Abstract
The natural history of limited-stage peripheral T-cell lymphomas PTCL remains poorly defined. Therefore, we examined outcomes in patients with the most common PTCL subtypes (PTCL, NOS, AITL, ALCL) and limited-stage disease. In this retrospective, multicenter study, 75 patients with limited-stage disease were identified. The median event-free (EFS) and overall survival (OS) observed were 2.1 and 6.5 years, respectively. In a landmark analysis excluding patients with primary refractory disease, no significant benefit was observed for patients undergoing consolidative radiation therapy. With the exception of patients undergoing salvage hematopoietic stem cell transplantation, survival following disease relapse or progression was poor, thus highlighting the need for improved therapeutic strategies.
Keywords: Peripheral T-cell lymphoma, radiation, prognosis
INTRODUCTION
Peripheral T-cell lymphomas (PTCL) account for approximately 10% of non-Hodgkin lymphomas (NHL) in North America. Many patients with these aggressive NHL present with advanced stage disease and extranodal involvement. Among those with PTCL, not otherwise specified (PTCL, NOS), approximately 70% present with advanced stage (III/IV) disease. Extranodal disease is common, being observed in one or more sites in ≈ 60–80% and ≈25–30% of patients, respectively (1–4). Not surprisingly, the natural history and optimal treatment, particularly the potential role of radiation therapy, for patients with limited stage PTCL is poorly understood.
Following initial anthracycline-based chemotherapy, patients with limited-stage PTCL frequently undergo consolidation with radiation therapy. The rationale for this approach is an extrapolation of studies performed in the more common aggressive B-cell NHL(5–7). Unfortunately, there is a paucity of data addressing the merits of consolidative radiation therapy in PTCL. Retrospective studies addressing this question are limited by a small sample size, the inclusion of various T- and NK-cell NHL subtypes, including extranodal NK/T cell lymphomas, for which the role of radiation therapy is established, and fail to account for the relatively high rates of primary refractory disease that may preclude subsequent consolidation with radiation therapy. Some of these studies appear to support consolidation with radiation therapy(2, 8–10), while others do not(11, 12). Therefore, we sought to examine outcomes at diagnosis and at the time of relapse in limited stage PTCL. In this retrospective multicenter study, we identified patients with the most common nodal PTCL histologies, including PTCL, NOS, angioimmunoblastic T-cell lymphoma (AITL), and anaplastic large cell lymphoma (ALCL), initially presenting with limited-stage (Ann Arbor stage I/II) disease. A landmark analysis including only those patients that achieved a response to induction chemotherapy was performed to examine the role of consolidative radiation therapy in limited-stage PTCL. Survival after relapse or progression among these patients was evaluated.
PATIENTS AND METHODS
Consecutive patients 18 years of age or older with biopsy-confirmed PTCL, NOS, angioimmunoblastic T-cell NHL (AITL), or anaplastic large cell lymphoma (ALCL) who were evaluated at the Mayo Clinic (between 1994 and 2009) or the University of Michigan (between 1988 and 2011) were considered for study inclusion. Patients with primary cutaneous disease were excluded. Patients with limited stage disease (Ann Arbor stage I/II), based on a complete staging evaluation (CT and/or PET, and bone marrow examination) were included in this study. Study approval was granted by the Institutional Review Board of each respective institution, in accordance with U.S. federal regulations and the Declaration of Helsinki. Clinical characteristics, including Ann Arbor stage (stage I vs. II), LDH, performance status, and treatments received, including radiation, were retrospectively collected. Diagnostic biopsy specimens were reviewed (A.L.F., N.G.B., M.S.L.) and classified in accordance with the 2008 World Health Organization classification whenever possible. Of the 75 cases examined, the lack of tissue availability precluded reclassification per the 2008 WHO classification in 17 cases (23%). EFS was calculated from the time of initial diagnosis to the time of progression, relapse, second-line therapy, or death from any cause. OS was calculated from the time of initial diagnosis to the time of death. Patients who did not experience any of these events were censored at the time of last contact. EFS and OS were estimated using the Kaplan-Meier method and two-tailed log-rank test (13). The Cox proportional hazards model was used to evaluate dichotomized variables. All data were analyzed using JMP 8 software (SAS, Cary, NC).
RESULTS
The median age at diagnosis for this cohort of 75 limited-stage PTCL patients was 52 years (range, 19–88 years). Median follow up following diagnosis was 17 months for all patients, and 20 months for surviving patients. As summarized in Table 1, systemic ALCL (sALCL) was the most common subtype (46%), followed by PTCL, NOS (39%), and AITL (15%). Among sALCL cases, 40% were ALK+, 54% were ALK-, and ALK status was unknown in 6%. The clinical characteristics of these patients are summarized in Table 1. High-risk features were infrequently observed. Extranodal disease was observed in 37 patients, and most commonly involved the skin, nasopharynx/sinuses, or gastrointestinal tract. Patients with primary cutaneous disease were excluded from this cohort. Most patients were <60 years of age (68%) and had stage I disease (53%). Consequently, at least 60% of patients were considered low-risk by the International Prognostic Index (IPI) (14). Most patients (68%) were initially treated with anthracycline-based multiagent chemotherapy regimens, most commonly CHOP, and 3% of patients received non-anthracycline-based regimens. A minority of patients received either palliative regimens (9%), underwent surgical resection (1%), or were not treated (15%). Initial treatment was unknown in three patients.
Table 1.
Patient characteristics (n=75).
| Characteristic | No. | (%) |
|---|---|---|
| Age, years | ||
| >60 | 24 | 32 |
| Gender | ||
| Male | 44 | 59 |
| Vital status | ||
| Alive | 50 | 67 |
| Deceased | 25 | 33 |
| Histology | ||
| PTCL, NOS | 29 | 39 |
| AITL | 11 | 15 |
| ALCL | 35 | 46 |
| IPI | ||
| 0–1 | 45 | 60 |
| 2–3 | 13 | 17 |
| 4–5 | 0 | 0 |
| Missing | 17 | 23 |
| Ann Arbor stage | ||
| I | 40 | 53 |
| II | 35 | 47 |
| Missing | 0 | 0 |
| Front-line treatment | ||
| Multiagent | 53 | 71 |
| Anthracycline-based | 51 | 68 |
| Non-Anthracycline | 2 | 3 |
| Other | ||
| HD-Mtx | 1 | 1 |
| Palliative* | 6 | 8 |
| Resection | 1 | 1 |
| None/observation | 11 | 15 |
| Unknown | 3 | 4 |
| Stem-cell transplant | ||
| Autologous | 5 | 7 |
| Allogeneic | 3 | 4 |
Palliative therapy includes steroids, retinoids, and radiation
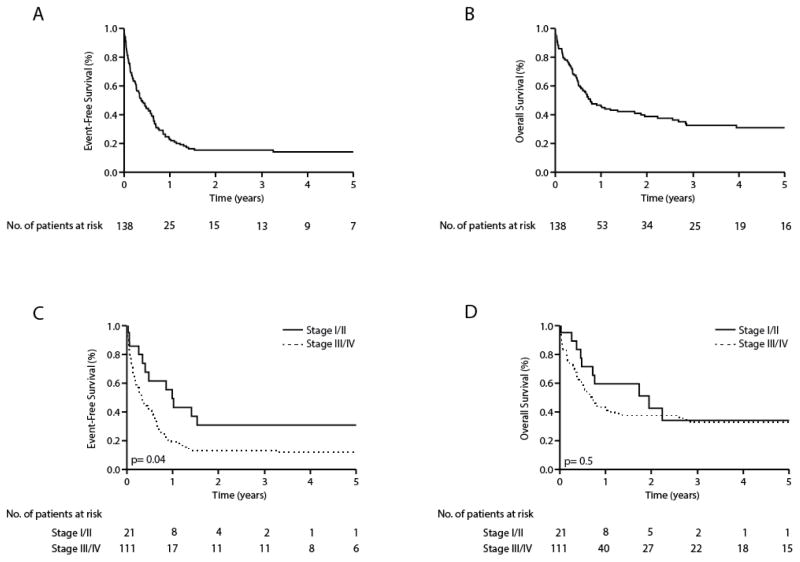
In this cohort, 36 events and 24 deaths were observed. The median EFS and OS were 2.1 (0.9-NR, 95% CI) and 6.5 (3.1-NR, 95% CI) years, respectively, for the entire cohort. The estimated 5-year overall survival was 51% (Figure 1). As summarized in Table 2, the prognostic significance of age, ECOG performance status, LDH, Ann Arbor stage (I vs. II) and histology (ALCL vs. PTCL, NOS/AITL) were examined on univariate analysis. Stage II disease was associated with inferior EFS, but only age, ECOG performance status, and LDH were associated with significantly inferior EFS and OS. On multivariate analysis, only age was independently associated with inferior EFS/OS (Table 2).
Figure 1.
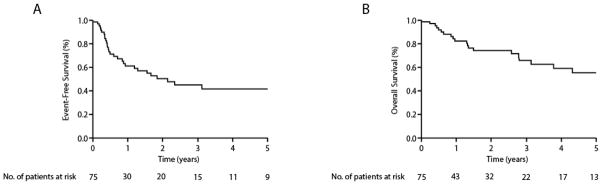
Event-free (a) and overall (b) survival in limited stage PTCL (PTCL-NOS, AITL, and ALCL).
Table 2.
Univariate and multivariate analyses for event-free (EFS) and overall survival (OS).
| Prognostic factors | Univariate | |||
|---|---|---|---|---|
| EFS
|
OS
|
|||
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Age >60 | 2.89 (1.38–5.98) | 0.005 | 6.27 (2.72–15.65) | <0.0001 |
| ECOG PS >1 | 3.99 (1.38–10.22) | 0.01 | 8.70 (2.64–28.79) | 0.0007 |
| LDH >normal | 3.38 (1.28–8.65) | 0.01 | 3.72 (1.43–9.65) | 0.008 |
| Stage II | 2.75 (1.28–6.38) | 0.009 | 1.44 (0.61–3.40) | 0.4 |
| PTCL, NOS/AITL histology | 0.66 (0.32–1.35) | 0.2 | 0.74 (0.33–1.67) | 0.5 |
| Multivariate | ||||
|---|---|---|---|---|
| EFS
|
OS
|
|||
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Age >60 | 3.43 (1.05–11.12) | 0.04 | 6.57 (1.90–26.19) | 0.003 |
| ECOG PS >1 | 1.97 (0.46–8.94) | 0.4 | 2.96 (0.67–15.72) | 0.2 |
| LDH >normal | 1.46 (0.37–5.31) | 0.6 | 1.12 (0.26–4.09) | 0.9 |
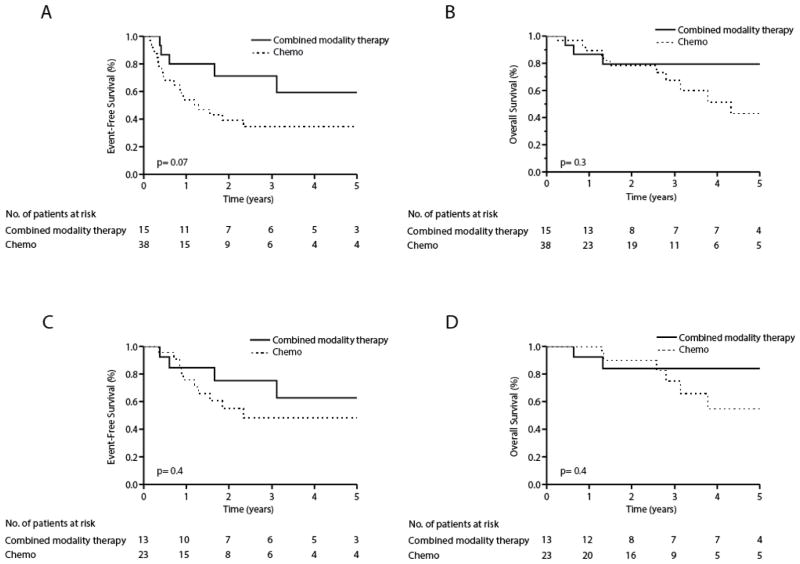
In order to examine the role of radiation therapy, we compared EFS and OS in patients who initially received multiagent chemotherapy alone (n=38) with those who underwent combined modality therapy (CMT) with consolidative radiation therapy (n=15). The clinical characteristics of patients undergoing CMT are summarized in Table 3. Chemotherapy alone was associated with a median EFS and OS of 1.3 and 4.3 years, respectively, and was not significantly different from the median EFS (not reached) and OS (not reached) observed in patients consolidated with radiation therapy (Figure 2a,b). Given the prevalence of primary refractory disease in PTCL, a subset of patients for whom radiation was intended never received radiation due to disease progression. The inclusion of these patients likely overestimates any apparent benefit associated with radiation. 47% of patients receiving chemotherapy alone experienced disease progression while on therapy or failed to achieve a complete or partial remission. Therefore, a landmark analysis excluding these patients was performed. With the exception of age, no significant differences in clinical characteristics between patients with chemosensitive disease who did or did not undergo consolidative radiation therapy were observed (Table 4). When only patients who initially responded to front-line chemotherapy were stratified by consolidation with radiation therapy, any potential benefit associated with consolidative radiation therapy was no longer apparent (Figure 2c,d).
Table 3.
Patient Characteristics.
| Prognostic factors | Chemo (n=38) | CMT (n=15) | P-value | ||
|---|---|---|---|---|---|
| No. | (%) | No. | (%) | ||
| Age, in years | |||||
| Age ≤60 | 24 | 63 | 13 | 87 | 0.08 |
| Age >60 | 14 | 37 | 2 | 13 | |
| LDH status | |||||
| Normal LDH | 22 | 58 | 8 | 53 | 0.3 |
| LDH > normal | 5 | 13 | 4 | 27 | |
| Unknown | 11 | 29 | 3 | 20 | |
| ECOG Performance status | |||||
| ≤1 | 22 | 58 | 12 | 81 | 0.9 |
| >1 | 4 | 11 | 1 | 6 | |
| Unknown | 12 | 31 | 2 | 13 | |
| Ann Arbor Stage | |||||
| I | 17 | 55 | 7 | 47 | 0.5 |
| II | 21 | 45 | 8 | 53 | |
| IPI | |||||
| 0–1 risk factors | 21 | 55 | 12 | 81 | |
| 2–3 risk factors | 4 | 11 | 2 | 13 | 0.9 |
| 4–5 risk factors | 0 | 0 | 0 | 0 | |
| Unknown | 13 | 34 | 1 | 6 | |
| Histology | |||||
| PTCL, NOS | 12 | 32 | 7 | 47 | 0.7 |
| AITL | 4 | 11 | 2 | 13 | |
| ALCL | 22 | 58 | 6 | 40 | |
| Stem-cell transplant | |||||
| Autologous | 4 | 11 | 1 | 6 | 0.6 |
| Allogeneic | 2 | 5 | 1 | 6 | 0.8 |
| Front-line treatment | |||||
| Multiagent | 38 | 100 | 15 | 100 | 1.0 |
| Anthracycline-based | 37 | 97 | 14 | 94 | |
| Non-anthracycline | 1 | 3 | 1 | 6 | 0.5 |
| Cycles of chemotherapy | |||||
| < 4 cycles | 12 | 31 | 2 | 13 | 0.05 |
| ≥ 4 cycles | 6 | 16 | 6 | 40 | |
| Unknown | 20 | 53 | 7 | 47 | |
Figure 2.
Event-free (a, c) and overall (b, d) survival of limited stage PTCL (PTCL-NOS, AITL, ALCL) patients who received front-line combination chemotherapy with (—) or without (·····) radiotherapy. A landmark analysis, including those who achieved a complete or partial remission following front-line combination chemotherapy, is shown (c, d).
Table 4.
Patient Characteristics for Landmark Analysis.
| Prognostic factors | Chemo (n=23)
|
CMT (n=13)
|
P-value | ||
|---|---|---|---|---|---|
| No. | (%) | No. | (%) | ||
| Age, in years | |||||
| Age ≤60 | 15 | 65 | 12 | 92 | 0.05 |
| Age >60 | 8 | 35 | 1 | 8 | |
| LDH status | |||||
| Normal LDH | 14 | 61 | 7 | 54 | 0.3 |
| LDH > normal | 2 | 9 | 3 | 23 | |
| Unknown | 7 | 30 | 3 | 23 | |
| ECOG Performance status | |||||
| ≤1 | 13 | 57 | 10 | 77 | 0.5 |
| >1 | 1 | 4 | 1 | 8 | |
| Unknown | 9 | 39 | 2 | 15 | |
| Ann Arbor Stage | |||||
| I | 13 | 57 | 6 | 46 | 0.9 |
| II | 10 | 43 | 7 | 54 | |
| IPI | |||||
| 0–1 risk factors | 11 | 48 | 11 | 84 | |
| 2–3 risk factors | 1 | 4 | 1 | 8 | 1.0 |
| 4–5 risk factors | 0 | 0 | 0 | 0 | |
| Unknown | 11 | 48 | 1 | 8 | |
| Histology | |||||
| PTCL, NOS | 8 | 35 | 7 | 54 | 0.7 |
| AITL | 3 | 13 | 1 | 8 | |
| ALCL | 12 | 52 | 5 | 38 | |
| Stem-cell transplant | |||||
| Autologous | 4 | 17 | 1 | 8 | 0.4 |
| Allogeneic | 2 | 9 | 1 | 8 | 0.9 |
| Front-line treatment | |||||
| Multiagent | 23 | 100 | 13 | 100 | 1.0 |
| Anthracycline-based | 22 | 96 | 12 | 92 | |
| Non-anthracycline | 1 | 4 | 1 | 8 | 0.7 |
| Cycles of chemotherapy | |||||
| < 4 cycles | 3 | 13 | 2 | 15 | 0.6 |
| ≥ 4 cycles | 4 | 17 | 5 | 38 | |
| Unknown | 16 | 70 | 6 | 46 | |
Among this cohort of limited-stage PTCL patients, 21 patients relapsed or had refractory disease following initial multiagent chemotherapy. The median age at relapse was 56 years (range 29–86 years), and ALCL was the most common histologic subtype (53%) observed, followed by PTCL, NOS (33%) and AITL (14%). Survival after first relapse or progression among these patients was compared with that observed among 111 patients with advanced-stage disease who similarly received initial multiagent chemotherapy. Among patients with advanced-stage disease, the median age at relapse was 59 years (range 19–91 years), and PTCL, NOS was the most common histologic subtype (42%) observed, followed by AITL (29%) and ALCL (29%). The median time to relapse or progression was 10 months (range 2–37 months) for limited-stage patients and 7 months (range 0.2–114 months) for advanced-stage patients. The EFS/OS for both limited- and advanced-stage patients following first relapse or progression is shown in Figure 3(a, b). Among limited-stage patients, 81% received systemic therapies at relapse/progression, including single-agents (29%) or combination chemotherapy (52%). Six patients (29%) ultimately underwent allogeneic (n=3) or autologous (n=3) hematopoietic stem cell transplantation. A similar proportion of patients with advanced-stage disease received single-agent (18%) or multiagent (55%) therapies. Of these, 37 (33%) patients underwent allogeneic (n=12) or autologous (n=25) hematopoietic stem cell transplantation (HSCT). The median EFS and OS following first relapse/progression for limited-stage patients was 12 and 23 months, respectively (Figure 3c, d). The median EFS and OS following relapse/progression was 3 and 6 months, respectively, among patients with initially advanced-stage disease. With the exception of HSCT for patients with chemosensitive disease, treatment of relapsed/refractory PTCL is largely palliative. Among patients with relapsed/refractory PTCL who did not undergo salvage HSCT a median EFS and OS of 3 and 6 months, respectively, were observed.
Figure 3.
Event-free (a) and overall (b) survival of limited and advanced staged PTCL (PTCL-NOS, AITL, ALCL) patients following first relapse or progression. Event-free (c) and overall (d) survival following first relapse or progression stratified by initial stage.
DISCUSSION
The PTCLs are relatively rare and are commonly associated with advanced-stage disease. Therefore, a prospective, randomized-controlled trial addressing the role of radiation therapy among patients with limited-stage disease is not feasible. For this reason, we sought to examine the potential role of radiation therapy and the natural history of limited-stage PTCL in this retrospective, multicenter study. Previous studies have reported improved survival for patients undergoing consolidative radiation therapy (2, 8–10). Unfortunately, most of these studies fail to adjust for selection bias by including patients with primary refractory disease. Therefore, patients undergoing consolidation with radiation therapy are those with chemosensitive disease. In order to address this limitation, we performed a landmark analysis including only patients achieving a partial or complete remission with initial chemotherapy, and were unable to detect any meaningful improvement in EFS/OS for patients undergoing consolidation with radiation therapy.
This study has several important limitations, including a small sample size, particularly for patients receiving CMT. Patients receiving their initial treatment or subsequent evaluations at other institutions were not excluded from this study, and may contribute to the relatively short median follow up observed in this study conducted at two large academic centers. Staging evaluations in this cohort were largely performed with computerized tomography (CT) imaging. In contrast to 18F-fluorodeoxyglucose positron emission tomography (PET) imaging, the use of CT imaging for disease staging likely underestimates the extent of disease involvement in approximately 50% of patients (15). Therefore, it is likely that many of the patients included in this cohort may have had more extensive disease. This may be supported by the observation that all of the relapses observed in patients who had received radiation occurred at distant sites outside of the radiation field. As in diffuse large B-cell lymphoma (16), PET imaging following initial chemotherapy may identify patients with a suboptimal response who may preferentially benefit from consolidative radiation, as complete remissions (CR) may be achieved following radiation therapy among limited-stage PTCL patients failing to achieve a CR with initial chemotherapy (8). The role of radiation therapy in advanced-stage and bulky PTCL was not addressed in this study. While improved EFS/OS was not observed for patients undergoing consolidative radiation therapy in this study, the possibility remains that PET imaging may identify selected patients with limited-stage PTCL for whom consolidative radiation therapy may be reasonable and beneficial. Future studies will be needed to address this question.
With the exception of hematopoietic stem-cell transplantation (HSCT), treatment following disease relapse or progression is largely palliative. A recent report from the British Columbia Cancer Agency described outcomes in 153 PTCL patients who did not undergo HSCT following first relapse or progression (17). Mak et. al. observed a median PFS and OS (5.5 and 3.1 months, respectively) that is nearly identical to the median EFS and OS observed in this study (3 and 6 months, respectively). Survival, particularly overall survival, following first relapse/progression was not markedly influenced by initial disease stage. The incorporation of HSCT was associated with improved survival following first relapse/progression.
Collectively, the findings of this retrospective study further confirm the poor outcomes associated with relapsed/refractory PTCL and further highlight the need for improved therapies in both the front-line and salvage settings.
Acknowledgments
This work was supported in part by grants from the Leukemia & Lymphoma Society, the Leukemia Research Foundation, and the NCI (K08CA172215).
Footnotes
CONFLICT-OF-INTEREST AND DISCLOSURE
The authors declare no competing financial interests.
References
- 1.Vose J, Armitage J, Weisenburger D. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26:4124–4130. doi: 10.1200/JCO.2008.16.4558. [DOI] [PubMed] [Google Scholar]
- 2.Weisenburger DD, Savage KJ, Harris NL, Gascoyne RD, Jaffe ES, MacLennan KA, Rudiger T, Pileri S, Nakamura S, Nathwani B, Campo E, Berger F, Coiffier B, Kim WS, Holte H, Federico M, Au WY, Tobinai K, Armitage JO, Vose JM. Peripheral T-cell lymphoma, not otherwise specified: a report of 340 cases from the International Peripheral T-cell Lymphoma Project. Blood. 2011;117:3402–3408. doi: 10.1182/blood-2010-09-310342. [DOI] [PubMed] [Google Scholar]
- 3.Savage KJ, Chhanabhai M, Gascoyne RD, Connors JM. Characterization of peripheral T-cell lymphomas in a single North American institution by the WHO classification. Ann Oncol. 2004;15:1467–1475. doi: 10.1093/annonc/mdh392. [DOI] [PubMed] [Google Scholar]
- 4.Gallamini A, Stelitano C, Calvi R, Bellei M, Mattei D, Vitolo U, Morabito F, Martelli M, Brusamolino E, Iannitto E, Zaja F, Cortelazzo S, Rigacci L, Devizzi L, Todeschini G, Santini G, Brugiatelli M, Federico M Intergruppo Italiano L. Peripheral T-cell lymphoma unspecified (PTCL-U): a new prognostic model from a retrospective multicentric clinical study. Blood. 2004;103:2474–2479. doi: 10.1182/blood-2003-09-3080. [DOI] [PubMed] [Google Scholar]
- 5.Miller TP, Dahlberg S, Cassady JR, Adelstein DJ, Spier CM, Grogan TM, LeBlanc M, Carlin S, Chase E, Fisher RI. Chemotherapy alone compared with chemotherapy plus radiotherapy for localized intermediate- and high-grade non-Hodgkin’s lymphoma. N Engl J Med. 1998;339:21–26. doi: 10.1056/NEJM199807023390104. [DOI] [PubMed] [Google Scholar]
- 6.Horning SJ, Weller E, Kim K, Earle JD, O’Connell MJ, Habermann TM, Glick JH. Chemotherapy with or without radiotherapy in limited-stage diffuse aggressive non- Hodgkin’s lymphoma: Eastern Cooperative Oncology Group study 1484. J Clin Oncol. 2004;22:3032–3038. doi: 10.1200/JCO.2004.06.088. [DOI] [PubMed] [Google Scholar]
- 7.Leitch HA, Gascoyne RD, Chhanabhai M, Voss NJ, Klasa R, Connors JM. Limited-stage mantle-cell lymphoma. Ann Oncol. 2003;14:1555–1561. doi: 10.1093/annonc/mdg414. [DOI] [PubMed] [Google Scholar]
- 8.Zhang XM, Li YX, Wang WH, Jin J, Wang SL, Liu YP, Song YW, Ren H, Fang H, Zhou LQ, Chen B, Qi SN, Liu QF, Lu NN, Liu XF, Yu ZH. Favorable outcome with doxorubicin-based chemotherapy and radiotherapy for adult patients with early stage primary systemic anaplastic large-cell lymphoma. Eur J Haematol. 2013;90:195–201. doi: 10.1111/ejh.12060. [DOI] [PubMed] [Google Scholar]
- 9.Zhang XM, Li YX, Wang WH, Jin J, Wang SL, Liu YP, Song YW, Fang H, Ren H, Zhou LQ, Liu XF, Yu ZH. Survival advantage with the addition of radiation therapy to chemotherapy in early stage peripheral T-cell lymphoma, not otherwise specified. Int J Radiat Oncol Biol Phys. 2013;85:1051–1056. doi: 10.1016/j.ijrobp.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 10.Fernandes AT, Svoboda J, Nasta SD, Wilson LD, Plastaras JP. Radiation Therapy in Peripheral T-cell Lymphoma of the Head and Neck. Blood (ASH Annual Meeting Abstracts) 2013;122:1795. [Google Scholar]
- 11.Sakata K, Fuwa N, Kodaira T, Aratani K, Ikeda H, Takagi M, Nishio M, Satoh M, Nakamura S, Satoh H, Hareyama M. Analyses of dose-response in radiotherapy for patients with mature T/NK-cell lymphomas according to the WHO classification. Radiother Oncol. 2006;79:179–184. doi: 10.1016/j.radonc.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 12.Lee HK, Wilder RB, Jones D, Ha CS, Pro B, Rodriguez MA, Romaguera JE, Cabanillas F, Rodriguez J, Cox JD. Outcomes using doxorubicin-based chemotherapy with or without radiotherapy for early-stage peripheral T-cell lymphomas. Leukemia & Lymphoma. 2002;43:1769–1775. doi: 10.1080/1042819021000006277. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 14.A predictive model for aggressive non-Hodgkin’s lymphoma. The International Non-Hodgkin’s Lymphoma Prognostic Factors Project. N Engl J Med. 1993;329:987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 15.Casulo C, Schoder H, Feeney J, Lim R, Maragulia J, Zelenetz AD, Horwitz S. 18F-fluorodeoxyglucose positron emission tomography in the staging and prognosis of T cell lymphoma. Leuk Lymphoma. 2013;54:2163–2167. doi: 10.3109/10428194.2013.767901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Held G, Murawski N, Ziepert M, Fleckenstein J, Poschel V, Zwick C, Bittenbring J, Hanel M, Wilhelm S, Schubert J, Schmitz N, Loffler M, Rube C, Pfreundschuh M. Role of radiotherapy to bulky disease in elderly patients with aggressive B-cell lymphoma. J Clin Oncol. 2014;32:1112–1118. doi: 10.1200/JCO.2013.51.4505. [DOI] [PubMed] [Google Scholar]
- 17.Mak V, Hamm J, Chhanabhai M, Shenkier T, Klasa R, Sehn LH, Villa D, Gascoyne RD, Connors JM, Savage KJ. Survival of patients with peripheral T-cell lymphoma after first relapse or progression: spectrum of disease and rare long-term survivors. J Clin Oncol. 2013;31:1970–1976. doi: 10.1200/JCO.2012.44.7524. [DOI] [PubMed] [Google Scholar]