Abstract
Biomedical textiles and fiber-based implants (BTFIs) have been in routine clinical use to facilitate healing for nearly five decades. Amongst the variety of biomaterials used, silk-based biomaterials (SBBs) have been widely used clinically viz. sutures for centuries and are being increasingly recognized as a prospective material for biomedical textiles. The ease of processing, controllable degradability, remarkable mechanical properties and biocompatibility have prompted the use of SBBs for various BTFIs for extracorporeal implants, soft tissue repair, healthcare/hygiene products and related needs. The present review focuses on BTFIs from the perspective of types and physical and biological properties, and this discussion is followed with an examination of the advantages and limitations of BTFIs from SBBs. The review covers progress in surface coatings, physical and chemical modifications of SBBs for BTFIs and identifies future needs and opportunities for the further development for BTFIs using SBBs.
Keywords: Biomedical textiles, implants, devices, silk-based biomaterials, medical applications, surface modifications
1. Introduction
Biomedical textiles and fiber-based implants (BTFIs) manufactured using textile-forming technologies have been in routine clinical use to facilitate healing for nearly five decades [1, 2]. Biomedical textiles are defined as fibrous textile structures prepared from synthetic or natural materials that are used either in an internal or external (inside or outside the body) biological environment as a medical device to improve the health and medical condition of the patient [1, 3]. These devices include non-implantable materials from wound dressings, implantable materials like vascular grafts, heart valves and sutures to wearable medical implants and polymer sensors. Medical textiles serve biomedical applications throughout the body and can provide solutions for clinical applications including general surgery, orthopedic, cardiovascular and cosmetic surgeries, tissue engineering, bariatric, dental, and veterinary needs [4–15].
Various natural and synthetic fibers are used to construct biomedical textiles. Silk, as unique natural biological proteins made by silkworms and spiders offer remarkable mechanical properties [16]. Silk-based biomaterials (SBBs) have been more widely used clinically as sutures for centuries and are being increasingly recognized as a prospective material for biomedical textiles. Because of the availability of large quantities of material from the textile industry, the ease of processing, controllable degradability, remarkable mechanical properties and biocompatibility, SBBs have been explored and engineered for the fabrication of various BTFIs such as sutures, arterial grafts, heart valves, hernia and prolapsed repair meshes, heart implants supports, and prosthetic ligaments and tendons, among other devices [5, 6, 8, 17–21].
In order to further expand the applications of SBBs for versatile biomedical textiles, there has been interest in the regeneration of silk solutions and fibers from native silk fibers to gain even further control of properties. However, applications of regenerated silk fibers have been limited in part due to the inferior mechanical properties when compared to the native fibers. Usually, regenerated silk fibers exhibit 0.1–0.2 cN/tex (tex stands for linear density of fibers) in tensile strength while raw silk fibers from Bombyx mori, which are composed of fibroin (SF) and sericin (SS), and degummed silk fiber, mainly SF with SS removed, show 0.3–0.4 cN/tex [22–26]. Furthermore, the flexibility of regenerated silk fibers is poor compared to that of raw silk fibers. Such poor mechanical properties have been a primary reason that regenerated silk fibers are seldom used in industrial applications for BTFIs. Numerous efforts have been conducted to improve the mechanical properties of the regenerated silk materials, but to date the properties remain inferior to their native counterparts [27]. Additionally, the biological properties of fabricated BTFIs, such as biocompatibility, thrombogenicity and antimicrobial behavior, have been enhanced with many surface modification techniques, including plasma treatment, surface coatings, chemical grafting, and encapsulation of nanoparticles [28–32].
The number of publications on the use of SBBs for biomedical textiles is increasing, however, few review papers [10–13, 17] about SBBs for biomedical textiles have been published. The present review provides a comprehensive overview of progress in SBBs for BTFIs and identifies opportunities for further development. The present review focuses on the types, compositions and physical and biological properties of BTFIs, followed by an examination of the advantages and limitations of BTFIs prepared from SBBs. The review then covers progress with surface coatings and physical and chemical modifications of SBBs for BTFIs and concludes with a discussion of critical issues, future needs and opportunities for further development for BTFIs using SBBs.
2. Structure, function and properties of silk fibers
2.1 Silkworm fibers
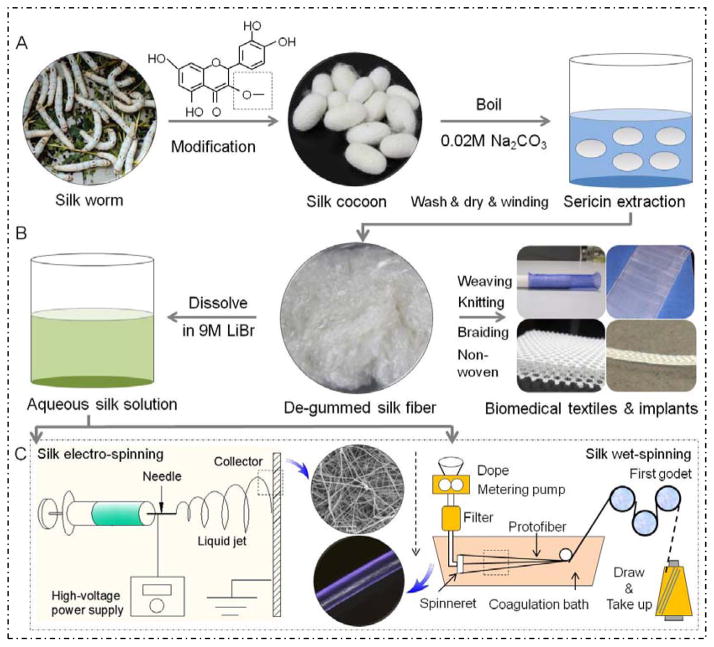
Silkworm silk has been used commercially as biomedical sutures for decades, and in textile production for centuries [10, 33]. Silkworm silk fibers are composed of at least two fibroin protein microfilaments (known as brins) embedded in a glue-like glycoprotein coating (Figure 1a) [34]. The brins are fibroin filaments consisting of bundles (diameter 100 nm) of nanofibrils (individual diameter 5 nm) that are aligned with the long axis of the fiber [35, 36]. The nanofibrils possess three proteinaceous components: a heavy chain (H-chain) fibroin, light chain (L-chain) fibroin and P25 protein [33]. The L-chain is more hydrophilic and elastic than the H-chain, and the P25 protein has the function to maintain the integrity of the complex [37]. The fibroin filaments are coated with sericins (glue-like glycoproteins, 25 – 30% wt of the fiber) so that the twin filaments stick together to form the composite material or cocoon (Figure 1a) [38]. The sericin proteins are coated on the surface of fibers so that the cocoon can be isolated and protected from the outside (Figure 1a) [39, 40]. The sericin needs to be removed using extraction/washing after harvesting the silk cocoons in order to generate purified silk fibroin for medical use [38].
Figure 1.
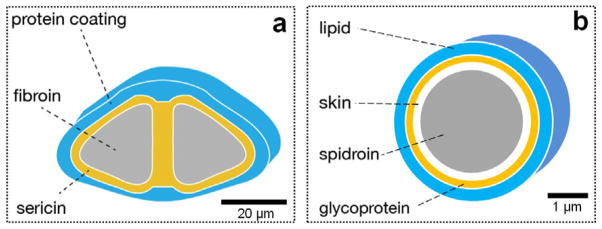
Cross-section diagrams of silkworm (a) and spider silk fibers (b): a and b are schematic diagrams [33].
2.2 Spider fibers
Spider silk fibers posses excellent high-tensile strength and moderate elasticity, with proteins produced in the major ampullate (MA) silk gland. The structure of MA silk is a “core-shell” multilayer and the diamter is around 1–20 μm depending on the spider species (Figure 1b) [41, 42]. There are two typical proteins mainly composed of glycine, alanine and glycine in the core filament [43]. These two proteins are distributed inhomogeneously [41, 42]. The β-sheet structures of MA silk contribute to their remarkable mechanical performance such as high-tensile strength and the formation of turns and spirals provide the elasticity or flexibility [44–47]. The multilayer stucture of the silk fibers consisits of a core, a spidroin-like protein (skin), glycoprotein, and a lipid coat (Figure 1b) [41, 42]. Allmeling et al. (2013) reviewed a large number of textile, technical and biomedical applications for spider silk [48]. However, spider silk have not been domesticated or commercialized because spiders have a predatory nature so they can not be raised in high densities, and the level of silk production by spiders is much lower than silkworm [11].
2.3 Mechanical properties
Data on the mechanical properties of silk fibers is provided in Table 1 and shows that silk fibers possess remarkable ductility and toughness in terms of balancing elongation, modulus and breaking strength [11, 49]. Both the strength and elasticity of the spider silk are very high compared to other synthetic or natural fibers, including classic high performance fibers like nylon and aramid [50, 51]. Particularly, the strength-to-density ratio of spider silk is about 10 times higher than steel [52], and shows remarkable strain hardening behavior which is important for energy absorption [53]. Stress-strain curves of wild-type silkworm silk and spider or dragline silk show similar behavior in terms of strain hardening [54, 55]. Hence, silk can be employed for tissue grafts or scaffolds with high load bearing applications due to this excellent mechanical performance. However, most current silk-based BTFIs are not matched well to tissues in terms of mechanical perfomance, or interactions between the materials and the native tissue interface. Futher, different silk or differently processed silk, may provide different types of SBBs with different mechanical properties to match mechanical requirements for various BTFIs. The relative bending rigidity for fiber stiffness can be calculated using equation 1[56].
Table 1.
| Fiber | Density (g/cm3) | Tenacity cN/denier (MPa) | Breaking strain (%) | Initial modulus N/tex (GPa) | Relative bending Rigidity1 |
|---|---|---|---|---|---|
| Raw silk2 | 1.34 | 4.2(507) | 20–24 | 7.02 (9~10) | 5.2 |
| De-gummed silk3 | 1.27 | (610~690) | 4–16 | /(15~17) | - |
| Spider silk4 | 1.31 | (875~972) | 17–18 | /(11~13) | - |
| Cellulose, rayon | 1.50 | 2.6 (351) | 18 | 6.12 (9.2) | 4.1 |
| Nylon | 1. 14 | 7.5 (770) | 25 | 3.15 (3.6) | 2.8 |
| Polyester | 1.38 | 6.3 (782) | 16 | 11.7 (16.2) | 8.5 |
| Polypropylene | 0.91 | 7.0 (573) | 18 | 7.20 (6.6) | 7.9 |
| Polyurethane | 1.20 | 0.4(43) | 500 | 0.01 (.012) | 0.01 |
| Aramid (para) | 1.44 | 27.5 (3564) | 2.4 | 88.2 (127) | 61.3 |
| Steel cord | 7.85 | 3.0 (2120) | 8 | 24.5 (192) | 3.1 |
| Spectra 1000 | 0.97 | 36 (3142) | 3.3 | 112 (109) | 115.5 |
| PBO | 1.56 | 40 (5616) | 2.5 | 183 (286) | 117.3 |
Note:
caculated using equation 1;
raw silk are the silk fibers naturally produced from silk worm coated in sericin;
de-gummed silk are individual fibroin fibers following extraction of sericin;
Nephila clavipes silk produced naturally and through controlled silking. “-” means the relate items were unreported in the literatures.
| (1) |
Where: tensile modulus (E), linear density (d), density (ρ), fiber cross-section (ε, ε=1 for circular, <1 for flat or elliptical, >1 for hollow fiber), β0 is a constant, and the units are shown in Table 1.
3. Sources, processing and regeneration of silk for biomedical textiles
3.1 Sources of silk proteins
This review focuses on silkworm and spider silk since they are the most commonly studied silk [57]. B. mori silkworms have been domesticated by humans for industrial production over centuries. Alternative options for silk are time consuming and yield lower amounts of protein fiber than the silkworm cocoon [33]. Since the spider silk cannot be commercialized, recombinant DNA methods are utilized to produce spider silk-like proteins [58]. Recombinant DNA technologies have been utilized to generate many variants of silk proteins, including intruducing specific spider silk or silkworm silk DNA sequences into chimaeric/hybrid proteins to improve solubility, cell interactions and biomineralization [59, 60].
3.2. Processing silk proteins for biomedical textiles
Figure 2 shows a schematic of sources, processing and regeneration of SBBs for BTFIs. Some additional properties like fluorescence can be incorporated into the fibers by feeding silk larvae with the plant quercetin, which becomes exported to the cocoon [61]. Silk proteins can be dissolved into solution to produce various SBBs including regenerated fibers, fibrous mats and scaffolds using wet/electro spinning or hand-drawing process. Selecting the proper solvent is also important to modulate the interactions of the silk proteins in concentrated aqueous solutions, often consisting of strong acids, ionic liquids, fluorinated solvents and salts [62–64]. The solvents should break the strong intermolecular hydrogen bonded stacks of β-sheets and denature the protein. Also aqueous solutions of kosmotropic salts, such as potassium phosphate, and alcohols are usually used to induce β-sheet formation in the SBBs, to decrease solubility in water and improve mechanical properties [10, 62–64].
Figure 2.
Schematic of sources, processing and regeneration of silk-based biomaterials for biomedical textiles and fiber-based implants: (A) illustrates silk production or modification from worm to cocoon, and then purification; (B) the degummed or purified fibers processed into various BTFIs, or formed into aqueous silk fibroin solution; (C) the aqueous silk solution used for silk fiber regeneration using electro-spinning and wet-spinning [4, 266].
3.3. Regeneration of silk proteins for biomedical textiles
Although the naturally spun silk from B. mori silkworm have impressive properties such as luster strength, wear resistance and anti-wrinkle properties, photo-induced yellowing can limit further applications [65]. Hence, substantial physical, chemical and even genetic treatments have been used for the modification of native silk fibers [66–71]. The silk fibers can be easily produced from aqueous protein solutions using a natural process in which the temperature and pressure in the spinning container are lower, and no noxious solvent are used in the spinning bath [72]. Spinning silk proteins with other components have been utilized to regenerate silk fibers with new properties [33]. Moreover, efforts have been made to utilize waste materials, including unreelable dupion (two-strand) cocoons to produce specialty materials [11]. Electrospinning [73–76] and wet spinning [25, 77–82] have been widely used for silk fiber spinning. One of the advantages of silk electrospinning is that fibrous yarn or membranes with nano/micro fibers can be fabricated for implants and tissue engineering [11, 73]. The wet-spun silk fibers can be woven/knitted/braided for various BTFIs due to the mechanical properties. In order to fabricate silk fibers with better mechanical properties, larger scale industrial utility are needed, however, has not yet been demonstrated [83, 84].
3.3.1 Electro-spun silk-based fibers
Silk fibroin has been used to generate fibrous silk membranes from reprocessed native silk fibers or by electrospinning [2]. Electro-spun fibrous membranes are of interest as biomaterials because the increased surface area is favorable for cell adhesion [85]. 3-D constructs of nano-fibers have been used as blood vessel grafts and nerve guides [86, 87]. Adding type I calf skin collagen and chitin into B. mori fibroin seperately, the electrospun fibers supported cell adhesion and the proliferation of human epidermal keratinocytes (NHEK) and fibroblasts (NHEF) in vitro. Interestingly, electrospun fibers composed of unmixed B. mori fibroin and type I calf skin collagen or B. mori fibroin and chitin (prepared by electrospinning from two syringes simultaneously and subsequently cross-linked with glutaraldehyde) showed higher levels of cell adhesion and proliferation for NHEK cells in vitro [88–90]. The mechanical properties of electrospun SF scaffolds were enhanced by uniformly dispersing hydroxyapatite (HAp) nanoparticles within the SF nanofibers [91].
Electrospinning has also been utilized to produce spider silk nano fibers [92]. One of the advantages is that the diameter and pore size of electrospun spider silk fibers can be controlled from 50 to 1,100 nm by adjusting SF nanostructure and concentration [92, 93]. Araneus diadematus fibroin 3 (ADF-3) recombinant spider-silk protein was employed to generate electrospun spider silk fibers with a diameter of ~40 μm. These fibers had comparable toughness and modulus but lower tenacity than natural A. diadematus dragline silk [94]. Arcidiacono et al. (2002) reported an electrospinning method for producing spider silk fibers from genetically engineered protein with 10 – 60 μm diameter, comparable to native spider silk due to molecular orientation. The spinning solution was dialyzed in urea containing Tris buffer and salts [95]. Also, electrospun N. clavipes major ampullate spider proteins I (MaSpI) generated silk fibers (diameter ~300 nm) using a solvent HFIP (hexafluoroisopropanol) and the properties of the fibers were improved by controlling conformation and molecular alignment [96]. Wang et al. (2013) reported that electro-netting can overcome bottlenecks with electrospinning to provide a versatile method for generating spider-web-like nano-nets with ultrafine fiber diameters less than 20 nm [97].
3.3.2 Wet-spun silk-based fibers
Fiber spinning technologies including melt and solution spinning (dry and wet spinning) are widely used for fiber production in the textile industry. The temperature in the melt spinning process is above 200°C, too high to fabricate regenerated silk fibers. In contrast, wet spinning is better to fabricate regenerated silk fibers with micrometer scale diameters [98, 99]. Compared to native silk fibers, the morphology, diameter, components (other natural or synthetic polymers) and mechanical properties can be tailored according to specific applications. Wet spinning is one of oldest methods used for making fibers and was used for producing rayon (cellulose fiber, formula (C6H10O5)n). The polymeric raw material is converted into a soluble derivative and extruded through a spinneret into a chemical bath where the extrudate coagulates and regenerates into a filament. A diagram of a typical wet spinning process is illustrated in Figure 2C.
In recent decades there have been numerous efforts to develop useful processes for silk fiber formation using wet spinning, including approaches where sericin may played an important role in the re-crystallization process of SF [27, 100–102]. Experimental wet-spinning of SF has mostly been carried out by injection from a hypodermic syringe [25, 65, 77, 103] or with a simplified industrial wet-spinning device [79, 80, 98]. Dry-jet wet-spinning is new technique for spinning of fibers, which offers useful options for both dry spinning (using hot air as the coagulation medium) and wet spinning (using the solution as coagulation medium). During the dry-jet wet-spinning process, the orientation of the fibroin molecules increases due to drawing in the hot air [26, 104–106]. Phillips et al. (2005) examined the feasibility of spinning silk fibers from an ionic liquid solution [65]. Chang et al. (2013) investigated how SS promoted molecular chain orientation of SF resulting in improved mechanical properties. X-ray diffraction analysis and Herman’s orientation coefficient confirmed that improvements in tensile properties were mainly due to an increase in molecular orientation induced by sericin during the drawing process. One possible hypothesis is that the sericin performed a lubrication benefit. An increase in chain orientation of SF by SS is important for developing β-sheet structures [27]. Zheng et al. (2010) demonstrated that the water-collecting ability of capture silk of the cribellate spider Uloborus walckenaerius was the result of a unique fiber structure that forms after wetting, with the ‘wet-rebuilt’ fibres characterized by periodic spindle-knots consisting of random nanofibrils and separated by joints of aligned nanofibrils [107].
4. Applications of silk-based biomaterials for biomedical textiles and fiber-based implants
Biomedical textiles and fiber-based implants (BTFIs) are used for first aid, clinical and hygene needs. BTFIs offer more choice and flexibility in design than many traditional materials and allow manufacturers to expand their portfolios with biologically-based minimally invasive products. Even treatments and implants that typically have been engineered with metals and hard plastics now are often shifting towards soft implants and fixation methods. SBBs as regenerated or modified fibres and yarns can be woven, knitted or braided into textile structures and used for a wide range of medical applications, including bone grafting, fusions and motion-preserving spinal repair, and treatments for cartilage, joints, ligaments, tendons and other soft tissues [1, 29].
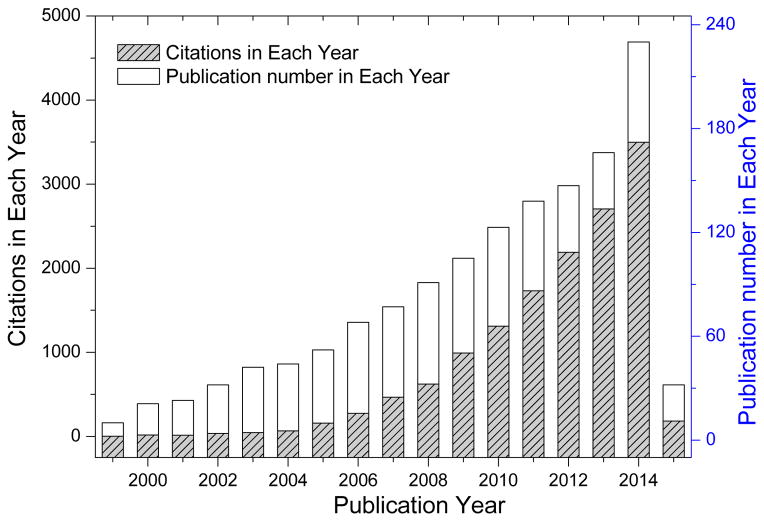
The applications of different SBBs for producing BTFIs is illustrated in Table 2, which focuse on non-implantable materials, implantable materials, extracorporeal implants and healthcare/hygiene products, respectively. Examples of products are wound dressings, sutures, nerve conduits, ligaments and vascular prosthesis, etc. As shown in Figure 3, the number of publications and citations on the use of SBBs for biomedical textiles is rapidly increasing. However, few review papers [10–13, 17] about SBBs used for biomedical textiles have been published. The present review provides a comprehensive overview of progress in the SBBs for BTFIs and identifies opportunities for further developments.
Table 2.
List of biomedical textiles made from silk-based biomaterials: categories, types of materials and applications
| Categories | Product applications | Textile technologies | Materials used | Ref. |
|---|---|---|---|---|
| Non-implantable materials | Wound dressings Orthopaedic bandages/textiles |
Non-woven/knitting Knitting/woven |
Fibrous silk film/composites Silk fabric |
[111, 112, 267, 268] [191] |
|
| ||||
| Implantable materials | Surgical sutures | Mono/multifilament braided | Silk thread or a titanium clip | [11, 147, 269–271] |
Soft-tissue artificial implants
|
Knitting and/or braiding Woven/warp and/or weft knitting Non-woven |
RGD-modified silk multi-fiber rope B. mori SF net Poly(L-lactide-co-ε-caprolacton e) (PLCL)/collagen and or SF fibers |
[18–20] [138, 149, 152, 154, 155, 272, 273] [173, 274] [275, 276] |
|
Orthopedic implants
|
Non-woven/electrospinning Braiding |
SF matrix of bone protein & nHAP Modified silk ligature twisted wire |
[162, 277] [163, 278] |
|
Vascular implants
|
Non-woven/braiding/woven Woven/knitting/braiding/eletrospinning |
3-dimentional SF scaffold PET/silk yarns with sericin coating Multifilament silk yarn/SF coating |
[175, 183] [5, 181, 279] |
|
Gastrointestinal stents
|
Weft/warp knitting/braiding/non -woven coating | Drug-loaded SF coating | [164, 280] | |
|
| ||||
| Extracorporeal implants | Artificial kidneys/lungs Artificial liver |
Silk film from B. mori SF Non-woven/fiber blending |
Silk-polylysine block copolymers with plasmid DNA Silk fibroin/collagen blend films |
[173, 281] [190] |
|
| ||||
| Healthcare/hygiene products | Hospital/surgical textile fabrics (gowns/wear) Face masks |
Non-woven/weft knitting/warp knitting/woven/braiding/textile finishing | SF finishing agents; coated with antimicrobial substances | [191–195] |
Figure 3.
Publications and citations on silk-based biomedical textiles and silk implants and silk devices (data searched from SciFinder and Web of Science, Thomson Reuters, searched topic: silk medical textiles OR silk implants OR silk device, Time-span: 1999–2015).
4.1 Non-implantable materials
Non-implantable materials in BTFIs are used for external applications on the body including wound dressings, orthopedic bandages, pressure garments and prosthetic socks, among other examples. Textile scientists often try to provide bio-functions in silk fabrics as new wound dressings. Fibrous mats prepared via non-weaving and electrospinning can be used for wound dressings [108]. Blending silver nanoparticles into B. mori fibroin fibers can provide antibacterial wound dressings [109]. Xia et al. (2009) demonstrated combinations of B. mori fibroin films and titanium dioxide nanoparticles to inhibit bacteria [110]. A wax-coated silk fibroin woven fabric was introduced as a non-adhesive layer with a sponge of sericin and glutaraldehyde crosslinked silk fibroin/gelatin fabricated as a bioactive layer. This prototype two-layered wound dressing showed greater wound size reduction, epithelialization, and collagen formation than the control group [111, 112].
4.2 Implantable materials
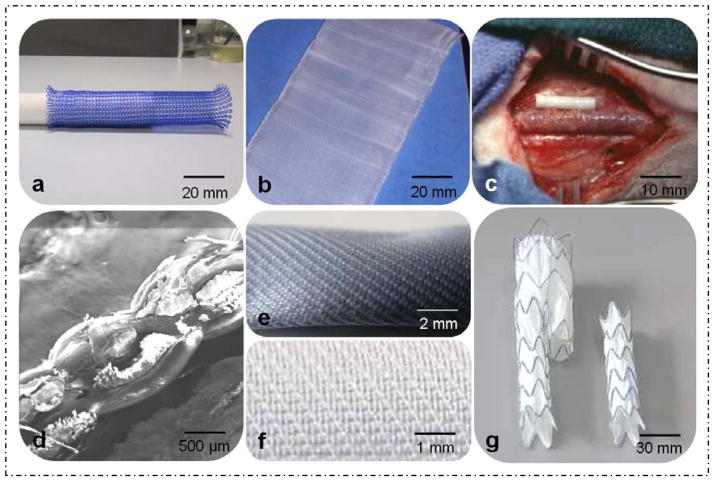
Implantable materials in BTFIs are used for repairs to the body for wound closure (sutures) or replacement surgery (soft-tissue implants, vascular prosthesis, artificial tendon/ligaments, as examples). Biocompatibility is one of most critical aspects if the biomedical textile materials are designed for in vivo use. Figure 4 shows specific samples of BTFIs in generated in our labs using natural or synthetic polymers including silk or medical polyester (PET) mono or multi-filaments.
Figure 4.
Prototype samples for BTFIs using natural or synthetic polymers including silk or polyester mono or multi-filaments: (a) weft-knitted stent, (b) woven chest wall, (c) artificial nerve, (d) cross-section of multi-layer woven vascular prosthesis, (e) two-layer vascular prosthesis, (f) surface of endo-vascular graft membrane, (g) endo-vascular graft for abdominal aneurysms.
4.2.1 Sutures
Natural silk fibers have been used as sutures for wound closure for centuries due to their strength, handling and biocompatibility. One of most important properties of sutures is the mechanical properties with knot strength as one of tests for wound sutures according to standards: USP (United States Pharmacopeia) and EP (European Pharmacopeia). However, regenerated silk sutures can be brittle with low knot strength but with reasonable breaking strength. Adding 50 wt% poly(vinyl alcohol) into silk fibroin increased tenacity and elongation at break of the fibers and the knot strength increased three-fold over pure silk wet-spun fibers, thus making these systems suitable as sutures for wounds [26]. Gulrajani et al. (2008) identified that B. mori fibroin fibers coated with silver may be beneficial for antimicrobial sutures [113].
4.2.2 Soft-tissue artificial implants
1) Artificial skin
As the largest organ in human body, skin plays the role as a barrier to infectious pathogens and microorganisms [114]. Ideally, a graft should cover and protect a wound bed without a negative immune response, enhance the healing process, lessening the pain of the patient and result in little or no scar formation [17]. During the past few decades, many attempts have been employed to mimic human skin using various SBBs [114]. The results showed that silk supported human keratinocytes and fibroblasts [85] and non-weaving or electrospinning technologies were the most commonly utilized material formats for engineering artificial skin. For example, eletrospun SF scaffolds (fiber diameters 30 to 120 nm) [115], water vapor-treated SF nanofiber matrices [116] and formic acid crosslinked SF based 3D nonwoven scaffolds [117] have been investigated in vitro using human oral keratinocytes (NHOK) and fibroblasts. Also, blending of SF and natural carbohydrate based polymers, such as SF/alginate [118][143], electropsun chitin/SF (25% chitin and 75% SF) [88], biomimetic nanostructured collagen/SF hybrid matrix [90] and intermolecular cross-linked recombinant human-like collagen (RHLC) with fibroin [119] have been studied in vivo for potential use for skin regeneration.
Wendt et al. (2011) constructed a bilayered skin equivalent containing fibroblasts and keratinocytes on woven native dragline silk to demonstrate the utility of spider silk for the enhancement of skin regeneration [120]. Min et al. (2004) compared three forms of SF matrices: woven (microfiber), non-woven (nanofiber), film form, and related cell culture responses by human oral keratinocytes (NHOK) to the conformation of the protein. The results suggested that the SF non-woven nanofiber matrices were preferred for skin scaffolds [121]. Water vapor-treated silk nano-fibrous mats showed better cell adhesion and dispersion of human keratinocytes and fibroblasts, which suggested that selecting the appropriate processing route is important for silk-based biomaterial designs [116]. These findings suggested that SBBs artificial skin with blending materials and multilayer micro structures may offer improved performance than pure silk for skin tissue regeneration. The complicated structure of native skin tissue and the mechanical performance require multilayer scaffolding using a bio-mimimetic approach.
2) Artificial nerve guides
The human nervous system includes the central nervous system (CNS) and peripheral nervous system (PNS) [17, 122, 123]. Peripheral nerves are capable of self-healing from minor injuries, but fail to regenerate critical sized gaps after lesion/trauma [124]. Recent advances in neurobiology and biomaterials, including the use of collagen and silk fibers, provide optimism for new treatments for nerve injuries [17, 125]. Silk promoted neuronal outgrowth at interfaces [126] and was useful in helping to assess neural signals and stimulate neurons [127]. Allmeling et al. (2011) described the use of nerve constructs consisting of decellularized vein grafts filled with spider silk fiber guides to bridge a 6.0 cm tibial nerve defects in adult sheep [128].
Usually, silk fibers in monofilament, multifilament and non-woven formats are woven, knitted and braided or combined into “wire-rope” scaffolds with a micro-porous structure [18–21, 127, 129, 130]. SBBs nerve scaffolds prepared from non-woven or fiber enhanced methods demonstrated good biocompatibility and appropriate mechanical performance in vitro and in vivo [131, 132]. For example, Chen et al. (2007) conducted a 6-month follow up after implantation of prototype nerve guide conduits reinforced with oriented silk fibers in rats. The results suggested these silk-based nerve scaffolds as candidates for nerve tissue engineering [133, 134]. Tang et al. (2009) also demonstrated SF fiber nerve scaffolds with feasibility as CNS substitutes due to good biocompatibility when studied with primary cultured hippocampal neurons [135]. Roloff et al. (2014) developed an in vitro crossed silk fiber array to visualize direct cellular interactions between spider silk and neurons in vitro [136, 137]. Silk films were used to modulate the conductance of astroglia and sacrificial silk fibroin films (dissolution times of 15–20 min) were used for neural interfaces [138].
Electrospining has been widely used for nerve scaffolds including electrospun silk nanofibers [139] or nano fibrous membranes. The silk has been also blended with other biopolymers including chitosan or poly(l-lactic acid-co-caprolactone) [140, 141], poly(lactic-co-glycolic acid) (PLGA) [142], chitosan or PLGA-SF-collagen [143], loaded with glial cell line-derived neurotrophic factor and nerve growth factor [144], blended with B. mori silk fibroin (BmSF) and Antheraea pernyi silk fibroin (ApSF) as examples [145]. However, only limited size defects (10–13 mm) have been successfully bridged in rat or chicken animal models [131, 144].
3) Artificial ligament and tendon
Scaffold engineering for ligaments and tendons should include systems that are biodegradable, exhibit sufficient mechanical strength, elasticity, toughness and structural integrity, and promote the regeneration of new ligamentous and tendon tissue [122, 146, 147]. Many reports using SBBs for ligament tissue engineering have appeared due to biocompatibility, slow degradability, and mechanical capabilities [11, 13]. For example, some spider silk dragline fibers are lightweight at 1.3 g/cm3, high strength up to 4.8 GPa, and exhibit elasticity up to 35% [148]. Both woven and braided artificial ACLs can be designed with similar mechanical properties to those of native ACLs in the human body [149]. Bone marrow derived mesenchymal stem cells (MSCs) can attach, proliferate and differentiate on these materials [149–151]. The results showed that the cells within the scaffolds generated tissues that resembled native ACL mechanically and histologically. Additional options have included silk fibers with a crosslinked collagen matrix [21, 152], electrospun PLGA fibers coated with silk [153], and the addition of basic fibroblast growth factor and transforming growth factor-β (TGF-β) within the scaffold to stimulate biochemical pathways for ACL regeneration [154]. For example, silk scaffolds modified with arginine-glycine-aspartic acid (RGD) significantly increased collagen production [155]. Fang et al. (2009) employed A. pernyi silk scaffolds to repair defects in the Achilles tendon using a rabbit model [156]. The results also suggested the possibility of using non-mulberry silk as tendon scaffolds in BTFIs. Hohlrieder et al. (2013) developed a bioreactor for the physical stimulation of ACL grafts prepared from SF, indicating that physical stimuli modulated cell functions [138]. Hennecke et al. (2013) reported that braided spider silk was suitable for sutures in flexor tendon repair, providing similar tensile behaviour and improved fatigue properties compared with conventional suture materials [147]. Ghiasi et al.(2014) used both texturing and coating method comprised of coated silk fibroin nanofibers onto the surface of textured silk yarn to improve the properties of a tendon and ligament scaffold. The results demonstrated that the nano-coated textured silk yarn can be a promising construct for engineered scaffolds in tendon and ligament tissue engineering [157]. Chen et al. (2014) developed engineered tendons constructed with the tendon-specific transcription factor scleraxis (SCX) in hESC-derived mesenchymal stem cells (hESC-MSCs) and knitted silk-collagen sponge scaffolds, demonstrating an ability of promote tendon regeneration [158].
4.2.3 Orthopaedic implants
Orthopaedic implants are used for hard tissue applications to repair bones, as well as utility for fixation plates to stabilize fractures [159]. Usually, metal and hard plastic materials are used for such implants to ensure structural integrity [160]. For example, braided surgical cables composed of steel filaments ranging from 13 – 130 mm are used to stabilize fractured bones or to secure orthopaedic implants [161]. The field is currently shifting more towards soft implants and fixation methods. Li et al. (2006) fabricated silk fiber scaffolds containing bone morphogenetic protein 2 (BMP-2) and/or nanoparticles of hydroxyapatite (nHAP) prepared via electrospinning for in vitro bone formation from human MSCs [162]. The results showed that electrospun silk-based scaffolds were useful for bone tissue engineering [162]. Kim et al. (2012) reported a modified silk ligature twisted with wire for treatment of advanced periodontitis using a less invasive approach, reducing unnecessary interventions compared to previously established techniques. The results illustrated that alveolar bone was significantly lower in the silk and the wire ligature group than the other groups on day 60 (p < 0.05), suggesting that experimental periodontitis induced by the silk and wire ligature were effective for treating periodontitis in canine models [163].
4.2.4 Vascular implants
Woven, knitted or nonwoven stents made of synthetic polymers are commonly used for transplants and bypasses of the abdominal aorta in the treatment of occlusive vascular disease and aneurysmectomy [164–168]. These systems generally fall short of meeting the biological challenges at the blood-material interface and fail at the microvascular scale (< 6 mm inner diameter) [17, 169]. SBBs have been clinically used for vascular tissue regeneration as flow diverting implants and stents [170, 171]. Previous studies showed that non-woven silk fiber mesh scaffolds from silkworm cocoons (B. mori) possessed an anti-thrombotic surface with good resistance to high shear stress and blood flow [85, 172], thus good biocompatibility and supportive of intercellular contact with endothelial cells [173, 174]. The outgrowth of endothelial cells isolated and expanded from heterogeneous human peripheral blood cultures has been used in combination with silk fiber meshes and demonstrated as a suitable autologous cell source for endothelialization of 3-D silk scaffolds [175].
The implantation of vascular grafts of silk composites consisting of B. mori and transgenic silkworm silk into rat abdominal aorta provided patency (ca. 85%) after one year [176]. These compositions offered good tensile strength to meet vascular requirements. Composites of silk and human-like-collagen or warp knitted silk-poly(ethylene glycol diglycoldiglycidyl ether) were used for vascular constructs and avoided early thrombosis [177]. Silk grafts with small diameters, < 3 mm, and 0.15 mm thick, were employed to reconstruct an intra-cranial aneurysm artery [178]. The burst strength of these silk prostheses was 811 mm Hg, lower than gold standard saphenous veins (1,800 mm Hg) [179, 180]. In order to simulate the multilayer structure of human blood vessels, multilayer grafts were fabricated. A multilayer silk protein graft was designed to assess potential as small diameter vessels. A nanofibrous silk coating as the outer layer supported the proliferation of smooth muscle cells and fibroblasts. Silk fiber-reinforced films contained heparin as the internal layer to provide low thrombogenicity. The results suggested good cytocompatibility and hemocompatibility in vitro [5].
Most commercial vascular grafts and prosthesis currently consist of polyesters (Dacron®) and use weaving and knitting technology. In order to improve biocompatibility, the surface of these products is often modified using silk fibroin and other proteins such as collagen. Yang et al. (2014) developed a seamless tubular prostheses made from a combination of polyester and silk yarns [181]. The results showed that the polyester/silk woven samples had better mechanical properties and improved cytocompatibility compared to commercial expanded polytetrafluoroethylene (e-PTFE) implants. These prostheses were prepared in different weaves using a modified rigid rapier loom [7, 182] and it was demonstrated that small caliber arterial prostheses could be manufactured [181]. The selection of suitable cell sources remains a challenge for vascular reconstruction. The space between adjacent silk fibers cannot be filled after seeding primary human endothelial cells (HPMEC-ST1.6R) and endothelial cells (ISO-HAS-1) [183]. Silk modifications to improve anti-coagulant activity [188], to enhance endothelium coverage and to co-culture endothelial cells and smooth muscle cells [89] are all important issues for the development for native vascular tissues.
4.2.5 Gastrointestinal stents
Gastrointestinal stents can be divided into different regions: esophagus, small intestine, gallbladder and large intestine. In previous studies, biodegradable and or drug-loaded stents have been demonstrated as an option to treat intestinal obstructions and stenosis [184, 185]. The major advantages of biodegradable stents are that they avoid long-term complications and do not require removal, thus avoiding further surgeries and potential morbidity [186, 187]. A few studies have been conducted using silk-based intestinal stents. Ni et al. (2008) constructed a silk-based stent for the treatment of tracheal defects in rabbits. The results showed that a new fibroblast layer formed (240 – 302 μm thick), and no foreign-body granuloma or macro-phage infiltration was evident [188]. Zang et al. (2011) developed a scaffold composed of silk and chitosan which supported the formation of an epithelial lining for tracheal stents [189].
4.3 Extracorporeal implants
Extracorporeal implants are artificial organs that are used for blood purification and include artificial kidney, artificial liver, and mechanical lung. The function and performance of these implants benefit from fibrous materials and textile technologies. Table 2 illustrates the functions of each device and the materials used in their manufacture. The function of the artificial kidney is achieved by circulating the blood through a fibrous membrane with bundles of hollow fibers or fibrous 3-D structures. Many layers of needle-punched fabrics composed of hollow fibers with different densities are designed for the rapid and efficient removal of rwastes [161]. For the mechanical lung, micro-porous membranes with high permeability to gases but low permeability to liquids are utilized, and function in the same manner as the natural lung by allowing oxygen contact with the patient’s blood [161]. The artificial liver can be made from hollow fibers or membranes similar to those used for the artificial kidney. Usually, hollow fibers consist of regenerated cellulose composite fibers. There is little reported on the use of silk-based hollow fibers for artificial organs, although silk has been reported as a biomimetic coating to promote liver cell adhesion and differentiation [190]. Cirillo et al. (2004) developed SF-collagen blend films to investigate rat liver cell adhesion and metabolism. The results suggested that silk was a suitable substrate for liver cell attachment and culture, and a potential alternative to collagen as a biomimetic coating because collagen may expose the host to risks of cross-species infections due to contamination with prions [190].
4.4 Healthcare/hygiene biomedical textiles
Healthcare and hygiene biomedical textiles have been widely used in clinical applications for the operating theatre and the hospital ward for hygiene, and the care and safety of patients and staff. Table 2 illustrates biomedical textiles used in healthcare and hygiene applications made from or treated with SBBs. These systems include fiber-based materials [191–195]. Silk-based BTFIs used in the operating theatre include surgeon’s gowns, caps and masks, patient drapes, and cover cloths of various sizes. The advantages of silk-based BTFIs include softness, biocompatibility, mechanical performance, and multi-functions such as antibacterial properties of the silk fabric. Ricci et al. (2004) evaluated the effectiveness of a special silk fabric (MICROAIR DermaSilk®) in the treatment of young children affected by atopic dermatitis (AD) with acute lesions at the time of examination. The results showed a significant decrease in the severity of AD in the children involved [193]. Sha et al. (2012; 2013) developed a nano-TiO2 photocatalytic silk mask paper with new functions, such as for the degradation of volatile organic compounds (VOCs), by combining the advantages of silk fibers and nano-TiO2 [194, 195].
5. Physical and chemical modifications
For biomedical textiles, the surface properties in contact with biological systems are important, including features such as topography, hydrophilicity/hydrophobicity and electrostatics, as these features can impact thrombogenicity, antimicrobial behavior and biocompatibility. Surface modifications are methods to modify and optimize the surface properties of materials and components [196], including the introduction of new functional groups, increases in surface energy or wettability, increases in hydrophobicity, improvement of chemical inertness, surface cross-linking and many other options [196]. There are a multitude of surface modification methods available to engineer custom designed interfaces, including micro and nanostructuring of surfaces via lithographic techniques, imprinting and laser micromachining; shot peening, laser ablation, plasma spraying, gas plasma treatments, ion bombardment, chemical etching, chemical and physical vapor deposition, as well as coatings of proteins, polymers, ceramics and other molecular-level self-assembled coatings [164, 197]. A number of surface modification techniques have been used for SBBs as BTFIs including plasma activation, plasma polymerization, chemical grafting, and polymer encapsulation of nanoparticles [1, 109, 166, 198–201]. The optimal surface will vary depending on the particular application, such as the use for the medical implant. More significantly, the optimal surface will vary with time, raising interest in dynamic surfaces, as well as smart surfaces which react to a changing local biological environment. Therefore, modifications and biotechnology approaches may facilitate more biocompatible silk-based BTFIs.
5.1 Surface coatings
Silk protein can be used as biocompatible coatings for BTFIs to improve or inhibit cell attachment, cell proliferation and anticoagulant properties. Silk can be produced in powder, solution and fibrous membrane formats with various surface properties to modulate compatibility in vitro and in vivo. B. mori silk possesses good antibacterial properties and inhibits the attachment of Staphylococcus epidermis Gram-positive bacteria in vitro when coated on poly(propylene) and poly(amide) films [201]. Moreover, several antibacterial nanoparticles such as titanium dioxide and silver nanoparticles were blended into SF [109], which then showed significant inhabitation of the proliferation of Escherichia. coli, Pseudomonas. aeruginosa and S. aureus [110].
Anticoagulant coatings are often used for biomedical implants, for example, on the surface of artificial warp-knitted prostheses to reduce blood penetration through the wall. Combinations of B. mori silk and poly(acrylic acid) modified with tetramethylpyrazine [199] or carboxymethyl keratin [198] showed better antithrombotic features than either of these polymers alone due to the interactions. Furthermore, the release of heparin from fibrous membranes of B. mori silk and Pellethane™ elastomeric particles can be controlled via adjusting drug-loading, ratios, and fiber diameter in the membrane, as well as the density and thickness [200].
Hybrid silk scaffolds were coated by hydroxyapatite (HA) using an alternate soaking technology to progressively reduce the hydrophobicity of the silk surface [202]. The osteoinductivity of HA-coated silk scaffolds resulted in osteogenic differentiation of bone marrow mesenchymal stem cells, and osteoblast growth and maturity. These HA-coatings demonstrated utility for fabricating osteoinductive ends for silk-based ligament grafts to enhance graft-to-host bone integration [202]. Jiang et al. (2014) found that hydroxyapatite (HAP) crystals grew under guidance from silk fibroin polyethylene terephthalate (PET) surfaces. The study showed that the combined SF and HAP coating on the surface of PET artificial ligaments induced graft osseointegration in bone tunnels.[203]. Zhang et al. (2014) used poly(lactide-co-glycolide) (PLGA, 10:90) fibers to adjust the degradation rate of the scaffolds and filled them with collagen to reserve space for cell ingrowth. A silk fibroin-PLGA (36:64) mesh scaffold was prepared using weft-knitting, filled with type I collagen, and incubated with rabbit autologous bone marrow-derived mesenchymal stem cells (MSCs). These findings suggested that tissue engineered tendons could be constructed using weft-knitted silk fibroin-PLGA fiber meshes/collagen matrices seeded with MSCs for rabbit achilles tendon repairs [204]. Li et al. (2013) studied the effect of multiple silk coatings to improve the mechanical and biological properties of biphasic calcium phosphate (BCP) scaffolds. The results showed that multiple silk coatings on BCP ceramic scaffolds achieved a significant improvement of the mechanical properties of the scaffolds and positively influence osteogenesis by hMSCs over time[205]
5.2 Physical modifications
Surface modifications include physical adsorption or chemical immobilization of silk proteins or ligands. Compared to chemical modifications, physical modification of SBBs can also provide suitable substrates to alter cell attachment and impact cell proliferation. Low temperature radiofrequency (RF) plasma treatment is an effective technique to modify the surface properties of natural silk fibers, without influencing their bulk properties [206–209]. Nitrogen, oxygen, and argon are often used in cold plasma implants. RF plasma treatment is a non-hazardous and environmentally friendly technique and has the advantage over wet chemical treatment in terms of reduction in energy consumption, avoidance of solvents and reduced treatment time [210]. Several investigations have been conducted on RF plasma treatment of silk fibers in order to modify wetting, dying and printing properties, tensile properties, shrink resistance, flame retardance, anti-bacterial properties, hydrophilicity and hydrophobicity (Table 3) [211–216].
Table 3.
Physical modifications of silk-based biomaterials for BTFIs
| Approaches | General practice | Findings | Ref. |
|---|---|---|---|
| Thermal treatment |
|
|
[282] |
| Plasma treatment using Ar/cold oxygen |
|
|
[207, 210, 283] |
| γ-ray/Raman irradiation treatment |
|
|
[284, 285] |
| Ultrasonic |
|
|
[286] |
| Sericin finishing treatment |
|
|
[287] |
Gogoi et al. (2011) investigated the surface modification of muga silk fibers to improve their tensile strength and hydrophobicity using Argon plasma treatment [207]. Zheng et al. (2012) conducted a study using titania sols by sol-gel methods and applied the sols with or without cold oxygen plasma on degummed B. mori silk fabrics. The results revealed that processing sequence of cold oxygen plasma and titania sols, and curing conditions, had significant impacts on the crystalline, thermal, UV resistant characteristics of silk fabrics [210]. Although numerous attempts using plasma technologies have been employed in modifying polymer surfaces [212], the mechanism of interactions between plasma and surfaces is complex and difficult to fully understand [210].
5.3 Chemical modifications
Chemical modifications may be passive or active compared to physical modifications [217]. Table 4 illustrates the usual chemical modification methods for silk-based BTFIs. In previous studies, SBB surfaces were modified with integrin recognition sequence [arginine-glycine-aspartic acid-serine (RGDS)] [218], poly(ethylene glycol) (PEG) [219] and horseradish peroxidase (HRP) [220]. Other available methods like carbodiimide chemistry modifications (using amine or carboxyl groups) using glucose oxidase [221], 1,2-cyclohexanedione [222], arginine-glycine-aspartic acid (RGD) [222–224], parathyroid hormone (PTH) [225, 226] and bone morphogenetic protein-2 (BMP-2) [227] have been coupled to silk to alter cell adhesion and proliferation, including human Saos-2 osteoblasts, tenocytes [223], hMSCs and fibroblasts.
Table 4.
Chemical modifications of silk-based biomaterials for BTFIs
| Approaches | General practice | Findings | Ref. |
|---|---|---|---|
| Chemical assembly of TiO2-Ag nano particles |
|
|
[288] |
| Silk grafting with chitosan or vinyl phosphate |
|
|
[289, 290] |
| Iodine treatment |
|
|
[291, 292] |
| Crosslinking reaction of nano TiO2 and chitosan |
|
|
[293] |
| chemically modified with sodium hydroxide or poly(pyrrole) |
|
|
[294, 295] |
| Emulsion graft copolymerization |
|
|
[296] |
| Sol-gel crosslinking |
|
|
[297] |
The diversity of chemical groups on silk proteins enables site-specific reactions, which allow the addition of unique chemical moieties [228]. Interestingly, an engineered spider silk protein (eADF4(C16)) provided a biomimetic coating (due to ECM related features) on engineered spider silk films that improved cell adhesion[229]. Remero et al. (2013) demonstrated that covalent attachment of negatively charged, hydrophilic sulfonic acid groups to silk protein can promote pyrrole absorption and polymerization to form conductive, interpenetrating networks of pyrrole and silk that did not delaminate. A variety of small molecule sulfonic acid dopants were utilized to further increase the conductivity and long-term stability of the Ppy network [230]. An engineered recombinant spider silk protein eADF4(C16) was modified with the integrin recognition sequence arginine-glycine-aspartic acid (RGD) by genetic (fusing the genetic sequence encoding GRGDSPG) and chemical (using the cyclic peptide c(RGDfK)) approaches [231]. The attachment and proliferation of BALB/3T3 mouse fibroblasts were significantly improved on the films with the RGD-modified silk proteins. Interestingly, the genetic hybrid protein (with linear RGD sequence) showed similar or slightly better cell adhesion than the silk chemically modified with cyclic RGD peptide [231].
Vepari et al. (2006) found that solution pH, hydrophobicity and pI of the protein determined how the silk surface attracted or repelled the attachment of proteins [220]. Modifying the amino acid side chain chemistry can be used to add functions to silk. However, the total content of modifiable amino acid side chain groups for chemical modifications is limited, for example, about 3.3% of the amino acids offer carboxyl side chains, which is much lower than bovine collagen (ca. 9.5%) [10]. The possible reason for the differentiation of cells on RGD-coupled silk matrices is that the increased cell density improved cell-cell interactions [222]. The above findings identified surface modifications with adhesion ligand and specific growth factors or morphogens contributed to the utility of silk for biological studies and offer routes to the further selection of chemical enhancements of SBBs in BTFIs. Murphy et al. (2008, 2010) found out additional chemical modifications have also been developed to exploit the higher percentage of tyrosines (>5%) in the silk, allowing additional modifications as above but with a higher degree of substitution.
6. Critical issues, opportunities and future needs
6.1 Critical issues
6.1.1 Improvement of the mechanical properties of regenerated silk fibers
Whether the silk material is to be woven, knitted and braided into biomedical textile products or used as an individual fiber in biomedical applications, it is important to produce long lengths of fibrous material or fiber in sufficient quantity and with acceptable mechanical performance (i.e., similar to the native silkworm or spider silk fiber). Silkworm silk have been reengineered into silk fiber materials using wet-spinning, electrospinning and microfluidic approaches, which might be appropriate for spider-silk proteins as well [12, 232, 233]. The advantages and limitations of each system will determine their use in specific applications or their commercial exploitation [234, 235]. For example, wet-spun fibers usually offer higher mechanical properties than electrospun and microfluidic fibers. Hence, wet-spun fibers are more suitable for weaving, knitting and braiding production. Although native silk fibers possess remarkable mechanical properties, most regenerated silk fibers are relatively weak and brittle when compared to native fibers. The tensile strength and elongation at break of the native fibers are 0.5 – 0.6 GPa and 10 – 40%, respectively, in comparison, that of silk fibrous films (0.02 GPa and 2%, respectively) [55, 236]. The reason for this difference may be the lack of appropriate hierarchical and secondary structures in the regenerated silk materials [149, 237]. The first critical issue is that the mechanical properties of regenerated silk materials need to be improved by control of specific structures during regeneration [98, 238, 239]. Once the mechanical properties of regenerated silk materials are significantly improved or can be tailored according to different applications, silk fiber materials will have more comprehensive applications in BTFIs. However, many studies have not been expanded to large scale industrialization. As discussed above, wet spinning is better to fabricate regenerated silk fibers with micrometer scale diameters. Development of appropriate solvents for disolving SF but not destroying the stucture is important. Currently, melt-spinning is mostly widely used in industry and the melt-spun fibers have the high performance in terms of physical, mechanical properties and low production cost, however, melt spinning of silk proteins is not currently considered due to the challenge of degradation under such conditions. Thus new studies on creative approaches to melt-spun SF at low temperature need to be investigated.
6.1.2 Biomechanics of silk-based biomedical textiles and fiber-based implants
Dimensions, structural designs, materials properties and processing methods influence the mechanical performance of BTFIs [138, 240–243]. Therefore, the biomechanical properties of silk-based BTFIs are important to consider. Usually, biomechanical performance, stress distribution and deformation behavior of medical implants have been investigated by different models including structural mechanical models such as the Bernoulli-Euler beam theory and the Coulomb torsion theory [138, 244, 245]. Numerical modeling is also often adopted, including finite element model (FEM) analyses to expand stents by imposing pressure to the internal stent struts [246] or by enlarging a rigid cylinder inside the stents for displacement control [247]. Although much progress has been reported, few studies have focused on the effect of biomedical textile structures and processing parameters on the mechanical properties of silk-based biomedical textiles [181, 240, 248–250].
6.1.3 Multifunctional features of silk-based biomedical textiles and fiber-based implants
1) Controlled drug release
It is important for a drug system to have sustainable and controllable release profiles to improve efficiency and minimize systemic side effects [33, 251]. Biomedical textiles provide good opportunities for the combined use of textile and pharmaceutical technologies to develop novel drug delivery systems. Silk-based drug delivery systems (DDS) can be employed in BTFIs due to their biocompatibility and highly tunable morphologies [252, 253]. However, challenges remain regarding drug-loading capability, control of release, the structures (micro-crystal, amorphous, particulate) of the loaded fibers and the possible interaction between the drug and material during spinning or processing. There are also problems with the relationship between the structure of the silk-based fibers and their drug release profiles, the release mechanism of drugs from silk-based BTFIs, and mathematical models for the prediction of drug release.
The forms of SBBs used for DDS are commonly particles, solution, sponges, films, microspheres and hydrogels [254]. For silk-based biomedical textiles, electrospinning or fiber-based composites are usually used for DDS. Core-sheath nanofibers composed of poly (ε-caprolactone) (PCL) and SF blends generated via emulsion electrospinning showed long-term drug release [255]. In contrast, core-sheath fibers displayed continuous release kinetics (the cumulative release percentage of FITC is 62.2 ± 4.2% at 80 h). These results indicated that core-sheath structured PCL/SF nanofibrous scaffolds with sustained drug release have potential utility in tissue engineering [255]. A fusion polypeptide comprised of a fibrous protein domain and a mineralization domain was used to form organic-inorganic composites which could be loaded with drugs [257]. Silk fibroin solution was combined with a therapeutic agent to form materials, where the control of the release of the therapeutic agent could be tuned based on silk protein conformation (content of beta sheet crystals) [258]. Single material carriers or layer-by-layer depositions can be used to load different therapeutic agents or different concentrations of these agents in each layer. The silk fibroin composition can comprise a fiber-based structure, hydrogel, coating or microsphere [258].
2) Controlled degradation
Compared to other commercial synthetic biomaterials such as polyglycolides and polylactides, SBBs or recombinant silk protein-based materials present a rare combination of desirable properties for sustained drug delivery, including biocompatibility, robust mechanical properties, aqueous-based purification and processing options without chemical cross-linkers, compatibility with common sterilization methods, controllable and surface-mediated biodegradation into non-inflammatory by-products and utility in drug stabilization [158, 259]. As evidenced by a number of sustained release silk-based formulations described in a recent review by Yucel et al. (2014), silk plays a role in stabilizing small molecule or biological therapeutics as a function of adsorption, covalent attachment, entrapment, and/or encapsulation [259]. For example, the degradation products of the synthetic biomaterials can be absorbed via metabolic pathways, but the release of acidic by-products can induce a rapid decrease of mechanical properties during early degradation due to acidic hydrolysis [260, 261]. In contrast, one of advantages of SBBs is that they can maintain appropriate mechanical properties over a long time due to the surface mediated enzymatic degradation of the silk, as opposed to the bulk hydrolysis of the synthetic systems. In particular, the slow degradation and load-bearing capacity are important for tissue engineering. Despite the above advantages, it is also important to understand the mechanisms and correlation of silk degradation with mechanical properties [10, 158].
In general, silk is slowly absorbed in vivo and defined as a non-degradable biomaterial according to the definition made by US Pharmacopeia. This is because silk fibers retain more than 50% of their mechanical properties after two months of implantation in vivo [10, 262]. In a rat model with subcutaneous implantation, silk fibers lost 29% of their tensile strength at 10 days, 73% at 30 days and 83% within 70 days post-implantation (Table 5) [11, 263]. Silk degrade via the action of proteases. The rate of silk degradation depends upon the structure, morphology, and mechanical and biological conditions at the location of implantation. Usually, silk degradation can be regulated by changing crystallinity, pore size, porosity and molecular weight distribution (MWD) [264]. In previous studies, regenerated silk fibroin biomaterials degraded much faster than native fibers due to the secondary structure of silk from preparation of regenerated silk materials [265]. The results demonstrated that a high content of β-sheet structure leads to a low degradation rate. The random coil regions in the silk fibroin material are degraded, whereas the crystal regions remain stable. Hence, these findings indicate that it is possible to control the degradation rate of a silk fibroin scaffold by controlling the content of β-sheet structure for a specific devlice or tissue engineering application [265].
Table 5.
| Suture | Time post-implantation
|
||
|---|---|---|---|
| 10 days | 30 days | 70 days | |
| Black braided silk | 29% | 73% | 83% |
| Polyglycolic acid (PGA) | 65% | 92% | 99% |
| Multifilament nylon | 16% | 19% | 22% |
| Monofilament nylon | 3% | 7% | 16% |
6.2 Opportunities and Future needs
A shift in population demographics, including a growing elderly population and higher obesity, affect BTFIs development. Active patients increasingly consult with surgeons about treatment options and minimally invasive surgical techniques that return them to health in a shorter time than traditional more invasive surgical procedures. To meet the increasing demand in the society, biomedical textile engineering aims to develop a holistic and integrative approach of designing and engineering BTFIs to meet biomedical and healthcare needs. SBBs, in particular, are being re-engineered with flexible and compliant textile structures to minimize the loss of natural movement. For example, the global orthobiologics market is estimated to more than double from US$ 4.3 billion in 2009 to US$ 9.6 billion by 2016 [30]. US sales of advanced DDSs have continued to grow 15.6% annually, reaching $153.5 billion by 2011 [30]. The competitive orthopaedic soft tissue repair market also is expected to grow from US$ 920 million to US$ 1.6 billion in the same time period [29].
On a worldwide basis, biomedical textile stents made from synthetic polymers using textile-forming technologies have been in routine clinical use for nearly five decades and have been implanted in hundreds of thousands of patients. Although a number of areas of study are developing in the novel silk-based BTFIs, these include developments in polymer science and advanced textile solutions such as non-weaving, braiding, knitting and weaving technologies, there remain many questions for silk-based BTFIs to progress to clinical applications. First, new development needs to exploit the biocompatibility, mechanical properties, fiber varieties and fabric-forming techniques of SBBs which are required to improve silk-based BTFIs with increased value and end-use performance in the biomedical textile field. Furthermore, the development of silk-based BTFIs is a multidisciplinary field which needs in-depth research involving biology, medicine, material engineering and mathematics.
7. Conclusions
The textile industry has demonstrated its competiveness in the field of biomedical and healthcare, with the development and advanced manufacturing of medical textiles, smart textiles and multifunctional textiles. BTFIs are very important in all aspects of medicine and surgery and the range and extent of applications to which these materials are used is a reflection of their enormous versatility. Among the variety of materials used for BTFIs, SBBs have been widely used clinically such as for sutures for centuries and are increasingly recognized as a prospective material for biomedical textiles.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (NSFC) with project code 51273138 and the National Institutes of Health (P41 EB002520). We would like to thank the support of State Key Laboratory for Modification of Chemical Fibers and Polymer Materials, Donghua University with project code LK1421, Guangdong Provincial Department of Science and Technology with project codes 2012B050800002 and Natural Science Foundation for Young Scholars of Soochow University.
Contributor Information
Dr. Gang Li, National Engineering Laboratory for Modern Silk, College of Textile and Clothing Engineering, Soochow University, Suzhou 215123, P.R. China
Prof. Yi Li, Institute of Textiles and Clothing, The Hong Kong Polytechnic University, Hong Kong, China
Prof. Guoqiang Chen, National Engineering Laboratory for Modern Silk, College of Textile and Clothing Engineering, Soochow University, Suzhou 215123, P.R. China
Prof. Jihuan He, National Engineering Laboratory for Modern Silk, College of Textile and Clothing Engineering, Soochow University, Suzhou 215123, P.R. China
Prof. Yifan Han, Department of Applied Biology and Chemical Technology, The Hong Kong Polytechnic University, Hong Kong, China
Prof. Xiaoqin Wang, Email: wangxiaoqin@suda.edu.cn, National Engineering Laboratory for Modern Silk, College of Textile and Clothing Engineering, Soochow University, Suzhou 215123, P.R. China
Prof. David L. Kaplan, Email: david.kaplan@tufts.edu, Department of Biomedical Engineering, Tufts University, 4 Colby St, Room 153, Medford, MA 02155, USA
References
- 1.King MW. Can Text J. 1991;108:24. [Google Scholar]
- 2.Daniele MA, Boyd DA, Adams AA, Ligler FS. Adv Healthc Mater. 2015;4:11. doi: 10.1002/adhm.201400144. [DOI] [PubMed] [Google Scholar]
- 3.Williams DF. Definitions in biomaterials: proceedings of a consensus conference of the European Society for Biomaterials. Elsevier Science Ltd; Chester, England: 1987. [Google Scholar]
- 4.Li G, Chen Y, Hu J, Wu X, Hu J, He X, Li J, Zhao Z, Chen Z, Li Y. Biomaterials. 2013;34:9451. doi: 10.1016/j.biomaterials.2013.08.055. [DOI] [PubMed] [Google Scholar]
- 5.Liu S, Dong C, Lu G, Lu Q, Li Z, Kaplan DL, Zhu H. Acta Biomater. 2013;9:8991. doi: 10.1016/j.actbio.2013.06.045. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Blasioli DJ, Kim HJ, Kim HS, Kaplan DL. Biomaterials. 2006;27:4434. doi: 10.1016/j.biomaterials.2006.03.050. [DOI] [PubMed] [Google Scholar]
- 7.Li G, Liu Y, Lan P, Li Y, Li Y. J Text I. 2013;104:1017. [Google Scholar]
- 8.Mandal BB, Grinberg A, Gil ES, Panilaitis B, Kaplan DL. Proc Natl Acad Sci U S A. 2012;109:7699. doi: 10.1073/pnas.1119474109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang M, Yu JH, Kaplan DL, Rutledge GC. Macromolecules. 2006;39:1102. [Google Scholar]
- 10.Vepari C, Kaplan DL. Prog Polym Sci. 2007;32:991. doi: 10.1016/j.progpolymsci.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Altman GH, Diaz F, Jakuba C, Calabro T, Horan RL, Chen J, Lu H, Richmond J, Kaplan DL. Biomaterials. 2003;24:401. doi: 10.1016/s0142-9612(02)00353-8. [DOI] [PubMed] [Google Scholar]
- 12.Kluge JA, Rabotyagova O, Leisk GG, Kaplan DL. Trends Biotechnol. 2008;26:244. doi: 10.1016/j.tibtech.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Kim HJ, Vunjak-Novakovic G, Kaplan DL. Biomaterials. 2006;27:6064. doi: 10.1016/j.biomaterials.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 14.Li G, Li Y, Lan P, He X, Hu H. Text Res J. 2013;83:2129. [Google Scholar]
- 15.Lu Q, Zhang X, Hu X, Kaplan DL. Macromol Biosci. 2010;10:289. doi: 10.1002/mabi.200900258. [DOI] [PubMed] [Google Scholar]
- 16.Omenetto FG, Kaplan DL. Science. 2010;329:528. doi: 10.1126/science.1188936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kasoju N, Bora U. Adv Healthc Mater. 2012;1:393. doi: 10.1002/adhm.201200097. [DOI] [PubMed] [Google Scholar]
- 18.Fan H, Liu H, Toh SL, Goh JCH. Biomaterials. 2009;30:4967. doi: 10.1016/j.biomaterials.2009.05.048. [DOI] [PubMed] [Google Scholar]
- 19.Lu HH, Cooper JA, Jr, Manuel S, Freeman JW, Attawia MA, Ko FK, Laurencin CT. Biomaterials. 2005;26:4805. doi: 10.1016/j.biomaterials.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 20.Fan H, Liu H, Wong EJW, Toh SL, Goh JCH. Biomaterials. 2008;29:3324. doi: 10.1016/j.biomaterials.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 21.Chen X, Qi Y, Wang L, Yin Z, Yin G, Zou X, Ouyang H. Biomaterials. 2008;29:3683. doi: 10.1016/j.biomaterials.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 22.Ki CS, Lee KH, Baek DH, Hattori M, Um IC, Ihm DW, Park YH. J Appl Polym Sci. 2007;105:1605. [Google Scholar]
- 23.Matsumoto K, Uejima H, Iwasaki T, Sano Y, Sumino H. J Appl Polym Sci. 1996;60:503. [Google Scholar]
- 24.Marsano E, Corsini P, Arosio C, Boschi A, Mormino M, Freddi G. Int J Biol Macromol. 2005;37:179. doi: 10.1016/j.ijbiomac.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Um IC, Ki CS, Kweon H, Lee KG, Ihm DW, Park YH. Int J Biol Macromol. 2004;34:107. doi: 10.1016/j.ijbiomac.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 26.Lee KH, Baek DH, Ki CS, Park YH. Int J Biol Macromol. 2007;41:168. doi: 10.1016/j.ijbiomac.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 27.Ki CS, Park YH. Fiber Polym. 2013;14:1460. [Google Scholar]
- 28.Tungjitpornkull S, Chaochanchaikul K, Sombatsompop N. J Thermoplast Compos Mater. 2007;20:535. [Google Scholar]
- 29.King MW, Gupta BS, Guidoin R. Biotextiles as medical implants. Elsevier; New York: 2013. [Google Scholar]
- 30.Koslosky JM, Shorrock S. Eur Med Device Technol. 2012;3:20. [Google Scholar]
- 31.Thwe MM, Liao K. Composites, Part A. 2002;33:43. [Google Scholar]
- 32.Gloria A, Ronca D, Russo T, D’Amora U, Chierchia M, De Santis R, Nicolais L, Ambrosio L. J Appl Biomater Biomech. 2010;9:151. doi: 10.5301/JABB.2011.8569. [DOI] [PubMed] [Google Scholar]
- 33.Hardy JG, Scheibel TR. Prog Polym Sci. 2010;35:1093. [Google Scholar]
- 34.Shao Z, Vollrath F. Nature. 2002;418:741. doi: 10.1038/418741a. [DOI] [PubMed] [Google Scholar]
- 35.Putthanarat S, Stribeck N, Fossey SA, Eby RK, Adams WW. Polymer. 2000;41:7735. [Google Scholar]
- 36.Putthanarat S, Eby RK, Adams WW, Liu GF. J Macromol Sci, Part A: Pure ApplChem. 1996;33:899. [Google Scholar]
- 37.Inoue S, Tanaka K, Arisaka F, Kimura S, Ohtomo K, Mizuno S. J Biol Chem. 2000;275:40517. doi: 10.1074/jbc.M006897200. [DOI] [PubMed] [Google Scholar]
- 38.Kundu SC, Dash BC, Dash R, Kaplan DL. Prog Polym Sci. 2008;33:998. [Google Scholar]
- 39.Nirmala X, Kodrik D, Žurovec M, Sehnal F. Eur J Biochem. 2001;268:2064. doi: 10.1046/j.1432-1327.2001.02084.x. [DOI] [PubMed] [Google Scholar]
- 40.Nirmala X, Mita K, Vanisree V, Žurovec M, Sehnal F. Insect Mol Biol. 2001;10:437. doi: 10.1046/j.0962-1075.2001.00282.x. [DOI] [PubMed] [Google Scholar]
- 41.Sponner A, Vater W, Monajembashi S, Unger E, Grosse F, Weisshart K. PloS one. 2007;2:e998. doi: 10.1371/journal.pone.0000998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sponner A, Unger E, Grosse F, Weisshart K. Nat Mater. 2005;4:772. doi: 10.1038/nmat1493. [DOI] [PubMed] [Google Scholar]
- 43.Gatesy J, Hayashi C, Motriuk D, Woods J, Lewis R. Science. 2001;291:2603. doi: 10.1126/science.1057561. [DOI] [PubMed] [Google Scholar]
- 44.Holland GP, Creager MS, Jenkins JE, Lewis RV, Yarger JL. J Am Chem Soc. 2008;130:9871. doi: 10.1021/ja8021208. [DOI] [PubMed] [Google Scholar]
- 45.Holland GP, Jenkins JE, Creager MS, Lewis RV, Yarger JL. Chem Commun. 2008:5568. doi: 10.1039/b812928b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holland GP, Lewis RV, Yarger JL. J Am Chem Soc. 2004;126:5867. doi: 10.1021/ja031930w. [DOI] [PubMed] [Google Scholar]
- 47.Holland GP, Jenkins JE, Creager MS, Lewis RV, Yarger JL. Biomacromolecules. 2008;9:651. doi: 10.1021/bm700950u. [DOI] [PubMed] [Google Scholar]
- 48.Allmeling C, Radtke C, Vogt PM. Spider Ecophysiology. Springer; 2013. Technical and biomedical uses of nature’s strongest fiber: spider silk; p. 475. [Google Scholar]
- 49.Cunniff PM, Fossey SA, Auerbach MA, Song JW, Kaplan DL, Adams WW, Eby RK, Mahoney D, Vezie DL. Polym Adv Technol. 1994;5:401. [Google Scholar]
- 50.Vollrath F. Curr Biol. 2005;15(10):364. doi: 10.1016/j.cub.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 51.Vollrath F, Knight DP. Nature. 2001;410:541. doi: 10.1038/35069000. [DOI] [PubMed] [Google Scholar]
- 52.Giesa T, Arslan M, Pugno NM, Buehler MJ. Nano Lett. 2011;11:5038. doi: 10.1021/nl203108t. [DOI] [PubMed] [Google Scholar]
- 53.Du N, Yang Z, Liu XY, Li Y, Xu HY. Adv Funct Mater. 2011;21:772. [Google Scholar]
- 54.Zhang Y, Yang H, Shao H, Hu X. J Biomed Biotechnol. 2010;2010:683962. doi: 10.1155/2010/683962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rajkhowa R, Gupta VB, Kothari VK. J Appl Polym Sci. 2000;77:2418. [Google Scholar]
- 56.Hearle JWS, Morton WE. Physical properties of textile fibres. Elsevier, Woodhead Publishing Ltd; Cambridge: 2008. [Google Scholar]
- 57.Sutherland TD, Young JH, Weisman S, Hayashi CY, Merritt DJ. Annu Rev Entomol. 2010;55:171. doi: 10.1146/annurev-ento-112408-085401. [DOI] [PubMed] [Google Scholar]
- 58.Vendrely C, Scheibel T. Macromol Biosci. 2007;7:401. doi: 10.1002/mabi.200600255. [DOI] [PubMed] [Google Scholar]
- 59.Hardy J, Scheibel T. Biochem Soc Trans. 2009;37:677. doi: 10.1042/BST0370677. [DOI] [PubMed] [Google Scholar]
- 60.Weisman S, Haritos VS, Church JS, Huson MG, Mudie ST, Rodgers AJ, Dumsday GJ, Sutherland TD. Biomaterials. 2010;31:2695. doi: 10.1016/j.biomaterials.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 61.Kusurkar TS, Tandon I, Sethy NK, Bhargava K, Sarkar S, Singh SK, Das M. Sci Rep. 2013:3. doi: 10.1038/srep03290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hardy JG, Römer LM, Scheibel TR. Polymer. 2008;49:4309. [Google Scholar]
- 63.Hardy JG, Scheibel TR. J Polym Sci A Polym Chem. 2009;47:3957. [Google Scholar]
- 64.Fu C, Shao Z, Fritz V. Chem Commun. 2009:6515. doi: 10.1039/b911049f. [DOI] [PubMed] [Google Scholar]
- 65.DeáLong HC. J Mater Chem. 2005;15:4206. [Google Scholar]
- 66.Arai T, Freddi G, Innocenti R, Kaplan DL, Tsukada M. J Appl Polym Sci. 2001;82:2832. [Google Scholar]
- 67.Arai T, Freddi G, Innocenti R, Tsukada M. J Appl Polym Sci. 2003;89:324. [Google Scholar]
- 68.Qing F-L, Ji M, Lu R, Yan K, Mao Z. J Fluor Chem. 2002;113:139. [Google Scholar]
- 69.Prachayawarakorn J, Boonsawat K. J Appl Polym Sci. 2007;106:1526. [Google Scholar]
- 70.Cai Z, Jiang G, Qiu Y. J Appl Polym Sci. 2004;91:3579. [Google Scholar]
- 71.Tsukada M, Imai T, Freddi G, Lenka S, Kasai N. J Appl Polym Sci. 1998;69:239. [Google Scholar]
- 72.Viney C, Bell FI. Curr Opin Solid State Mater Sci. 2004;8:165. [Google Scholar]
- 73.Jin H-J, Fridrikh SV, Rutledge GC, Kaplan DL. Biomacromolecules. 2002;3:1233. doi: 10.1021/bm025581u. [DOI] [PubMed] [Google Scholar]
- 74.Zarkoob S, Eby RK, Reneker DH, Hudson SD, Ertley D, Adams WW. Polymer. 2004;45:3973. [Google Scholar]
- 75.Ohgo K, Zhao C, Kobayashi M, Asakura T. Polymer. 2003;44:841. [Google Scholar]
- 76.Sukigara S, Gandhi M, Ayutsede J, Micklus M, Ko F. Polymer. 2003;44:5721. [Google Scholar]
- 77.Lock RL, inventor. U.S.5252285. Google Patents 1993
- 78.Trabbic KA, Yager P. Macromolecules. 1998;31:462. [Google Scholar]
- 79.Yao J, Masuda H, Zhao C, Asakura T. Macromolecules. 2002;35:6. [Google Scholar]
- 80.Zhao C, Yao J, Masuda H, Kishore R, Asakura T. Biopolymers. 2003;69:253. doi: 10.1002/bip.10350. [DOI] [PubMed] [Google Scholar]
- 81.Liivak O, Blye A, Shah N, Jelinski LW. Macromolecules. 1998;31:2947. doi: 10.1016/s0141-8130(98)00085-3. [DOI] [PubMed] [Google Scholar]
- 82.Ha S-W, Tonelli AE, Hudson SM. Biomacromolecules. 2005;6:1722. doi: 10.1021/bm050010y. [DOI] [PubMed] [Google Scholar]
- 83.Zhou GQ, Chen X, Shao ZZ. ProgChem. 2006;18:933. [Google Scholar]
- 84.Yan J, Zhou G, Knight DP, Shao Z, Chen X. Biomacromolecules. 2009;11:1. doi: 10.1021/bm900840h. [DOI] [PubMed] [Google Scholar]
- 85.Zhang X, Reagan MR, Kaplan DL. Adv Drug Deliv Rev. 2009;61:988. doi: 10.1016/j.addr.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lovett ML, Cannizzaro CM, Vunjak-Novakovic G, Kaplan DL. Biomaterials. 2008;29:4650. doi: 10.1016/j.biomaterials.2008.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang X, Wang X, Keshav V, Wang X, Johanas JT, Leisk GG, Kaplan DL. Biomaterials. 2009;30:3213. doi: 10.1016/j.biomaterials.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yoo CR, Yeo I-S, Park KE, Park JH, Lee SJ, Park WH, Min B-M. Int J Biol Macromol. 2008;42:324. doi: 10.1016/j.ijbiomac.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 89.Park KB, Do YS, Kang WK, Choo SW, Han YH, Suh SW, Lee SJ, Park KS, Choo IW. Radiology. 2001;219:679. doi: 10.1148/radiology.219.3.r01jn21679. [DOI] [PubMed] [Google Scholar]
- 90.Yeo I-S, Oh J-E, Jeong L, Lee TS, Lee SJ, Park WH, Min B-M. Biomacromolecules. 2008;9:1106. doi: 10.1021/bm700875a. [DOI] [PubMed] [Google Scholar]
- 91.Zhao L, Xu YL, He M, Zhang WX, Li M. Compos Interfaces. 2014;21:869. [Google Scholar]
- 92.Zhang F, Zuo B, Fan Z, Xie Z, Lu Q, Zhang X, Kaplan DL. Biomacromolecules. 2012;13:798. doi: 10.1021/bm201719s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lang G, Jokisch S, Scheibel T. J Vis Exp. 2013:e50492. doi: 10.3791/50492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lazaris A, Arcidiacono S, Huang Y, Zhou JF, Duguay F, Chretien N, Welsh EA, Soares JW, Karatzas CN. Science. 2002;295:472. doi: 10.1126/science.1065780. [DOI] [PubMed] [Google Scholar]
- 95.Arcidiacono S, Mello CM, Butler M, Welsh E, Soares JW, Allen A, Ziegler D, Laue T, Chase S. Macromolecules. 2002;35:1262. [Google Scholar]
- 96.Stephens JS, Fahnestock SR, Farmer RS, Kiick KL, Chase DB, Rabolt JF. Biomacromolecules. 2005;6:1405. doi: 10.1021/bm049296h. [DOI] [PubMed] [Google Scholar]
- 97.Wang XF, Ding B, Sun G, Wang MR, Yu JY. Prog Mater Sci. 2013;58:1173. doi: 10.1016/j.pmatsci.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ha S-W, Tonelli AE, Hudson SM. Biomacromolecules. 2005;6:1722. doi: 10.1021/bm050010y. [DOI] [PubMed] [Google Scholar]
- 99.Kinahan ME, Filippidi E, Koster S, Hu X, Evans HM, Pfohl T, Kaplan DL, Wong J. Biomacromolecules. 2011;12:1504. doi: 10.1021/bm1014624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lee KH. Macromol Rapid Commun. 2004;25:1792. [Google Scholar]
- 101.Ki CS, Um IC, Park YH. Polymer. 2009;50:4618. [Google Scholar]
- 102.Ki CS, Kim JW, Oh HJ, Lee KH, Park YH. Int J Biol Macromol. 2007;41:346. doi: 10.1016/j.ijbiomac.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 103.Um IC, Kweon H, Lee KG, Ihm DW, Lee J-H, Park YH. Int J Biol Macromol. 2004;34:89. doi: 10.1016/j.ijbiomac.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 104.Zhu Z, Ohgo K, Watanabe R, Takezawa T, Asakura T. J Appl Polym Sci. 2008;109:2956. [Google Scholar]
- 105.Marsano E, Corsini P, Canetti M, Freddi G. Int J Biol Macromol. 2008;43:106. doi: 10.1016/j.ijbiomac.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 106.Marsano E, Canetti M, Conio G, Corsini P, Freddi G. J Appl Polym Sci. 2007;104:2187. [Google Scholar]
- 107.Zheng Y, Bai H, Huang Z, Tian X, Nie FQ, Zhao Y, Zhai J, Jiang L. Nature. 2010;463:640. doi: 10.1038/nature08729. [DOI] [PubMed] [Google Scholar]
- 108.Gil ES, Panilaitis B, Bellas E, Kaplan DL. Adv Healthc Mater. 2013;2:206. doi: 10.1002/adhm.201200192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kang M, Jung R, Kim H-S, Youk JH, Jin H-J. J Nanosci Nanotechnol. 2007;7:3888. doi: 10.1166/jnn.2007.056. [DOI] [PubMed] [Google Scholar]
- 110.Xia Y, Gao G, Li Y. J Biomed Mater Res B Appl Biomater. 2009;90:653. doi: 10.1002/jbm.b.31331. [DOI] [PubMed] [Google Scholar]
- 111.Kanokpanont S, Damrongsakkul S, Ratanavaraporn J, Aramwit P. Int J Biol Macromol. 2013;55:88. doi: 10.1016/j.ijbiomac.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 112.Kanokpanont S, Damrongsakkul S, Ratanavaraporn J, Aramwit P. Int J Pharm. 2012;436:141. doi: 10.1016/j.ijpharm.2012.06.046. [DOI] [PubMed] [Google Scholar]
- 113.Gulrajani ML, Gupta D, Periyasamy S, Muthu SG. J Appl Polym Sci. 2008;108:614. [Google Scholar]
- 114.Groeber F, Holeiter M, Hampel M, Hinderer S, Schenke-Layland K. Adv Drug Deliv Rev. 2011;63:352. doi: 10.1016/j.addr.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 115.Min B-M, Lee G, Kim SH, Nam YS, Lee TS, Park WH. Biomaterials. 2004;25:1289. doi: 10.1016/j.biomaterials.2003.08.045. [DOI] [PubMed] [Google Scholar]
- 116.Min BM, Jeong L, Lee KY, Park WH. Macromol Biosci. 2006;6:285. doi: 10.1002/mabi.200500246. [DOI] [PubMed] [Google Scholar]
- 117.Dal Pra I, Chiarini A, Boschi A, Freddi G, Armato U. Int J Mol Med. 2006;18:241. [PubMed] [Google Scholar]
- 118.Roh DH, Kang SY, Kim JY, Kwon Y-B, Kweon HY, Lee KG, Park YH, Baek R-M, Heo CY, Choe J. J Mater Sci Mater Med. 2006;17:547. doi: 10.1007/s10856-006-8938-y. [DOI] [PubMed] [Google Scholar]
- 119.Khor HL, Ng KW, Htay AS, Schantz J-T, Teoh SH, Hutmacher DW. J Mater Sci Mater Med. 2003;14:113. doi: 10.1023/a:1022059511261. [DOI] [PubMed] [Google Scholar]
- 120.Wendt H, Hillmer A, Reimers K, Kuhbier JW, Schäfer-Nolte F, Allmeling C, Kasper C, Vogt PM. PloS one. 2011;6:e21833. doi: 10.1371/journal.pone.0021833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Min BM, Jeong L, Nam YS, Kim JM, Kim JY, Park WH. Int J Biol Macromol. 2004;34:223. doi: 10.1016/j.ijbiomac.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 122.Kundu B, Rajkhowa R, Kundu SC, Wang X. Adv Drug Deliv Rev. 2013;65:457. doi: 10.1016/j.addr.2012.09.043. [DOI] [PubMed] [Google Scholar]
- 123.Schmidt CE, Leach JB. Annu Rev Biomed Eng. 2003;5:293. doi: 10.1146/annurev.bioeng.5.011303.120731. [DOI] [PubMed] [Google Scholar]
- 124.Bartels V. Handbook of medical textiles. Elsevier; 2011. [Google Scholar]
- 125.Sherrell PC, Thompson BC, Wassei JK, Gelmi AA, Higgins MJ, Kaner RB, Wallace GG. Adv Funct Mater. 2014;24:769. [Google Scholar]
- 126.Benfenati V, Stahl K, Gomis-Perez C, Toffanin S, Sagnella A, Torp R, Kaplan DL, Ruani G, Omenetto FG, Zamboni R. Adv Funct Mater. 2012;22:1871. doi: 10.5301/JABFM.2012.10448. [DOI] [PubMed] [Google Scholar]
- 127.Fattahi P, Yang G, Kim G, Abidian MR. Adv Mater. 2014;26:1846. doi: 10.1002/adma.201304496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Radtke C, Allmeling C, Waldmann KH, Reimers K, Thies K, Schenk HC, Hillmer A, Guggenheim M, Brandes G, Vogt PM. PloS one. 2011;6:e16990. doi: 10.1371/journal.pone.0016990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chen JL, Yin Z, Shen WL, Chen X, Heng BC, Zou XH, Ouyang HW. Biomaterials. 2010;31:9438. doi: 10.1016/j.biomaterials.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 130.Vaquette C, Viateau V, Guerard S, Anagnostou F, Manassero M, Castner DG, Migonney V. Biomaterials. 2013;34:7048. doi: 10.1016/j.biomaterials.2013.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Huang W, Begum R, Barber T, Ibba V, Tee NCH, Hussain M, Arastoo M, Yang Q, Robson LG, Lesage S. Biomaterials. 2012;33:59. doi: 10.1016/j.biomaterials.2011.09.030. [DOI] [PubMed] [Google Scholar]
- 132.Yang Y, Chen X, Ding F, Zhang P, Liu J, Gu X. Biomaterials. 2007;28:1643. doi: 10.1016/j.biomaterials.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 133.Chen X, Yang Y, Wu J, Zhao Y, Ding F, Gu X. Prog Nat Sci. 2007:17. [Google Scholar]
- 134.Yang Y, Ding F, Wu J, Hu W, Liu W, Liu J, Gu X. Biomaterials. 2007;28:5526. doi: 10.1016/j.biomaterials.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 135.Tang X, Ding F, Yang Y, Hu N, Wu H, Gu X. J Biomed Mater Res A. 2009;91:166. doi: 10.1002/jbm.a.32212. [DOI] [PubMed] [Google Scholar]
- 136.Roloff F, Strauß S, Vogt PM, Bicker G, Radtke C. BioMed Res Int. 2014:2014. doi: 10.1155/2014/906819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Vogt P, Allmeling C, Reimers K. Google Patents. 2014. Implant of cross-linked spider silk threads. [Google Scholar]
- 138.Hohlrieder M, Teuschl AH, Cicha K, van Griensven M, Redl H, Stampfl J. Biomed Mater Eng. 2013;23:225. doi: 10.3233/BME-130746. [DOI] [PubMed] [Google Scholar]
- 139.Xu S, Yan X, Zhao Y, Wang W, Yang Y. J Appl Polym Sci. 2011;119:3490. [Google Scholar]
- 140.Wang CY, Zhang KH, Fan CY, Mo XM, Ruan HJ, Li FF. Acta Biomater. 2011;7:634. doi: 10.1016/j.actbio.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 141.Wei Y, Gong K, Zheng Z, Wang A, Ao Q, Gong Y, Zhang X. J Mater Sci Mater Med. 2011;22:1947. doi: 10.1007/s10856-011-4370-z. [DOI] [PubMed] [Google Scholar]
- 142.Wu G, Dong C, Wang G, Gao W, Fan H, Xiao W, Zhang L. Chin J Repar Reconstr Surg. 2009;23:1007. [PubMed] [Google Scholar]
- 143.Wang G, Hu X, Lin W, Dong C, Wu H. In Vitro Cell Dev Biol Anim. 2011;47:234. doi: 10.1007/s11626-010-9381-4. [DOI] [PubMed] [Google Scholar]
- 144.Madduri S, Papaloizos M, Gander B. Biomaterials. 2010;31:2323. doi: 10.1016/j.biomaterials.2009.11.073. [DOI] [PubMed] [Google Scholar]
- 145.Zhang F, Zuo BQ, Zhang HX, Bai L. Polymer. 2009;50:279. [Google Scholar]
- 146.Cooper JA, Lu HH, Ko FK, Freeman JW, Laurencin CT. Biomaterials. 2005;26:1523. doi: 10.1016/j.biomaterials.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 147.Hennecke K, Redeker J, Kuhbier JW, Strauss S, Allmeling C, Kasper C, Reimers K, Vogt PM. PloS one. 2013;8:e61100. doi: 10.1371/journal.pone.0061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rising A, Nimmervoll H, Grip S, Fernandez-Arias A, Storckenfeldt E, Knight DP, Vollrath F, Engström W. Zoolog Sci. 2005;22:273. doi: 10.2108/zsj.22.273. [DOI] [PubMed] [Google Scholar]
- 149.Altman GH, Horan RL, Lu HH, Moreau J, Martin I, Richmond JC, Kaplan DL. Biomaterials. 2002;23:4131. doi: 10.1016/s0142-9612(02)00156-4. [DOI] [PubMed] [Google Scholar]
- 150.Liu H, Fan H, Toh SL, Goh JCH. Biomaterials. 2008;29:1443. doi: 10.1016/j.biomaterials.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 151.Hairfield-Stein M, England C, Paek HJ, Gilbraith KB, Dennis R, Boland E, Kosnik P. Tissue Eng. 2007;13:703. doi: 10.1089/ten.2006.0203. [DOI] [PubMed] [Google Scholar]
- 152.Panas-Perez E, Gatt CJ, Dunn MG. J Mater Sci Mater Med. 2013;24:257. doi: 10.1007/s10856-012-4781-5. [DOI] [PubMed] [Google Scholar]
- 153.Sahoo S, Lok Toh S, Goh H, Cho J. J Biomed Mater Res B Appl Biomater. 2010;95:19. doi: 10.1002/jbm.b.31678. [DOI] [PubMed] [Google Scholar]
- 154.Moreau JE, Chen J, Horan RL, Kaplan DL, Altman GH. Tissue Eng. 2005;11:1887. doi: 10.1089/ten.2005.11.1887. [DOI] [PubMed] [Google Scholar]
- 155.Chen J, Altman GH, Karageorgiou V, Horan R, Collette A, Volloch V, Colabro T, Kaplan DL. J Biomed Mater Res A. 2003;67:559. doi: 10.1002/jbm.a.10120. [DOI] [PubMed] [Google Scholar]
- 156.Fang Q, Chen D, Yang Z, Li M. Mater Sci Eng C. 2009;29:1527. [Google Scholar]
- 157.Ghiasi M, Naghashzargar E, Semnani D. Nano Hybrids. 2014;7:35. [Google Scholar]
- 158.Gagner JE, Kim W, Chaikof EL. Acta Biomater. 2014;10:1542. doi: 10.1016/j.actbio.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sun L, Parker ST, Syoji D, Wang X, Lewis JA, Kaplan DL. Adv Healthc Mater. 2012;1:729. doi: 10.1002/adhm.201200057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Li J, Baker B, Mou X, Ren N, Qiu J, Boughton RI, Liu H. Adv Healthc Mater. 2014;3:469. doi: 10.1002/adhm.201300562. [DOI] [PubMed] [Google Scholar]
- 161.Horrocks AR, Anand SC. Handbook of technical textiles. Elsevier; 2000. [Google Scholar]
- 162.Li C, Vepari C, Jin H-J, Kim HJ, Kaplan DL. Biomaterials. 2006;27:3115. doi: 10.1016/j.biomaterials.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 163.Kim SE, Lee ER, Lee Y, Jeong M, Park YW, Ahn JS, Ahn JT, Seo K. J Vet Sci. 2012;13:193. doi: 10.4142/jvs.2012.13.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Li G, Lan P, Li Y, Hu H. J Fiber Bioeng Inform. 2011;3:187. [Google Scholar]
- 165.Kannan RY, Salacinski HJ, Sales K, Butler P, Seifalian AM. Biomaterials. 2005;26:1857. doi: 10.1016/j.biomaterials.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 166.King MW, Marois Y, Guidoin R, Ukpabi P, Deng X, Martin L, Pǎris E, Douville Y. J Biomed Mater Res. 1995;29:595. doi: 10.1002/jbm.820290507. [DOI] [PubMed] [Google Scholar]
- 167.Chen Y, Ding X, Li Y, King MW, Gao J, Zhao X. J Text I. 2012;103:106. [Google Scholar]
- 168.Lovett M, Eng G, Kluge JA, Cannizzaro C, Vunjak-Novakovic G, Kaplan DL. Organogenesis. 2010;6:217. doi: 10.4161/org.6.4.13407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ravi S, Chaikof EL. Regen Med. 2010;5:107. doi: 10.2217/rme.09.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Leonardi M, Cirillo L, Toni F, Dall’olio M, Princiotta C, Stafa A, Simonetti L, Agati R. Interv Neuroradiol. 2011;17:306. doi: 10.1177/159101991101700305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Causin F, Pascarella R, Pavesi G, Marasco R, Zambon G, Battaglia R, Munari M. Interv Neuroradiol. 2011;17:357. doi: 10.1177/159101991101700313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Enomoto S, Sumi M, Kajimoto K, Nakazawa Y, Takahashi R, Takabayashi C, Asakura T, Sata M. J Vasc Surg. 2010;51:155. doi: 10.1016/j.jvs.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 173.Unger RE, Wolf M, Peters K, Motta A, Migliaresi C, James Kirkpatrick C. Biomaterials. 2004;25:1069. doi: 10.1016/s0142-9612(03)00619-7. [DOI] [PubMed] [Google Scholar]
- 174.Bondar B, Fuchs S, Motta A, Migliaresi C, Kirkpatrick CJ. Biomaterials. 2008;29:561. doi: 10.1016/j.biomaterials.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 175.Fuchs S, Motta A, Migliaresi C, Kirkpatrick CJ. Biomaterials. 2006;27:5399. doi: 10.1016/j.biomaterials.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 176.Nakazawa Y, Sato M, Takahashi R, Aytemiz D, Takabayashi C, Tamura T, Enomoto S, Sata M, Asakura T. J Biomater Sci Polym Ed. 2011;22:195. doi: 10.1163/092050609X12586381656530. [DOI] [PubMed] [Google Scholar]
- 177.Yagi T, Sato M, Nakazawa Y, Tanaka K, Sata M, Itoh K, Takagi Y, Asakura T. J Artif Organs. 2011;14:89. doi: 10.1007/s10047-011-0554-z. [DOI] [PubMed] [Google Scholar]
- 178.Soffer L, Wang X, Zhang X, Kluge J, Dorfmann L, Kaplan DL, Leisk G. J Biomater Sci Polym Ed. 2008;19:653. doi: 10.1163/156856208784089607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Nishibe T, Kondo Y, Muto A, Dardik A. Vascular. 2007;15:356. doi: 10.2310/6670.2007.00053. [DOI] [PubMed] [Google Scholar]
- 180.Orban JM, Wilson LB, Kofroth JA, El-Kurdi MS, Maul TM, Vorp DA. J Biomed Mater Res A. 2004;68:756. doi: 10.1002/jbm.a.20110. [DOI] [PubMed] [Google Scholar]
- 181.Yang X, Wang L, Guan G, King MW, Li Y, Peng L, Guan Y, Hu X. J Biomater Appl. 2014;28:676. doi: 10.1177/0885328212472216. [DOI] [PubMed] [Google Scholar]
- 182.Li G, Li YL, Chen XW, Ding XWL. J Donghua Univ Nat Sci. 2009;35:264. [Google Scholar]
- 183.Unger RE, Peters K, Wolf M, Motta A, Migliaresi C, Kirkpatrick CJ. Biomaterials. 2004;25:5137. doi: 10.1016/j.biomaterials.2003.12.040. [DOI] [PubMed] [Google Scholar]
- 184.Li G, Li Y, Lan P, Li J, Zhao Z, He X, Zhang J, Hu H. J Biomed Mater Res A. 2014;102A:982. doi: 10.1002/jbm.a.34759. [DOI] [PubMed] [Google Scholar]
- 185.Tan YP, Li G, Wu XJ, He XW, Ke J, Zhang J, Lan P. Chin J Bases Clin General Surg. 2012;19:610. [Google Scholar]
- 186.Saito Y, Tanaka T, Andoh A, Minematsu H, Hata K, Tsujikawa T, Nitta N, Murata K, Fujiyama Y. Dig Dis Sci. 2008;53:330. doi: 10.1007/s10620-007-9873-6. [DOI] [PubMed] [Google Scholar]
- 187.Stivaros S, Williams L, Senger C, Wilbraham L, Laasch H-U. Eur Radiol. 2010;20:1069. doi: 10.1007/s00330-009-1662-5. [DOI] [PubMed] [Google Scholar]
- 188.Ni Y, Zhao X, Zhou L, Shao Z, Yan W, Chen X, Cao Z, Xue Z, Jiang JJ. Otolaryngol--Head Neck Surg. 2008;139:256. doi: 10.1016/j.otohns.2008.03.028. [DOI] [PubMed] [Google Scholar]
- 189.Zang M, Zhang Q, Davis G, Huang G, Jaffari M, Ríos CN, Gupta V, Yu P, Mathur AB. Acta Biomater. 2011;7:3422. doi: 10.1016/j.actbio.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 190.Cirillo B, Morra M, Catapano G. Int J Artif Organs. 2004;27:60. doi: 10.1177/039139880402700112. [DOI] [PubMed] [Google Scholar]
- 191.Li G, Liu H, Li T, Wang J. Mater Sci Eng C. 2012;32:627. [Google Scholar]
- 192.Lu W, Yang XM. Adv Mater. 2012;459:649. [Google Scholar]
- 193.Ricci G, Patrizi A, Bendandi B, Menna G, Varotti E, Masi M. Br J Dermatol. 2004;150:127. doi: 10.1111/j.1365-2133.2004.05705.x. [DOI] [PubMed] [Google Scholar]
- 194.Sha L-Z, Zhao H-F, Xiao G-N. Fiber Polym. 2013;14:976. [Google Scholar]
- 195.Sha L, Zhao H. Fiber Polym. 2012;13:1159. [Google Scholar]
- 196.Gold J. Eur Cell Mater. 2005;10:2. [Google Scholar]
- 197.Jiao Y-P, Cui F-Z. Biomed Mater. 2007;2:R24. doi: 10.1088/1748-6041/2/4/R02. [DOI] [PubMed] [Google Scholar]
- 198.Lee KY, Kong SJ, Park WH, Ha WS, Kwon IC. J Biomater Sci Polym Ed. 1998;9:905. doi: 10.1163/156856298x00235. [DOI] [PubMed] [Google Scholar]
- 199.Lian X, Wang S, Xu G, Lin N, Li Q, Zhu H. Appl Surf Sci. 2008;255:480. [Google Scholar]
- 200.Liu X-Y, Zhang C-C, Xu W-L, Ouyang C-x. Mater Lett. 2009;63:263. [Google Scholar]
- 201.Cassinelli C, Cascardo G, Morra M, Draghi L, Motta A, Catapano G. Int J Artif Organs. 2006;29:881. [PubMed] [Google Scholar]
- 202.He P, Sahoo S, Ng KS, Chen K, Toh SL, Goh JCH. J Biomed Mater Res Part A. 2013;101:555. doi: 10.1002/jbm.a.34333. [DOI] [PubMed] [Google Scholar]
- 203.Jiang J, Wan F, Yang J, Hao W, Wang Y, Yao J, Shao Z, Zhang P, Chen J, Zhou L. Int J Nanomedicine. 2014;9:4569. doi: 10.2147/IJN.S69137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Zhang W, Yang Y, Zhang K, Li Y, Fang G. Connect Tissue Res. 2014:1. doi: 10.3109/03008207.2014.976309. [DOI] [PubMed] [Google Scholar]
- 205.Li JJ, Gil ES, Hayden RS, Li C, Roohani-Esfahani S-I, Kaplan DL, Zreiqat H. Biomacromolecules. 2013;14:2179. doi: 10.1021/bm400303w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Hodak SK, Supasai T, Paosawatyanyong B, Kamlangkla K, Pavarajarn V. Appl Surf Sci. 2008;254:4744. [Google Scholar]
- 207.Gogoi D, Choudhury AJ, Chutia J, Pal AR, Dass NN, Devi D, Patil DS. Appl Surf Sci. 2011;258:126. [Google Scholar]
- 208.Sangprasert W, Lee VS, Boonyawan D, Tashiro K, Nimmanpipug P. J Mol Struct. 2010;963:130. [Google Scholar]
- 209.Demura M, Takekawa T, Asakura T, Nishikawa A. Biomaterials. 1992;13:276. doi: 10.1016/0142-9612(92)90050-x. [DOI] [PubMed] [Google Scholar]
- 210.Zheng C, Chen G, Qi Z. Plasma Chem Plasma Process. 2012;32:629. [Google Scholar]
- 211.Chan CM, Ko TM, Hiraoka H. Surf Sci Rep. 1996;24:1. [Google Scholar]
- 212.Inbakumar S. Journal of Physics: Conference Series. 1. IOP Publishing; Effect of plasma treatment on surface of protein fabrics; p. 208/012111. [Google Scholar]
- 213.Chu PK, Chen JY, Wang LP, Huang N. Mater Sci Eng R Rep. 2002;36:143. [Google Scholar]
- 214.Kim KS, Ryu CM, Park CS, Sur GS, Park CE. Polymer. 2003;44:6287. [Google Scholar]
- 215.Abbasi AR, Morsali A. Ultrason Sonochem. 2010;17:704. doi: 10.1016/j.ultsonch.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 216.Suanpoot P, Kueseng K, Ortmann S, Kaufmann R, Umongno C, Nimmanpipug P, Boonyawan D, Vilaithong T. Surf Coat Technol. 2008;202:5543. [Google Scholar]
- 217.Caliari SR, Gonnerman EA, Grier WK, Weisgerber DW, Banks JM, Alsop AJ, Lee JS, Bailey RC, Harley BA. Adv Healthcare Mater. 2015;4:58. doi: 10.1002/adhm.201400252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Pierschbacher MD, Ruoslahti E. Nature. 1984;309:30. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- 219.Gotoh Y, Tsukada M, Minoura N, Imai Y. Biomaterials. 1997;18:267. doi: 10.1016/s0142-9612(96)00137-8. [DOI] [PubMed] [Google Scholar]
- 220.Vepari CP, Kaplan DL. Biotechnol Bioeng. 2006;93:1130. doi: 10.1002/bit.20833. [DOI] [PubMed] [Google Scholar]
- 221.Demura M, Asakura T. J Memb Sci. 1991;59:39. [Google Scholar]
- 222.Gotoh Y, Tsukada M, Minoura N. J Biomed Mater Res. 1998;39:351. doi: 10.1002/(sici)1097-4636(19980305)39:3<351::aid-jbm2>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 223.Fini M, Motta A, Torricelli P, Giavaresi G, Nicoli Aldini N, Tschon M, Giardino R, Migliaresi C. Biomaterials. 2005;26:3527. doi: 10.1016/j.biomaterials.2004.09.040. [DOI] [PubMed] [Google Scholar]
- 224.Jin H-J, Park J, Valluzzi R, Cebe P, Kaplan DL. Biomacromolecules. 2004;5:711. doi: 10.1021/bm0343287. [DOI] [PubMed] [Google Scholar]
- 225.Dinerman AA, Cappello J, Ghandehari H, Hoag SW. Biomaterials. 2002;23:4203. doi: 10.1016/s0142-9612(02)00164-3. [DOI] [PubMed] [Google Scholar]
- 226.Dinerman AA, Cappello J, Ghandehari H, Hoag SW. J Control Release. 2002;82:277. doi: 10.1016/s0168-3659(02)00134-7. [DOI] [PubMed] [Google Scholar]
- 227.Megeed Z, Haider M, Li D. J Control Release. 2004;94:433. doi: 10.1016/j.jconrel.2003.10.027. [DOI] [PubMed] [Google Scholar]
- 228.Kundu B, Kurland NE, Bano S, Patra C, Engel FB, Yadavalli VK, Kundu SC. Prog Polym Sci. 2014;39:251. [Google Scholar]
- 229.Hardy JG, Pfaff A, Leal-Egaña A, Müller AH, Scheibel TR. Macromol Biosci. 2014 doi: 10.1002/mabi.201400020. [DOI] [PubMed] [Google Scholar]
- 230.Romero IS, Schurr ML, Lally JV, Kotlik MZ, Murphy AR. ACS applied materials & interfaces. 2013;5:553. doi: 10.1021/am301844c. [DOI] [PubMed] [Google Scholar]
- 231.Wohlrab S, Müller S, Schmidt A, Neubauer S, Kessler H, Leal-Egaña A, Scheibel T. Biomaterials. 2012;33:6650. doi: 10.1016/j.biomaterials.2012.05.069. [DOI] [PubMed] [Google Scholar]
- 232.Sofia S, McCarthy MB, Gronowicz G, Kaplan DL. J Biomed Mater Res. 2001;54:139. doi: 10.1002/1097-4636(200101)54:1<139::aid-jbm17>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 233.Xu Y, Shao H, Zhang Y, Hu X. J Mater Sci. 2005;40:5355. [Google Scholar]
- 234.Corsini P, Perez-Rigueiro J, Guinea GV, Plaza GR, Elices M, Marsano E, Carnasciali MM, Freddi G. J Polym Sci B Polym Phys. 2007;45:2568. [Google Scholar]
- 235.Zuo B, Liu L, Wu Z. J Appl Polym Sci. 2007;106:53. [Google Scholar]
- 236.Rajkhowa R, Levin B, Redmond SL, Li LH, Wang L, Kanwar JR, Atlas MD, Wang X. J Biomed Mater Res A. 2011;97:37. doi: 10.1002/jbm.a.33021. [DOI] [PubMed] [Google Scholar]
- 237.Gellynck K, Verdonk PCM, Van Nimmen E, Almqvist KF, Gheysens T, Schoukens G, Van Langenhove L, Kiekens P, Mertens J, Verbruggen G. J Mater Sci Mater Med. 2008;19:3399. doi: 10.1007/s10856-008-3474-6. [DOI] [PubMed] [Google Scholar]
- 238.Zuo B, Dai L, Wu Z. J Mater Sci. 2006;41:3357. [Google Scholar]
- 239.Jiang C, Wang X, Gunawidjaja R, Lin YH, Gupta MK, Kaplan DL, Naik RR, Tsukruk VV. Adv Funct Mater. 2007;17:2229. [Google Scholar]
- 240.Kawaguchi S, Nakamura T, Shimizu Y, Masuda T, Takigawa T, Liu Y, Ueda H, Sekine T, Matsumoto K. Biomaterials. 2001;22:3085. doi: 10.1016/s0142-9612(01)00056-4. [DOI] [PubMed] [Google Scholar]
- 241.Claes LE. Clinical materials. 1992;10:41. doi: 10.1016/0267-6605(92)90083-6. [DOI] [PubMed] [Google Scholar]
- 242.Fan S, Zhang Y, Shao H, Hu X. Int J Biol Macromol. 2013;56:83. doi: 10.1016/j.ijbiomac.2013.01.033. [DOI] [PubMed] [Google Scholar]
- 243.Dumoulin C, Cochelin B. J Biomech. 2000;33:1461. doi: 10.1016/s0021-9290(00)00098-1. [DOI] [PubMed] [Google Scholar]
- 244.Van Der Merwe H, Daya Reddy B, Zilla P, Bezuidenhout D, Franz T. J Biomech. 2008;41:1302. doi: 10.1016/j.jbiomech.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 245.De Beule M, Van Impe R, Verhegghe B, Segers P, Verdonck P. Technol Health Care. 2006;14:233. [PubMed] [Google Scholar]
- 246.Hall GJ, Kasper EP. J Biomech Eng. 2006;128:751. doi: 10.1115/1.2264382. [DOI] [PubMed] [Google Scholar]
- 247.He J, Guo N, Cui S. Iran Polym J. 2011;20:713. [Google Scholar]
- 248.Gil ES, Park SH, Hu X, Cebe P, Kaplan DL. Macromol Biosci. 2013;14:257. doi: 10.1002/mabi.201300321. [DOI] [PubMed] [Google Scholar]
- 249.Thitiwuthikiat P, Kanokpanont S. Adv Mater. 2012;506:381. [Google Scholar]
- 250.Nakajima K, Miura M. Jpn Agric Res Q. 2013;47:175. [Google Scholar]
- 251.Langer RS, Peppas NA. Biomaterials. 1981;2:201. doi: 10.1016/0142-9612(81)90059-4. [DOI] [PubMed] [Google Scholar]
- 252.Hofmann S, Wong Po Foo CT, Rossetti F, Textor M, Vunjak-Novakovic G, Kaplan DL, Merkle HP, Meinel L. J Control Release. 2006;111:219. doi: 10.1016/j.jconrel.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 253.Wang X, Zhang X, Castellot J, Herman I, Iafrati M, Kaplan DL. Biomaterials. 2008;29:894. doi: 10.1016/j.biomaterials.2007.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Pritchard EM, Kaplan DL. Expert opin on drug delivery. 2011;8:797. doi: 10.1517/17425247.2011.568936. [DOI] [PubMed] [Google Scholar]
- 255.Li L, Li H, Qian Y, Li X, Singh GK, Zhong L, Liu W, Lv Y, Cai K, Yang L. Int J Biol Macromol. 2011;49:223. doi: 10.1016/j.ijbiomac.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 256.Yucel T, Lovett ML, Giangregorio R, Coonahan E, Kaplan DL. Biomaterials. 2014;35:8613. doi: 10.1016/j.biomaterials.2014.06.030. [DOI] [PubMed] [Google Scholar]
- 257.Kaplan DL, Huang J, Po W, Cheryl F, Naik R, George A. Fibrous protein fusions and use the thereof in the formation of advanced organic/inorganic composition materials. 20, 140, 379, 094. US Patent. 2014
- 258.Kaplan DL, Meinel L. Google Patents. 2013. Silk-based drug delivery system. [Google Scholar]
- 259.Yucel T, Lovett ML, Kaplan DL. J Control Release. 2014 doi: 10.1016/j.jconrel.2014.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Gunatillake PA, Adhikari R. Eur Cell Mater. 2003;5:1. doi: 10.22203/ecm.v005a01. [DOI] [PubMed] [Google Scholar]
- 261.Nair LS, Laurencin CT. Prog Polym Sci. 2007;32:762. [Google Scholar]
- 262.Horan RL, Antle K, Collette AL, Wang Y, Huang J, Moreau JE, Volloch V, Kaplan DL, Altman GH. Biomaterials. 2005;26:3385. doi: 10.1016/j.biomaterials.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 263.Bucknall TE, Teare L, Ellis H. Eur Surg Res. 1983;15:59. doi: 10.1159/000128334. [DOI] [PubMed] [Google Scholar]
- 264.Minoura N, Tsukada M, Nagura M. Biomaterials. 1990;11:430. doi: 10.1016/0142-9612(90)90100-5. [DOI] [PubMed] [Google Scholar]
- 265.Hu Y, Zhang Q, You R, Wang L, Li M. Adv Mater Sci Eng. 2012:1. [Google Scholar]
- 266.Li G, Chen Y, Cai Z, Li J, Wu X, He X, Zhao Z, Lan P, Li Y. J Mater Sci. 2013;48:6186. [Google Scholar]
- 267.Schneider A, Wang XY, Kaplan DL, Garlick JA, Egles C. Acta Biomater. 2009;5:2570. doi: 10.1016/j.actbio.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Wharram SE, Zhang X, Kaplan DL, McCarthy SP. Macromol Biosci. 2010;10:246. doi: 10.1002/mabi.200900274. [DOI] [PubMed] [Google Scholar]
- 269.Foschi D, Corsi F, Cellerino P, Rizzi A, Morandi E, Trabucchi E. Eur Surg Res. 2001;33:16. doi: 10.1159/000049687. [DOI] [PubMed] [Google Scholar]
- 270.Viju S, Thilagavathi G. Fiber Polym. 2012;13:782. [Google Scholar]
- 271.Janiga PK, Elayarajah B, Rajendran R, Rammohan R, Venkatrajah B, Asa S. J Ind Text. 2012;42:176. [Google Scholar]
- 272.Kardestuncer T, McCarthy MB, Karageorgiou V, Kaplan D, Gronowicz G. Clin Orthop Relat Res. 2006;448:234. doi: 10.1097/01.blo.0000205879.50834.fe. [DOI] [PubMed] [Google Scholar]
- 273.Yan X, Zhao Y, Wang W, Gu X, Yang Y. Adv Stud Biol. 2009;1:119. [Google Scholar]
- 274.Han F, Liu S, Liu X, Pei Y, Bai S, Zhao H, Lu Q, Ma F, Kaplan DL, Zhu H. Acta Biomater. 2014;10:921. doi: 10.1016/j.actbio.2013.09.026. [DOI] [PubMed] [Google Scholar]
- 275.Ilhan-Sarac O, Akpek EK. Curr Opin Ophthalmol. 2005;16:246. doi: 10.1097/01.icu.0000172829.33770.d3. [DOI] [PubMed] [Google Scholar]
- 276.Myung D, Koh W, Bakri A, Zhang F, Marshall A, Ko J, Noolandi J, Carrasco M, Cochran JR, Frank CW. Biomed Microdevices. 2007;9:911. doi: 10.1007/s10544-006-9040-4. [DOI] [PubMed] [Google Scholar]
- 277.Takeuchi A, Ohtsuki C, Miyazaki T, Tanaka H, Yamazaki M, Tanihara M. J Biomed Mater Res A. 2003;65:283. doi: 10.1002/jbm.a.10456. [DOI] [PubMed] [Google Scholar]
- 278.Duan X, Tu Q, Zhang J, Ye J, Sommer C, Mostoslavsky G, Kaplan D, Yang P, Chen J. J Cell Physiol. 2011;226:150. doi: 10.1002/jcp.22316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Liu H, Ding X, Bi Y, Gong X, Li X, Zhou G, Fan Y. Macromol Biosci. 2013;13:755. doi: 10.1002/mabi.201200470. [DOI] [PubMed] [Google Scholar]
- 280.Kukreja N, Onuma Y, Daemen J, Serruys PW. Pharmacol Res. 2008;57:171. doi: 10.1016/j.phrs.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 281.Numata K, Subramanian B, Currie HA, Kaplan DL. Biomaterials. 2009;30:5775. doi: 10.1016/j.biomaterials.2009.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Ramgopal G, Ramani R, Ramachandra P, Ranganathaiah C. J Appl Polym Sci. 1997;63:395. [Google Scholar]
- 283.Gogoi D, Chutia J, Choudhury AJ, Pal AR, Patil D. J Theor Biol Appl Phys. 2012;6:1. [Google Scholar]
- 284.Wang JN, Liu ZW, Yang YX, Huang HY. Text Res J. 2012;82:1799. [Google Scholar]
- 285.Jurasekova Z, Domingo C, Garcia-Ramos JV, Sanchez-Cortes S. J Raman Spectrosc. 2008;39:1309. [Google Scholar]
- 286.Yuksek M, Kocak D, Beyit A, Merdan N. Adv Environ Biol. 2012;6:801. [Google Scholar]
- 287.Kongdee A, Bechtold T, Teufel L. J Appl Polym Sci. 2005;96:1421. [Google Scholar]
- 288.Li G, Liu H, Zhao H, Gao Y, Wang J, Jiang H, Boughton RI. J Colloid Interface Sci. 2011;358:307. doi: 10.1016/j.jcis.2011.02.053. [DOI] [PubMed] [Google Scholar]
- 289.Ferrero F, Periolatto M, Burelli S, Carletto RA. Fiber Polym. 2010;11:185. [Google Scholar]
- 290.Guan J, Chen G. Fiber Polym. 2008;9:438. [Google Scholar]
- 291.Khan MR, Gotoh Y, Miura M, Morikawa H, Nagura M. J Polym Sci B Polym Phys. 2006;44:3418. [Google Scholar]
- 292.Khan MMR, Gotoh Y, Morikawa H, Miura M. Text Res J. 2009;79:1305. [Google Scholar]
- 293.Lu YH, Lin H, Chen YY, Wang C, Hua YR. Fiber Polym. 2007;8:1. [Google Scholar]
- 294.Wang L, Li C, Senna M. J Nanopart Res. 2006;9:919. [Google Scholar]
- 295.Romero IS, Schurr ML, Lally JV, Kotlik MZ, Murphy AR. ACS Appl Mater Interfaces. 2013;5:553. doi: 10.1021/am301844c. [DOI] [PubMed] [Google Scholar]
- 296.Li Z-x, Jin F-f, Cao B-w, Wang X-f. Chin J Polym Sci. 2008;26:353. [Google Scholar]
- 297.Hou A, Chen H. Mater Sci Eng B. 2010;167:124. [Google Scholar]