Abstract
IMPORTANCE
Major depressive disorder (MDD) has been linked to imbalanced communication among large-scale brain networks, as reflected by abnormal resting-state functional connectivity (rsFC). However, given variable methods and results across studies, identifying consistent patterns of network dysfunction in MDD has been elusive.
OBJECTIVE
To investigate network dysfunction in MDD through the first meta-analysis of rsFC studies.
DATA SOURCES
Seed-based voxel-wise rsFC studies comparing MDD with healthy individuals (published before June 30, 2014) were retrieved from electronic databases (PubMed, Web-of-Science, EMBASE), and authors contacted for additional data.
STUDY SELECTION
Twenty-seven datasets from 25 publications (556 MDD adults/teens; 518 controls) were included in the meta-analysis.
DATA EXTRACTION AND SYNTHESIS
Coordinates of seed regions-of-interest and between-group effects were extracted. Seeds were categorized into “seed-networks” by their location within a priori functional networks. Multilevel kernel density analysis of between-group effects identified brain systems in which MDD was associated with hyperconnectivity (increased positive, or reduced negative, connectivity) or hypoconnectivity (increased negative, or reduced positive, connectivity) with each seed-network.
RESULTS
MDD was characterized by hypoconnectivity within the frontoparietal network (FN), a set of regions involved in cognitive control of attention and emotion regulation, and hypoconnectivity between frontoparietal systems and parietal regions of the dorsal attention network (DAN) involved in attending to the external environment. MDD was also associated with hyperconnectivity within the default network (DN), a network believed to support internally-oriented and self-referential thought, and hyperconnectivity between FN control systems and regions of DN. Finally, MDD groups exhibited hypoconnectivity between neural systems involved in processing emotion or salience and midline cortical regions that may mediate top-down regulation of such functions.
CONCLUSIONS AND RELEVANCE
Reduced connectivity within frontoparietal control systems, and imbalanced connectivity between control systems and networks involved in internal- or external-attention, may reflect depressive biases towards internal thoughts at the cost of engaging with the external world. Meanwhile, altered connectivity between neural systems involved in cognitive control and those that support salience or emotion processing may relate to deficits regulating mood. These findings provide an empirical foundation for a neurocognitive model in which network dysfunction underlies core cognitive and affective abnormalities in depression.
Keywords: depression, connectivity, meta-analysis, network, resting-state
Major Depressive Disorder (MDD) is a psychiatric illness with devastating social, personal, and medical consequences.1,2 Moreover, MDD is ubiquitous, affecting more than 16 million people in the United States3 and 350 million people worldwide4 each year. Although significant progress has been made in understanding MDD and developing treatments, much is unknown about the pathophysiology of the disease, and rates of recurrence remain high.5 Exploring the neurobiological signature of MDD from new perspectives has the potential to transform current conceptualizations of the disease and sharpen the search for treatment targets.6
Recently, researchers have become increasingly interested in the role of abnormal communication among large-scale functional brain networks in the pathophysiology of MDD.6,7 Functional networks can be defined as distributed sets of brain regions that exhibit correlated activity either at rest, i.e., resting-state functional connectivity (rsFC), or during task performance.8,9 The recruitment of a highly-synchronized network, either in response to task demands or at rest, is believed to reflect distinct cognitive or emotional processes or mental states (e.g., mind-wandering),10–12 although these relationships are complex and remain a rapidly evolving field of study. Of particular relevance are networks putatively related to processes affected in depression, such as the frontoparietal network (FN), involved in top-down regulation of attention and emotion; the default network (DN) and dorsal attention network (DAN), involved in internally- or externally-oriented attention, respectively; and the affective network (AN) and ventral attention network (VAN) (sometimes together called the salience network13), involved in processing emotion or monitoring for salient events.14–16 For example, abnormal communication within FN may underlie deficits in cognitive control, which are commonly observed in depression17 and may contribute to symptoms such as difficulty concentrating or regulating emotions. Likewise, aberrant communication between FN and DN may reflect ongoing rumination, or an underlying bias for control systems to allocate resources towards internal thoughts at the cost of engaging with the external world.18 Hence, specific patterns of network dysfunction may contribute to core deficits in cognitive and affective functioning that are believed to underlie clinical symptoms.
Investigation of functional networks has surged in recent years, in particular in the domain of rsFC. Initial findings support the view that MDD is characterized by abnormal rsFC,19 but inconsistency in the location and nature of effects makes it difficult to unify this research. Variability across studies may emerge for several reasons, including small sample sizes or differences in the networks selected for study. For example, studies using seed-based rsFC,20 the most common analytic strategy, vary considerably in the location of seed regions-of-interest (ROIs). Although a spatially extensive set of seed ROIs provides a comprehensive view of rsFC across the brain, organizing results into a coherent model of network functioning is challenging. A theoretically-informed strategy for categorizing seed ROIs and related findings, e.g., by the location of seed ROIs within functional networks, would help organize the diverse set of findings and allow for a direct test of replication across studies. Meta-analysis is arguably the most powerful tool for synthesizing this research, as it is capable of evaluating whether effects are robust across differences in methodological details and disentangling consistent effects from false positives.21,22 However, although rsFC abnormalities related to MDD have been reviewed,19 meta-analysis of this burgeoning literature has never been performed.
The present study aimed to fill this important gap by conducting a meta-analysis of seed-based rsFC studies, and unifying findings in a neurocognitive model of depression. Primary analyses tested for consistency in the location of brain systems exhibiting depression-related hyper- or hypoconnectivity with seed ROIs, which in turn were categorized within a priori networks. Based on evidence for broad deficits in cognitive control in MDD,17 it was predicted that seed ROIs located within the frontoparietal network would exhibit reduced connectivity with other areas of FN. In addition, based on the central role of ruminative, self-referential thinking in cognitive models of depression,23,24 it was predicted that seed ROIs located within the default network would exhibit increased connectivity with other DN regions, and increased connectivity with prefrontal regions of FN involved in directing attention. Secondary analyses tested whether rsFC abnormalities were moderated by seed anatomy, or by demographic or clinical factors.
Methods
Literature Search
A comprehensive literature search was conducted in Web-of-Science, PubMed, and EMBASE, using the keywords rest*(-ing), connect*(-ivity), and depress*(-ion, -ive). Manual searches were conducted within the references sections of empirical and review papers, and for publications that cited those papers. Original fMRI studies using whole-brain seed-based rsFC to compare MDD with healthy control (HC) groups were eligible for inclusion (other rsFC methods (e.g., ICA) adopt a distinct statistical approach that cannot be aggregated with seed-based data). If a published study did not report whole-brain effects, or did not provide seed ROI or peak effect coordinates, authors were contacted for this information. Exclusion criteria were: (1) no HC group or no current MDD group; (2) non-seed-based method; (3) whole-brain results could not be retrieved or did not survive correction (meta-analyses of fMRI data test for consistency in the spatial location of significant effects across studies;22 thus, only studies that reported group differences in rsFC were eligible for inclusion); (4) entirely overlapping sample and seed ROIs reported in another publication; or (5) seed ROI or peak effect coordinates could not be retrieved (eFig. 1). Publications reporting on the same sample but using different seed ROIs were coded as a single study; publications in which distinct MDD groups were each compared to a single HC group were coded as distinct studies, and supplementary analyses were conducted to address the issue of partial nonindependence.25 These searches and inclusion criteria yielded a sample of 27 studies from 25 publications26–50 reporting on 556 individuals with MDD and 518 controls (eTables 1–2).
Data Extraction and Coding
The present meta-analysis was coordinate-based,21,22,51 but here, coordinates reflected the locations of significant group differences in functional connectivity at the time series level. Data extraction and coding included the following: first, coordinates for the center-of-mass of each seed ROI (91 seeds), and the peak of each significant between-group effect (346 effects), were extracted for each study and converted to Montreal Neurological Institute space as needed.52 If the seed ROI was an anatomical region from a mask or standard brain atlas, center-of-mass was calculated to obtain a representative coordinate. Second, each seed ROI was categorized into a “seed-network” based on the location of its center-of-mass within a priori rsFC networks defined by a previous whole-brain network parcellation in 1,000 participants14–16 (eTable 3). This network parcellation was selected given its full coverage of cortex, cerebellum, and striatum; its definition in a large sample; its replication across an independent sample; and its close correspondence with networks derived from alternative rsFC analytic strategies and task-based patterns of co-activation.53,54
Effects were also categorized based on the direction of effect, i.e., hyper- or hypoconnectivity in MDD groups. In previous studies, hyperconnectivity (MDD>HC) has been defined as larger positive, or reduced negative, rsFC in MDD compared with HC groups; hypoconnectivity (MDD<HC) has been defined as larger negative, or reduced positive, rsFC in MDD compared with HC groups. Because the distinction between enhanced and weakened connectivity was inconsistently reported in the studies reviewed here, it was not possible to test these forms of rsFC abnormality separately. However, when reported in the original publication, patterns of abnormal rsFC related to stronger or weaker connectivity in MDD are noted in the results.
Multilevel Kernel Density Analysis
Multilevel kernel density analysis (MKDA)22 was performed using the MKDA toolbox (http://www.wagerlab.colorado.edu), a Matlab (Mathworks, Natick, MA) toolbox that incorporates tools from Statistical Parametric Mapping (http://www.fil.ion.ucl.ac.uk/spm/).
Coordinates for peak effects from each study and seed-network comparison were convolved with a spherical kernel (r=15mm55,56) and thresholded at a maximum value of 1, yielding an indicator map in which a value of 1 indicated a significant effect in the neighborhood and a value of 0 indicated no significant effect. Next, the density of effects across studies was computed by averaging the indicator maps, weighted by study sample size.21 The resulting density maps showed the proportion of studies in which hyper- or hypoconnectivity with each seed-network was observed in MDD within 15mm of each voxel. Differences between density maps were calculated to test for directional effects, e.g., either consistent hyperconnectivity or hypoconnectivity in MDD (unless otherwise noted, all effects were specific to one direction).
A Monte Carlo simulation was performed to establish the Family-Wise Error Rate (FWER) threshold used to correct for multiple comparisons. In this simulation, the locations of significant effects from indicator maps were randomized within a gray-matter mask in 15,000 iterations, yielding an estimate of the maximum density of effects predicted to occur by chance. A FWER threshold of p<0.05 was met when the density statistic exceeded the maximum null in 95% of the Monte Carlo maps. Density maps can be thresholded based on height (density at that voxel exceeds the maximum expected over the entire brain by chance) or extent (density at multiple contiguous voxels exceeds the maximum expected in a cluster of that size by chance). Because these thresholds provide complementary information, both are reported. Findings are discussed in terms of within-network abnormalities (effects fall within the same functional network as seed ROIs) or between-network abnormalities (effects fall outside the functional network in which seed ROIs are located).
Post-hoc Analyses
Three categories of post-hoc tests were conducted. First, jackknife analyses were conducted to assess whether the inclusion of any partially non-independent study disproportionately affected the results.25 To accomplish this, the density statistic for each significant cluster was iteratively recalculated leaving out each partially non-independent study, and a chi-square test or Fisher’s exact test was performed between the original density statistic and the leave-one-out density statistic. Because these analyses failed to reveal disproportionate effects of any individual study, results reported here include all studies. Second, Fisher’s tests were conducted to investigate whether a specific anatomical region contributed more strongly to a significant effect than other regions of the same network. Although the primary analytic approach of grouping regions into functional networks made meta-analysis possible by boosting power across studies, this network-level approach made the assumption that distinct regions within each functional network show similar abnormalities in MDD. Therefore, post-hoc region-level analyses were conducted by calculating the likelihood of a particular effect for seeds in distinct anatomical regions of a functional network, and testing the difference in effect likelihood between regions. Third, analyses were performed to investigate moderation of effects by clinical and demographic factors (eTable 1), including severity of depression (mild/moderate/severe57–59), medication status (yes/no medication use in MDD group), or age (teen/adult/elder). For these analyses, proportion of studies within each clinical/demographic group reporting the effect was calculated, and differences in proportions were tested between groups.
Results
Within-Network Abnormalities
Hypoconnectivity within the frontoparietal network (FN)
MDD was associated with hypoconnectivity between FN seeds and bilateral posterior parietal cortex (PPC), regions involved in attending to goal-relevant stimuli or features of an internal representation60 (Fig. 1A, Table 1). Examining the original empirical studies revealed that, when reported, hypoconnectivity was related to weaker positive connectivity between FN seeds and PPC.26,27 Specifically, FN seeds in dorsolateral prefrontal cortex (DLPFC) or cerebellum exhibited hypoconnectivity with PPC, and post-hoc testing indicated that seeds in DLPFC were more likely than cerebellar seeds to exhibit hypoconnectivity with right PPC, Likelihood Ratio=5.29, p=0.04, although no differences were detected for the left PPC, p=0.53. Hypoconnectivity within FN was not moderated by age, depression severity, or medication status, p’s>0.17.
Figure 1. Results of meta-analysis.
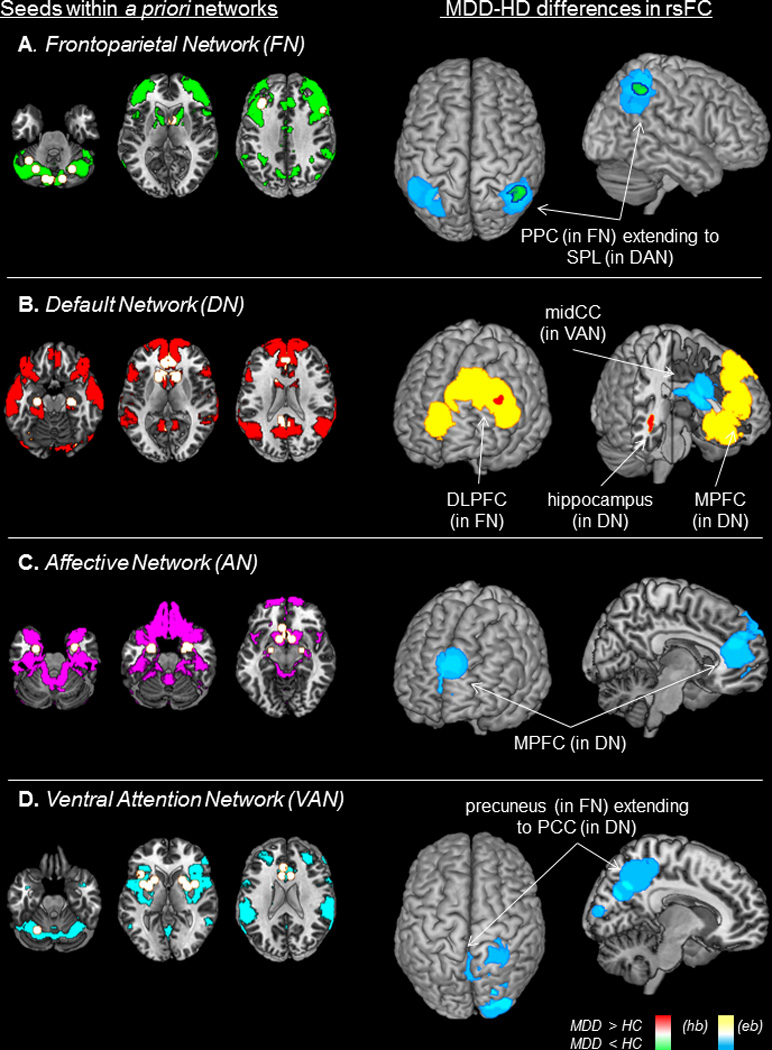
Seed regions-of-interest categorized by a priori functional network (left panels), and results of meta-analysis (right panels) showing regions in which abnormal resting-state functional connectivity (rsFC) was observed in individuals with Major Depressive Disorder (MDD) as compared to healthy controls (HC). (A) MDD individuals exhibited hypoconnectivity within the frontoparietal network (FN), between FN seeds and posterior parietal cortex (PPC); and hypoconnectivity between FN seeds and a region of superior parietal lobule (SPL) within the dorsal attention network (DAN). (B) MDD was associated with hyperconnectivity within the default network (DN), between DN seeds and medial prefrontal cortex (MPFC) and hippocampus; and hyperconnectivity between DN seeds and dorsolateral prefrontal cortex (DLPFC), a key hub of FN. (C) MDD was linked to hypoconnectivity between seeds in the affective network (AN) and regions of MPFC. (D) MDD was related to hypoconnectivity between VAN seeds and precuneus extending to occipital and posterior cingulate cortex (PCC), although post-hoc analyses also indicated hyperconnectivity between VAN and posterior regions. Shown here are results of both height-based (hb) thresholding (proportion of studies reporting an effect at that voxel exceeds chance) and extent-based (eb) thresholding (proportion of studies reporting an effect at contiguous voxels exceeds chance), all results significant at p<0.05, corrected for Family-Wise Error-Rate.
Table 1.
Results of meta-analysis of resting-state functional connectivity in Major Depressive Disorder.
| Seed network (and thresholding) |
Seed anatomy | Effect network | Effect anatomy | X | Y | Z | Voxels | max P |
|---|---|---|---|---|---|---|---|---|
| FN | caudate, cerebellum, DLPFC | |||||||
| MDD < HC (hb) | FN | r. PPC | 44 | −50 | 50 | 162 | 0.54 | |
| MDD < HC (eb) | FN/DAN | r. PPC/ SPL | 46 | −54 | 46 | 2074 | 0.40 | |
| MDD < HC (eb) | FN/DAN | l. PPC/ SPL | −42 | −52 | 48 | 2285 | 0.34 | |
| DN | ACC, caudate, cerebellum, hippocampus, IPL, MPFC, MTG, PCC | |||||||
| MDD > HC (hb) | DN | r. hippocampus/ MTG | 38 | −30 | −6 | 148 | 0.30 | |
| MDD > HC (eb) | DN | MPFC | −2 | 38 | 12 | 7456 | 0.20 | |
| MDD > HC (hb) | FN | l. DLPFC | −42 | 26 | 32 | 90 | 0.30 | |
| MDD > HC (eb) | FN | l. DLPFC | −38 | 26 | 36 | 784 | 0.29 | |
| MDD > HC (eb) | FN | l. DLPFC | −34 | 34 | 36 | 1125 | 0.18 | |
| MDD < HC (eb) | VAN | mid-cingulate/ thalamus/ putamen | −2 | 4 | 22 | 2476 | 0.26 | |
| AN | ACC, amygdala, NACC | |||||||
| MDD < HC (eb) | DN | MPFC | −2 | 46 | 16 | 3118 | 0.37 | |
| VAN | ||||||||
| MDD < HC (eb) | ACC,cerebellum, insula, putamen | FN/DN/Vis | precuneus/ PCC/ occipital cortex | 18 | −66 | 34 | 6194 | 0.32 |
Note: Results of comparison between individuals with Major Depressive Disorder (MDD) and healthy controls (HC). Thresholding was height-based (hb) or extent-based (eb) corrected to p<0.05; seed or effect networks included affective network (AN), dorsal attention network (DAN), default network (DN), frontoparietal network (FN), ventral attention network (VAN), or visual network (Vis); anatomical regions included anterior cingulate cortex (ACC), amygdala, caudate, cerebellum, dorsolateral prefrontal cortex (DLPFC), hippocampus, inferior parietal cortex (IPL), insula, medial prefrontal cortex (MPFC), middle temporal gyrus (MTG), nucleus accumbens (NACC), occipital cortex, posterior cingulate cortex (PCC), posterior parietal cortex (PPC), precuneus, putamen, superior parietal lobule (SPL), and thalamus. Coordinates are MNI standard stereotaxic space; Voxels = number of 1×1×1mm voxels; max P = maximum proportion of studies exhibiting the effect at the peak density, weighted by sample size.
Hyperconnectivity within the default network (DN)
MDD was characterized by hyperconnectivity between DN seeds and regions of hippocampus extending to middle temporal gyrus, and areas of medial prefrontal cortex (MPFC) (Fig. 1B). These areas are believed to support internal mentation, e.g., self-referential thinking and affective decision-making.61 When reported in the original studies, within-DN hyperconnectivity was related to enhanced positive connectivity in MDD.27,30,32,34,45 Post-hoc testing failed to reveal differences in the likelihood of hyperconnectivity as a function of seed anatomy, p’s>0.17. Neither age nor depression severity predicted DN hyperconnectivity, p’s>0.27, although trends emerged for greater likelihood of hyperconnectivity in unmedicated than medicated MDD between DN seeds and hippocampus, Likelihood Ratio=6.01, p=0.09, or MPFC, Likelihood Ratio=3.18, p=0.12.
Between-Network Abnormalities
Altered connectivity between the frontoparietal network (FN) and regions of dorsal attention network (DAN) or default network (DN) involved in externally- or internally-oriented attention
As reported above, MDD was associated with weaker rsFC between FN seeds and regions of bilateral parietal cortex; these clusters extended to regions of superior parietal lobule involved in attending to perceptual cues in the environment60 that fall within DAN (Fig. 1A). In addition, MDD was associated with hyperconnectivity between DN seeds and a region of left DLPFC believed to be critical for goal-directed regulation of attention and emotion60,62–64 (Fig. 1B). When reported, DN hyperconnectivity with lateral prefrontal regions was predominantly related to enhanced positive,32,34,45 but also weaker negative,31 connectivity in MDD. No differences were detected among anatomical regions of DN in the likelihood of hyperconnectivity with DLPFC, p’s>0.29, and effects were not moderated by clinical/demographic variables, p’s>0.19.
Altered connectivity between the affective network (AN) and regions of default network (DN) involved in mediating emotion regulation
Hypoconnectivity was observed between AN seeds and regions of MPFC involved in mediating emotion regulation65 (Fig. 1C). When reported, hypoconnectivity was related to both weaker positive (between nucleus accumbens (NACC) and MPFC35) and enhanced negative (between amygdala and MPFC46) connectivity. The likelihood of MPFC hypoconnectivity did not differ between anatomical regions of AN, p’s>0.33, and was not moderated by clinical/demographic variables, p’s>0.40.
Altered connectivity between the ventral attention network (VAN) and regions of frontoparietal network (FN) or default network (DN)
MDD was linked to hypoconnectivity between VAN seeds and regions of precuneus extending to occipital and posterior cingulate cortex (Fig. 1D), a functionally diverse set of regions involved in visual attention and internal thought.60,61 There was no difference in likelihood of hyper-versus hypoconnectivity, suggesting generally abnormal connectivity between VAN and posterior systems. Hypoconnectivity was also observed between DN seeds and a region of mid-cingulate extending to thalamus and putamen (Fig. 1B), areas involved in relaying information about salience and somatosensation.13,16 When reported, such hypoconnectivity was related to weaker positive connectivity in MDD.26,34 Post-hoc analyses failed to reveal differences among anatomical seeds in the likelihood of abnormal rsFC, p’s>0.17, or moderation by clinical/demographic variables, p’s>0.20.
Discussion
The present study provides the first meta-analytic evidence that individuals with MDD exhibit abnormal connectivity within and between brain networks involved in internally-(DN) or externally-(DAN) oriented attention, processing of emotion (AN) or salience (VAN), and goal-directed regulation of these functions (FN) (Fig. 2). These findings motivate a neurocognitive model in which network dysfunction is tightly linked to deficits regulating attention and mood.6,7,23 In this model, reduced coordination among brain systems critical for cognitive control, and altered communication between such control systems and other networks engaged for internal thought or emotional regulation, may underlie the biased cognitive style and persistent negative mood that characterize MDD.
Figure 2. A neurocognitive network model of Major Depressive Disorder.
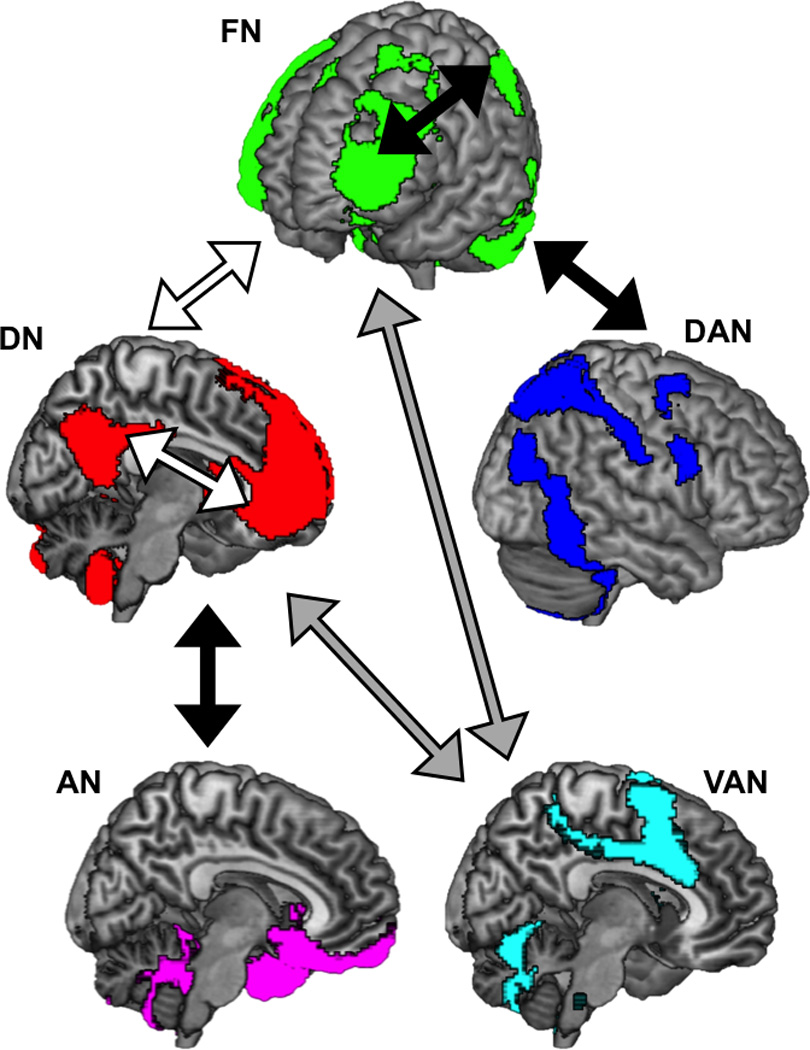
Reduced connectivity among regions of frontoparietal network (FN) may underlie general deficits in cognitive control, while increased connectivity between FN and default network (DN), and reduced connectivity between FN and dorsal attention network (DAN), may reflect biases towards ruminative thoughts at the cost of attending to the external world. Meanwhile, reduced connectivity between the affective network (AN) and MPFC regions that mediate top-down regulation may reflect impaired ability to up- or down-regulate emotions or arousal, whereas abnormal connectivity between the ventral attention network (VAN) and posterior regions may reflect altered or biased salience monitoring. (Black arrows represent hypoconnectivity in MDD; white arrows represent hyperconnectivity in MDD; gray arrows represent generally abnormal (both hypo- and hyper-) connectivity in MDD).
Reduced connectivity was observed in MDD among frontoparietal systems involved in cognitive control, and imbalanced connectivity was observed between control systems and regions engaged for externally-directed attention or internal mentation. These findings converge with theoretical models in which depression is defined by the tendency to become mired in negative rumination,24 which in turn stems from abnormal communication among brain regions supporting goal-directed control of attention, emotion, and self-referential thought.23 A coordinate-based search of prior studies (using BrainMap.org66) indicated that the same areas of DLPFC that exhibited hypoconnectivity with external-attention systems, and hyperconnectivity with internal-attention systems, have been implicated in top-down control of cognitive functions.62–64 Critically, overlapping regions of DLPFC have been shown to exhibit abnormal activity in depressed individuals exerting cognitive control.67 Meanwhile, regions of MPFC that were hyperconnected with other DN systems in the present meta-analysis have been implicated in functions such as self-referential thinking68 and autobiographical memory retrieval,69 and have been shown to be hyperactive in depressed individuals instructed to direct attention away from self-focused thinking.70 Hence, the present patterns of poorly coordinated or imbalanced network functioning in MDD may reflect weaknesses in cognitive control that contribute to both general deficits in goal-directed behavior, and specific biases towards internal thought at the cost of attending to the external world.
The present meta-analysis also revealed hypoconnectivity in MDD between MPFC and limbic regions. This pattern, considered in light of reduced connectivity among frontoparietal systems, suggests abnormal communication among networks involved in emotion regulation. Previous research has indicated that successful up- or down-regulation of emotion relies on communication between lateral PFC regions responsible for top-down control, areas of MPFC that mediate regulation, and limbic regions involved in affective responses.65,71 Altered activity and connectivity in this circuit has been observed in depressed individuals during emotion regulation tasks.72 Here, abnormal connectivity between regulatory and affective systems appeared to stem from both blunted positive communication (between MPFC and NACC) and excessive negative communication (between MPFC and amygdala). Thus, hypoconnectivity between MPFC and regions of AN may stem from abnormalities in multiple subnetworks engaged for distinct facets of emotional processing.
Although mixed, the present meta-analysis also provides evidence for hypoconnectivity between brain systems involved in processing salience and regions supporting cognitive control or internal mentation. The VAN is believed to play a role in signaling when to allocate resources to cognitive control systems in response to salient events or sensory experiences.73 Accordingly, decreased connectivity between VAN and control systems could reflect reduced reorientation of attention in response to salient cues. However, the pattern of altered VAN connectivity observed here included both hypo- and hyperconnectivity, suggesting that the nature of VAN abnormality in MDD may depend on additional factors. For example, previous research showed that, in response to negative emotional distractors, depression was associated with hyperconnectivity between regions responsive to salience and regions involved in internal mentation.18 Thus, the nature of communication between networks involved in salience and attention may be affected by the presence of environmental cues that correspond to the content of internal thoughts.
Two general patterns emerged in this meta-analysis. First, the sources of abnormal connectivity within seed-networks tended to be spatially distributed, highlighting the importance of considering anatomical regions within functional networks. However, given the low frequency of any single seed ROI implemented across studies, the absence of anatomical specificity should be interpreted with caution. Second, network abnormalities were similar across demographic and clinical groups. However, these analyses could only compare differences in the likelihood (but not magnitude) of network abnormalities between clinical/demographic groups, and only for groups that were consistently identified across the original studies. Future studies investigating additional clinical constructs will provide a more nuanced view of rsFC in depression.
Several limitations warrant attention and suggest directions for future research. First, the present meta-analysis was necessarily limited to seed-based rsFC studies, and seed ROIs selected by those studies (eTable 3). Hence particular networks and anatomical regions were better represented than others. In addition, it was not possible to include findings from studies that adopted alternative analytic methods (e.g., ICA). Because relatively few prior studies have implemented these methods with MDD samples (eTable 2), separate meta-analyses for each analytic approach could not be conducted. However, as this literature grows, an important next step will be to test the replicability of rsFC abnormalities across other analytic methods and network parcellations.
Second, because rsFC is a rapidly evolving field, standards for data acquisition and processing varied considerably between studies reviewed here (eTable 4). Differences in motion correction, or instructions to rest with eyes open versus closed, may substantially affect results.74 Unfortunately, it was not possible to test the moderating effects of such variables due to low frequency of studies within methodological categories, but these effects merit future investigation.
Third, an important question unanswered by the present meta-analysis is the extent to which aberrant functional connectivity could be related to structural abnormalities.23 For example, decreased cortical thickness has been associated with altered functional connectivity in depressed adults.75 Future studies that integrate structural and functional perspectives may provide a more comprehensive view of neurobiological abnormalities in mood disorders.
Fourth, it is unclear to what extent depression-related abnormalities in rsFC would persist during performance of other tasks. Resting-state connectivity appears to reflect both static (e.g., related to anatomical connections) and dynamic (e.g., related to changing goals or states of arousal) components, but the precise contribution of these components to rsFC is unknown.74 Abnormal rsFC in MDD may be a transient consequence of internally-biased attention, related to ruminating while resting in the scanner, rather than a persistent cause for biased or poorly controlled attention when engaged in other tasks. To disentangle these non-mutually-exclusive possibilities, studies will be required that compare network functioning at rest and during tasks that challenge attention and mood regulation.
Conclusions
This study provides the first meta-analytic evidence for large-scale network dysfunction in MDD, including imbalanced connectivity among networks involved in regulating attention to the internal or external world, and decreased connectivity between networks involved in regulating or responding to emotion or salience. These findings are consistent with a neurocognitive model of MDD in which abnormal communication among functional networks may mediate the core cognitive and affective biases that characterize this serious disorder.
Supplementary Material
Acknowledgements
Over the past three years, Dr. Pizzagalli has received honoraria/consulting fees from Advanced Neuro Technology North America, AstraZeneca, Otsuka Pharmaceutical, Pfizer, and Servier for activities unrelated to this project.
Funding/Support:
This project was partially supported by NIMH grants R01 MH068376 and R01 MH101521 awarded to Dr. Pizzagalli. Dr. Kaiser is partially supported by The Phyllis and Jerome Lyle Rappaport Mental Health Research Fellowship (McLean Hospital). Drs. Wager and Andrews-Hanna were supported by R01 MH076136 awarded to Dr. Wager.
Role of the Sponsor:
The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of data; preparation, review, or approval of manuscript; or decision to submit the manuscript for publication.
Other contributions:
We thank the authors of the included studies, with special thanks to authors who generously shared unpublished data from whole-brain analyses and seed ROI and peak coordinates for inclusion in this meta-analysis. We also thank Franziska Goer, B.A., Center for Depression, Anxiety, and Stress Research, for providing inter-rater checks on the database.
Footnotes
Conflicts of Interest:
No part of this study constitutes a conflict of interest for the corresponding author or coauthors.
Author Contributions:
Drs. Kaiser and Pizzagalli had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Kaiser, Pizzagalli.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Kaiser.
Critical revision of the manuscript: All authors.
Statistical analysis: Kaiser.
Obtained funding: Pizzagalli.
Administrative, technical, or material support: Andrews-Hanna, Pizzagalli, Wager.
Study supervision: Pizzagalli.
Disclosures:
All other authors have no financial interests to disclose.
References
- 1.Kessler RC. The costs of depression. Psychiatric Clinics of North America. 2012 Mar;35(1):1–14. doi: 10.1016/j.psc.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Merikangas KR, Ames M, Cui L, et al. The impact of comorbidity of mental and physical conditions on role disability in the US adult household population. Archives of General Psychiatry. 2007 Oct;64(10):1180–1188. doi: 10.1001/archpsyc.64.10.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Substance Abuse and Mental Health Services Administration S. Results from the 2012 National Survey on Drug Use and Health: Mental Health Findings. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2013. [Google Scholar]
- 4.World Health Organization W. World Health Statistics. Geneva, Switzerland: WHO Press; 2010. [Google Scholar]
- 5.Kessler RC, Petukhova M, Sampson NA, Zaslavsky AM, Wittchen HU. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. International Journal of Methods in Psychiatric Research. 2012 Sep;21(3):169–184. doi: 10.1002/mpr.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pizzagalli DA. Frontocingulate dysfunction in depression: Toward biomarkers of treatment response. Neuropsychopharmacology. 2011 Jan;36(1):183–206. doi: 10.1038/npp.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: Implications for neurocircuitry models of depression. Brain Structure & Function. 2008 Sep;213(1–2):93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic Resonance in Medicine. 1995 Oct;34(4):537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 9.Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences of the United States of America. 2003 Jan;100(1):253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buckner RL, Krienen FM. The evolution of distributed association networks in the human brain. Trends in Cognitive Sciences. 2013 Dec;17(12):648–665. doi: 10.1016/j.tics.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 11.Chang C, Liu ZM, Chen MC, Liu X, Duyn JH. EEG correlates of time-varying BOLD functional connectivity. Neuroimage. 2013 May;72:227–236. doi: 10.1016/j.neuroimage.2013.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shirer WR, Ryali S, Rykhlevskaia E, Menon V, Greicius MD. Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cerebral Cortex. 2012 Jan;22(1):158–165. doi: 10.1093/cercor/bhr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience. 2007 Feb;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BTT. The organization of the human cerebellum estimated by intrinsic functional connectivity. Journal of Neurophysiology. 2011 Nov;106(5):2322–2345. doi: 10.1152/jn.00339.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi EY, Yeo BTT, Buckner RL. The organization of the human striatum estimated by intrinsic functional connectivity. Journal of Neurophysiology. 2012 Oct;108(8):2242–2263. doi: 10.1152/jn.00270.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yeo BTT, Krienen FM, Sepulcre J, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology. 2011 Sep;106(3):1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Snyder HR. Major Depressive Disorder is associated with broad impairments on neuropsychological measures of executive function: A meta-analysis and review. Psychological Bulletin. 2013 Jan;139(1):81–132. doi: 10.1037/a0028727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaiser RH, Andrews-Hanna JR, Spielberg JM, et al. Distracted and down: Neural mechanisms of affective interference in subclinical depression. Social Cognitive and Affective Neuroscience. 2014 doi: 10.1093/scan/nsu100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang L, Hermens DF, Hickie IB, Lagopoulos J. A systematic review of resting-state functional-MRI studies in major depression. Journal of Affective Disorders. 2012 Dec;142(1–3):6–12. doi: 10.1016/j.jad.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 20.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Reviews Neuroscience. 2007 Sep;8(9):700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 21.Wager TD, Lindquist M, Kaplan L. Meta-analysis of functional neuroimaging data: current and future directions. Social Cognitive and Affective Neuroscience. 2007 Jun;2(2):150–158. doi: 10.1093/scan/nsm015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wager TD, Lindquist MA, Nichols TE, Kober H, Van Snellenberg JX. Evaluating the consistency and specificity of neuroimaging data using meta-analysis. Neuroimage. 2009 Mar;45(1):S210–S221. doi: 10.1016/j.neuroimage.2008.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hasler G, Northoff G. Discovering imaging endophenotypes for major depression. Molecular Psychiatry. 2011 Jun;16(6):604–619. doi: 10.1038/mp.2011.23. [DOI] [PubMed] [Google Scholar]
- 24.Holtzheimer PE, Mayberg HS. Stuck in a rut: rethinking depression and its treatment. Trends in Neurosciences. 2011 Jan;34(1):1–9. doi: 10.1016/j.tins.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Etkin A, Wager TD. Functional neuroimaging of anxiety: A meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. American Journal of Psychiatry. 2007 Oct;164(10):1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alalade E, Denny K, Potter G, Steffens D, Wang LH. Altered cerebellar-cerebral functional connectivity in geriatric depression. Plos One. 2011 May;6(5) doi: 10.1371/journal.pone.0020035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alexopoulos GS, Hoptman MJ, Kanellopoulos D, Murphy CF, Lim KO, Gunning FM. Functional connectivity in the cognitive control network and the default mode network in late-life depression. Journal of Affective Disorders. 2012 Jun;139(1):56–65. doi: 10.1016/j.jad.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alexopoulos GS, Hoptman MJ, Yuen G, et al. Functional connectivity in apathy of late-life depression: A preliminary study. Journal of Affective Disorders. 2013 Jul;149(1–3):398–405. doi: 10.1016/j.jad.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andreescu C, Tudorascu DL, Butters MA, et al. Resting state functional connectivity and treatment response in late-life depression. Psychiatry Research-Neuroimaging. 2013 Dec;214(3):313–321. doi: 10.1016/j.pscychresns.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berman MG, Peltier S, Nee DE, Kross E, Deldin PJ, Jonides J. Depression, rumination and the default network. Social Cognitive and Affective Neuroscience. 2011 Oct;6(5):548–555. doi: 10.1093/scan/nsq080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cao XH, Liu ZF, Xu C, et al. Disrupted resting-state functional connectivity of the hippocampus in medication-naive patients with major depressive disorder. Journal of Affective Disorders. 2012 Dec;141(2–3):194–203. doi: 10.1016/j.jad.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 32.Connolly CG, Wu J, Ho TC, et al. Resting-state functional connectivity of subgenual anterior cingulate cortex in depressed adolescents. Biological Psychiatry. 2013 Dec;74(12):898–907. doi: 10.1016/j.biopsych.2013.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cullen KR, Gee DG, Klimes-Dougan B, et al. A preliminary study of functional connectivity in comorbid adolescent depression. Neuroscience Letters. 2009 Sep;460(3):227–231. doi: 10.1016/j.neulet.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davey CG, Harrison BJ, Yucel M, Allen NB. Regionally specific alterations in functional connectivity of the anterior cingulate cortex in major depressive disorder. Psychological Medicine. 2012 Oct;42(10):2071–2081. doi: 10.1017/S0033291712000323. [DOI] [PubMed] [Google Scholar]
- 35.Furman D, Hamilton JP, Gotlib IH. Frontostriatal functional connectivity in major depressive disorder. Biology of Mood and Anxiety Disorders. 2011;1(1):11. doi: 10.1186/2045-5380-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gabbay V, Ely BA, Li QY, et al. Striatum-based circuitry of adolescent depression and anhedonia. Journal of the American Academy of Child and Adolescent Psychiatry. 2013 Jun;52(6):628–641. doi: 10.1016/j.jaac.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo WB, Liu F, Xue ZM, et al. Abnormal resting-state cerebellar-cerebral functional connectivity in treatment-resistant depression and treatment sensitive depression. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2013 Jul;44:51–57. doi: 10.1016/j.pnpbp.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 38.Guo WB, Liu F, Xue ZM, et al. Decreased interhemispheric coordination in treatment-resistant depression: A resting-state fMRI study. Plos One. 2013 Aug;8(8) doi: 10.1371/journal.pone.0071368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo WB, Liu F, Dai Y, et al. Decreased interhemispheric resting-state functional connectivity in first-episode, drug-naive major depressive disorder. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2013 Mar;41:24–29. doi: 10.1016/j.pnpbp.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 40.Guo WB, Liu F, Liu JR, et al. Is there a cerebellar compensatory effort in first-episode, treatment-naive major depressive disorder at rest? Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2013 Oct;46:13–18. doi: 10.1016/j.pnpbp.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 41.Hamilton JP, Chen G, Thomason ME, Schwartz ME, Gotlib IH. Investigating neural primacy in Major Depressive Disorder: multivariate Granger causality analysis of resting-state fMRI time-series data. Molecular Psychiatry. 2011 Jul;16(7):763–772. doi: 10.1038/mp.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Horn DI, Yu C, Steiner J, et al. Glutamaterigic and resting-state functional connectivity correlates of severity in major depression - the role of pregenual anterior cingulate cortex and anterior insula. Frontiers in Systems Neuroscience. 2010;4:10. doi: 10.3389/fnsys.2010.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kenny ER, O'Brien JT, Cousins DA, et al. Functional connectivity in late-life depression using resting-state functional magnetic resonance imaging. American Journal of Geriatric Psychiatry. 2010 Jul;18(7):643–651. doi: 10.1097/JGP.0b013e3181cabd0e. [DOI] [PubMed] [Google Scholar]
- 44.Lui S, Wu QZ, Qiu LH, et al. Resting-state functional connectivity in treatment-resistant depression. American Journal of Psychiatry. 2011 Jun;168(6):642–648. doi: 10.1176/appi.ajp.2010.10101419. [DOI] [PubMed] [Google Scholar]
- 45.Ma CQ, Ding JR, Li J, et al. Resting-state functional connectivity bias of middle temporal gyrus and caudate with altered gray matter volume in Major Depression. Plos One. 2012 Sep;7(9) doi: 10.1371/journal.pone.0045263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pannekoek JN, van der Werff SJA, Meens PHF, et al. Aberrant resting-state functional connectivity in limbic and salience networks in treatment-naive clinically depressed adolescents. Journal of Child Psychology and Psychiatry. 2014 Dec;55(12):1317–1327. doi: 10.1111/jcpp.12266. [DOI] [PubMed] [Google Scholar]
- 47.Sheline YI, Price JL, Yan ZZ, Mintun MA. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proceedings of the National Academy of Sciences of the United States of America. 2010 Jun;107(24):11020–11025. doi: 10.1073/pnas.1000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tahmasian M, Knight DC, Manoliu A, et al. Aberrant intrinsic connectivity of hippocampus and amygdala overlap in the fronto-insular and dorsomedial-prefrontal cortex in major depressive disorder. Frontiers in Human Neuroscience. 2013 Oct;7 doi: 10.3389/fnhum.2013.00639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang Y, Kong L, Wu F, et al. Decreased functional connectivity between the amygdala and the left ventral prefrontal cortex in treatment-naive patients with major depressive disorder: a resting-state functional magnetic resonance imaging study. Psychological Medicine. 2013 Sep;43(9):1921–1927. doi: 10.1017/S0033291712002759. [DOI] [PubMed] [Google Scholar]
- 50.Ye T, Peng J, Nie BB, et al. Altered functional connectivity of the dorsolateral prefrontal cortex in first-episode patients with major depressive disorder. European Journal of Radiology. 2012 Dec;81(12):4035–4040. doi: 10.1016/j.ejrad.2011.04.058. [DOI] [PubMed] [Google Scholar]
- 51.Salimi-Khorshidi G, Smith SM, Keltner JR, Wager TD, Nichols TE. Meta-analysis of neuroimaging data: A comparison of image-based and coordinate-based pooling of studies. Neuroimage. 2009 Apr;45(3):810–823. doi: 10.1016/j.neuroimage.2008.12.039. [DOI] [PubMed] [Google Scholar]
- 52.Brett M, Christoff K, Cusack R, Lancaster J. Using the Talairach atlas with the MNI template. Neuroimage. 2001 Jun;13(6):S85–S85. [Google Scholar]
- 53.Smith SM, Fox PT, Miller KL, et al. Correspondence of the brain's functional architecture during activation and rest. Proceedings of the National Academy of Sciences of the United States of America. 2009 Aug;106(31):13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smith SM. The future of FMRI connectivity. Neuroimage. 2012 Aug;62(2):1257–1266. doi: 10.1016/j.neuroimage.2012.01.022. [DOI] [PubMed] [Google Scholar]
- 55.Nee DE, Wager TD, Jonides J. Interference resolution: Insights from a meta-analysis of neuroimaging tasks. Cognitive Affective & Behavioral Neuroscience. 2007 Mar;7(1):1–17. doi: 10.3758/cabn.7.1.1. [DOI] [PubMed] [Google Scholar]
- 56.Thayer JF, Ahs F, Fredrikson M, Sollers JJ, Wager TD. A meta-analysis of studies of the neural correlates of heart rate variability: Importance of the amygdala and the medial prefrontal cortex. Psychophysiology. 2010;47:S7-S7. [Google Scholar]
- 57.Beck AT, Steer RA, Ball R, Ranieri WF. Comparison of Beck Depression Inventories-IA and -II in psychiatric outpatients. Journal of Personality Assessment. 1996 Dec;67(3):588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 58.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry. 1979;134(APR):382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 59.Zimmerman M, Martinez JH, Young D, Chelminski I, Dalrymple K. Severity classification on the Hamilton depression rating scale. Journal of Affective Disorders. 2013 Sep;150(2):384–388. doi: 10.1016/j.jad.2013.04.028. [DOI] [PubMed] [Google Scholar]
- 60.Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. Journal of Neurophysiology. 2008 Dec;100(6):3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain's default network. Neuron. 2010 Feb;65(4):550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fales CL, Barch DM, Rundle MM, et al. Altered emotional interference processing in affective and cognitive-control brain circuitry in major depression. Biological Psychiatry. 2008 Feb;63(4):377–384. doi: 10.1016/j.biopsych.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kerns JG. Anterior cingulate and prefrontal cortex activity in an FMR1 study of trial-to-trial adjustments on the Simon task. Neuroimage. 2006 Oct;33(1):399–405. doi: 10.1016/j.neuroimage.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 64.Veltman DJ, Rombouts S, Dolan RJ. Maintenance versus manipulation in verbal working memory revisited: an fMRI study. Neuroimage. 2003 Feb;18(2):247–256. doi: 10.1016/s1053-8119(02)00049-6. [DOI] [PubMed] [Google Scholar]
- 65.Ochsner KN, Ray RD, Cooper JC, et al. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004 Oct;23(2):483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 66.Laird AR, Lancaster JL, Fox PT. BrainMap - The social evolution of a human brain mapping database. Neuroinformatics. 2005;3(1):65–77. doi: 10.1385/ni:3:1:065. [DOI] [PubMed] [Google Scholar]
- 67.Dichter GS, Felder JN, Smoski MJ. Affective context interferes with cognitive control in unipolar depression: An fMRI investigation. Journal of Affective Disorders. 2009 Apr;114(1–3):131–142. doi: 10.1016/j.jad.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: Relation to a default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001 Mar;98(7):4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim H. A dual-subsystem model of the brain's default network: Self-referential processing, memory retrieval processes, and autobiographical memory retrieval. Neuroimage. 2012 Jul;61(4):966–977. doi: 10.1016/j.neuroimage.2012.03.025. [DOI] [PubMed] [Google Scholar]
- 70.Johnson MK, Nolen-Hoeksema S, Mitchell KJ, Levin Y. Medial cortex activity, self-reflection and depression. Social Cognitive and Affective Neuroscience. 2009 Dec;4(4):313–327. doi: 10.1093/scan/nsp022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008 Sep;59(6):1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: Counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. Journal of Neuroscience. 2007 Aug;27(33):8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: From environment to theory of mind. Neuron. 2008 May;58(3):306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Buckner RL, Krienen FM, Yeo BTT. Opportunities and limitations of intrinsic functional connectivity MRI. Nature Neuroscience. 2013 Jul;16(7):832–837. doi: 10.1038/nn.3423. [DOI] [PubMed] [Google Scholar]
- 75.van Tol MJ, Li M, Metzger CD, et al. Local cortical thinning links to resting-state disconnectivity in Major Depressive Disorder. Psychological Medicine. 2014 Jul;44(10):2053–2065. doi: 10.1017/S0033291713002742. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


