Abstract
The purpose of bone tissue engineering is to employ scaffolds, cells, and growth factors to facilitate healing of bone defects. The aim of this study was to assess the viability and osteogenic differentiation of primary human osteoblasts and adipose tissue–derived mesenchymal stem cells from various donors on titanium dioxide (TiO2) scaffolds coated with an alginate hydrogel enriched with enamel matrix derivative. Cells were harvested for quantitative reverse transcription polymerase chain reaction on days 14 and 21, and medium was collected on days 2, 14, and 21 for protein analyses. Neither coating with alginate hydrogel nor alginate hydrogel enriched with enamel matrix derivative induced a cytotoxic response. Enamel matrix derivative–enriched alginate hydrogel significantly increased the expression of osteoblast markers COL1A1, TNFRSF11B, and BGLAP and secretion of osteopontin in human osteoblasts, whereas osteogenic differentiation of human adipose tissue–derived mesenchymal stem cells seemed unaffected by enamel matrix derivative. The alginate hydrogel coating procedure may have potential for local delivery of enamel matrix derivative and other stimulatory factors for use in bone tissue engineering.
Keywords: Enamel matrix derivative, human adipose tissue–derived mesenchymal stem cells, primary human osteoblasts, alginate hydrogel, TiO2 scaffold
Introduction
Bone tissue engineering (BTE) aims to support the body’s own regenerative potential to restore structure and functionality of damaged bone tissue by means of progenitor cells, osteoconductive matrices, and growth factors.1 A promising cell candidate for this purpose is the abundant human adipose tissue–derived mesenchymal stem cell (hAD-MSC).2 Factors may be incorporated into a matrix in order to direct mesenchymal stem cells (MSCs) toward osteogenic differentiation and ensure differentiation following implantation.3 Previous studies have demonstrated enhanced osteogenic differentiation of MSCs when cultured in an extracellular matrix–like environment.4,5 Incorporation of factors into alginate hydrogel–coated titanium dioxide (TiO2) scaffolds, like simvastatin, was recently demonstrated to enhance osteogenic differentiation of both human osteoblasts (hOBs) and hAD-MSCs.6,7 Also incorporation of proteins, like amelogenin, which is the major component of enamel matrix derivative (EMD), has been demonstrated to enhance osteogenic differentiation of bone marrow–derived MSCs.8
In this study, a TiO2 scaffold coated with an EMD-enriched alginate hydrogel was designed. Previous studies have reported the TiO2 scaffold to have properties which may have potential for BTE purposes, such as porosity, interconnectivity, and compressive strength.9 EMD is commercially available, and in clinical use as Emdogain®, and contains extracellular matrix derivatives from embryonic porcine tooth enamel. It is used in periodontal regeneration where it facilitates formation of new cementum, periodontal ligament, and bone.10,11 Furthermore, it has been shown to enhance differentiation of osteoblasts.12,13 Hence, primary hOBs can be used for proof of concept, to verify the osteogenic effect of EMD. The hypothesis of this study was that cells seeded on TiO2 scaffolds would demonstrate osteogenic differentiation when EMD was added to the subsequent alginate hydrogel coating.
The aim of the study was to assess the viability and osteogenic differentiation of hAD-MSCs and primary hOBs seeded on porous TiO2 scaffolds subsequently coated with an alginate hydrogel enriched with EMD.
Materials and methods
Scaffold production
Porous TiO2 scaffolds, 4 mm in height and 9 mm in diameter, were produced by polymer sponge replication as previously described.9 The scaffolds were sterilized by autoclaving at 121°C for 20 min.
MSCs
The hAD-MSCs were isolated from liposuction material from abdominal regions of three healthy female donors (aged 36, 50, and 61 years). The donors provided informed consent and collection and storage of adipose tissue, and hAD-MSCs were approved by the regional ethics committee for medical research. The isolation and in vitro expansion of hAD-MSCs were carried out as previously described.14 The hAD-MSCs were cultured in medium consisting of Dulbecco’s modified Eagle’s medium (DMEM)/F12 (Gibco/BRL, Carlsbad, CA, USA) supplemented with 10% human platelet lysate plasma (PLP),15 2 IU/mL heparin (Wockhardt, Wrexham, UK), and 1% penicillin/streptomycin. Cells were subcultured at confluence and expanded for 4–5 passages.
Characterization of hAD-MSCs by flow cytometry
The hAD-MSCs were validated as MSCs by flow cytometry and differentiation assays as previously described.15 The following fluorochrome-conjugated antibodies were used for cell surface marker staining: CD14/PE (Diatec, Oslo, Norway), CD19/APC (Diatec), CD45/PE (eBioscience, San Diego, CA, USA), CD105/APC (Diatec), HLA-DR/APC (Diatec), IgG/PE (Southern Biotech, Birmingham, AL, USA), CD34/APC (BD Biosciences, San Jose, CA, USA), CD73/PE, CD90/PE (BD Biosciences), and CD44/PE (Southern Biotech).
Osteogenic differentiation
Osteogenic differentiation of hAD-MSCs was performed at passage 7 as follows. Cells were seeded at 3.5 × 104 cells per well in a 12-well plate and differentiated in a medium consisting of DMEM/F12 (Gibco) containing 10% PLP, 2 IU/mL heparin and 1% penicillin/streptomycin, 10 mM β-glycerophosphate, 10 nM dexamethasone, and 150 µM l-ascorbic acid-2-phosphate. The medium was changed every 3–4 days. Cells were harvested after 21 days of culture for use in quantitative reverse transcription polymerase chain reaction (qRT-PCR) and staining. For alizarin red staining, the cells were washed with phosphate buffered saline (PBS), fixed for 1 h with 1% paraformaldehyde (PFA), and rinsed with PBS. Mineralization was observed by staining with 40 mM Alizarin Red S (pH 4.2) for 5 min. The qRT-PCR was done for RUNX2 and ALPL (Table 1).
Table 1.
List of TaqMan probes used in qRT-PCR.
| Gene symbol | Gene name | TaqMan assay no. |
|---|---|---|
| GAPDH | Plyceraldehyde-3-phosphate dehydrogenase | Hs99999905_m1 |
| COL1A1 | Collagen type I alpha 1 | Hs00164004_m1 |
| TNFRSF11B | Osteoprotegerin | Hs00900360_m1 |
| SPP1 | Osteopontin | Hs00959010_m1 |
| BGLAP | Osteocalcin | Hs01587814_g1 |
| PPARG | Peroxisome proliferator-activated receptor gamma | Hs01115513_m1 |
| SOX9 | SRY (sex determining region Y)-box 9 | Hs00165814_m1 |
| COL2A1 | Collagen type II alpha 1 | Hs00264051_m1 |
| ACAN | Aggrecan | Hs00202071_m1 |
| RUNX2 | Runt-related transcription factor 2 | Hs00231692_m1 |
| ALPL | Alkaline phosphatase | Hs00758162_m1 |
qRT-PCR: quantitative reverse transcription polymerase chain reaction.
Adipogenic differentiation
Adipogenic differentiation of hAD-MSCs was performed at passage 7. Cells were seeded at 3.5 × 104 cells per well in a 12-well plate and cultured in DMEM/F12 (Gibco) containing 10% PLP, 2 IU/mL heparin and 1% penicillin/streptomycin, 10 mg/mL insulin (Novo Nordisk, Bagsvaerd, Denmark), 0.5 mM 1-methyl-3 isobutylxanthine, 1 µM dexamethasone, and 100 µM indomethacin (Dumex-Alpharma, Copenhagen, Denmark). The medium was changed every 3–4 days. Cells were harvested after 21 days of culture for use in qRT-PCR and Oil Red O staining. The qRT-PCR was done for PPARG (Table 1).
Chondrogenic differentiation
For chondrogenic differentiation, 1 × 106 cells were imbedded in 100 µL alginate.15 Chondrogenic differentiation was induced by DMEM/F12 (Gibco) supplemented with 2.8 g/L glucose, 1 mM sodium pyruvate (Gibco), 0.1 mM ascorbic acid-2-phosphate, 0.1 µM dexamethasone, 1% ITS (insulin 25 µg/mL, transferrin 25 µg/mL, and sodium selenite 25 ng/mL), 1.25 mg/mL human serum albumin (Octapharma, Jessheim, Norway), 500 ng/mL bone morphogenic protein-2 (Wyeth Pharmaceuticals, Taplow, UK), 25 ng/mL recombinant human transforming growth factor-β1 (R&D Systems, Minneapolis, MN, USA), and 200 mg/mL insulin-like growth factor 1 (IGF1; Sigma–Aldrich, St. Louis, MO, USA). Medium was changed every 3–4 days. Cells were harvested after 21 days of culture for use in qRT-PCR. The qRT-PCR was done for SOX9, COL2A1, and ACAN (Table 1).
Alizarin red staining
For alizarin red staining, the cells were washed with PBS, fixed for 1 h with 1% PFA, and rinsed with PBS. Mineralization was observed by staining with 40 mM Alizarin Red S (pH 4.2) for 5 min.
Oil Red O staining
For Oil Red O staining, the cells were washed with PBS, fixed for 15 min with 4% PFA, and washed in 50% isopropanol. Lipid droplets were visualized by staining the cells for 10 min with Oil Red O, followed by washing with isopropanol.
Osteoblasts
The hOBs (Cambrex Bio Science, Walkersville, MD, USA) from three male donors (aged 10, 16, and 41 years), two from femur and one from tibia, were cultured in osteoblast culture medium supplemented with 10% fetal bovine serum, 0.1% gentamicin sulfate, amphotericin-B, and ascorbic acid (Lonza, Walkersville, MD, USA). The hOBs from the femur donors were subcultured till passages 6 and 8, and the hOBs from tibia were propagated till passage 9.
Cell seeding and coating
Cell seeding was performed using an agitated seeding method in order to ensure a homogeneous cell distribution throughout the scaffold.16 Scaffolds were presoaked in culture medium and placed in 24-well plates, after which 1 mL cell suspension was added at a density of 2 × 105 cells/mL. After seeding, the plates were agitated on an orbital shaker at 200 r/min for 6 h at 37°C in humid conditions. Following agitation, the cell-seeded scaffolds were transferred to new culture plates in 1 mL culture medium and incubated overnight for 18 h at 37°C in a humidified atmosphere of 5% CO2. The next day, the cell-seeded scaffolds were coated with EMD alginate (EMD group) using a self-gelling alginate system.17 Scaffolds were washed twice in a 4.6% d-Mannitol solution and subsequently centrifuged at 300 × g for 1 min to remove excess 4.6% d-Mannitol. Scaffolds were then immersed in a freshly made solution consisting of three parts: 1% (w/v) Pronova ultrapure (UP) low viscosity, high-G sodium alginate (LVG) sodium alginate (FMC BioPolymer, Sandvika, Norway) in 4.6% d-Mannitol, 2% (w/v) Novamatrix calcium-oligoG-alginate (FMC BioPolymer), and 50 µg/mL EMD (Lot number: EMD 9121; Institut Straumann, Basel, Switzerland) in 0.003% (w/v) acetic acid in 4.6% d-Mannitol. The scaffolds were left to incubate at room temperature for 10 min to allow the alginate solution to gel. Subsequently, the scaffolds were centrifuged at 300 × g for 1 min to remove excess alginate solution. The alginate-coated scaffolds were stabilized in a 50 mM CaCl2 solution and transferred to new culture plates in 1 mL culture medium and maintained at 37°C in a humidified atmosphere of 5% CO2 for up to 21 days. To assess the effect of EMD and alginate coating, two more groups were included. One group of scaffolds was coated with alginate without EMD (alginate group). The other control group was washed twice in 4.6% d-Mannitol, centrifuged at 300 × g for 1 min, as for the other groups, and subsequently transferred to new culture plates in 1 mL culture medium (control group). Triplicates of each donor, each treatment, and for two harvest time points were included, rendering a total of 108 cell-seeded scaffolds (54 hOBs and 54 hAD-MSCs). The culture medium was changed every second day and collected for analyses. The cells in scaffolds were harvested after 14 and 21 days of culture for use in qRT-PCR.
Visualization of cells and alginate coating
The alginate coating was visualized by periodic acid–Schiff (PAS) staining. Scaffolds were cut in half and fixed in 4% PFA/4.6% d-Mannitol for 15 min prior to staining. Fixed scaffolds were washed with distilled water and oxidized in 1% periodic acid solution (Sigma–Aldrich) for 5 min. Then, scaffolds were rinsed with distilled water and placed into Schiff reagent (Sigma–Aldrich) for 15 min. Subsequently, scaffolds were incubated in PBS containing 1.25% bovine serum albumin (BSA) and 0.2% Triton X-100 for 30 min followed by incubation with mouse anti-human Pan-Cadherin (1:2000, I-8H5; MP Biomedicals, Santa Ana, CA, USA) for 1 h at room temperature. After washing in PBS containing 2.5% BSA and 0.05% Tween 20, the scaffolds were incubated in secondary antibody, Alexa Fluor 488 rabbit anti-goat IgG (1:250) (Invitrogen Life Technologies, Paisley, UK), for 30 min at room temperature. Finally, scaffolds were incubated in PBS containing 4′,6-diamidino-2-phenylindole (DAPI) at a concentration of 5 mg/mL for nuclear labeling. Stained scaffolds were placed on a coverslip and covered with Dako fluorescent mounting medium (Dako, Glostrup, Denmark). Confocal laser scanning microscopy (CLSM) was performed on a FluoView 1000 (Olympus, Center Valley, PA, USA). Alexa Fluor 488 and DAPI were detected using specific filters for the respective fluorophores, and the PAS staining was detected with a filter for Alexa-546.18 For macroscopic evaluation of PAS staining, scaffolds were photographed using a SONY NEX-5N camera (Sony, Tokyo, Japan).
Lactate dehydrogenase activity in culture medium and acridine orange/ethidium bromide staining for viability assessment
The cytotoxicity was estimated based on the lactate dehydrogenase (LDH) activity in the culture medium collected every second day up to 14 days with a cytotoxicity detection kit (Roche Diagnostics, Mannheim, Germany) according to the manufacturer’s instructions. The absorbance was measured in a plate reader (Biochrom Asys Expert 96 Microplate Reader; Biochrom, Holliston, MA, USA).
Viability of hAD-MSCs was evaluated by acridine orange/ethidium bromide (AO/EB) staining at day 2 after alginate coating. Scaffolds were cut in half to reveal the center of the scaffold and incubated with AO/EB staining solution in culture medium (1:10) for 5 min. Fluorescence images were captured using CLSM. The AO and EB were both excited using a 488-nm laser. The emitted fluorescence for AO was acquired at 500–560 nm, and for EB, the emission was acquired at 600–700 nm using a virtual channel setting.19 The scaffold surfaces were visualized using CLSM in reflection mode.
Analysis of messenger RNA expression
Total RNA was isolated from scaffolds using Qiagen RNA mini-kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocols. The complementary DNA (cDNA) was synthesized with RevertAid First Strand cDNA Synthesis Kit (Fermentas, St. Leon-Rot, Germany) using random primers. The qRT-PCR was performed in the Applied Biosystems 7300 Real-Time System (Life Technologies) with TaqMan Universal PCR Master Mix and TaqMan Gene Expression Assays (Applied Biosystems, Paisley, UK). The qRT-PCR was done for GAPDH, COL1A1, TNFRSF11B, SPP1, and BGLAP. List of probes used in qRT-PCR is provided in Table 1. The qRT-PCR analysis was performed in duplicate for EMD and control groups at days 14 and 21. Relative messenger RNA (mRNA) levels were calculated by the comparative CT method.20
Quantification of secreted proteins
Multianalyte profiling of protein levels in culture medium was performed on the Luminex 200 system (Luminex, Austin, TX, USA) employing xMAP technology. Acquired fluorescence data were analyzed by xPONENT 3.1 software (Luminex). The amount of osteoprotegerin (OPG), osteopontin (OPN), and osteocalcin (OC) in the culture medium was measured using the human bone panel kit (Millipore, Billerica, MA, USA) in triplicates after 2 and 14 days for hAD-MSCs and hOBs, and after 21 days in triplicates for hAD-MSCs and duplicates for hOBs. All analyses were performed according to the manufacturer’s protocols.
Statistics
The data obtained by gene expression and protein secretion analyses were compared groupwise using the Holm–Sidak test following a parametric one-way analysis of variance (ANOVA). Whenever the equal variance and/or the normality test failed, a Kruskal–Wallis one-way ANOVA on ranks was performed (SigmaPlot 12.0; Systat Software, San Jose, CA, USA). A probability of ⩽0.05 was considered significant.
Results
Characterization of hAD-MSCs
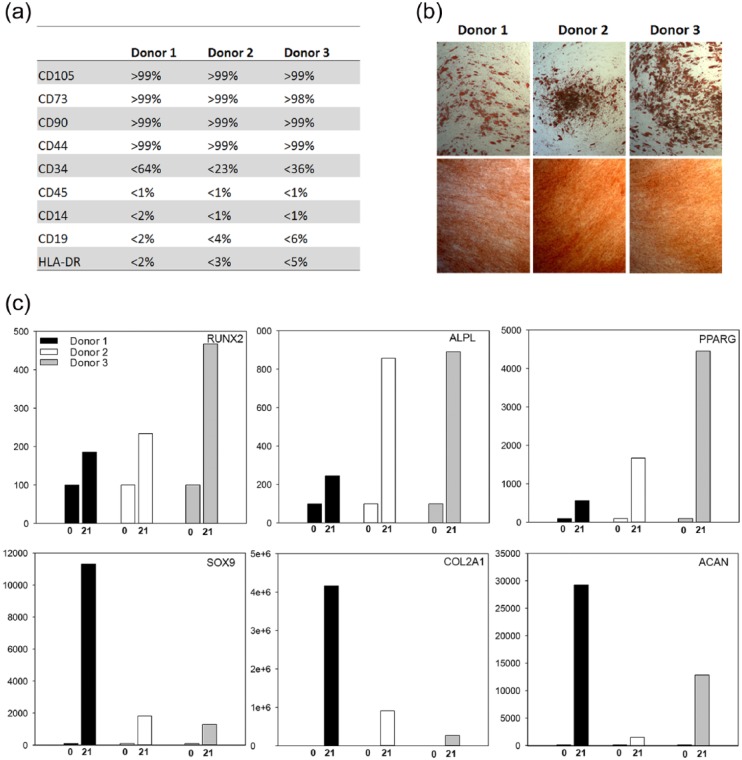
Surface antigen profiles obtained at passage 5 were concurrent with those of hAD-MSCs. Furthermore, cells differentiated into adipogenic and osteogenic lineages at passage 7 as verified by extensive staining of lipid droplets (Oil Red O) and calcium deposits (Alizarin Red) as well as by up-regulation of PPARG (adipogenic) and RUNX2 and ALPL (osteogenic) expression by real-time RT-PCR analysis. Cells also differentiated into the chondrogenic lineage with up-regulation of the chondrogenic markers (SOX9, COL2A1, ACAN) (Figure 1).
Figure 1.
(a) Surface marker profiles of hAD-MSCs as measured by flow cytometry before differentiation (passage 5). (b) Light microscopy pictures of stained osteogenic and adipogenic differentiated hAD-MSCs on day 21: upper panel—adipogenic differentiated cells stained with Oil Red O; lower panel—osteogenic differentiation of hAD-MSCs stained with Alizarin Red. (c) Real-time RT-PCR analysis of RUNX2 and ALPL expression in osteogenic differentiated cells, PPARG in adipogenic differentiated cells, and SOX9, COL2A1, and ACAN in chondrogenic differentiated cells after 0 and 21 days of inductive culture.
Characterization of cell-seeded and alginate-coated scaffolds
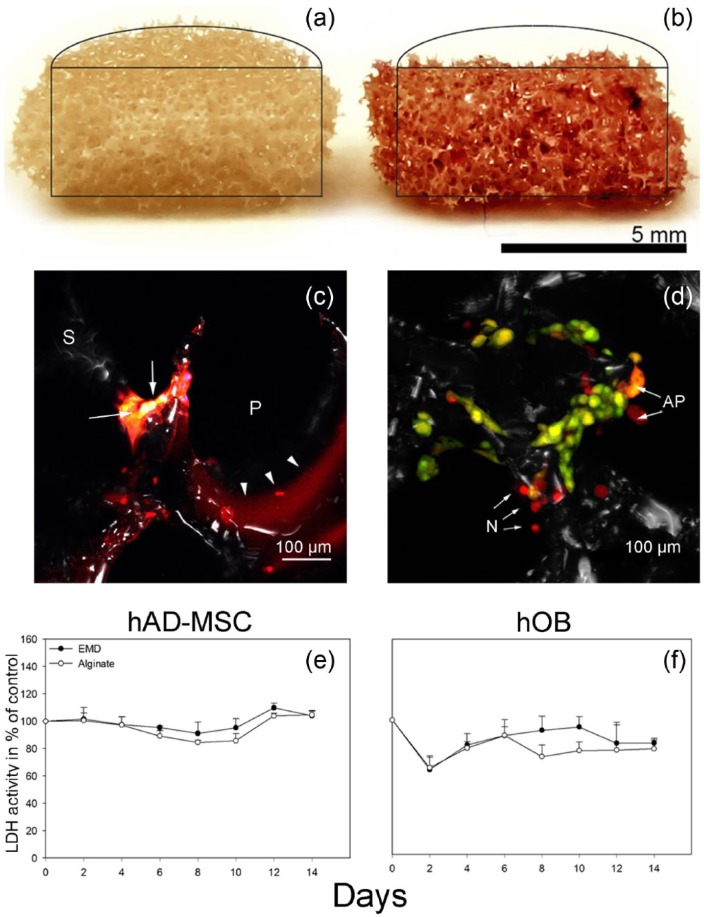
PAS/Pan-Cadherin double staining revealed that the alginate coating left an even distribution of alginate hydrogel throughout the scaffolds (Figure 2). Confocal microscopy further confirmed that cells as well as alginate were attached to the struts leaving the pores in the scaffolds unplugged (Figure 2).
Figure 2.
PAS/Pan-cadherin double staining of (a) hAD-MSC-seeded uncoated and (b and c) alginate-coated scaffolds. The alginate is distributed throughout the scaffold as visualized by the PAS staining (red) depicted in the cross–sectional view (b). The alginate (arrowheads) coats the struts (S) without filling the pores (P), and pancadherin (yellow) stained cells (arrows) attach to the scaffold struts (S). The images are representative of the respective groups. (d) AO/EB staining of hAD-MSCs 2 days after alginate coating. The majority of cells are viable (green), and only few necrotic cells (N) and cells undergoing apoptosis (AP) are present. The image is representative of the respective group. LDH activity measured in culture medium from (e) hAD-MSCs and (f) hOBs cultured on (e) EMD (EMD) and alginate (Alginate) scaffolds. The result is presented in percentage of control, uncoated scaffold. Values represent the mean of three donors (six parallels per donor) + SD.
Cell viability
The LDH activity in the culture medium from hAD-MSC cultures did not change during the initial culture period for either EMD or alginate groups. However, a 10% higher activity was detected at day 12 in the medium from EMD group when compared to control group (Figure 2). In the medium from hOB-seeded scaffolds, a lower LDH activity was observed for both EMD and alginate groups compared to control group at days 2 and 4 (Figure 2). The low cytotoxicity was verified by live/dead staining demonstrating that the majority of cells were viable at day 2 after alginate coating (Figure 2).
Analysis of mRNA expression
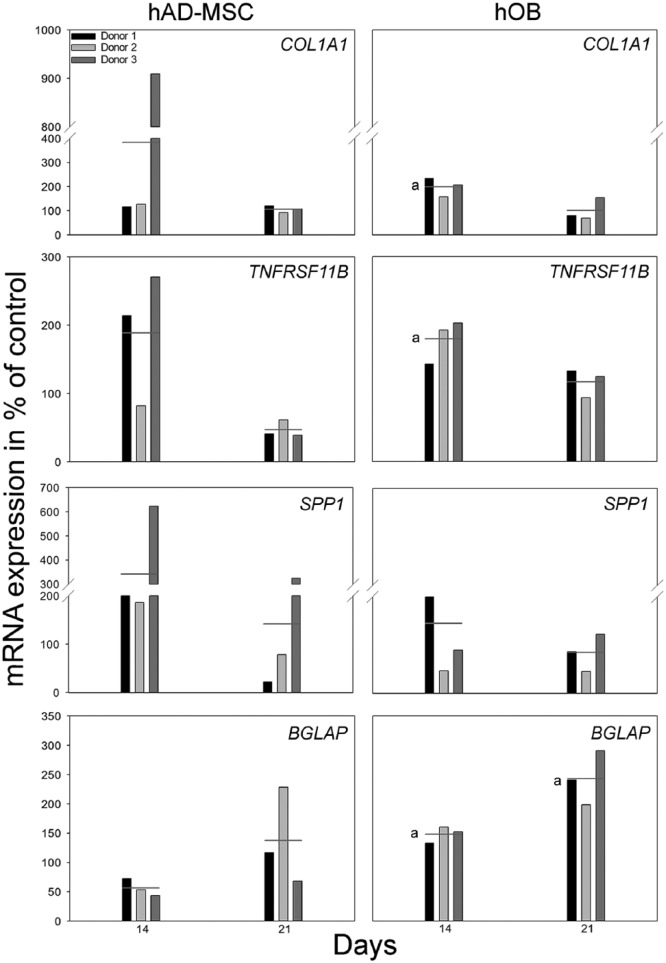
Relative quantification of the mRNA expression in hAD-MSCs showed no significant differences between groups at any time points (Figure 2). For hOB-seeded scaffolds, COL1A1 and TNFRSF11B expression was significantly up-regulated in EMD group after 14 days of culture compared to alginate group (p = 0.011 and p = 0.013, respectively). Also, the expression of BGLAP was significantly higher in the EMD group after 14 (p = 0.004) and 21 (p = 0.006) days of culture when compared to the alginate group. No significant differences were observed in the expression of SPP1 mRNA at any of the time points when comparing the experimental groups (Figure 3).
Figure 3.
The effect of EMD on relative mRNA expression levels for COL1A1, TNFRSF11B, SPP1, and BGLAP after 14 and 21 days of culture for hAD-MSCs and hOBs on scaffolds. The result is presented in percentage of control, alginate group, normalized to reference gene GAPDH, and shown for each individual hAD-MSC and hOB donor, respectively (D1, D2, D3). Horizontal lines indicate the average mRNA expression of three donors (one parallel per donor). (a) indicates significant (p ⩽ 0.05) difference compared to alginate group.
Quantification of secreted proteins
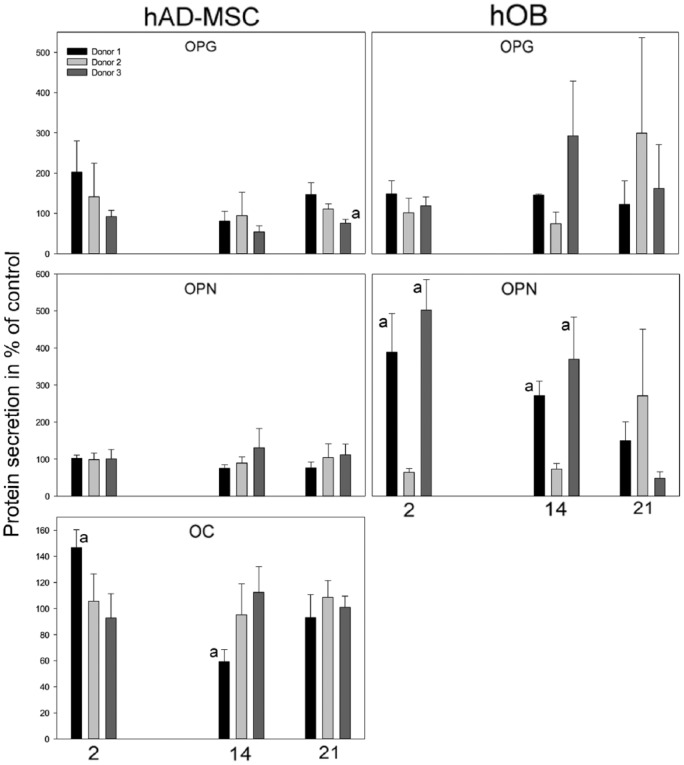
Few and inconsistent differences were observed between the EMD and alginate groups in the hAD-MSC cultures when comparing the average content of OPG, OPN, and OC in the culture medium from the three donors (Figure 4). In donor 1, OC was significantly higher in the EMD group at day 2 as compared to the alginate group (0 = 0.009), but at day 14 the opposite was found (p = 0.02). No differences were observed for OPN, and for OPG, a difference was only found in donor 3 at day 21 where the concentration was lower in the EMD group (p = 0.01).
Figure 4.
Secretion of OPG, OPN, and OC to culture medium from hAD-MSCs and hOBs cultured on EMD scaffolds at days 2, 14, and 21. The result is presented in percentage of control, alginate group, and shown for each individual hAD-MSC and hOB donor, respectively (D1, D2, D3). (a) indicates significant (p ⩽ 0.05) difference compared to alginate group.
In the hOBs, the average content of OPN in the culture medium of the EMD group was significantly increased at days 2 (p = 0.01, p = 0.002) and 14 (p = 0.007, p = 0.02) for both donors 1 and 3, respectively. For donor 2, however, the alginate group demonstrated a higher level of OPN in the cell medium at day 2 only (p = 0.03). No further significant differences were observed between EMD and alginate groups when comparing the average content of OPG and OC in the culture medium from the hOB cultures. The content of OC in the culture medium from the hOB cultures was not detected at a high enough level to allow for quantification. The low level of detectable OC in the medium was most likely caused by repeated freeze thaw cycles during storage.
Variations were seen in the content of proteins in the culture medium when comparing the effect of EMD on the various donors (Figure 4). Significant donor differences were observed for hAD-MSCs in the secretion of OPG between donor 1 and donor 3 at day 21 (p = 0.01) and in the secretion of OC between donor 1 and donor 3 at days 2 (p = 0.03) and 14 (p = 0.04). However, donor differences were also observed for the hOBs in the secretion of OPG at day 14 between donor 2 and donor 3 (p = 0.004) and in the secretion of OPN between donor 2 and 3 at days 2 (p = 0.03) and 14 (p = 0.04).
Discussion
The alginate hydrogel coating of the cell-seeded TiO2 scaffolds did not induce a cytotoxic response. The expression of the osteoblast markers COL1A1, TNFRSF11B, and BGLAP and the secretion of OPN were significantly higher in hOBs when cultured on scaffolds with EMD-enriched alginate, whereas the hAD-MSCs seemed unaffected by the EMD.
With an average pore size of 400 µm and an interconnectivity of more than 90% through 200 µm connections,9,21 the TiO2 scaffold may have sufficient space for coating applications. A subtle coating without compromising porosity is desired to allow capillary ingrowth, sufficient cell nutrients, and oxygen supply. Insufficient oxygen delivery to the center of three-dimensional scaffolds may inhibit cell survival,22 and hypoxia has furthermore demonstrated to reduce osteogenic differentiation of adipose-derived mesenchymal cells.23 Except for the first time points, negligible differences were observed across groups with respect to the LDH activity in the medium. This indicates that the open-pore scaffold structure was sufficiently preserved by the alginate hydrogel and the EMD. Larsen et al.24 recently demonstrated the impact of the distance between cells immobilized in alginate to the cell medium interface in terms of oxygen diffusion. The findings from this study suggested that the distance between the immobilized cells to the cell medium interface was small enough to maintain viability. This is in agreement with a previous study reporting high cell viability in alginate hydrogel for 21 days.25
An initial decline of LDH activity was observed in the medium from the hOBs, but not from the hAD-MSCs. The centrifugation steps in conjunction with the coating procedure may have contributed to the removal of loosely adhered cells. The authors cannot soundly explain why this seemed to affect only one cell type. However, no differences were observed between the groups for the hOBs at day 6 onward.
Neither gene expression nor protein analysis indicated that EMD influenced the osteogenic differentiation of hAD-MSCs. This is in agreement with a previous study that evaluated mRNA expression of human umbilical cord MSCs at early time points.13 In that study, EMD was shown to up-regulate osteogenic markers only when osteogenic supplements were present in the culture media. Recently, the impact of donor age on the differentiation potential of hAD-MSCs was demonstrated.26 The effect of donor age was described to mainly influence chondrogenic differentiation. Differences in donor age could explain some of the large donor variations observed in this study, especially when it comes to the chondrogenic differentiation. The lack of osteogenic response in the hAD-MSCs, when cultured on scaffolds with EMD, may also in part be attributed to the relatively high donor age. However, there was no trend of age-related changes in expression and secretion in either cell type.
In contrast to the hAD-MSCs, the hOBs cultured on scaffolds with EMD did significantly up-regulate the expression of the early osteoblast markers (COL1A1, TNFRS11B) after 14 days of culture and the late osteoblast marker BGLAP after both 14 and 21 days. EMD stimulated the secretion of OPN to the culture medium at early time points for donors 1 and 3, whereas an increase was observed for donor 2 at a much later time point. The stimulatory effects of EMD on osteoblast differentiation are in agreement with previous reports.12,27
The finding that EMD incorporated in the alginate hydrogel could enhance the osteogenic expression suggests a potential for the TiO2 scaffold and the alginate hydrogel coating in BTE. As previously demonstrated for simvastatin,6,7 the results of this study indicate that also substantially larger proteins such as EMD may exert cellular effects when included in an alginate hydrogel. Based on our data, care must be taken when interpreting results from studies based on cells from only one donor and with a narrow window of observations. The huge variation in differentiation potential between primary cells from donors may lead to contradictory results.
The alginate hydrogel may have potential as a vehicle for in situ delivery of factors involved in cell differentiation. The clinical potential of the method described, in vitro cell expansion on the TiO2 scaffold followed by alginate hydrogel coating prior to implantation, enables differentiation factors to act in situ. Few previous studies have attempted to modify osteogenic differentiation of cells adhered to a scaffold by encapsulation of an alginate hydrogel. Although the hypothesized effect of EMD was not accomplished for the hAD-MSCs in this study, other factors may be added to the alginate hydrogel to regulate differentiation of cells. Recent studies have demonstrated effects of peptides,25,28 statins,9 proteins,29 and biomaterials30 on cell differentiation in alginate hydrogels. Further in vitro studies need to be conducted in order to find the most appropriate factor for MSC differentiation in conjunction with TiO2 scaffolds and alginate hydrogel. Future studies should include more donors and samples in order to more fully elucidate the impact of donor variation and differentiation.
In conclusion, low cytotoxicity and significant up-regulation of the osteoblast markers COL1A1, TNFRSF11B, and BGLAP and secretion of OPN in hOBs demonstrated that the post-seeding alginate hydrogel coating was tolerated by the cells and functioned as a vehicle for in situ delivery of proteins such as EMD. Hence, the alginate coating procedure may have a potential for local delivery of factors to enhance osteogenic differentiation of cells in a porous scaffold.
Acknowledgments
The authors thank Teres Colosseum, Oslo, for kindly providing liposuction material and Novamatrix for providing the alginate. Furthermore, the authors would like to thank Hege Brincker Fjerdingstad (Norwegian Center for Stem Cell Research, Oslo University Hospital) and Aina-Mari Lian (Department of Biomaterials, Faculty of Dentistry, University of Oslo) for technical assistance.
Helen Pullisaar and Anders Verket Contributed equally.
Footnotes
Declaration of conflicting interests: The authors declare that there is no conflict of interest.
Funding: This work was supported by the Research Council of Norway, grants 201596 and 228415. The open-access fee was generously provided by the University of Oslo’s Publication fund.
References
- 1. Drosse I, Volkmer E, Capanna R, et al. Tissue engineering for bone defect healing: an update on a multi-component approach. Injury 2008; 39(Suppl. 2): S9–20. [DOI] [PubMed] [Google Scholar]
- 2. Kolk A, Handschel J, Drescher W, et al. Current trends and future perspectives of bone substitute materials—from space holders to innovative biomaterials. J Craniomaxillofac Surg 2012; 40(8): 706–718. [DOI] [PubMed] [Google Scholar]
- 3. Bose S, Roy M, Bandyopadhyay A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol 2012; 30(10): 546–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Salasznyk RM, Williams WA, Boskey A, et al. Adhesion to vitronectin and collagen I promotes osteogenic differentiation of human mesenchymal stem cells. J Biomed Biotechnol 2004; 2004(1): 24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mizuno M, Kuboki Y. Osteoblast-related gene expression of bone marrow cells during the osteoblastic differentiation induced by type I collagen. J Biochem 2001; 129(1): 133–138. [DOI] [PubMed] [Google Scholar]
- 6. Pullisaar H, Tiainen H, Landin MA, et al. Enhanced in vitro osteoblast differentiation on TiO2 scaffold coated with alginate hydrogel containing simvastatin. J Tissue Eng. Epub ahead of print 26 November 2013. DOI: 10.1177/2041731413515670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pullisaar H, Reseland JE, Haugen HJ, et al. Simvastatin coating of TiO2 scaffold induces osteogenic differentiation of human adipose tissue-derived mesenchymal stem cells. Biochem Biophys Res Commun 2014; 447(1): 139–144. [DOI] [PubMed] [Google Scholar]
- 8. Tanimoto K, Huang YC, Tanne Y, et al. Amelogenin enhances the osteogenic differentiation of mesenchymal stem cells derived from bone marrow. Cells Tissues Organs 2012; 196(5): 411–419. [DOI] [PubMed] [Google Scholar]
- 9. Tiainen H, Lyngstadaas SP, Ellingsen JE, et al. Ultra-porous titanium oxide scaffold with high compressive strength. J Mater Sci Mater Med 2010; 21(10): 2783–2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rathe F, Junker R, Chesnutt BM, et al. The effect of enamel matrix derivative (Emdogain) on bone formation: a systematic review. Tissue Eng Part B Rev 2009; 15(3): 215–224. [DOI] [PubMed] [Google Scholar]
- 11. Needleman IG, Worthington HV, Giedrys-Leeper E, et al. Guided tissue regeneration for periodontal infra-bony defects. Cochrane Database Syst Rev 2006; 2: Cd001724. [DOI] [PubMed] [Google Scholar]
- 12. Reseland JE, Reppe S, Larsen AM, et al. The effect of enamel matrix derivative on gene expression in osteoblasts. Eur J Oral Sci 2006; 114(Suppl. 1): 205–211; discussion 254–256, 381–382. [DOI] [PubMed] [Google Scholar]
- 13. Ramis JM, Rubert M, Vondrasek J, et al. Effect of enamel matrix derivative and of proline-rich synthetic peptides on the differentiation of human mesenchymal stem cells toward the osteogenic lineage. Tissue Eng Part A 2012; 18(11–12): 1253–1263. [DOI] [PubMed] [Google Scholar]
- 14. Szoke K, Beckstrom KJ, Brinchmann JE. Human adipose tissue as a source of cells with angiogenic potential. Cell Transplant 2012; 21(1): 235–250. [DOI] [PubMed] [Google Scholar]
- 15. Jakobsen RB, Østrup E, Zhang X, et al. Analysis of the effects of five factors relevant to in vitro chondrogenesis of human mesenchymal stem cells using factorial design and high throughput mRNA-profiling. PLoS ONE 2014; 9(5): e96615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Takahashi Y, Tabata Y. Homogeneous seeding of mesenchymal stem cells into nonwoven fabric for tissue engineering. Tissue Eng 2003; 9(5): 931–938. [DOI] [PubMed] [Google Scholar]
- 17. Melvik JE, Dornish M, Onsoyen E, et al. Self-gelling alginate systems and uses thereof. Patent WO2006044342 A3, 2007. [Google Scholar]
- 18. Li Z, Ohno N, Terada N, et al. Application of periodic acid-Schiff fluorescence emission for immunohistochemistry of living mouse renal glomeruli by an “in vivo cryotechnique.” Arch Histol Cytol 2006; 69(3): 147–161. [DOI] [PubMed] [Google Scholar]
- 19. Mironova EV, Evstratova AA, Antonov SM. A fluorescence vital assay for the recognition and quantification of excitotoxic cell death by necrosis and apoptosis using confocal microscopy on neurons in culture. J Neurosci Methods 2007; 163(1): 1–8. [DOI] [PubMed] [Google Scholar]
- 20. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔC(T)) Method. Methods 2001; 25(4): 402–408. [DOI] [PubMed] [Google Scholar]
- 21. Sabetrasekh R, Tiainen H, Lyngstadaas SP, et al. A novel ultra-porous titanium dioxide ceramic with excellent biocompatibility. J Biomater Appl 2011; 25(6): 559–580. [DOI] [PubMed] [Google Scholar]
- 22. Volkmer E, Drosse I, Otto S, et al. Hypoxia in static and dynamic 3D culture systems for tissue engineering of bone. Tissue Eng Part A 2008; 14(8): 1331–1340. [DOI] [PubMed] [Google Scholar]
- 23. Malladi P, Xu Y, Chiou M, et al. Effect of reduced oxygen tension on chondrogenesis and osteogenesis in adipose-derived mesenchymal cells. Am J Physiol Cell Physiol 2006; 290(4): C1139–C1146. [DOI] [PubMed] [Google Scholar]
- 24. Larsen BE, Sandvik JA, Karlsen J, et al. Oxygen consumption in T-47D cells immobilized in alginate. Cell Prolif 2013; 46(4): 469–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Duggal S, Fronsdal KB, Szoke K, et al. Phenotype and gene expression of human mesenchymal stem cells in alginate scaffolds. Tissue Eng Part A 2009; 15(7): 1763–1773. [DOI] [PubMed] [Google Scholar]
- 26. Choudhery MS, Badowski M, Muise A, et al. Donor age negatively impacts adipose tissue-derived mesenchymal stem cell expansion and differentiation. J Transl Med 2014; 12: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Miron RJ, Bosshardt DD, Gemperli AC, et al. In vitro characterization of a synthetic calcium phosphate bone graft on periodontal ligament cell and osteoblast behavior and its combination with an enamel matrix derivative. Clin Oral Investig 2014; 18(2): 443–451. [DOI] [PubMed] [Google Scholar]
- 28. Rubert M, Monjo M, Lyngstadaas SP, et al. Effect of alginate hydrogel containing polyproline-rich peptides on osteoblast differentiation. Biomed Mater 2012; 7(5): 055003. [DOI] [PubMed] [Google Scholar]
- 29. Poldervaart MT, Wang H, Van der Stok J, et al. Sustained release of BMP-2 in bioprinted alginate for osteogenicity in mice and rats. PLoS ONE 2013; 8(8): e72610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zeng Q, Han Y, Li H, et al. Bioglass/alginate composite hydrogel beads as cell carriers for bone regeneration. J Biomed Mater Res B Appl Biomater 2014; 102(1): 42–51. [DOI] [PubMed] [Google Scholar]