Abstract
To address the functionality of diabetic adipose-derived stem cells in tissue engineering applications, adipose-derived stem cells isolated from patients with and without type II diabetes mellitus were cultured in bioreactor culture systems. The adipose-derived stem cells were differentiated into adipocytes and maintained as functional adipocytes. The bioreactor system utilizes a hollow fiber–based technology for three-dimensional perfusion of tissues in vitro, creating a model in which long-term culture of adipocytes is feasible, and providing a potential tool useful for drug discovery. Daily metabolic activity of the adipose-derived stem cells was analyzed within the medium recirculating throughout the bioreactor system. At experiment termination, tissues were extracted from bioreactors for immunohistological analyses in addition to gene and protein expression. Type II diabetic adipose-derived stem cells did not exhibit significantly different glucose consumption compared to adipose-derived stem cells from patients without type II diabetes (p > 0.05, N = 3). Expression of mature adipocyte genes was not significantly different between diabetic/non-diabetic groups (p > 0.05, N = 3). Protein expression of adipose tissue grown within all bioreactors was verified by Western blotting.The results from this small-scale study reveal adipose-derived stem cells from patients with type II diabetes when removed from diabetic environments behave metabolically similar to the same cells of non-diabetic patients when cultured in a three-dimensional perfusion bioreactor, suggesting that glucose transport across the adipocyte cell membrane, the hindrance of which being characteristic of type II diabetes, is dependent on environment. The presented observation describes a tissue-engineered tool for long-term cell culture and, following future adjustments to the culture environment and increased sample sizes, potentially for anti-diabetic drug testing.
Keywords: Adipocytes, adipogenesis, bioreactor, cell and tissue culture, cell cultures, three-dimensional, diabetes research, mesenchymal stem cells, stem cells—adipose, tissue culture models, tissue engineering
Introduction
Type II diabetes and obesity have recently become epidemics in the developed countries. According to the Center for Disease Control, diabetes affects 25.8 million people in the United States, or 8.3% of the country’s population, and 35.7% of American adults are considered to be obese.1,2 As the nation’s obesity rate continues to escalate, further understanding on the role of adipose tissue in disease states becomes more significant.
Both types I and II diabetes mellitus are characterized by the lack of insulin-stimulated glucose transport from blood to tissue, a consequence of β-cell failure in the pancreas.3 Simply, type I diabetes mellitus is a result of β-cell apoptosis activated by cytokines produced by invading immune cells. Type II diabetes is characterized by insulin resistance, a condition in which the body is able to produce insulin but the adipose, liver, and muscles are unable to absorb glucose. This results in an elevated level of blood glucose, signaling β-cells to increase insulin production. Over time, the β-cells cannot maintain the amount of insulin necessary for glucose uptake; additionally, the insulin receptors on adipocytes become exhausted.4,5 Type II diabetes is preventable and reversible through lifestyle changes including diet and exercise regulation. Patients diagnosed with prediabetes and type II diabetes are prescribed to first make the necessary lifestyle changes, then to combine with oral medications. If blood sugar control is not improved, oral medication in combination with insulin is prescribed.
Adipose-derived stem cells (ASCs) are widely accepted as an attractive cell source in the field of tissue engineering and regenerative medicine due to multipotent characteristics, ease of isolation, and attainability.6–8 Clinically, autologous ASCs have been added to whole fat grafts to promote graft volume retention and to support formation of new vascularization within breast reconstruction and craniofacial defects.9–13 Human adult ASCs are typically isolated from discarded adipose tissue from elective cosmetic plastic surgeries14–16 and cultured on the two-dimensional (2D) surface of standard tissue culture flasks with static medium. ASCs are stored, motionless, at 37°C with exposure to 5% carbon dioxide to ensure stable physiological pH. Traditional, 2D culture of ACSs does not allow long-term lipid accumulation and, therefore, maintenance of adipocytes.16 In order to study mature adipose tissue long-term, a three-dimensional culture model is necessary.
Some work has been accomplished culturing adipocytes three-dimensionally in a scaffold or via ceiling culture on a tissue culture flask;16 however, long-term maintenance (i.e. months) and three-dimensional perfusion of adipocytes had not been acknowledged in the literature until Gerlach et al.17 described adipocyte culture within a hollow fiber–based bioreactor for 60 days. Human ASCs have been isolated and differentiated in three-dimensional bioreactors previously; however, most work has studied differentiation into bone, rather than adipocytes, including cultures within spinner flask bioreactors18–20 on scaffolds21 and perfusion systems.22,23 The dynamic perfusion bioreactor has been used to culture various tissue types, including neuronal,24 hepatic,25 and embryonic.26–30 The cells in such bioreactor cultures grow and attach to an interconnected network of porous, polymeric fibers inside the bioreactor chamber while nutrient medium is continuously recirculated throughout the system.
The multi-compartment bioreactor system utilized in our presented study involves two interwoven networks of porous hollow fiber membrane polysulfone bundles intended for medium perfusion and a third interwoven network of oxygenation hollow fibers on which the cells are seeded and cultured, providing a decentralized oxygenation and uniform nutrient and gas exchange with physiological gradients to the tissue (Figure 1). The extracapillary space forms a compartment in which cells are inoculated; the total cell compartment inside the three-dimensional hollow fiber–based bioreactor volume is 8 mL. Run via external perfusion systems and pumps, the continuous media perfusion and gas flow through the system can be tailored and have been optimized, based on several previous reports on such bioreactors used for hepatocyte progenitors and embryonic stem cell cultures.26–30 The bicarbonate buffer system and carbon dioxide gas exchange regulate pH of the entire cell compartment volume with negligible shear stress, creating a permitting environment for sustained, long-term cultures of tens of millions of cells without mechanical stimuli.17 Previous work further explaining the development of such bioreactor system as applied to adipocyte culture is featured in Gerlach et al.17
Figure 1.
Hollow fiber–based bioreactor setup. (a) Photograph of an 8 mL-volume hollow fiber–based bioreactor used; (b) bioreactors incorporated onto the perfusion system and pumps as utilized in the presented work; and (c) schematic of nutrient/gas flow in and out of the bioreactor cell compartments through the porous, hollow fiber membranes.
The described study aims to develop an ex vivo process to study the metabolic activity and adipogenesis of human ASCs derived from patients with type II diabetes mellitus to determine viability within the tissue engineering field. The ASCs are assessed within a hollow fiber–based bioreactor under three-dimensional dynamic perfusion to function as a potential model for drug therapy, specifically type II diabetes mellitus. The presented work includes the analysis and comparison of metabolic behavior and functionality of human ASCs isolated from adult patients with type II diabetes and non-diabetic patients.
Materials and methods
Adipose stem cell isolation
Adipose tissue was harvested from the abdominal depots of type II diabetic female patients (39 and 62 years old, body mass index (BMI) of 27.4 and 32.0, respectively) and from female patients without diabetes (55 and 25 years old, BMIs of 25.5 and 28.6, respectively), all undergoing elective plastic surgery at the University of Pittsburgh Medical Center in Pittsburgh, PA, USA. Tissue was collected under a human studies exempt protocol approved by the University of Pittsburgh Institutional Review Board (IRB). The University of Pittsburgh waived the need for written informed consent from the participants under an exempt review and approval process. De-identified specimens were obtained from discarded surgical tissue under an approved process that did not require subjects to provide written or verbal informed consent. Samples were not pooled between diabetic and non-diabetic patients rather; cells from two separate diabetic samples were combined in one instance to provide sufficient number of cells required for optimal inoculation and cell-to-cell communication within the bioreactor (Table 1, Bioreactor 4). Non-diabetic patients were not prediabetic and did not have insulin resistance at time of adipose tissue harvesting.
Table 1.
Patient age, gender and BMI relative to diabetic state at time of ASC isolation used for bioreactors.a
| Bioreactor | Patient age (years) | Gender | BMI | Diabetic? | Patient | Cell passage |
|---|---|---|---|---|---|---|
| 1 | 35 | Female | 28.6 | No | 1 | 3 |
| 2 | 55 | Female | 25.5 | No | 2 | 3 |
| 3 | 55 | Female | 25.5 | No | 2 | 3 |
| 4 | 62 | Female | 32.0 | Yes | 3 | 5 |
| 4 | 39 | Female | 27.4 | Yes | 4 | 5 |
| 5 | 39 | Female | 27.4 | Yes | 4 | 5 |
| 6 | 39 | Female | 27.4 | Yes | 4 | 5 |
BMI: body mass index; ASC: adipose-derived stem cell.
Two patients had diabetes at time of ASC isolation and two patients did not have diabetes at time of ASC isolation. Three bioreactors were seeded with ASCs isolated from the diabetic patients, and three bioreactors were seeded with ASCs acquired from the non-diabetic patients.
Abdominal adipose tissue was placed in 50-mL centrifuge tubes at 10 g per tube and soaked in 1 mg/mL of freshly prepared collagenase (Type II collagenase; Worthington Biochemical Product Catalog; Lakewood, NJ, USA). The tissue was finely minced, vortexed, and shaken at 37°C for 35 min until a fatty supernatant layer became apparent. Tubes were again vortexed and filtered through double-layered gauze (J&J Steri-Pad Gauze Pads; New Brunswick, NJ, USA) into sterile 50-mL centrifuge tubes. Digested specimens were then centrifuged at 1000 r/min (6449 × g), 4°C for 10 min, fatty layers aspirated, and the resulting pellets suspended in erythrocyte lysing buffer. The solution was once again centrifuged at 1000 r/min (6449 × g), 4°C for 10 min, and the pellet, containing the stromal vascular fraction, was resuspended in Dulbecco’s modified Eagle’s medium (DMEM/F12) with 10% v/v fetal bovine serum and 1% v/v penicillin/streptomycin, plated, and stored at 37°C at 5% CO2. Cells were expanded in two dimensions using growth medium with low serum (Promocell Preadipocyte Growth Medium; Heidelberg, Germany), passaged at confluency, and characterized as previously described by our laboratory report.14
Bioreactor inoculation
The bioreactors are prototyped by Stem Cell Systems (Berlin, Germany) and contain three independent hollow fiber membrane systems, interwoven into repetitive subunits, forming a cell compartment that houses 8.0 × 107 cells with a volume of 8 mL (Figure 1). The capillary network serves three functions: cell oxygenation/carbon dioxide removal, medium inflow, and medium outflow via counter-current flow operation of two independent membrane systems. As a result of interweaving and high-performance mass exchange via counter-current medium flow operation, decentralized gas supply and medium exchange with low gradients is provided to the cultures. The medium fiber membrane systems are made of polyethersulfone capillary systems (Membrana, Wuppertal, Germany) with a molecular weight cutoff of 400,000 g/mol and gas is supplied by hydrophobic multi-laminate hollow fiber membrane systems (MHF; Mitsubishi, Tokyo, Japan).
A total of six bioreactor experiments were performed: three bioreactors cultured with ASCs from females diagnosed with type II diabetes mellitus at time of surgery, and three bioreactors cultured with ASCs from healthy, non-obese, females without type II diabetes. Bioreactor sterilization was performed with ethylene oxide gas and degassed with air. Before cells were introduced into the system, a continuous phosphate buffered solution flush was run within the bioreactor for 72 h, followed by priming with DMEM/F12 media flush for 24 h prior to inoculation. Cell suspensions of 8.0 × 107 total cells per bioreactor were inoculated and cultured throughout a time period of 6 weeks. The cell compartments were continuously perfused with culture media through the polyethersulfone hollow fiber bundles at a feed rate of 4 mL/h in combination with a recirculation loop at a rate of 20 mL/min. Waste medium was removed from the circuit at 4 mL/h. The flows of compressed air and carbon dioxide in the gas compartment were maintained at 20 mL/min. Partial pressures of oxygen, carbon dioxide, and acid/base status within the bioreactor were measured daily, and the carbon dioxide content was adjusted throughout culture time to maintain the medium pH within the range of 7.35–7.45.
Cell culture
Cells were inoculated into the bioreactor cell compartments via a suspension of 8.0 × 107 cells/mL of medium. Cell passages at time of inoculation for each experiment were either passage 3 or 5 and are described in Table 1. Upon completion of a 21-day expansion period of the ASCs within the bioreactor cell compartment by perfusion of ASC plating media (DMEM/F12 as described above), to initiate three-dimensional adipogenic differentiation, the feed media were changed for the following 14 days to adipogenic media containing DMEM/F12, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), biotin, pantothenate, human insulin, dexamethasone, 3-isobutyl-1-methylxanthine (IBMX), peroxisome proliferator–activated receptor (PPAR)-γ agonist, and antibiotics (ZenBio, Research Triangle Park, NC, USA). Adipocytes were maintained for 7 days with feed media containing DMEM/F12, HEPES, fetal bovine serum, biotin, pantothenate, human insulin, dexamethasone, and antibiotics (ZenBio). Cells were not further passaged within the bioreactor, rather, cell fate was influenced by changes in culture medium.
In parallel, ASCs in a 2D control group were cultured in 175-cm2 tissue culture flasks with ASC plating medium containing 10% v/v fetal bovine serum and 1% v/v penicillin/streptomycin, plated, and stored at 37°C at 5% CO2. 2D control ASCs were cultured for 3 weeks, with static media changes every other day and passaged at 80% confluency. 2D controls were stored for gene and protein assessment.
Metabolic activity analysis
Glucose production and consumption of the cells throughout culture within the bioreactors were assessed under a dynamic open-circuit system. A sampling volume of 2 mL was taken daily via a luer-lock sample port; glucose and lactate dehydrogenase (LDH) levels were measured (YSI 2300 STATE Plus Glucose & Lactate Analyzer; YSI Life Sciences, Yellow Springs, OH, USA; Quantichrom Lactate Dehydrogenase Kit; BioAssay Systems, Hayward, CA, USA).
Glucose production/consumption rates of the cells were determined by a previously established protocol considering various parameters including measured glucose concentration within the recirculation sample, total system volume, baseline medium concentrations, flow rate of nutrients through the system, and time points of measurements.26–30
Tumor necrosis factor-α functional testing
After 7 days of maintenance, adipocytes within all six bioreactor cultures underwent exposure to 10 ng/mL direct injection of tumor necrosis factor (TNF)-α (Roche Applied Sciences, San Francisco, CA, USA) intended to inhibit glucose uptake by the adipocytes. TNF-α exposure lasted for 24 h and glucose production was measured every 30 min. Feed inlet and waste outlet flow rates were set to 10 mL/h to ensure that the entire circuit volume was replaced every hour. Throughout TNF-α exposure, media containing 10 ng/mL dosage of TNF-α and no insulin were delivered to the tissue with intention of hindering glucose consumption of the adipocytes.
Once glucose consumption/production had stabilized after 24 h of TNF-α delivery, all six bioreactors were stimulated with human insulin as a “recovery” (Sigma–Aldrich, St. Louis, MO, USA) by a 5-µM direct injection in addition to delivering feed medium containing insulin to maintain a steady influx of insulin for an 8-h period. After the 8-h stimulation period, feed medium was returned to non-insulin-containing medium and glucose consumption measurements were continued for another 20 h to confirm adipocyte metabolism had returned to baseline before bioreactor disassembly.
Immunohistochemistry and histology
Upon termination of each bioreactor culture, tissue/fiber samples were extracted from the bioreactors and placed in 10% w/v buffered formalin (Thermo Scientific, Pittsburgh, PA, USA) and stored at 4°C in the formalin for fixed histology samples. Samples were stained with AdipoRed Assay Reagent to analyze lipid inclusion (Lonza, Walkersville, MD, USA). Samples were protected from light and incubated at room temperature with AdipoRed staining dilutions according to Lonza protocol for 40 min, then exposed to 4′,6-diamidino-2-phenylindole (DAPI) (0.6 µg/mL; Invitrogen, Carlsbad, CA, USA) and AlexaFluor 488 Phalloidin (6.6 µM; Invitrogen) to stain for nuclei and F-actin, respectively, for 10 min at room temperature. Immunofluorescence was captured from an Olympus Fluoview 1000 Upright Confocal Microscope (Olympus America, Melville, NY, USA).
Additionally, samples were paraffin embedded, stained with hematoxylin and eosin and Masson’s Trichrome, and imaged under bright field microscopy of an Olympus Provis Light Microscope (Olympus America) for architectural assessment and to determine matrix formation.
Scanning electron microscopy
Bioreactor explants stored at 4°C in formalin were air dried and placed onto metal stubs covered in double-sided copper tape. The fibers were then gold-sputtered to a density of 3.5 mm from Cressington 108 auto sputter-coater (Cressington, Watford, UK) and imaged with a JEM-6330f Scanning Electron Microscope (Jeol, Peabody, MA, USA). Scope was operated at 5 kV acceleration.
Gene expression
Tissue samples from all six bioreactor cultures were stored in RNAlater Stabilization Reagent (Qiagen, Valencia, CA, USA) at 4°C for quantitative polymerase chain reaction (qPCR). Messenger RNA (mRNA) was collected using Qiagen RNeasy mini Kit and reverse-transcribed into complementary DNA (cDNA) using First Strand Transcription Kit (Invitrogen), both according to the manufacturer’s protocol. PCR primers were designed using Invitrogen’s Vector NTI and synthesized by Invitrogen.
qPCR was performed in triplicate in 96-well optical plates and the primer sequences are as follows: PPAR-γ forward: 5′-CGAGAAGGAGAAGCTGT TGG-3′; PPAR-γ reverse: 5′-TCAGCGGGAAGGACTTTATGTATG-3′; fatty acid binding protein (FABP)4 forward: 5′-AGCACCATAACCTTAGATGGGG-3′; FABP4 re-verse: 5′-CGTGGAAGTGACGCCTTTCA-3′; glyceraldehyde 3-phosphate dehydrogenase (GAPDH) forward: 5′-ACAGTCAGCCGCATCTTCT-3′; GAPDH reverse: 5′-ACGACCAAATCCGTTCACT-3′; where GAPDH was applied as a housekeeping gene.
Protein expression
Cells were scraped from bioreactor fibers stored in RNAlater at 4°C using Cell Scrapers from BD Falcon (Franklin Lakes, NJ, USA), centrifuged for 10 min at 2000 r/min (12,989 × g), resuspended in sample buffer (NuPAGE LDS Sample Buffer; Invitrogen), and boiled for 5 min in water. A total of 10% w/v sodium dodecyl sulfate (SDS)-based gels were made and loaded with protein marker, 5 µg of positive and negative controls (whole fat sample and 2D ASCs, respectively), and equal sample amounts of cells from diabetic and non-diabetic bioreactors. Gels were run, electrophoretically transferred to membranes (Immobilon Transfer Membranes, Sandwiches, and Blotting Filter Paper; Millipore, Billerica, MA, USA), and blocked with 5% w/v milk (Skim Milk Powder; EMD Chemicals Inc., Darmstadt, Germany) for 1 h. Membranes were stored overnight at 4°C in rabbit polyclonal anti-PPAR-γ (Abcam, Cambridge, MA, USA) at 1:1000 dilution, or mouse polyclonal anti-GAPDH (Abcam) at 1:1000 dilution. After overnight primary antibody incubation, membranes were washed three times with buffer solution (Bio-Rad, Hercules, CA, USA) containing Tween-20 followed by soaking in rabbit or mouse polyclonal secondary antibodies (Jackson Immuno Research, West Grove, PA, USA) at 1:50,000 dilution for 60 min at room temperature. Membranes were then washed three times with buffer solution containing Tween-20 (Acros Organics, Geel, Belgium) and detected using SuperSignal detection agent (SuperSignal West Femto Maximum Sensitivity Substrate; Thermo Scientific). Membranes were taken into a dark room where they were developed onto X-ray film (Blue X-Ray Film; Phenix Research Products, Candler, NC, USA) with a phosphor screen (Storage Phosphor Screen; Kodak, Rochester, NY, USA) and processed (X-OMAT 2000 Processor; Kodak).
Statistical methods
All results are presented as mean ± standard deviation. Unpaired, two-tailed t-tests were performed to assess differences in metabolic activity between treatment groups. One-way analysis of variance (ANOVA) followed by Games–Howell post hoc testing was used to determine differences in gene expression between groups. All data were found to be normally distributed and variances were homogenous. Statistical significance is determined at p values less than 0.05.
Results
Metabolic activity compared between ASCs from patients with and without type II diabetes mellitus within bioreactors
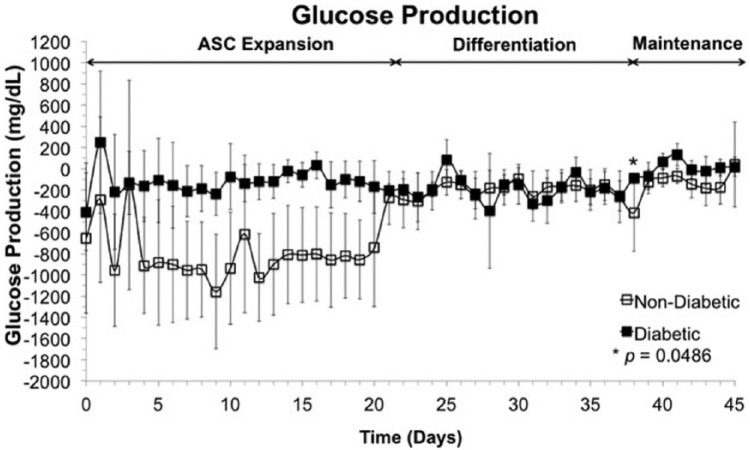
Figure 2 represents average glucose production within the bioreactors of the cells and tissue using aforementioned protocol during ASC expansion (days 0–21), ASC differentiation into adipocytes (days 22–36), and adipocyte maintenance (days 37–45) within all six bioreactors, where solid squares signify glucose production/consumption of cells from diabetic patients and empty squares denote cells from non-diabetic patients. LDH was measured daily to monitor damage to plasma membrane. No increase in LDH concentration within the recirculation media was observed throughout bioreactor culture nor was any significant difference in LDH concentration between diabetic and non-diabetic ASCs.
Figure 2.
Metabolic activity. Glucose production/consumption trends of three bioreactors seeded with ASCs from non-diabetic patients (empty squares) and three bioreactors seeded with ASCs from type II diabetic patients (filled squares) over 45 days of culture. Days 0–21 represent a time period in which ASCs were allowed to expand within the bioreactor; throughout days 22–36, ASCs were influenced to differentiate into adipocytes by a medium change; adipocytes were maintained from days 37 to 45. Glucose consumption is mostly not significantly different (*p = 0.0498, N = 3) within tissue derived from diabetic patients as to tissue derived from patients without diabetes.
Over the entire 45-day culture, glucose production of ASCs from diabetic patients is no different—with the exception of one time point, which had a p value of 0.0498—within the bioreactor from glucose production of ASCs from non-diabetic patients, up to a confidence interval of 95% with a N value of 3. Throughout bioreactor culture, the average glucose production/consumption of tissue from diabetic samples appeared to remain steady, whereas tissue from non-diabetic samples exhibited sharply decreased glucose consumption upon differentiation. Such change in glucose uptake could be due to the cells preparing for differentiation, saturated and storing glucose for lipid accumulation.31–33
TNF-α and insulin functional testing over a 52-h time period
Figure 3 depicts the average glucose production of all six bioreactors (three each from diabetic and non-diabetic groups) before, during, and after insulin stimulation of the three-dimensional adipose tissue. The results indicate no significant difference between glucose consumption/production of adipocytes generated from diabetic ASCs and those from non-diabetic ASCs within the hollow fiber–based bioreactor (p > 0.05, N = 3) before and after insulin stimulation. During insulin stimulation, bioreactors containing samples from diabetic patients experienced less glucose consumption than those from non-diabetic patients in 3 of the 16 time points measured, with an N value of 3 and p values of 0.010, 0.043, and 0.023.
Figure 3.
Adipocyte functionality. All six bioreactors were stimulated with TNF-α for 24 h, and glucose consumption was measured and noted to be hindered. After reaching a steady glucose consumption rate, insulin was introduced to the system and the functional adipocytes were able to recover from the TNF-α dosage as glucose consumption increased. Tissue from diabetic patients (filled squares) functions with mostly no significant difference (*p < 0.05, N = 3) to tissue from patients without diabetes (empty squares) when stimulated with TNF-α and insulin.
Immunohistochemistry/histology and scanning electron microscopy of end-point extracted tissue samples
The imaging results of the tissue generated within the bioreactors are shown in Figures 4–6. Macroscopic tissue formation was observed in and around the hollow fiber membranes upon bioreactor disassembly (Figures 4 and 5). Regarding both groups of adipose tissue—that differentiated from ASCs obtained from diabetic patients and that from non-diabetic patients—histological analyses revealed closely associated adipocytes throughout all bioreactors and confocal fluorescent imaging demonstrated AdipoRed lipid formation after 45 days of culture within the dynamic perfusion systems, while DAPI and AlexaFluor 488 Phalloidin highlight nuclei and F-actin, respectively.
Figure 4.
Histology. End-point (45 days) histology from bioreactors containing adipose tissue generated from patients (a, b) without or (c, d) with type II diabetes mellitus, where (a) and (c) are H&E, and (b) and (d) are Masson’s Trichrome; all were captured at 40× magnification; scale bar = 100 µm.
Figure 5.

Immunohistochemistry. End-point (45 days) AdipoRed/DAPI/Phalloidin immunohistochemistry from bioreactors containing adipose tissue generated from patients (a) without or (b) with type II diabetes mellitus at 20×; scale bar = 100 µm.
Figure 6.
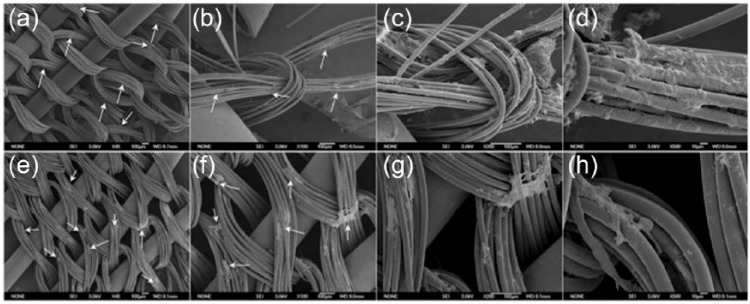
Scanning electron micrographs. Scanning electron microscopy (SEM) images of adipocytes on fibers extracted from bioreactors after 45 days of culture inoculated with ASCs from (a–d) patients without type II diabetes mellitus and (e–h) patients with type II diabetes mellitus. Adipose tissue is indicated by white arrows at 45× and 100×.
SEM images of the samples removed from all bioreactors reveal cell growth on and among the hollow fibers (Figure 6). White arrows on Figure 6 indicate adipocytes.
Gene and protein expression of adipose tissue generated within bioreactors
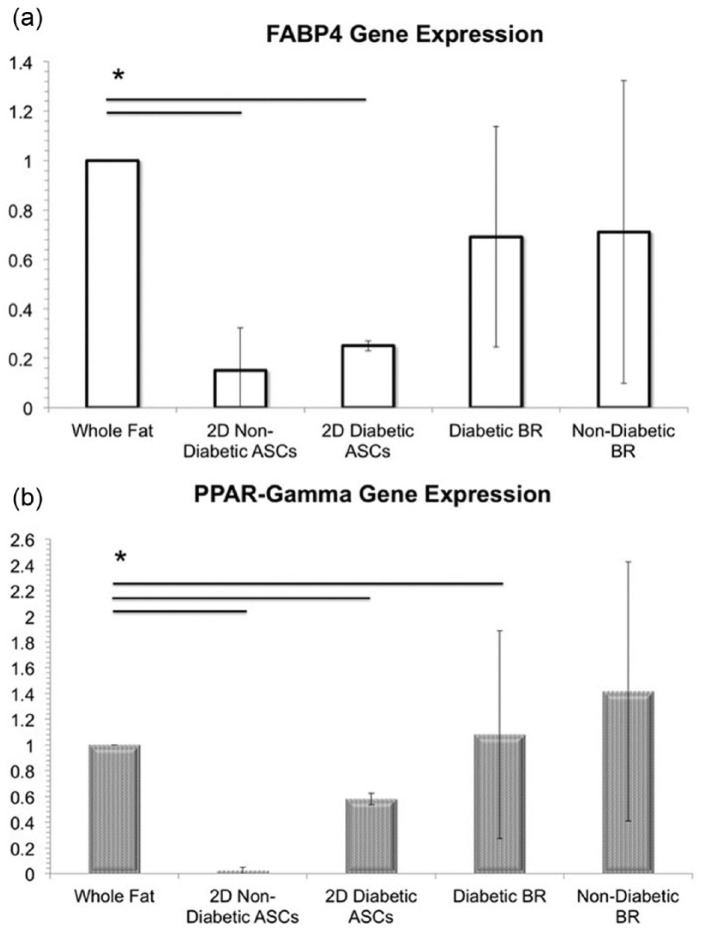
The results obtained from qPCR confirm FABP4 and PPAR-γ gene expression of the adipose tissue generated within all six of the bioreactors, with no significant difference in FABP4 gene expression between adipose differentiated from diabetic and non-diabetic ASCs as determined by one-way ANOVA (F(4, 10), p > 0.05) (Figure 7). A Games–Howell post hoc test confirmed that FABP4 gene expression in whole fat was statistically significantly higher than FABP4 gene expression in ASCs (p < 0.05) and was not statistically different from FABP4 gene expression in samples extracted from both diabetic and non-diabetic bioreactor cultures (p > 0.05, N = 3) (Figure 7). Meanwhile, PPAR-γ gene expression was found to be statistically significantly higher in whole fat compared to both 2D ASC controls and samples extracted from the diabetic bioreactor culture (p < 0.05, N = 3), whereas PPAR-γ gene expression was not significantly different in samples extracted from the non-diabetic bioreactor culture (p > 0.05, N = 3) (Figure 7).
Figure 7.
Gene expression. (a) Fatty acid binding protein-4 (FABP4, solid white bars) and (b) peroxisome proliferator–activated receptor-γ (PPAR-γ, dotted white bars) gene expression is significantly higher (*p < 0.05,) in whole fat than in ASCs grown in 2D culture. PPAR-γ gene expression was higher in whole fat compared to both 2D ASC controls and samples extracted from the diabetic bioreactor culture (p < 0.05, N = 3), whereas, PPAR-γ gene expression was not significantly different in samples extracted from the non-diabetic bioreactor culture (p > 0.05, N = 3).
PPAR-γ and GAPDH protein expression was exhibited by adipose tissue from bioreactors with diabetic and non-diabetic ASCs as well as whole fat samples (Figure 8). While housekeeping protein, GAPDH, confirmed the presence of protein in all samples, including 2D ASCs, 2D ASCs did not express PPAR-γ protein, reaffirming the maturity of adipose tissue cultured within the three-dimensional, hollow fiber–based, dynamic perfusion bioreactors.
Figure 8.
Protein expression. Images obtained from Western blots measuring protein expression of PPAR-γ or GAPDH of (a) adipose tissue cultured within the bioreactors over 45 days from diabetic patients and adipose tissue cultures within the bioreactors from non-diabetic patients or (b) ASCs cultured in two-dimensional tissue culture flasks as a negative control, native human whole fat sample as a positive control.
Discussion
With obesity rapidly approaching epidemic status in the developed countries, followed by type II diabetes mellitus, the need to further understand cellular and molecular mechanisms underlying such diseases becomes more apparent. A key influence in these diseases, but still often overlooked as an endocrine-functioning organ, is adipose tissue. Adipose tissue possesses attractive qualities as a human stem cell source to tissue engineers in regenerative medicine. Furthermore, purity of ASCs and variance between patients have been well characterized;34–36 however, traditional, 2D culture of ACSs does not allow long-term lipid accumulation and, therefore, maintenance of adipocytes.16
Previously, our laboratory has established a three-dimensional perfusion bioreactor culture system, providing an environment suitable for long-term maintenance of adipocytes within a hollow fiber–based, dynamic mass exchange bioreactor that provides decentralized oxygenation.17 With the intention of serving as a potential tool for anti-diabetic drug discovery, the presented tissue engineering study discusses, to the best of our knowledge—for the first time—differentiation of ASCs isolated from diabetic tissue into functional adipocytes within a hollow fiber–based, dynamic, three-dimensional perfusion bioreactor.
The similar metabolic trends observed between diabetic and non-diabetic tissues led the authors to hypothesize that ACSs, once removed from elevated glucose and insulin-leveled environments of type II diabetic patients, retain no “memory” of the diseased state, confirming insulin/insulin-receptor binding to be environment-dependent. While ASCs from diabetic patients consumed less glucose than ASCs from non-diabetic patients during the first 3 weeks of bioreactor culture, the results were not significantly different (p > 0.05, N = 3). After the “ASC expansion” period, ASCs were exposed to a change in feed media—to induce differentiation into adipocytes—during this phase and the “adipocyte maintenance” phase; glucose consumption was expected to not increase, indicating differentiation and maintenance rather than ASC proliferation. To evaluate adipocyte functionality within the bioreactor of both tissue types (diabetic and non-diabetic), after exposure to TNF-α, insulin was introduced to all bioreactors and glucose uptake was observed to increase over an 8-h period and eventually return to the inhibited TNF-α level. Throughout insulin stimulation, three statistically significant time points were observed between the diabetic and non-diabetic groups (p < 0.05, N = 3), indicating that adipocytes from diabetic patients may not recover with insulin as functionally as adipocytes from non-diabetic patients. While the dissimilarity is small, the disparity in glucose consumption may reflect the ability of tissue from diabetic ASCs to recover after 24 h of inhibited glucose uptake.16,37 However, after the 8 h of insulin stimulation, glucose consumption was inhibited to the same degree between the two groups (p > 0.05, N = 3).
While the cells studied within this report are derived from different patients within the two experimental groups, previous work in our laboratory has been conducted establishing ASCs obtained from different patients within certain age, and BMI ranges hold inconsequential differences between proliferation and differentiation capabilities.34,38–40 No prominent, qualitative difference was noted between architecture and formation of adipocytes differentiated from diabetic versus non-diabetic ASCs when cultured and differentiated in the hollow fiber bioreactors for 45 days. When stained with AdipoRed, DAPI, and Phalloidin AlexaFluor 488, lipid-loaded vacuoles indicate mature adipose tissue upon the extracted hollow membrane fibers from all bioreactors, including those inoculated with cells from diabetic patients (Figures 5 and 6). In Figure 5, the Phalloidin indicates a less organized lipid-storing tissue from the bioreactors seeded with ASCs from diabetic patients. No quantitative variance was detected of adipocyte gene marker, FABP4, expressed between adipocytes differentiated from diabetic patients, non-diabetic patients, and whole fat samples (Figure 7). PPAR-γ protein expression presence exhibited through Western blotting was confirmed from diabetic and non-diabetic bioreactor samples (Figure 8(a)) as well as native human whole fat samples and 2D ASCs (Figure 8(b)). The authors acknowledge that protein band strength in the Western blotting is not equal between groups and samples extracted for Western blotting were taken at random from the bioreactors. While housekeeping protein, GAPDH, showed stronger presence in whole fat samples than in samples extracted from bioreactor fibers, it should be noted that the presented work remains a proof-of-concept pilot study.
Conclusion
In conclusion, human ACSs derived from adults with type II diabetes mellitus were cultured within three-dimensional, hollow fiber–based membrane, dynamic perfusion bioreactors for 45 days and metabolic behavior was assessed along with gene and protein expression. Glucose consumption following TNF-α exposure increased during insulin influence, confirming functionality of the generated adipocytes. Masson’s Trichrome, H&E, and AdipoRed images in addition to FABP4 and PPAR-γ gene expression and PPAR-γ protein expression of the functional adipocytes show no major difference between adipose tissue generated in three dimensions when isolated from patients with or without type II diabetes mellitus, raising the question of whether the ASCs retain a diseased state when cultured in vitro and how environment affects functionality of human adipose cells in three-dimensional culture—a critical issue in tissue engineering. Following massive weight loss and change in diet and exercise, patients with type II diabetes mellitus experience regression of the insulin-stimulated uptake and lowered blood glucose levels.37 As type II diabetes mellitus is reversible in vivo, the authors support the hypothesis that human-derived ASCs experience plasticity in culture, dependent on environment. The presented proof-of-concept work provides a first step to advance better understanding of adipose tissue as a whole-body endocrine organ and the potential role of diabetic ASCs in tissue engineering experiments. Current in vitro studies are being conducted in our laboratory to optimize nutrient concentrations within medium as well as further address constituents of diabetic environments. Future studies include optimizing medium components, engineering a long-term diabetic model of adipocytes in a bioreactor, and utilizing the model as a drug discovery tool for various factors addressing diabetes, obesity, and fat volume retention.
Acknowledgments
The authors would like to acknowledge the following members of the Adipose Stem Cell Center and The Bioreactor Groups at the University of Pittsburgh, specifically: Meghan McLaughlin, Eva Schmelzer, Dan McKeel, Brian Phillips, and Sun Jung Oh for their technical assistance; Deanna Rhoads for embedding, sectioning, and staining all H&E and Masson’s Trichrome samples; John Fernstrom for his expertise in data interpretation and suggestions for future project design; Latha Satish and Fang Liu for their expertise in Western Blot protocols. All confocal, bright field, and SEM images were captured at the Center for Biologic Imaging at the University of Pittsburgh.
Footnotes
Declaration of conflicting interests: The authors declare that there is no conflict of interest.
Funding: This study was supported by the National Institute of Health (1R01DK089190-01 (to K.G.M.)).
References
- 1. Center for Disease Control. Adult obesity facts, 13 August 2012, http://www.cdc.gov/obesity/data/adult.html (accessed 6 November 2012).
- 2. Center for Disease Control. National diabetes fact sheet, 2011, http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf (accessed 6 November 2012).
- 3. Cnop M, Welsh N, Jonas JC, et al. Mechanisms of pancreatic β-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes 2005; 54(2): S97–S107. [DOI] [PubMed] [Google Scholar]
- 4. Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr 2006; 83: 461S–4615S. [DOI] [PubMed] [Google Scholar]
- 5. Marcelino H, Veyrat-Durebex C, Summermatter S, et al. A role for adipose tissue de novo lipogenesis in glucose homeostasis during catch-up growth. Diabetes 2013; 62(2): 362–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Choi JH, Gimble JM, Kaplan DL, et al. Adipose tissue engineering for soft tissue regeneration. Tissue Eng Part B Rev 2010; 16(4): 413–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chung MT, Liu C, Hyun JS, et al. CD90 (Thy-1)-positive selection enhances osteogenic capacity of human adipose-derived stromal cells. Tissue Eng Part A 2013; 19(7–8): 989–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Minteer DM, Marra KG, Rubin JP. Adipose-derived mesenchymal stem cells: biology and potential applications. Mesenchymal Stem Cells - Basics and Clinical Application I. Berlin: Springer, 2013, pp. 59–71. [DOI] [PubMed] [Google Scholar]
- 9. Moseley TA, Zhu M, Hedrick MH. Adipose-derived stem and progenitor cells as fillers in plastic and reconstructive surgery. Plast Reconstr Surg 2006; 118: 121S–128S. [DOI] [PubMed] [Google Scholar]
- 10. Kølle S-FT, Fischer-Nielsen A, Mathiasen AB, et al. Enrichment of autologous fat grafts with ex-vivo expanded adipose tissue-derived stem cells for graft survival: a randomized placebo-controlled trial. Lancet 2013; 382(9898): 1113–1120. [DOI] [PubMed] [Google Scholar]
- 11. Pelto J, Bjorninen M, Palli A, et al. Novel polypyrrole-coated polylactide scaffolds enhance adipose stem cell proliferation and early osteogenic differentiation. Tissue Eng Part A 2013; 19(7–8): 882–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yoshimura K, Sato K, Aoi N, et al. Cell-assisted lipotransfer for cosmetic breast augmentation: supportive use of adipose-derived stem/stromal cells. Aesthetic Plast Surg 2008; 32: 48–55; discussion 56–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhu M, Zhou Z, Chen Y, et al. Supplementation of fat grafts with adipose-derived regenerative cells improves long-term graft retention. Ann Plast Surg 2010; 64: 222–228. [DOI] [PubMed] [Google Scholar]
- 14. Brayfield CA, Marra KG, Rubin JP. Adipose stem cells for soft tissue regeneration. Handchir Mikrochir Plast Chir 2010; 42: 124–128. [DOI] [PubMed] [Google Scholar]
- 15. Eberli D, Atala A. Tissue engineering using adult stem cells. Methods Enzymol 2006; 420: 287–302. [DOI] [PubMed] [Google Scholar]
- 16. Fischbach C, Spru T, Weiser B, et al. Generation of mature fat pads in vitro and in vivo utilizing 3-D long-term culture of 3T3-L1 preadipocytes. Exp Cell Res 2004; 300: 54–64. [DOI] [PubMed] [Google Scholar]
- 17. Gerlach JC, Lin YC, Brayfield CA, et al. Adipogenesis of human adipose-derived stem cells within three-dimensional hollow fiber-based bioreactors. Tissue Eng Part C Methods 2012; 18(1): 54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Botchwey EA, Pollack SR, Levine EM, et al. Bone tissue engineering in a rotating bioreactor using a microcarrier matrix system. J Biomed Mater Res 2001; 55(2): 242–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Detamore MS, Athanasiou KA. Use of a rotating bioreactor toward tissue engineering the temporomandibular joint disc. Tissue Eng 2011; 11(7–8): 1188–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Girdler NM. In vitro synthesis and characterization of a cartilaginous meniscus grown from isolated temporomandibular chondroprogenitor cells. Scand J Rheumatol 1998; 27: 446–453. [DOI] [PubMed] [Google Scholar]
- 21. Mauney JR, Nguyen T, Gillen K, et al. Engineering adipose-like tissue in vitro and in vivo utilizing human bone marrow and adipose-derived mesenchymal stem cells with silk fibroin 3D scaffolds. Biomaterials 2007; 28: 5280–5290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bancroft GN, Sikavitsas VI, Mikos AG. Design of a flow perfusion bioreactor system for bone tissue-engineering applications. Tissue Eng 2003; 9(3): 549–554. [DOI] [PubMed] [Google Scholar]
- 23. Liao J, Guo X, Grande-Allen KJ, et al. Bioactive polymer/extracellular matrix scaffolds fabricated with a flow perfusion bioreactor for cartilage tissue engineering. Biomaterials 2010; 31(34): 8911–8920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brayfield CA, Marra KG, Leonard JP, et al. Excimer laser channel creation in polyethersulfone hollow fibers for compartmentalized in vitro neuronal cell culture scaffolds. Acta Biomater 2008; 4(2): 244–255. [DOI] [PubMed] [Google Scholar]
- 25. Miki T, Ring A, Gerlach JC. Three-dimensional dynamic perfusion culture conditions promote hepatic differentiation of human embryonic stem cells. Tissue Eng Part C Methods 2011; 17(5): 557–568. [DOI] [PubMed] [Google Scholar]
- 26. Gerlach JC, Hout M, Edsbagge J, et al. Dynamic 3D culture promotes spontaneous embryonic stem cell differentiation in vitro. Tissue Eng Part C Methods 2010; 16(1): 115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gerlach JC, Lübberstedt M, Edsbagge J, et al. Interwoven four-compartment capillary membrane technology for three-dimensional perfusion with decentralized mass exchange to scale up embryonic stem cell culture. Cells Tissues Organs 2010; 192(1): 39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ring A, Gerlach JC, Peters G, et al. Hepatic maturation of human fetal hepatocytes in four-compartment three-dimensional perfusion culture. Tissue Eng Part C Methods 2010; 16(5): 835–845. [DOI] [PubMed] [Google Scholar]
- 29. Schmelzer E, Triolo F, Turner ME, et al. Three-dimensional perfusion bioreactor culture supports differentiation of human fetal liver cells. Tissue Eng Part A 2010; 16(6): 2007–2016. [DOI] [PubMed] [Google Scholar]
- 30. Stachelscheid H, Wulf-Goldenberg A, Eckert K, et al. Teratoma formation of human embryonic stem cells in three-dimensional perfusion culture bioreactors. J Tissue Eng Regen Med 2012; 7(9): 729–741. [DOI] [PubMed] [Google Scholar]
- 31. Kissebah AH, Vydelingum N, Murray R, et al. Relation of body fat distribution to metabolic complications of obesity. J Clin Endocrinol Metab 1982; 54: 254–260. [DOI] [PubMed] [Google Scholar]
- 32. Lofgren P, Hoffstedt J, Myden M, et al. Major gender differences in the lipolytic capacity of abdominal subcutaneous fat cells in obesity observed before and after long-term weight reduction. J Clin Endocrinol Metab 2002; 87: 764–771. [DOI] [PubMed] [Google Scholar]
- 33. Ramis J, Salinas R, Garcia-Sanz JM, et al. Depot- and gender-related differences in the lipolytic pathway of adipose tissue from severely obese patients. Cell Physiol Biochem 2006; 17: 173–180. [DOI] [PubMed] [Google Scholar]
- 34. Schipper BM, Marra KG, Zhang W, et al. Regional anatomic and age effects on cell function of human adipose-derived stem cells. Ann Plast Surg 2008; 60(5): 538–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rubin JP, Marra KG. Soft tissue reconstruction. Methods Mol Biol 2011; 702: 395–400. [DOI] [PubMed] [Google Scholar]
- 36. Zimmerlin L, Donnenberg VS, Pfeifer ME, et al. Stromal vascular progenitors in adult human adipose tissue. Cytometry A 2010; 77(1): 22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mikus CR, Oberlin DJ, Libla JL, et al. Lowering physical activity impairs glycemic control in healthy volunteers. Med Sci Sports Exerc 2012; 44(2): 225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Aksu AE, Rubin JP, Dudas JR, et al. Role of gender and anatomical region on induction of osteogenic differentiation of human adipose-derived stem cells. Ann Plast Surg 2008; 60(3): 306–322. [DOI] [PubMed] [Google Scholar]
- 39. Dudas JR, Marra KG, Cooper GM, et al. The osteogenic potential of adipose-derived stem cells for the repair of rabbit calvarial defects. Ann Plast Surg 2006; 56(5): 543–548. [DOI] [PubMed] [Google Scholar]
- 40. Kokai LE, Rubin JP, Marra KG. The potential of adipose-derived adult stem cells as a source of neuronal progenitor cells. Plast Reconstr Surg 2005; 116(5): 1453–1460. [DOI] [PubMed] [Google Scholar]