Holz A. Measles virus infection in a transgenic model: virus-induced immunosuppression and central nervous system disease. Cell. 1999;98:629–640. doi: 10.1016/s0092-8674(00)80050-1. [DOI] [PubMed] [Google Scholar]
- 62.Carette J.E., Raaben M., Wong A.C., Herbert A.S., Obernosterer G., Mulherkar N., Kuehne A.I., Kranzusch P.J., Griffin A.M., Ruthel G. Ebola virus entry requires the cholesterol transporter Niemann-Pick C1. Nature. 2011;477:340–343. doi: 10.1038/nature10348. [DOI] [PMC free article] [PubMed] [Google Scholar]


