Abstract
Agents targeting insulin-like growth factor 1 receptor (IGF-1R) are being actively examined in clinical trials. Although there has been some initial success of single agent targeting IGF-1R, attempts in later studies failed due to resistance. This study aimed to understand the effects of programmed cell death 4 (Pdcd4) on the chemosensitivity of the IGF-1R inhibitor, OSI-906, in colorectal cancer (CRC) cells and the mechanism underlying this impact. Using OSI-906 resistant and sensitive CRC cells, we found that the Pdcd4 level directly correlates with cell chemosensitivity to OSI-906. In addition, tumors derived from Pdcd4 knockdown cells resist the growth inhibitory effect of OSI-906 in a CRC xenograft mouse model. Moreover, Pdcd4 enhances the antiproliferative effect of OSI-906 in resistant cells through suppression of p70S6K1 activation. Knockdown of p70S6K1, but not p70S6K2, significantly increases the chemosensitivity of OSI-906 in cultured CRC cells. Furthermore, the combination of OSI-906 and PF4708671, a p70S6K1 inhibitor, efficiently suppresses the growth of OSI-906 resistant colon tumor cells in vitro and in vivo. Taken together, activation of p70S6K1 that is inhibited by Pdcd4 is essential for resistance to IGF-1R inhibitor in colon tumor cells, and the combinational treatment of OSI-906 and PF-4708671 results in enhanced antiproliferation effects in CRC cells in vitro and in vivo, providing a novel venue to overcome the resistance to IGF-1R inhibitor in treating colorectal cancer.
Keywords: Pdcd4, OSI-906, S6K, PF-4708671, IGF-1R/IR
Introduction
The insulin-like growth factor 1 receptor (IGF-1R) signaling pathway has been shown to stimulate cancer development and progression in various cancers, including colorectal cancer (CRC) (1–4). Conversely, inactivation of IGF-1R signaling inhibits tumor growth, reduces tumor metastasis, and enhances the antitumor effects of other cancer therapeutic agents (5–7). Upon binding with ligands (IGF-1, IGF-2, and insulin), IGF-1R leads to activation of downstream pathways, including the PI3-K/AKT and Ras/MAPK pathway (2). Several epidemiologic studies have shown that IGF-1R activation correlates with the risk of colon cancer (8–10). In addition, immunohistochemical studies revealed that IGF-1R is over-expressed in CRC tissues compared to adjacent normal tissues (8). Moreover, the higher expression level of IGF-1R is associated with a higher grade and stage in CRC patients (11). Thus, inhibition of the IGF-1R pathway may offer a promising strategy for CRC therapeutics.
OSI-906 (cis-3-[8-amino-1-(2-phenylquinolin-7-yl)imidazo[1,5-a]pyrazin-3-yl]-1-methyl cyclobutanol) is a small molecule that binds to the ATP-binding pocket of tyrosine kinase receptors, causing dual inhibition of both IGF-1R and insulin receptor (IR) (12). Tissue culture and xenograft studies have confirmed the antitumor properties of OSI-906 in several types of human cancer cells, including lung (13), colon (14, 15), liver (16), and ovarian (17). However, like other receptor tyrosine kinases that have been tested as therapeutic targets, tumor cells frequently develop resistance to IGF-1R inhibitors. Recently, in a study of 27 colon tumor cell lines responding to OSI-906 treatment, Pitts et al. classified cell lines with an IC50 ≤ 1.5 μmol/L as sensitive and cell lines with an IC50 ≥ 5.0 μmol/L as resistant (15). A similar result was also reported by Flanigan et al. using PQIP (cis-3-[3-(4-methyl-piperazin-l-yl)-cyclobutyl]-1-(2-phenyl-quinolin-7-yl)-imidazo[1,5-a]) pyrazin-8-ylamine), an OSI-906 derivative (14). Consistent with the cell culture system, OSI-906 showed robust antitumor activity in the GEO (sensitive cell) xenograft but did not significantly inhibit tumor growth in RKO (resistant cell) xenograft (14, 15). The mechanism that resistant cells deter the growth inhibition by OSI-906 is unknown.
Programmed cell death 4 (Pdcd4), a tumor suppressor, is frequently down-regulated in several cancerous tissues compared to adjacent normal tissues, including CRC (18). Immunohistochemical studies showed that a high Pdcd4 protein level correlates with good prognosis in CRC patients (18), suggesting that Pdcd4 expression level is an important factor for CRC patient survival. Overexpression of pdcd4 cDNA inhibits 12-O-tetraadecanoylphorbol-13-acetate (TPA)-induced transformation in mouse epidermal cells (19). Conversely, down-regulation of Pdcd4 expression by pdcd4 antisense DNA resulted in an increase in TPA-induced transformation (20). Consistent with these observations, transgenic mice overexpressing pdcd4 cDNA in the skin showed significant reduction in 7,12-dimethylbenz(a)anthracene (DMBA)/TPA induced skin papilloma formation and carcinoma incidence (21). Knockout of Pdcd4 in mice led to increased DMBA/TPA-induced papilloma (22). In addition, recent studies also demonstrated that Pdcd4 inhibited tumor invasion and metastasis. In CRC cells, ectopic expression of pdcd4 cDNA suppressed invasion (23, 24), while knockdown of Pdcd4 expression resulted in epithelial to mesenchymal transition (25), promoted invasion in cultured cells (26, 27), and increased liver metastasis when cells were orthotopically injected into nude mice (25). These findings suggest that Pdcd4 is able to inhibit both tumor promotion and progression stages.
In this study, we examined the effects of Pdcd4 expression level on OSI-906 sensitivity in CRC cells. We found that Pdcd4 enhances the chemosensitivity of OSI-906 in CRC cells through inactivation of p70S6K1. OSI-906 in combination with p70s6k1 siRNA or p70S6K1 kinase inhibitor, PF-4708671, sufficiently inhibits resistant cell growth in vitro and in vivo, suggesting that inhibition of both IGF-1R and p70S6K1 activation is a promising strategy to overcome the resistance to IGF-1R inhibitor for CRC therapeutics.
Materials and Methods
Drug
OSI-906 and BMS-754807 were purchased from Chemie Tek (Indianapolis, IN). PF-4708671 was obtained from Selleckchem (Houston, TX). All drugs were dissolved in DMSO at 10 mmol/L and stored at −20°C for in vitro studies. For in vivo studies, both OSI-906 and PF-4708671 were dissolved in 25 mmol/L tartaric acid.
Cell culture
The colon GEO and RKO cells were generously provided by Dr. Douglas Boyd (MD Anderson Cancer Center, Houston, TX), and the rest cell lines were purchased from American Type Culture Collection (ATCC, Manassas, VA). GEO, HT29, RKO, and HCT116 cells were grown in McCoy’s medium. LoVo, SW480, SW620, and Colo205 cells were cultured in RPMI-1640 medium. CaCo2 cells were cultured in MEM medium. All medium was supplemented with 10% FBS, 2 mM L-glutamine, and 100 U/mL penicillin-streptomycin. HT29-shLacZ (HT29-L), HT29-shPdcd4 (HT29-P), GEO-shLacZ (GEO-L), and GEO-shPdcd4 (GEO-P) cells were generated as described previously (26). Cells were incubated at 37°C in a humidified atmosphere of 5% CO2 in air. All cell lines were not tested and authenticated by the authors.
Over-expression of Pdcd4 and knockdown of S6K
For over-expression of Pdcd4, 5×105 cells were plated onto a 100 mm dish and transfected with 2.5 μg of pcDNA3.1-Pdcd4 plasmid (or 2.5 μg of pcNDA3.1 plasmid) using 7.5 μl of PolyJet™ DNA In Vitro Transfection Reagent (SignaGen Laboratories, Gaithersburg, MD) according to the manufacturer’s protocol. For knockdown of S6K, 3.5×105 cells were seeded onto a 60 mm dish and transfected with 11 μl of 10 μM p70s6k1 siRNA (or p70s6k2 siRNA) (Santa Cruz Biotechnology, Santa Cruz, CA) using Lipofectamine RNAiMAX Transfection Reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. After 48 hours, cells were collected for proliferation and Western blot analyses.
Cell proliferation and apoptosis assays
The effects of OSI-906 or the combination of OSI-906 and PF-4708671 on cell proliferation was determined with XTT and clonogenic assays. XTT assays were performed as described previously using Cell Proliferation Kit II (XTT) (Biotium, Inc., Hayward, CA) (28). For clonogenic assays, cells (1×103 cells/well) were seeded on a 6-well plate and subsequently treated with drugs as indicated in the figure legend. After one week of incubation, cells were stained with 1% crystal violet and the number of colonies was counted and recorded.
Apoptosis assays were performed using the Annexin V-FITC apoptosis kit (Biolegend, San Diego, CA). Briefly, 1×106 cells were washed with PBS twice and then re-suspended in Annexin V Binding Buffer. The cell suspension (100 μl) was subsequently added with 5 μl of FITC-Annexin V solution and 10 μl of propidium iodide solution. After incubation for 15 min at room temperature, 400 μl of Annexin V Binding Buffer was added to the cell suspension and subsequently assayed for apoptotic cell distribution using a FacsCalibur cell analyzer (BD Biosciences, San Jose, CA).
Western blot analysis
Western blot analysis was performed as described previously (26). The following antibodies were used: Pdcd4 (1:2500 dilution), phospho-p70S6K1(Thr389), phospho-AKT(Ser473), p70S6K1, p70S6K2 (1:500 dilution), AKT, phospho-MDM2, phospho-S6, S6, phospho-4E-BP1(Thr37/46), 4E-BP1, casepase-3. The antibodies without specific dilution factor were used at 1:1000 dilution. Pdcd4 antibody was generated as described previously (26). The p70S6K2 antibodies were purchased from Thermo Scientific (Rockford, IL), cleaved caspase 3 antibody was purchased from Abcam (Cambridge, MA), and the rest of the antibodies were purchased from Cell Signaling (Danvers, MA). The band intensity of the target protein was quantified using VisionWork LS image acquisition and analysis software (UVP, CA).
In vivo xenograft study
Five- to six-week-old female athymic nude mice (Hsd:Athymic Nude-Foxn1nu) were purchased from Harlan (Indianapolis, IN). Mice were maintained in the facility of University of Kentucky under specific pathogen-free conditions. Animal care procedures and experimental protocols were approved by the Institutional Animal Care and Use Committee based on guidelines from the National Institutes of Health. All mice were fed with a commercial diet, given water ad libitum and subjected to a 12 h-light/12 h-dark cycle. HT29-L, HT29-P, and HCT116 cells (1 × 106 cells) in a logarithmic growth phase were harvested and re-suspended in PBS. For each mouse, five million cells were injected subcutaneously into the flank using a 23-gauge needle. Mice were monitored daily for signs of toxicity and weighed twice weekly. Tumor size was evaluated every three days by caliper measurements. Tumor volume was calculated using the following formula: volume = (length×width2)/0.52. When tumors reached 150 to 300 mm3, mice were randomly assigned to the following groups (5 mice per group). For injection of HT29-L and HT29-P cells, mice were treated with vehicle (25 mmol/L tartaric acid) or OSI-906 (30 mg/kg) for 12 days. For injection of HCT116 cells, mice were treated with (i) vehicle (25 mmol/L tartaric acid); (ii) OSI-906 alone (30 mg/kg); (iii) PF-4708671 alone (60 mg/kg); and (iv) OSI-906 (30 mg/kg)+ PF-4708671 (60 mg/kg) and treated with drugs orally for 14 days. Vehicle and OSI-906 are given once per day and PF-4708671 is given once every other day. Twenty-four hours after the last treatment, the mice were sacrificed and the tumor weights were measured. It has been reported that treating mice with 25 to 75 mg/kg of OSI-906 per day significantly inhibits tumor growth without toxic effects (29). Thus, we chose the dose of OSI-906 at 30 mg/kg per day. The dosage of PF-4708671 at 60 mg/kg was chosen based on the amount of PF-4708671 we used for in vitro study and the fact that the IC50 of PF-4708671 is about twice that of IC50 of OSI-906 (29, 30). We treated mice with PF-4708671 every other day to reduce the potential toxicity. In fact, mice treated with OSI-906 (30 mg/kg), PF-4708671 (60 mg/kg), or OSI-906 (30 mg/kg)+ PF-4708671(60 mg/kg) for 14 days showed no evidence of toxicity, i.e. body weight loss more than 15%, decreased food intake, or diarrhea.
Immunohistochemical (IHC) analysis and TUNEL assay
The tissue samples were fixed with 4% neutral buffered paraformaldehyde, embedded in paraffin, and sectioned into 4-μm slices. IHC analysis with Ki-67 antibody was carried out as described previously (25). The TUNEL assay was performed using ApopTag Peroxidase In Situ Apoptosis Detection Kit (EMD Millipore, Billerica, MA) according to the manufacturer’s protocol. The apoptosis index and proliferation index were calculated as number of cells with positive TUNEL and Ki-67 staining per 500 cells counted × 100%, respectively.
Statistical analysis
For longitudinal tumor volume data, pairwise comparisons of interest were accomplished by linear mixed models on loge transformed % tumor volume with fixed effects of treatment group, time and their interaction and random effects of intercept and slope. The loge transformation was made on % tumor volume to improve the linearity assumption of the mean growth curve of % tumor volume. For tumor weight data, pairwise comparisons of interest were accomplished by two-sample t-tests. Adjustment in p-values due to multiple pairwise group comparisons was performed using the Holm’s procedure. Data are shown as the mean ± standard deviation (SD) with at least four replicates (n≥4) except semi-quantification of Western blot analyses (n≥2). Adjusted p-values less than 0.05 were considered statistically significant. All statistical modeling and tests were performed by SAS version 9.3 and R version 3.0.1.
Results
The level of Pdcd4 expression correlates with the chemosensitivity of OSI-906 in CRC cells
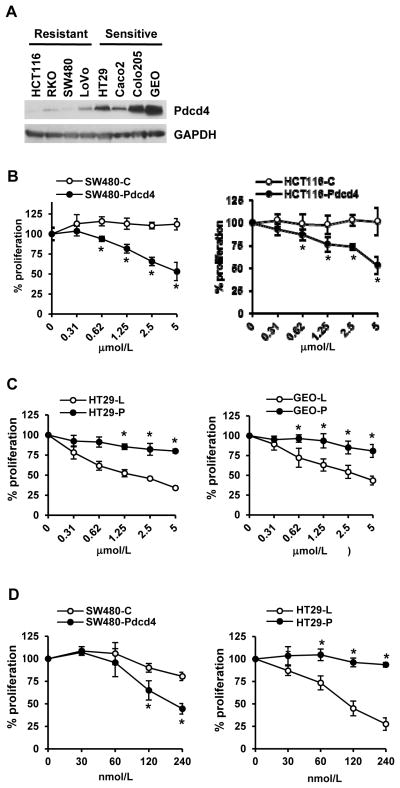
To test whether Pdcd4 expression correlates with the sensitivity to OSI-906, an IGF-1R/IR inhibitor, we examined the Pdcd4 expression levels in four OSI-906 sensitive and four resistant colon cell lines. As shown in Fig. 1A, the Pdcd4 protein level in sensitive cells (HT29, Caco2, Colo205, and GEO) is much higher than in resistant cells (HCT116, RKO, SW480, and LoVo), implying that the Pdcd4 expression level correlates with the chemosensitivity to OSI-906 in CRC cells.
Figure 1.
Expression level of Pdcd4 correlates with cell sensitivity to OSI-906. (A) Cell extracts from resistant (HCT116, RKO, SW480, and LoVo) and sensitive cells (HT29, Caco2, Colo205, and GEO) were analyzed with Western blot analysis to examine Pdcd4 expression level. (B) Over-expression of Pdcd4 in resistant cells enhances sensitivity to OSI-906. Cells were transfected with empty vector (SW480-C and HCT116-C) or pdcd4 expression plasmid (SW480-Pdcd4 and HCT116-Pdcd4). After 48 h, cells were plated onto 96-well plates and exposed to different concentrations of OSI-906. After 72 h, the cell viability was determined using XTT. The absorbance at 0 μmol/L is designed as 100%. Results are expressed as mean±SD (n=4). * indicates significant difference (P<0.05) versus control. (C) Knockdown of Pdcd4 in sensitive cells reduces sensitivity to OSI-906. Cells were transduced with lentiviral particles containing lacZ shRNA (HT29-L and GEO-L) or pdcd4 shRNA (HT29-P and GEO-P). Cells were treated and assayed as in (B). The absorbance at 0 μmol/L is designed as 100%. Results are expressed as mean±SD (n=4). * indicates significant difference (P<0.01) versus control. (D) Cells were plated onto 96-well plates and exposed to different concentrations of BMS-754807. After 72 h, the cell viability was determined using XTT. The absorbance at 0 nmol/L is designed as 100%. Results are expressed as mean±SD (n=4). * indicates significant difference (P<0.05) versus control. Each experiment was repeated at least twice. The representative data are shown.
If Pdcd4 expression level is crucial for tumor cell sensitivity to OSI-906, altering the Pdcd4 level should affect the chemosensitivity to OSI-906 treatment. First, we over-expressed pdcd4 cDNA in OSI-906 resistant SW480 and HCT116 cells and treated cells with OSI-906 from 0 to 5 μmol/L for 72 h. SW480 and HCT116 cells were chosen due to their low level of endogenous Pdcd4 (Fig. 1A). Over-expression of Pdcd4 enhanced the chemosensitivity of OSI-906 in SW480 and HCT116 cells (SW480-Pdcd4 and HCT116-Pdcd4) in both XTT assays (Fig. 1B) and clonogenic assays (Supplementary Fig. S1A). OSI-906 showed no significant effect on proliferation of cells expressing empty vector (SW480-C and HCT116-C). We also treated control and Pdcd4 over-expressing cells with OSI-906 at 5 μmol/L from 1 to 6 days. The Pdcd4 over-expressing cells treated with OSI-906 displayed slower proliferation than empty vector-expressing cells treated with OSI-906 (Supplementary Fig. S2). Next, we knocked down Pdcd4 expression in sensitive (HT29 and GEO) cells. Pdcd4 knockdown cells (HT29-P and GEO-P) exhibited resistance to OSI-906 comparing to control cells (HT29-L and GEO-L) (Fig. 1C and Supplementary Fig. S1B). Pdcd4 knockdown cells were generated by transducing cells with lentiviral particles containing pdcd4 shRNA as described previously (26). These results suggest that the level of Pdcd4 expression is critical in sensitizing tumor cells to the antiproliferatvie effect of OSI-906.
We also evaluated whether Pdcd4 enhances cell sensitivity to another potent IGF-1R/IR inhibitor, BMS-754807 (31). As shown in Fig. 1D, BMS-754805 inhibits proliferation more efficiently in SW480 cells with Pdcd4 over-expression than in control cells. Conversely, knockdown of Pdcd4 in HT29 cells abolished the growth inhibitory effect of BMS-754807. These results further demonstrate that Pdcd4 enhances cell sensitivity to IGF-1R inhibitor.
Tumors derived from Pdcd4 knockdown cells resist to OSI-906
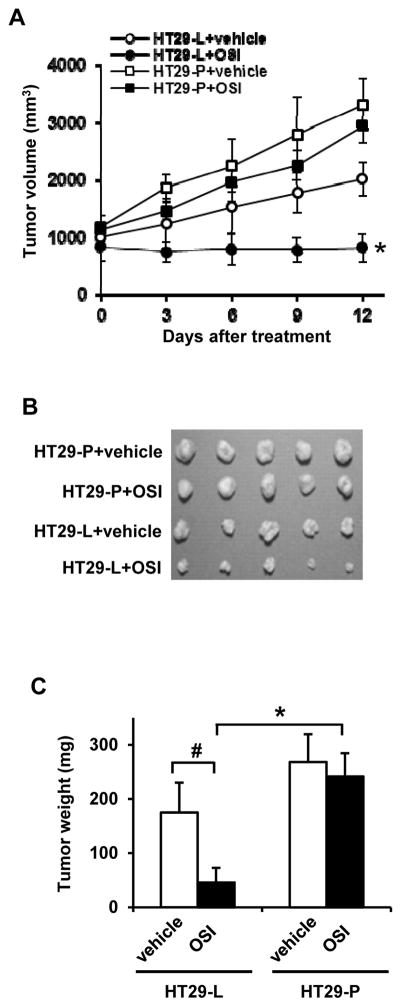
To further validate our in vitro results, we tested the efficacy of OSI-906 in inhibiting growth of tumors derived from HT29-L and HT29-P in vivo. As expected, OSI-906 (30 mg/kg, daily) dramatically inhibited tumor growth in mice injected with HT29-L (sensitive) cells (Fig. 2A, closed circle). However, OSI-906 did not significantly inhibit the growth of HT29-P (resistant) cells derived tumors (Fig. 2A, closed square) when compared to vehicle treated mice (Fig. 2A, open square) (P=0.639). The increased rate of tumor volume over time in HT29-L+OSI-906 is significantly slower than HT29-P+OSI-906 (P=0.045). In addition, treatment of OSI-906 for 12 days also showed significant decrease in tumor weight in HT29-L-derived tumors compared to vehicle-treated HT29-L-derived tumors (P=0.008) and OSI-906-treated HT29-P-derived tumors (P<0.001) (Figs. 2B and 2C). These findings are consistent with the results observed in vitro with OSI-906 treatment, in which cells with low Pdcd4 level resist to OSI-906 treatment.
Figure 2.
Tumors derived from Pdcd4 knockdown cells resist to OSI-906 treatment. Nude mice (5 mice per group) were subcutaneously injected with HT29-L or HT29-P cells and treated with vehicle (25 mmol/L tartaric acid) or OSI-906 (30 mg/kg) for 12 days. (A) Tumor volumes were measured every 3 day using a caliper. * indicates significant difference (P<0.001) versus HT29-L+vehicle (open circle) or HT29-P+OSI-906 (closed square) after 12-day treatment. (B) Image of tumors after 12-day treatment. (C) Average tumor weight of each group after 12-day treatment. * indicates significant difference (P<0.001) versus HT29-P+OSI; # indicates significant difference (P=0.008) versus HT29-L+vehicle.
Pdcd4 inhibits OSI-906 induced phosphorylation of p70S6K1 in CRC cells
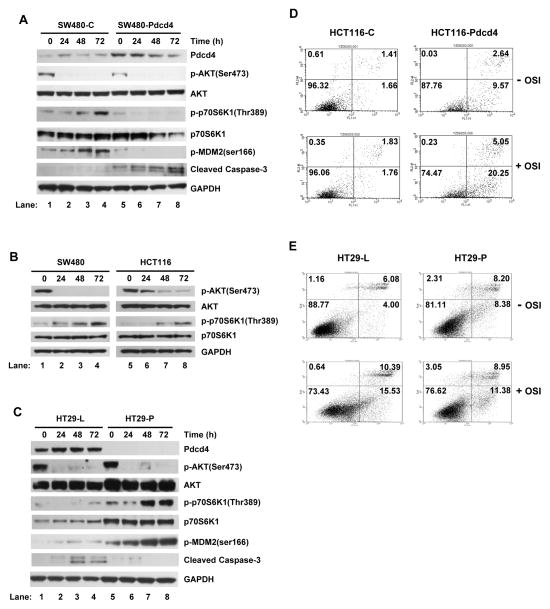
To understand the mechanism by which Pdcd4 affects the chemosensitivity of OSI-906 in CRC cells, SW480 cells expressing empty vector (SW480-C) and Pdcd4 (SW480-Pdcd4) were treated with 5 μmol/L, the minimal IC50 for the resistant cells (15), of OSI-906 for 0 to 72 h. The OSI-906 efficiently inhibits AKT phosphorylation in all tested cells, indicating that the drug functions properly (Fig. 3A). Interestingly, p70S6K1 phosphorylation was increased in SW480-C, but not in SW480-Pdcd4 cells with OSI-906 treatment for 48 and 72 h (Fig. 3A and Supplementary Fig. S3). The elevated p70S6K1 phosphorylation after OSI-906 treatment was also observed in parental SW480 and HCT116 cells (Fig. 3B and Supplementary Figs. S4 and S5). The increase in p70S6K1 phosphorylation in SW480-C cells correlates with the increase in phosphorylation of MDM2, a downstream target of p70S6K1 (32, 33). The activation of p70S6K1 is not likely through the feedback activation of AKT, since phosphorylation of AKT was inhibited after 48- and 72-h treatment of OSI-906. As seen in SW480 cells, over-expression of pdcd4 cDNA in HCT116 cells also inhibited OSI-906-induced phosphorylation of p70S6K1 (Supplementary Fig. S6). To further confirm that Pdcd4 inhibits OSI-906-induced phosphorylation of p70S6K1, we treated HT29-L and HT29-P cells with 5 μmol/L of OSI-906. As shown in Fig. 3C and Supplementary Fig. S7, Pdcd4 knockdown increased phosphorylation of both p70S6K1 and MDM2 after OSI-906 treatment. The elevated p70S6K1 phosphorylation upon OSI-906 treatment when Pdcd4 is knocked down was also observed in GEO cells, another OSI-906 sensitive cell line (Supplementary Figs. S8). These data indicate that prolonged treatment of OSI-906 increased p70S6K1 phosphorylation in resistant cells, which can be reversed by Pdcd4.
Figure 3.
Pdcd4 enhances cell sensitivity to OSI-906 via suppression of p70S6K1 phosphorylation. (A–C) Western blot analyses were performed using cell extracts from various cells treated with OSI-906 (5 μmol/L) for 0–72 h. (A) Over-expression of Pdcd4 in OSI-906-treated SW480 cells inhibits p70S6K1 phosphorylation and increases cleaved caspase 3 level. (B) Phosphorylation of p70S6K1 is increased in SW480 and HCT116 cells upon OSI-906 treatment. (C) Pdcd4 knockdown enhances p70S6K1 phosphorylation but decreases cleaved caspase 3 level in OSI-906-treated HT29 cells. (D and E) The Pdcd4 level correlates with OSI-906-induced apoptosis. Cells were treated with or without OSI-906 (5 μmol/L) for 72 h and the apoptotic cells were determined using Annexin V assays. Each experiment was repeated at least twice. The representative data are shown.
Consistent with the increase in cell survival signal (phospho-p70S6K1 and phospho-MDM2), the level of cleaved caspase 3 was barely detectable in SW480-C cells treated with OSI-906 for 48 and 72 h. However, the level of cleaved caspase 3 increased in SW480-Pdcd4 cells with same treatment (Fig. 3A, lanes 3 and 4 vs. 7 and 8, and Supplementary Fig. S3), suggesting that Pdcd4 inhibits phosphorylation of p70S6K1 leading to the increased apoptosis in response to OSI-906 treatment. Conversely, the cleaved caspase 3 level was barely detected in Pdcd4 knockdown cells (HT29-P) while it was increased in the control cells (HT29-L) after OSI-906 treatment for 48 and 72 h (Fig. 3C, lanes 3 and 4 vs. 7 and 8, and Supplementary Fig. S7). To verify that Pdcd4 enhances the apoptosis induced by OSI-906 treatment, Annexin V assay was performed. As shown in Fig. 3D, apoptosis increased approximately 12-fold in HCT116-Pdcd4 cells compared to that in control cells (HCT116-C) with OSI-906 treatment (20.25 vs. 1.76). Subjected to the same OSI-906 treatment, Pdcd4 knockdown (HT29-P) cells showed a slight decrease in apoptosis when compared to HT29-L cells (Fig. 3E, 15.53 vs. 11.38). These results suggest that Pdcd4 facilitates the apoptotic effects of OSI-906 treatment in cells.
Collectively, these findings indicate that CRC cells gain resistance to OSI-906, at least in part, through the activation of p70S6K1. These results also show that Pdcd4 inhibits OSI-906 induced p70S6K1 phosphorylation to render cells sensitive to OSI-906 induced apoptosis. We also noted that the total protein level of p70S6K1 was decreased in the Pdcd4 over-expressing cells but increased in the Pdcd4 knockdown cells (Fig. 3A and 3C, and Supplementary Figs.S3, and S6–S8). Since Pdcd4 has been demonstrated to interact with translation initiation factor 4A (eIF4A) and inhibit protein translation (34–37), it needs to further investigate whether Pdcd4 regulates p70S6K1 translation.
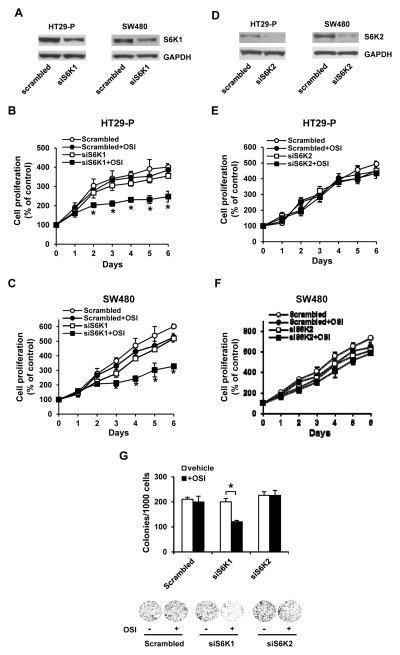
Knockdown of p70S6K1 boosts the sensitivity to OSI-906 in CRC cells
The data presented above suggest that p70S6K1 is the key regulator for OSI-906 chemosensitivity in CRC cells. We thus knocked down p70S6K1 in HT29-P and SW480 cells. The p70s6k1 siRNA successfully knocked down approximately 50% of p70S6K1 in both HT29-P and SW480 cells (Fig. 4A). Knockdown of p70S6K1 slightly reduced cell proliferation in both HT29-P and SW480 cells (open circle vs. open square in Figs. 4B and 4C). However, p70S6K1 knockdown cells treated with OSI-906 (5 μmol/L) showed a significant reduction of proliferation in HT29-P and SW480 cells comparing to the scrambled siRNA transfected cells (closed circle vs. closed square in Figs. 4B and 4C). In contrast, successful knockdown of p70S6K2 in HT29-P and SW480 cells (Fig. 4D) showed no effect on proliferation in the presence or absence of OSI-906 (Figs. 4E and 4F). In addition, colony formation significantly decreased following transfection with s6k1 siRNA but not s6k2 or scrambled siRNA (Fig. 4G). These results suggest that p70S6K1, but not p70S6K2, regulates the chemosensitivity of OSI-906 in CRC cells.
Figure 4.
Knockdown of p70S6K1 but not p70S6K2 enhances cell sensitivity to OSI-906. (A) Western blot analysis shows the effective knockdown of p70S6K1 in HT29-P and SW480 cells. (B and C) Knockdown of p70S6K1 enhances the cell sensitivity to OSI-906. Cells were treated with OSI-906 (5 μmol/L) for 0–6 days and the cell viability was determined using XTT. The absorbance at day 0 is designed as 100%. Results are expressed as mean±SD (n=4). * indicates significant difference (P<0.05) versus scrambled+OSI or siS6K1. (D) Western blot analysis shows the effective knockdown of p70S6K2 in HT29-P and SW480 cells. (E and F) Knockdown of p70S6K2 has no effects on cell sensitivity to OSI-906. The assay was carried out as in (B and C). The absorbance at day 0 is designed as 100%. Results are expressed as mean±SD (n=4). (G) Knockdown of S6K1 enhances the inhibitory effect of OSI-906 on colony formation. Cells were treated with or without OSI-906 (5 μmol/L) for 7 days. Data are expressed as the mean ± SD (n=3). * indicates significant difference (P<0.001). The representative images are shown in the lower panel. Each experiment was repeated at least twice. The representative data are shown.
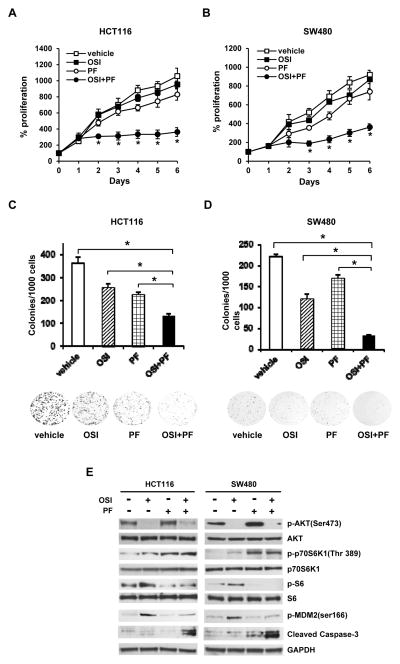
The combination of OSI-906 and PF-4708671, a p70S6K1 inhibitor, efficiently suppresses the growth of OSI-906 resistant CRC cells in vitro and in vivo
Since knockdown of p70S6K1 enhances cell sensitivity to OSI-906, we decided to test whether the combination of OSI-906 and a p70S6K1 inhibitor, PF-4708671, can efficiently inhibit the growth of OSI-906 resistant cells. We treated HCT116 cells with (i) vehicle (DMSO), (ii) OSI-906 (5 μmol/L), (iii) PF-4708671 (10 μmol/L), and (iv) OSI-906 (5 μmol/L)+ PF-4708671 (10 μmol/L) for various amounts of time. As shown in Fig. 5A, HCT116 cells treated with OSI-906 alone (closed square) or PF-4708671 alone (open circle) slightly inhibited cell growth. In contrast, proliferation in HCT116 cells was significantly inhibited after 2-day treatment with the combination of OSI-906 and PF-4708671 (closed circle). A similar result was also observed when SW480 cells were treated with the combination of OSI-906 and PF-4708671 (Fig. 5B). Colony formation also significantly reduced in OSI-906+PF-4708671 treated cells comparing to vehicle, OSI-906 alone, or PF-4708671 alone treated HCT116 or SW480 cells (Figs. 5C and 5D). OSI-906 and PF-4708671 efficiently inhibited phosphorylation of AKT and ribosomal protein S6, respectively, in both HCT116 and SW480 cells (Fig. 5E and Supplementary Figs. S9 and S10), indicating that both drugs function properly. It has been reported that PF-4708671 can inhibit the kinase activity of p70S6K1 as evidenced by decreased phosphorylation of ribosomal protein S6, even though p70S6K1 phosphorylation is increased (30). Cells treated with OSI-906+ PF-4708671 reduced OSI-906 induced p70S6K1 activation and MDM-2 phosphorylation, and consequently increased in levels of cleaved caspase 3 (Fig. 5E and Supplementary Figs. S9 and S10). These findings further support the notion that inhibition of p70S6K1 overcomes the OSI-906 resistance in CRC cells.
Figure 5.
The combination of OSI-906 and PF-4708671 significantly inhibits the growth of resistant CRC cells. (A and B) HCT116 (A) and SW480 (B) cells were treated with vehicle, OSI-906 (5 μmol/L) alone, PF-4708671 (10 μmol/L) alone, or OSI-906 (5 μmol/L)+PF-4708671 (10 μmol/L) for 0–6 days. Cell viability was determined using XTT. The absorbance at day 0 is designed as 100%. Results are expressed as mean±SD (n=4). * indicates significant difference (P<0.001) versus vehicle, OSI-90 alone, or PF-4708671 alone. (C and D) Clonogenic assay. HCT116 (C) and SW480 (D) cells were treated with vehicle, OSI-906 (5 μmol/L) alone, PF-4708671 (10 μmol/L) alone, or OSI-906 (5 μmol/L)+PF-4708671 (10 μmol/L) for 7 days. Data are expressed as the mean ± SD (n=3). * indicates significant difference (P<0.001). The representative images are shown in the lower panel. (E) Western blot analyses were performed using cell extracts from cells treated with vehicle, OSI-906 (5 μmol/L) alone, PF-4708671 (10 μmol/L) alone, or OSI-906 (5 μmol/L)+PF-4708671 (10 μmol/L) for 72 h. Each experiment was repeated at least twice. The representative data are shown.
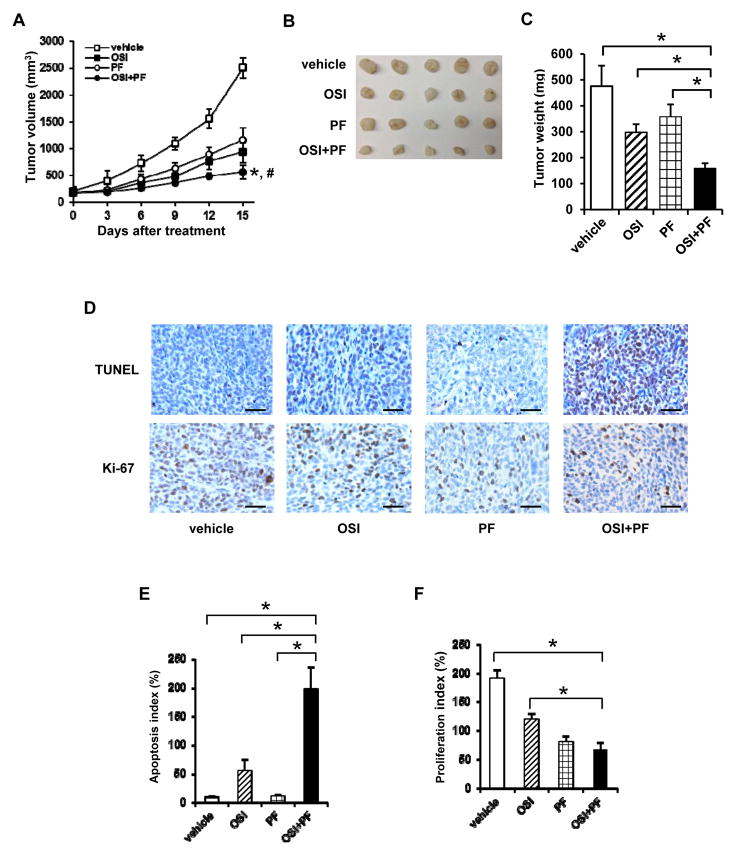
To further validate the results of in vitro study, we used the most stringent model of HCT116 cells derived tumor xenograft model, to test the efficacy of the combination of OSI-906 and PF-4708671 on inhibiting tumor growth in vivo. The tumor growth rate in mice treated with the combination of OSI-906+ PF-4708671 was significantly slower than that of OSI-906 alone (P=0.0189) or PF-4708671 alone (P=0.0165) treated mice (Fig. 6A, closed circle vs. closed square or open circle). The average tumor volume in the OSI-906 and PF-4708671 treated mice was approximately 50% of that in mice treated with OSI-906 (P=0.0056) or PF-4708671 alone (P<0.001) at the end of 15-day treatment (Fig. 6A). Similarly, the tumor weights from OSI-906 and PF-4708671 treated mice (filled column) was approximately 50% of that in mice treated with OSI-906 alone (slashed column, P<0.001) or PF-4708671 alone (gridded column, P<0.001) (Figs. 6B and 6C). Based on the data observed in Fig. 6A, the three-way interaction of two drugs and time in the linear mixed model is not significant (p=0.728), which suggests that OSI-906 and PF-4708671 were additive effect. To confirm that a combined treatment of OSI-906 and PF-4808671 enhances apoptosis and reduces proliferation in vivo, the tumor tissue sections were subjected to apoptosis and proliferation assays. The tumor tissues from OSI-906+PF-4708671 treated mice showed the most TUNEL positive staining and the least Ki-67 positive staining (Fig. 6D). Tumors from OSI-906 treated mice showed increased TUNEL staining. Interestingly, tumors from PF-4708671 treated mice showed no change in apoptosis but decrease in proliferation (Figs. 6D–6F). The apoptosis index and proliferation index of tumors from OSI-906+PF-4708671 treated mice were 197% and 76%, respectively (Figs. 6E and 6F). These findings suggest that the combination of OSI-906 and PF-4708671 overcomes the OSI-906 resistance and offers a feasible clinical testing for CRC treatment.
Figure 6.
The combination of OSI-906 and PF-4708671 significantly inhibits the growth of HCT116-derived tumor in nude mice. Nude mice (5 mice per group) were subcutaneously injected with HCT116 cells and treated with vehicle (25 mmol/L tartaric acid), OSI-906 (30 mg/kg) alone, PF-4708671 (60 mg/kg) alone, or OSI-906 (30 mg/kg)+PF-4708671 (60 mg/kg) for 15 days. (A) Tumor volumes were measured using calipers. * indicates significant difference (P<0.001) versus vehicle or PF-4708671 alone after 15-day treatment. # indicates significant difference (P<0.01) versus OSI-906 alone after 15-day treatment. (B) Image of tumors after 15-day treatment. (C) Average tumor weight of each group after 15-day treatment. * indicates significant difference (P<0.001) versus vehicle, OSI-906 alone, or PF-4708671 alone. (D) Tumor sections were examined for DNA fragmentation (TUNEL) or stained with Ki-76 antibody. The representative images are shown. The brown color indicates the positively stained cells. Scale bar: 50 μm. (E and F) The apoptosis index (E) and proliferation index (F) were calculated as number of TUNEL and Ki-67 positive cells per 500 cells counted, respectively. Two independent tumors were chosen with counting three areas each. The representative data are shown and expressed as the mean ± SD. * indicates significant difference (P<0.005).
Discussion
In this study, we have shown that Pdcd4 expression level plays a key role in sensitizing CRC cells to OSI-906 treatment (Figs. 1 and 2). We also found that Pdcd4 enhances OSI-906 chemosensitivity through inhibition of p70S6K1 activation in CRC cells (Fig. 3). Knockdown or inhibition of p70S6K1 has significantly increased the efficacy of OSI-906 in inhibiting CRC cells growth in cultured cells and xenograft (Figs. 4–6). Thus, the combination of OSI-906 and the p70S6K1 inhibitor, PF-4708671, will inhibit growth of both OSI-906 sensitive and resistant CRC cells. Results from our preclinical models offer a strong rationale for applying the combination of OSI-906 and p70S6K1 inhibitor, PF-4708671, to CRC therapeutics.
Our data suggested that the increased p70S6K1 phosphorylation in resistant cells (SW480 and HCT116) plays a critical role in OSI-906 resistance. The p70S6K, composed of p70S6K1 and p70S6K2 isoforms, is a serine/threonine kinase, which has been demonstrated to promote cell survival and protein translation (38, 39). It has been suggested that activation of p70S6K1 promotes cell survival through phosphorylation of MDM2 at Ser166 (33). Phosphorylation of MDM2 results in polyubiquitination and degradation of p53 and thereby inhibits p53-dependent apoptosis (40). In agreement with this scenario, resistant cells (SW480, HCT116, SW480-C, and HCT116-C) treated with OSI-906 for 48 and 72 h showed increase in phosphorylation of p70S6K1, concomitant increase in phosphorylation of MDM2, and decrease in cleaved caspase 3 and apoptosis (Fig. 3 and Supplementary Figs. S3–S8). The OSI-906-induced phosphorylation of p70S6K1 and MDM2 was inhibited and thereby cleaved caspase 3 and apoptosis was increased when Pdcd4 was over-expressed in the resistant cells. In addition to CRC cells, the elevation of phospho-p70S6K level was also observed in the IGF-1R inhibitor-resistant breast MCF7 cells (41). These findings collectively suggest that OSI-906 resistance in CRC cells is at least partially contributed by activation of p70S6K1.
It has been reported that p70S6K1 is able to phosphorylate Pdcd4 at Ser67 and subsequently leads to proteasome degradation of Pdcd4 (42). Interestingly, our data also suggest that Pdcd4 can regulate p70S6K1 phosphorylation since over-expression of Pdcd4 in the resistant cells (SW480 and HCT116) inhibited phosphorylation of p70S6K1 induced by OSI-906 treatment, while knockdown of Pdcd4 in the sensitive cells (HT29 and GEO) enhanced OSI-906-induced phosphorylation of p70S6K1 (Fig. 3 and Supplementary Figs. S3, and S6–S8). These findings reveal a feedback mechanism of the regulation between Pdcd4 and p70S6K1. We also noted that over-expression of Pdcd4 decreased p70S6K1 protein levels (Fig. 3A and Supplementary Figs. S3 and S6) and knockdown of Pdcd4 elevated p70S6K1 protein levels (Fig. 3C and Supplementary Figs. S7 and S8). Pdcd4 has been demonstrated to bind with eIF4A and inhibit protein translation, which preferentially inhibits translation of mRNA possessing secondary structures at 5′ untranslated region (5′UTR) with free energy (ΔG) higher than −44.8 kcal/mol (34, 35, 37). The ΔG value of the partial 5′UTR in p70S6K1 is −82.30 kcal/mol (as calculated with the mfold program). It is thus possible that Pdcd4 directly regulates the translation of p70S6K1, which needs further investigation.
The findings that knockdown or inhibition of p70S6K1 greatly enhanced the efficacy of OSI-906 in inhibiting the growth of colon tumor SW480 and HCT116 cells with mesenchymal phenotype (Figs. 4 and 5), suggesting that combinational targeting both IGF-1R and p70S6K1 is a promising strategy for CRC therapeutics. This concept was further supported by an HCT116 xenograft study in which the combination of OSI-906 and the p70S6K1 inhibitor PF-4708671 suppressed tumor growth significantly greater than either agent alone by the end of the 15-day study (Fig. 6). It is noteworthy that Pdcd4 protein level also increases in PF-4708671 treated SW480 and HCT116 cells (data not shown), which might be due to the prevention of Pdcd4 from proteasome degradation through inhibition of p70S6K1 activation (42). Elevation of Pdcd4 has been demonstrated to inhibit AP-1 and β-catenin dependent transcription (23, 26, 43). Thus, cells treated with PF-4708671 may inhibit p70S6K1 activity directly and inhibit AP-1 and β-catenin transactivation indirectly through Pdcd4. Since HCT116 cells are metastatic when cells were injected into nude mice (44), suppression of tumor growth in HCT116 xenograft by combined treatment of OSI-906 and PF-4708671 implies that this combination has a broad spectrum in inhibiting CRC, i.e. inhibiting both primary and metastatic colon tumor growth. The efficacy of the combined treatment of OSI-906 and PF-4708671 in inhibiting metastatic colon tumor growth needs to be further studied.
Supplementary Material
Acknowledgments
Grant support: NCI/NIH grant R01CA129015 (H.-S. Yang), NCRR/NCATS grant UL1TR000117 (H.-S. Yang), and NCI/NIH center core support grant P30CA177558 (L. Chen).
We thank Jennifer Rogers for critical reading and editing the manuscript.
Abbreviation
- IGF-1R
insulin-like growth factor 1 receptor
- CRC
colorectal cancer
- MAPK
mitogen-activated protein kinase
- TPA
12-O-tetraadecanoyl phorbol-13-acetate
- DMBA
7,12-dimethylbenz(a)anthracene
- eIF4A
translation initiation factor 4A
- 5′UTR
5′ untranslated region
Footnotes
Disclosure of potential conflicts of interest: No potential conflicts of interest were disclosed.
References
- 1.Sachdev D, Zhang X, Matise I, Gaillard-Kelly M, Yee D. The type I insulin-like growth factor receptor regulates cancer metastasis independently of primary tumor growth by promoting invasion and survival. Oncogene. 2010;29:251–62. doi: 10.1038/onc.2009.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8:915–28. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
- 3.Ma J, Pollak MN, Giovannucci E, Chan JM, Tao Y, Hennekens CH, et al. Prospective study of colorectal cancer risk in men and plasma levels of insulin-like growth factor (IGF)-I and IGF-binding protein-3. J Natl Cancer Inst. 1999;91:620–5. doi: 10.1093/jnci/91.7.620. [DOI] [PubMed] [Google Scholar]
- 4.Chan JM, Stampfer MJ, Giovannucci E, Gann PH, Ma J, Wilkinson P, et al. Plasma insulin-like growth factor-I and prostate cancer risk: a prospective study. Science. 1998;279:563–6. doi: 10.1126/science.279.5350.563. [DOI] [PubMed] [Google Scholar]
- 5.Baserga R, Hongo A, Rubini M, Prisco M, Valentinis B. The IGF-I receptor in cell growth, transformation and apoptosis. Biochim Biophys Acta. 1997;1332:F105–26. doi: 10.1016/s0304-419x(97)00007-3. [DOI] [PubMed] [Google Scholar]
- 6.Yuen JS, Macaulay VM. Targeting the type 1 insulin-like growth factor receptor as a treatment for cancer. Expert Opin Ther Targets. 2008;12:589–603. doi: 10.1517/14728222.12.5.589. [DOI] [PubMed] [Google Scholar]
- 7.Samani AA, Brodt P. The receptor for the type I insulin-like growth factor and its ligands regulate multiple cellular functions that impact on metastasis. Surg Oncol Clin N Am. 2001;10:289–312. viii. [PubMed] [Google Scholar]
- 8.Weber MM, Fottner C, Liu SB, Jung MC, Engelhardt D, Baretton GB. Overexpression of the insulin-like growth factor I receptor in human colon carcinomas. Cancer. 2002;95:2086–95. doi: 10.1002/cncr.10945. [DOI] [PubMed] [Google Scholar]
- 9.Hunt KJ, Toniolo P, Akhmedkhanov A, Lukanova A, Dechaud H, Rinaldi S, et al. Insulin-like growth factor II and colorectal cancer risk in women. Cancer Epidemiol Biomarkers Prev. 2002;11:901–5. [PubMed] [Google Scholar]
- 10.Kaaks R, Toniolo P, Akhmedkhanov A, Lukanova A, Biessy C, Dechaud H, et al. Serum C-peptide, insulin-like growth factor (IGF)-I, IGF-binding proteins, and colorectal cancer risk in women. J Natl Cancer Inst. 2000;92:1592–600. doi: 10.1093/jnci/92.19.1592. [DOI] [PubMed] [Google Scholar]
- 11.Hakam A, Yeatman TJ, Lu L, Mora L, Marcet G, Nicosia SV, et al. Expression of insulin-like growth factor-1 receptor in human colorectal cancer. Hum Pathol. 1999;30:1128–33. doi: 10.1016/s0046-8177(99)90027-8. [DOI] [PubMed] [Google Scholar]
- 12.Mulvihill MJ, Ji QS, Coate HR, Cooke A, Dong H, Feng L, et al. Novel 2-phenylquinolin-7-yl-derived imidazo[1,5-a]pyrazines as potent insulin-like growth factor-I receptor (IGF-IR) inhibitors. Bioorg Med Chem. 2008;16:1359–75. doi: 10.1016/j.bmc.2007.10.061. [DOI] [PubMed] [Google Scholar]
- 13.McKinley ET, Bugaj JE, Zhao P, Guleryuz S, Mantis C, Gokhale PC, et al. 18FDG-PET predicts pharmacodynamic response to OSI-906, a dual IGF-1R/IR inhibitor, in preclinical mouse models of lung cancer. Clin Cancer Res. 2011;17:3332–40. doi: 10.1158/1078-0432.CCR-10-2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flanigan SA, Pitts TM, Eckhardt SG, Tentler JJ, Tan AC, Thorburn A, et al. The insulin-like growth factor I receptor/insulin receptor tyrosine kinase inhibitor PQIP exhibits enhanced antitumor effects in combination with chemotherapy against colorectal cancer models. Clin Cancer Res. 2010;16:5436–46. doi: 10.1158/1078-0432.CCR-10-2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pitts TM, Tan AC, Kulikowski GN, Tentler JJ, Brown AM, Flanigan SA, et al. Development of an integrated genomic classifier for a novel agent in colorectal cancer: approach to individualized therapy in early development. Clin Cancer Res. 2010;16:3193–204. doi: 10.1158/1078-0432.CCR-09-3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao H, Desai V, Wang J, Epstein DM, Miglarese M, Buck E. Epithelial-mesenchymal transition predicts sensitivity to the dual IGF-1R/IR inhibitor OSI-906 in hepatocellular carcinoma cell lines. Mol Cancer Ther. 2012;11:503–13. doi: 10.1158/1535-7163.MCT-11-0327. [DOI] [PubMed] [Google Scholar]
- 17.King ER, Zu Z, Tsang YT, Deavers MT, Malpica A, Mok SC, et al. The insulin-like growth factor 1 pathway is a potential therapeutic target for low-grade serous ovarian carcinoma. Gynecol Oncol. 2011;123:13–8. doi: 10.1016/j.ygyno.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mudduluru G, Medved F, Grobholz R, Jost C, Gruber A, Leupold JH, et al. Loss of programmed cell death 4 expression marks adenoma-carcinoma transition, correlates inversely with phosphorylated protein kinase B, and is an independent prognostic factor in resected colorectal cancer. Cancer. 2007;110:1697–707. doi: 10.1002/cncr.22983. [DOI] [PubMed] [Google Scholar]
- 19.Yang HS, Jansen AP, Nair R, Shibahara K, Verma AK, Cmarik JL, et al. A novel transformation suppressor, Pdcd4, inhibits AP-1 transactivation but not NF-kappaB or ODC transactivation. Oncogene. 2001;20:669–76. doi: 10.1038/sj.onc.1204137. [DOI] [PubMed] [Google Scholar]
- 20.Cmarik JL, Min H, Hegamyer G, Zhan S, Kulesz-Martin M, Yoshinaga H, et al. Differentially expressed protein Pdcd4 inhibits tumor promoter-induced neoplastic transformation. Proc Natl Acad Sci U S A. 1999;96:14037–42. doi: 10.1073/pnas.96.24.14037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jansen AP, Camalier CE, Colburn NH. Epidermal expression of the translation inhibitor programmed cell death 4 suppresses tumorigenesis. Cancer Res. 2005;65:6034–41. doi: 10.1158/0008-5472.CAN-04-2119. [DOI] [PubMed] [Google Scholar]
- 22.Schmid T, Jansen AP, Baker AR, Hegamyer G, Hagan JP, Colburn NH. Translation inhibitor Pdcd4 is targeted for degradation during tumor promotion. Cancer Res. 2008;68:1254–60. doi: 10.1158/0008-5472.CAN-07-1719. [DOI] [PubMed] [Google Scholar]
- 23.Yang HS, Matthews CP, Clair T, Wang Q, Baker AR, Li CC, et al. Tumorigenesis suppressor Pdcd4 down-regulates mitogen-activated protein kinase kinase kinase kinase 1 expression to suppress colon carcinoma cell invasion. Mol Cell Biol. 2006;26:1297–306. doi: 10.1128/MCB.26.4.1297-1306.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leupold JH, Yang HS, Colburn NH, Asangani I, Post S, Allgayer H. Tumor suppressor Pdcd4 inhibits invasion/intravasation and regulates urokinase receptor (u-PAR) gene expression via Sp-transcription factors. Oncogene. 2007;26:4550–62. doi: 10.1038/sj.onc.1210234. [DOI] [PubMed] [Google Scholar]
- 25.Wang Q, Zhu J, Zhang Y, Sun Z, Guo X, Wang X, et al. Down-regulation of programmed cell death 4 leads to epithelial to mesenchymal transition and promotes metastasis in mice. Eur J Cancer. 2013:1761–70. doi: 10.1016/j.ejca.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Q, Sun Z, Yang HS. Downregulation of tumor suppressor Pdcd4 promotes invasion and activates both beta-catenin/Tcf and AP-1-dependent transcription in colon carcinoma cells. Oncogene. 2008;27:1527–35. doi: 10.1038/sj.onc.1210793. [DOI] [PubMed] [Google Scholar]
- 27.Wang Q, Sun ZX, Allgayer H, Yang HS. Downregulation of E-cadherin is an essential event in activating beta-catenin/Tcf-dependent transcription and expression of its target genes in Pdcd4 knockdown cells. Oncogene. 2010;29:128–38. doi: 10.1038/onc.2009.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo X, Li W, Wang Q, Yang HS. AKT Activation by Pdcd4 Knockdown Up-Regulates Cyclin D1 Expression and Promotes Cell Proliferation. Genes Cancer. 2011;2:818–28. doi: 10.1177/1947601911431082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mulvihill MJ, Cooke A, Rosenfeld-Franklin M, Buck E, Foreman K, Landfair D, et al. Discovery of OSI-906: a selective and orally efficacious dual inhibitor of the IGF-1 receptor and insulin receptor. Future Med Chem. 2009;1:1153–71. doi: 10.4155/fmc.09.89. [DOI] [PubMed] [Google Scholar]
- 30.Pearce LR, Alton GR, Richter DT, Kath JC, Lingardo L, Chapman J, et al. Characterization of PF-4708671, a novel and highly specific inhibitor of p70 ribosomal S6 kinase (S6K1) Biochem J. 2010;431:245–55. doi: 10.1042/BJ20101024. [DOI] [PubMed] [Google Scholar]
- 31.Carboni JM, Wittman M, Yang Z, Lee F, Greer A, Hurlburt W, et al. BMS-754807, a small molecule inhibitor of insulin-like growth factor-1R/IR. Mol Cancer Ther. 2009;8:3341–9. doi: 10.1158/1535-7163.MCT-09-0499. [DOI] [PubMed] [Google Scholar]
- 32.Lai KP, Leong WF, Chau JF, Jia D, Zeng L, Liu H, et al. S6K1 is a multifaceted regulator of Mdm2 that connects nutrient status and DNA damage response. EMBO J. 2010;29:2994–3006. doi: 10.1038/emboj.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fang J, Meng Q, Vogt PK, Zhang R, Jiang BH. A downstream kinase of the mammalian target of rapamycin, p70S6K1, regulates human double minute 2 protein phosphorylation and stability. J Cell Physiol. 2006;209:261–5. doi: 10.1002/jcp.20749. [DOI] [PubMed] [Google Scholar]
- 34.Yang HS, Cho MH, Zakowicz H, Hegamyer G, Sonenberg N, Colburn NH. A novel function of the MA-3 domains in transformation and translation suppressor Pdcd4 is essential for its binding to eukaryotic translation initiation factor 4A. Mol Cell Biol. 2004;24:3894–906. doi: 10.1128/MCB.24.9.3894-3906.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang HS, Jansen AP, Komar AA, Zheng X, Merrick WC, Costes S, et al. The transformation suppressor Pdcd4 is a novel eukaryotic translation initiation factor 4A binding protein that inhibits translation. Mol Cell Biol. 2003;23:26–37. doi: 10.1128/MCB.23.1.26-37.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang JH, Cho YH, Sohn SY, Choi JM, Kim A, Kim YC, et al. Crystal structure of the eIF4A-PDCD4 complex. Proc Natl Acad Sci U S A. 2009;106:3148–53. doi: 10.1073/pnas.0808275106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loh PG, Yang HS, Walsh MA, Wang Q, Wang X, Cheng Z, et al. Structural basis for translational inhibition by the tumour suppressor Pdcd4. EMBO J. 2009;28:274–85. doi: 10.1038/emboj.2008.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fenton TR, Gout IT. Functions and regulation of the 70kDa ribosomal S6 kinases. Int J Biochem Cell Biol. 2011;43:47–59. doi: 10.1016/j.biocel.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 39.Raught B, Peiretti F, Gingras AC, Livingstone M, Shahbazian D, Mayeur GL, et al. Phosphorylation of eucaryotic translation initiation factor 4B Ser422 is modulated by S6 kinases. EMBO J. 2004;23:1761–9. doi: 10.1038/sj.emboj.7600193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mayo LD, Donner DB. The PTEN, Mdm2, p53 tumor suppressor-oncoprotein network. Trends Biochem Sci. 2002;27:462–7. doi: 10.1016/s0968-0004(02)02166-7. [DOI] [PubMed] [Google Scholar]
- 41.Ekyalongo RC, Mukohara T, Kataoka Y, Funakoshi Y, Tomioka H, Kiyota N, et al. Mechanisms of acquired resistance to insulin-like growth factor 1 receptor inhibitor in MCF-7 breast cancer cell line. Invest New Drugs. 2013;31:293–303. doi: 10.1007/s10637-012-9855-1. [DOI] [PubMed] [Google Scholar]
- 42.Dorrello NV, Peschiaroli A, Guardavaccaro D, Colburn NH, Sherman NE, Pagano M. S6K1- and betaTRCP-mediated degradation of PDCD4 promotes protein translation and cell growth. Science. 2006;314:467–71. doi: 10.1126/science.1130276. [DOI] [PubMed] [Google Scholar]
- 43.Wang Q, Zhang Y, Yang HS. Pdcd4 knockdown up-regulates MAP4K1 expression and activation of AP-1 dependent transcription through c-Myc. Biochim Biophys Acta. 2012;1823:1807–14. doi: 10.1016/j.bbamcr.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cespedes MV, Espina C, Garcia-Cabezas MA, Trias M, Boluda A, Gomez del Pulgar MT, et al. Orthotopic microinjection of human colon cancer cells in nude mice induces tumor foci in all clinically relevant metastatic sites. Am J Pathol. 2007;170:1077–85. doi: 10.2353/ajpath.2007.060773. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.