Abstract
The steroid hydroxylation and carbon-carbon bond cleavage activities of cytochrome P450 17A1 (CYP17A1) are responsible for the production of glucocorticoids and androgens, respectively. The inhibition of androgen synthesis is an important strategy to treat androgen-dependent prostate cancer. We discuss the different enzymatic activities towards the various substrates of CYP17A1, demonstrating its promiscuity. Additionally, a novel interhelical interaction is proposed between the F-G loop and the B′-helix to explain the 16α-hydroxylase activity of human CYP17A1 with progesterone as the substrate. The techniques used by biochemists to study this important enzyme are also summarized.
1. Cytochrome P450 enzymes
Microsomal human cytochrome P450 17A1 (CYP17A1, 17α-hydroxylase, 17,20-lyase) belongs to the cytochrome P450 super family that contains a conserved cysteine residue, which provides the axial sulfur ligand attached to a heme prosthetic group. The P450 class of enzymes conducts a myriad of chemical transformations including oxidation, reduction, and non-redox reactions [1]. The prototypical reaction catalyzed by this family of enzymes is the oxygenation of C-H bonds to afford alcohol products. This process involves the two-electron reduction of a molecule of oxygen where one of the oxygen atoms goes to water and the other is incorporated into the alkane substrate [2]. The oxoiron active intermediate (compound I) responsible for C-H activation has been characterized by Mossbauer and UV-Vis spectroscopy [3]. The compound I intermediate was isolated through stopped flow by mixing CYP119 in one syringe and m-chloroperoxybenzoic acid in the other syringe (2:1 ratio) followed by freeze quenching with liquid ethane. Most P450 enzymes share a common 3-dimensional structure. Deisenhoefer and co-workers compared the crystal structures of the soluble bacterial enzymes P450cam (CYP101), P450terp and P450BM-3 and observed structural similarities in the three different proteins: 13 α-helices (A, B, B′, C-L), and 5 β-sheets (β1-β5) [4]. The x-ray crystal structures of several eukaryotic, membrane-bound P450 enzymes reveal the same basic fold with some variable areas. There are 57 human cytochrome P450 genes (that metabolize small molecules including fatty acids, xenobiotics, and steroids. Six of these (CYP11A, CYP11B1, CYP11B2, CYP17A1, CYP19A1, CYP21A2) are found in the steroid hormone biosynthesis pathway [5]. Crystal structures of CYP17A1 with azole inhibitors bound have been recently elucidated, and these structures can be used to increase our understanding of this enzyme [6]. This report will focus on the hydroxylation activity of CYP17A1, although the carbon-carbon (C-C) bond cleavage activity is also important and a topic of intense study [7]. The use of steroid analogs to show the diverse reactivity of this enzyme can enhance our understanding of the function of this important enzyme in human physiology and disease. The goal of this review is to educate the reader of the different approaches to study the enzymology of CYP17A1. The function of this enzyme may be elucidated with the information gained from biochemical studies.
2. CYP17A1 gene, physiology, and disease
The human CYP17A1 gene is localized to chromosome 10q24.3 [8]. This gene is expressed in the adrenals and gonads, with minor amounts in the brain, placenta, and heart [9]. The same mRNA and protein is expressed in the adrenal and gonad. The 17-hydroxylase activity of CYP17A1 is required for the production of the glucocorticoid cortisol, whereas the 17,20-lyase activity leads to androgens (and in turn estrogens). Most clinical mutations in CYP17A1 that have been reported result in the loss of both 17-hydroxylase and 17,20-lyase activities. The loss of all or most CYP17A1 activities leads to 17-hydroxylase/17,20-lyase deficiency (17OHD), a form of congenital adrenal hyperplasia. The phenotype of 17OHD consists primarily of hypertension and hypokalemia—from the accumulation of the mineralocorticoids 11-deoxycorticosterone (DOC) and corticosterone upstream of cortisol, plus sexual infantilism—from a lack of androgen and estrogen synthesis.
An interesting variant of 17OHD has been reported in a few patients, which results in the loss of solely the 17,20-lyase activity [10]. This loss of the C-C bond cleavage activity results in the loss of the production of 19-carbon androgens, which leads to undervirilization of 46,XY infants and pubertal failure in 46,XX children.
A more common condition involving CYP17A1 is not enzyme deficiency but rather androgen-dependent cancers and hyperplasias. Prostate cancer is typically androgen-dependent, and medical or surgical castration induces remission for most patients early in the course of the disease. Later, the disease progresses despite low testosterone (T) synthesis, a condition called castration-resistant prostate cancer (CRPC). CYP17A1 inhibitors ketoconazole, abiraterone acetate, and newer agents have been developed to treat these diseases [11, 12]. The androgen dependent-cancer patient usually takes the CYP17A1 inhibitor along with a glucocorticoid, prednisone, to lower adrenocorticotropin (ACTH) and prevent the accumulation of cortisol precursors that cause hypertension and hypokalemia [13].
3. CYP17A1 enzymology
3.1. Steroid substrates for CYP17A1
The first of two major physiologic steroid substrates of CYP17A1 contains the pregnenolone backbone (1) in the A/B-rings of the steroid, which consists of a 3β-hydroxy-Δ5,6 moiety. The other major steroid substrates contain the progesterone backbone (6), which is composed of a 3-keto-Δ4,5 enone group in the A/B-rings. In vivo, the 3-keto-Δ4,5 steroid backbone arises from the 3β-hydroxy-Δ5,6 moiety by the activity of 3β-hydroxysteroid dehydrogenase/isomerase enzymes (3βHSD, two isoenzymes in humans). In the crystal structure of abiraterone complexed with CYP17A1, the polar side chain of asparagine-202 hydrogen bonds to the 3β-hydroxyl group of abiraterone, which mimics the 3β-hydroxyl group in pregnenolone [6].
Although these two backbones are common to most steroidogenic pathways and lead to most active steroid hormones, the active site of CYP17A1 accommodates additional similar configurations of the steroid A/B-rings. The 3-keto, 5α-reduced steroid backbone (5α-dihydroprogesterone, 12), which arises from the action of 5α-reductases (2 major isoenzymes in humans) on the 3-keto-Δ4,5 steroids, is a viable substrate class for CYP17A1 [14]. This reduced steroid indicates that a double bond in the A/B ring system is not a structural requirement for substrates. More importantly, 5α-pregnan-3α-ol-20-one (allopregnanolone, 15), which is derived from 5α-dihydroprogesterone from the action of the reductive 3αHSD enzymes (aldo-keto reductase types 1C, AKR1C), is also a substrate for CYP17A1 [14]. The product of the 17,20-lyase reaction in this series is androsterone (17), which can be converted in two steps to dihydrotestosterone (DHT), the most potent endogenous androgen, without the intermediacy of T. This alternate or “backdoor” pathway to DHT significantly contributes to androgen production in certain pathologic states and explains some discrepancies between serum T concentrations and clinical parameters of androgen action. The positioning of the axial 3α-hydroxyl of allopregnanolone (15) within a trans-decalin system is vastly different than the equatorial 3β-hydroxyl of pregnenolone (1) in a twist-boat ring, yet both are excellent substrates for CYP17A1. This striking structural diversity demonstrates that the enzyme shows considerable tolerance for the orientation of the 3-hydroxy (or 3-keto) group, despite the hydrogen bonding seen in the crystal structures with bound 3β-hydroxy inhibitors.
3.2. Steroid products of CYP17A1
Pregnenolone (1) is the first 21-carbon steroid precursor found in the steroid biosynthesis pathway and is derived from cholesterol by the action of CYP11A1. CYP17A1 converts pregnenolone (1) exclusively to 17-hydroxypregnenolone (17Preg, 2) and also cleaves the C17–C20 carbon-carbon bond of 17Preg to dehydroepiandrosterone (DHEA, 3) (Figure 1). The presence of cytochrome b5 (b5) enhances the rate of the 17,20-lyase reaction to form DHEA from 17Preg by an order of magnitude. With pregnenolone substrate, the presence of b5 diverts 1–10% of the product to androsta-5,16-dien-3β-ol (5) (Fig. 1), depending on species and incubation conditions. With allopregnanolone substrate (15), CYP17A1 catalyzes the same reactions: 17-hydroxylation, 17,20-lyase, and andiene formation. While b5 stimulates the same two activities, androsterone (17) formation is very efficient and stimulated only 3-fold in the presence of b5. CYP17A1 primarily 17-hydroxylates progesterone (6) to 17-hydroxyprogesterone (17OHP, 7), but 20% of the product formed is 16α-hydroxyprogesterone (16OHP, 8), and a trace of DOC, which is 21-hydroxyprogesterone (9). The biological significance of 16OHP (8) has been studied [15], but its physiological role remains unclear. Although not discussed in the original report of 5α-dihydroprogesterone as a CYP17A1 substrate [14], the 16α-hydroxylated steroid appears to be a minor product, similar to the metabolism of progesterone. For both 3-ketosteroid substrate series, the 17,20-lyase reaction is very slow relative to the rate with 3-hydroxysteroids. Consequently, CYP17A1 shows considerable promiscuity: several substrates, each with different products; stimulation and product diversion via b5; and both hydroxylation and carbon-carbon bond cleavage chemistries. Some of these phenomena are very important in human physiology, such as b5 stimulation of androgen synthesis in specific steroidogenic cell types. Other processes might be important in disease states, such as the ordinarily minor 21-hydroxylation reaction in genetic 21-hydroxylase deficiency and a source of adrenal-derived mineralocorticoid.
Figure 1.
The substrates and products of CYP17A1.
3.3. Experimental systems and approaches
To understand the current state of knowledge, we provide a brief overview of the experimental systems and paradigms that enzymologists use to study CYP17A1 activities in vitro, in order to understanding the physiological roles of the enzyme and its variants. For example, how do we know that clinical mutations R358Q or R347H, which cause isolated 17,20-lyase deficiency, disrupt positive charges on the enzyme surface and impair interactions with POR and b5 [16]. The reactivity of CYP17A1 can be studied in vitro by expressing the native enzyme (or mutated variants) in mammalian cells or in yeast together with its redox partners human (or yeast) P450-oxidoreductase (POR) and/or b5 and isolating microsomes with the enzyme system [17]. Alternatively, modified and C-terminal polyhistidine tagged CYP17A1 enzyme is expressed in E. coli, purified using a Ni-NTA column [18], and studied in reconstituted assays with phospholipid and purified POR [18], with or without b5. The steroid substrate, which may be labeled with a stable isotope or radioisotope, is added in a small volume of water-miscible solvent. As the source of electrons, these incubations use NADPH or an NADPH generating system containing glucose-6-phosphate, glucose-6-phosphate dehydrogenase, and NADP+ [19]. For more detailed descriptions of experimental protocols, refer to the references cited within this section.
After extraction with an organic solvent, the products are resolved using high performance liquid chromatography (HPLC, typically reverse phase) and quantitated with UV-detection at 254 nm and/or scintillation counting when radiolabeled steroid is used [20]. Alternatively, liquid chromatography-mass spectrometry (LC-MS) may be employed, and this technique can assess for changes in isotopic labeling. For example, if the labeled carbon site is a methylene (-CH2-) and the labeling is stereospecific (-CHαDβ- or -CHβDα-), UV detection cannot distinguish between hydroxylated products with and without deuterium incorporated at that same carbon site. Thin-layer chromatography (TLC) analysis is another option: radiolabeled steroids should be used ([14C]- or [3H]- labeled) to detect steroids by phosphorimaging [16] or by scraping the sections containing the steroids from the TLC plates for quantifyication by liquid scintillation counting.
Each method has advantages and disadvantages. Although mass spectrometry is useful in measuring both the quantities and isotopic composition of products, some compounds ionize poorly and thus require derivatization, and the standardization is necessary to measure multiple compounds simultaneously with different ionization efficiencies. UV-detection is generally less sensitive than mass spectrometry and more vulnerable to interfering substances, and the compound must have a chromophore to be UV-active. For CYP17A1, the 3-keto-Δ4,5-series of steroids are amenable to UV-detection at 254 nm (ε~16,000 M−1cm−1) [21]. Steroids containing the 3β-hydroxy-Δ5,6-functional groups, in contrast, must be converted to the enone to enable UV-detection, and cholesterol oxidase is often used for this purpose. It is possible to detect the pregnenolone metabolites with UV-absorption in the far-UV such as 216 nm [22], but the absorbance is very weak. Radioactive substrates with [14C]- or [3H]-labels sites remote from the reacting carbon(s) offer high sensitivity and minimal interference but require exposure precautions and scintillation counting [20]. These radiolabeled compounds are expensive, and not all are commercially available. Liquid chromatography methods are time consuming [20], while the TLC method [16] allows one to simultaneously run multiple samples on each plate.
4. Approaches to study enzyme promiscuity
4.1. Synthesis of isotopically labeled substrates
To add to the arsenal of the enzymologist, kinetic isotope effects have been used to determine the bond-breaking step(s) [20]. Deuterium-labeled substrates have been used for decades to study mechanistic and energetic aspects of enzymatic reactions involving C-H bond-breaking chemistry, such as cytochrome P450-catalyzed hydroxylations [23]. These experiments require access to the substrate bearing deuterium label at the bonds where chemistry occurs. Methods to regioselectively and stereoselectively introduce deuterium only at the desired positions are preferable to base-catalyzed exchange (i.e. KOD in CH3OD). For example, in order to study the 17-hydroxylation of progesterone, these conditions would not only exchange the C17-proton but all C21-protons, as well as the C2-, C4-, and C6-protons. We have found that convenient access to the deuterated compounds is achieved with oxidative addition of zinc onto an alkyl bromide precursor, followed by quenching with a deuteron source in situ (Fig. 2) [20]. In most instances, we treat the brominated steroid precursor with zinc dust in deuterated acetic acid, the latter being generated inexpensively with D2O and acetic anhydride in diethyl ether or other aprotic solvent. The desired deuterium labeling is then confirmed with 1H NMR (Fig. 3) and MS. As shown in Fig. 3B, the deuterium incorporation might not reach 100% (presence of minor amounts of H-17 at a chemical shift of ~2.6 ppm) for various reasons, primarily: (i) the H-17 of progesterone was exchanged during the purification or handling process, or (ii) in the deuteration source (Fig. 2) is not 100% deuterated, from traces of atmospheric moisture in the solvent, reagents, and glassware. The isotopic abundance is measured by integrating the peaks of the protons in the NMR spectra and/or by measuring the exact masses of the desired deuterium-enriched compounds by MS.
Figure 2.

Strategy for deuterated compound synthesis, subjecting brominated precursors to zinc dust and deuterated acetic acid (AcOD).
Figure 3.
1H NMR spectra of deuterated progesterone analogs, which illustrate the labeled sites. The H-17 triplet (Panel C) collapses to a doublet with H-16α deuteration (Panel A, 19) and disappears with H-17 deuteration (Panel B, 20). The H-21 methyl singlet disappears with trideuteration at C21 (Panel C, 21). There is a trace amount of non-deuterated progesterone (H-17 proton at ~2.6 ppm) as shown in the NMR spectrum in Panel B, which can be integrated in order to determine the isotope abundance. CDCl3 was used as the solvent and the spectra were referenced to the CHCl3 singlet at 7.26 ppm.
This zinc-mediated chemistry does not require the bromine to be in the α-position relative to the C20-carbonyl (i.e. 17- or 21-position). Thus, 16α-bromopregnenolone acetate also undergoes regioselective and stereospecific deuteration at H-16α under these conditions, even though the bromine substituent is in the non-exchangeable β-position relative to the C20-carbonyl [20]. The hydroxylation activities of CYP17A1 were confirmed by incubation of regioselectively deuterated (either 16α-, 17-, or 21,21,21- positions) steroid substrates with purified enzyme from E. Coli in reconstituted assays and with microsomes from yeast expressing CYP17A1 and POR [20].
4.2. Metabolic switching
Metabolic switching occurs when the activated P450 enzyme (Fe=O) forms a complex with its substrate at the preferred reaction site, CA (H-CA), and the complex (i.e. Fe=O---H-CA) “switches” to an alternative C-H site, CB (i.e. Fe=O---H-CB). The most common approach to experimentally induce metabolic switching is substitution of deuterium or tritium for hydrogen on CA. Metabolic switching may be observed for any enzyme that hydroxylates more than one carbon position on a substrate to give rise to two or more possible products. Selective deuteration at the major reaction site decreases the rate of hydroxylation at that site (see section 4.3 below). While the overall substrate conversion to product remains unaffected, the proportion of the minor product increases [24]. Metabolic switching is usually not absolute in that the reaction at the primary site still occurs, but the proportion of products is variably changed. Deuteration will not induce metabolic switching if the energy barriers to reach alternative reaction sites are too high, rendering other sites of reaction inaccessible.
For CYP17A1, the metabolic switching phenomenon was nicely illustrated for [2H]-labeled progesterone substrates. Using 17-[2H]-progesterone as substrate (Fig. 3B, 20), the product distribution of 17OHP:16OHP shifted from 4:1 to 1:1, without significantly affecting the conversion from starting material to hydroxylated products. Conversely, 16α-[2H]-progesterone (Fig. 3A, 19) afforded a 9:1 ratio of 17OHP:16OHP products. In contrast, for 17-[2H]-pregnenolone substrate, the only product remained 17Preg [20]. Thus, CYP17A1 exhibits metabolic switching for some substrates but not others. Additionally, deuteration at the C17-position of progesterone increased CYP17A1-catalyzed hydroxylation at a previously unidentified position, C21, which yields deoxycorticosterone as the product.
4.3. Kinetic isotope effects (KIE)
4.3.1. KIE theory
The substitution of deuterium or tritium for hydrogen in the position of hydroxylation on the steroid ring might not only induce metabolic switching but also change the reaction kinetics, resulting in a KIE. Figure 4A illustrates the origin of the KIE and its use in studying CYP17A1 enzymology. The zero-point energy of the C-H bond is dependent on the reduced mass, based on Hooke’s law and the harmonic oscillator model. The substitution of deuterium for hydrogen in a C-H bond lowers the zero point energy by about 1.15 kcal/mol. In a symmetric transition state, the distances between the hydrogen atom and FeO (acceptor) and substrate (donor) are the same. Since this vibrational energy is excluded in a symmetrical transition state, the activation energy (Ea or ΔG‡) is greater for a C-D bond, and thus the reaction is slowed by a factor equal to the KIE. Metabolic switching is observed in the HPLC-UV analysis of the incubation extracts (Fig. 4B).
Figure 4.
(A) Hypothetical reaction coordinates for CYP17A1-catalyzed steroid 17- and 16α-hydroxylation reactions. The components in red illustrate the lowering of the zero point energy of the reacting bond when the deuterium label replaces the hydrogen at H-17. (B) HPLC UV-chromatograms, absorbance at 254 nm of products from CYP17A1 incubation with progesterone and the deuterated analogs, indicating metabolic switching (Fig. 3). Incubation conditions are described in reference [20].
4.3.2. Intermolecular KIE
The change in steady-state kinetics with labeled substrate (KIE) reflects the contribution of C-H bond breaking to the overall reaction rate. The magnitude of a primary KIE (KIE on the bond to be broken) varies from a maximum of ~7 if the transition state is symmetrical and C-H(D) bond-breaking step is fully rate limiting to the overall reaction rate to near 1 if the transition state is very asymmetrical and/or the C-H(D) bond-breaking step is much faster than one or more other microscopic steps, either chemical or physical [25]. A secondary KIE occurs when deuterium substitution changes the rate of the reaction, but the C-D bond is not broken. Most commonly, secondary KIEs result from changes in carbon hybridization, such as sp2 to sp3. However, the magnitude of the secondary KIE is not nearly as high as the primary KIE, in the range of 0.7–1.3 [26].
These experiments comparing the overall rate of the specific reaction involving C-H or C-D bond breaking are used to determine what is termed an intermolecular KIE. The experiment can be conducted by incubating the two different substrates (labeled and unlabeled) with the enzyme simultaneously in the same reaction tube, and the resulting products are measured using one or more techniques, which discriminate the contribution from two isotopically distinct substrates—such as UV detection, radiochemical detection, or mass spectrometry. For CYP17A1, we employed progesterone with a [3H]- or [14C]-label remote from the reaction site for the “unlabeled” (C-H) substrate plus the second progesterone substrate, which contained a deuterium at the reaction site and was used in >1,000-fold excess. The radiolabeled steroids were measured with scintillation counting, while the products from the deuterium-labeled progesterone were detected by UV-absorption at 254 nm [20]. An alternative approach is to label both substrates differently at remote site(s) with stable isotopes and to measure the products, using mass spectrometry to distinguish the products derived from each substrate.
Another approach for measuring KIE values is to separately determine the kinetic constants kcat, Vmax, and KM at steady state conditions, and this measurement is termed non-competitive kinetic isotope effect. These conditions require: (i) multiple incubations with varying substrate concentrations (several points ranging from 100 nM to 500 μM), (ii) uniform incubation times with adequate turnover at all concentrations to accurately measure products (i.e. ideally, each point should not surpass 20% conversion of starting material to product due to competing steps that may underestimate the rate such as product release), and (iii) use of a curve-fitting software such as GraphPad Prism to fit the v ((moles of product formed)*(moles of enzyme) −1*(time) −1) vs. [S] data to the Michaelis-Menten or other appropriate equation, to obtain these constants. Each approach has advantages and disadvantages related to cost, ease of synthesis, accuracy of determination, and confounding factors. Ideally, the data are obtained from more than one method to ensure internal consistency.
4.3.3. Intramolecular KIE
If C-H(D) bond-breaking is not significantly rate limiting, deuterium labeled substrates might still provide information on reaction energetics if more than one chemically equivalent hydrogen atom is subject to abstraction or if metabolic switching is involved, via the intramolecular KIE [27–29]. In the former case with more than one equivalent C-H bond (i.e. a -CH3 group, with 3 hydrogen atoms that are chemically equivalent due to free rotation), the site can be isotopically labeled with a single deuterium or tritium atom and subjected to hydroxylation chemistry. The intramolecular KIE is calculated from the isotopic composition of the products using mass spectrometry. For a methylene (-CH2-) group, similar calculations of intramolecular KIE apply, with the caveat that for an enzymatic reaction, the two hydrogen atoms might not be chemically equivalent if they are also diastereotopic. In the latter case in which metabolic switching occurs, the KIE is directly proportional to the change in product distribution with the deuterated substrate. For example, the 17OHP/16OHP product ratio for CYP17A1-catalyzed progesterone hydroxylation falls from 4:1 with natural abundance substrate to 1:1 with 17-[2H]-progesterone substrate (Fig. 3B, 20), yielding an intramolecular KIE of ~4 (Fig. 4B(ii), Fig. 5).
Figure 5.
Intramolecular KIE values can be determined by comparing the relative product distributions for the (A) natural abundance and the (B) deuterated substrates. For simplification purposes, the 16β- and 21- hydrogen abstraction steps are ignored. This figure illustrates the major difference in the rate of hydroxylation between (A) and (B) arises from the C-H abstraction steps (k8H, k8D, k12H, k12D), and these differences give rise to metabolic switching.
The C-H abstraction step is only one of several steps in the P450 reaction cycle and the C-H hydroxylation process, which includes substrate binding, electron transfers, oxygen activation, C-H abstraction, radical recombination, product release, redox partner binding, and any other enzyme conformational changes (Fig. 5). A comparison of the intermolecular and intramolecular KIE values reveals any “masking” of the intermolecular KIE, meaning a reduction in magnitude. The intramolecular KIE measurements are always higher than the intermolecular KIE values, since the intramolecular KIE values directly reflect the energetics of the C-H vs. C-D abstraction step without any masking. The intermolecular KIE value is masked or lowered when the transition state is asymmetrical or when C-H(D) bond breaking is not significantly rate-limiting. On the other hand, larger KIE values (~80) have been observed in the case where hydrogen atom tunneling is involved [30]. Hydrogen atom tunneling, or proton-coupled electron transfer, is invoked to explain anomalously large intramolecular KIE values, because in these cases the rate of the reaction is exquisitely sensitive to bond length.
5. Lesser activities of CYP17A1
5.1. Progesterone 16α-Hydroxylation and alanine-105
Baboon CYP17A1 has only 10% 16α-hydroxylase activity with progesterone substrate, compared to ~20% for human CYP17A1. This observation prompted the Swart laboratory to probe which residue(s) might be responsible for this difference in 16α-hydroxylase activity between the two species. In the original report, these investigators mutated a region in the F-G loop of human CYP17A1 to the sequence found in baboon CYP17A1 (AVIE to KIVH), but this change did not affect the 16α-hydroxylase activity. Instead, mutation of A105 to leucine, as found in the baboon enzyme, did not change 17-hydroxylase activity but raised the 17OHP:16OHP product ratio from 4:1 to 9:1, similar to baboon CYP17A1. Thus, residue 105 is the most important residue yet identified for maintaining the progesterone 16α-hydroxylase activity of human CYP17A1 [31]. The contributions of these differences in 16α-hydroxylase chemistry to adrenal physiologies among primates remains to be explored.
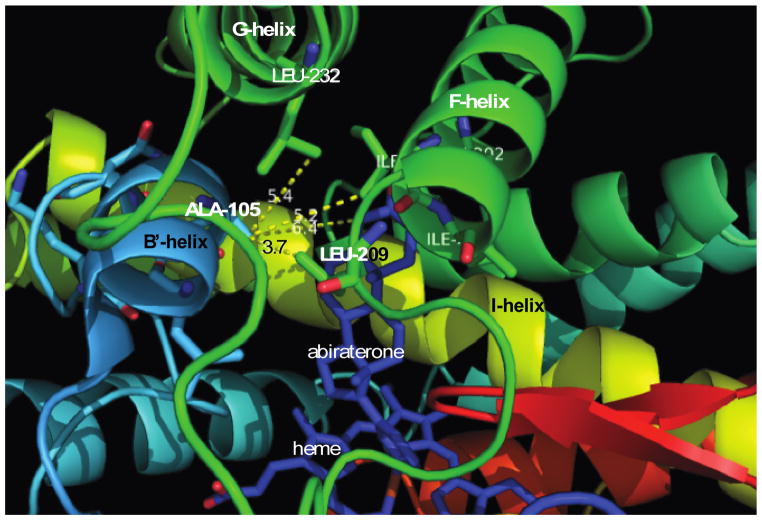
X-ray crystallography is another method employed by enzymologists to gain a structural understanding to rationalize enzymatic activity. Two crystal structures of CYP17A1 bound to either abiraterone or galeterone (TOK-001) were reported by Devore et al. [6], which provided insight to some key amino acid residues in the active site of CYP17A1 and the origins of its promiscuity for steroid hydroxylation. Alanine 105 is located on B′ helix of human CYP17A1. This amino acid residue does not seem to directly interact with substrate, because the structure of CYP17A1 with bound abiraterone shows that the alanine-105 side chain is 6.4 Å away from the C-4 position of abiraterone. Leucine-209 on the F-helix, however, is 3.7 Å away from the methyl group of alanine-105, suggesting a direct hydrophobic interaction (Fig. 6). The omnipresent F-G loop in P450 enzymes is viewed as the “lid” that opens and closes upon substrate entry and binding. These van der Waals forces between alanine-105 and leucine-209 might maintain the close interaction between the F-G loop and the steroid, which in turn pushes the steroid “down” so that C-16 of progesterone is exposed to the heme moiety. When leucine is substituted for alanine-105, the increased steric clash between leucine-105 and leucine-209 brings the F-G loop to a more “open” position, which in turn raises the steroid and C-16, while C-17 and C-21 swing closer to the heme center (Fig. 7). If this analysis is correct, double mutations of residues 105 and 209 might be used to validate the importance of this probable interaction. Other possible amino acid residues on the F-G loop that might directly interact with the side chain of leucine-105 are isoleucine-205 on the F-helix and leucine-232 on the G-helix, which are 5.2 and 5.4 Å away from alanine-105, respectively.
Figure 6.
Exploded view of CYP17A1 crystal structure with bound abiraterone. MacPymol software was used to visualize the structure. Alanine-105 residue interacts with leucine-205 of the F-helix. Heme and abiraterone = dark blue, B′-helix = light blue, I-helix = yellow, F-G loop = green. PDB: 3RUK.
Figure 7.

CYP17A1 mutation A105L. The introduction of leucine at residue 105 positions the substrate progesterone so that the 17- and 21- carbon positions are more exposed to the active oxoiron species (compound I) compared to the 16-position.
5.2. Progesterone 21-Hydroxylation
The use of selectively deuterated steroids not only allowed for calculation of KIEs for CYP17A1-catalyzed hydroxylations at these labeled positions but also confirmed a minor 21-hydroxylation activity with progesterone. When [17-2H]- or [16α-2H]-progesterone substrates (Fig. 3, 20 or 19) were incubated with CYP17A1, the 21-hydroxylation product (DOC) increased relative to 17OHP and 16OHP (Fig. 6). Conversely, when the [21,21,21-2H3]-progesterone (Fig. 3C, 21) was used as the substrate, the 21- hydroxylation product decreased relative to 17OHP and 16OHP. In other words, increasing the activation energy for one of the major products via deuterium labeling at that C-H bond favored formation of the minor product DOC, and vice versa. This 21-hydroxylase activity of CYP17A1 was further verified with mutation A105L [20], which has enhanced 21- hydroxylase activity and decreased 16α-hydroxylase activity (Fig. 8).
Figure 8.
CYP17A1 hydroxylation at C-16, C-17, and C-21, illustrating the abstraction of 4 non-equivalent hydrogen atoms among these reactions. The equilibrium arrows following the oxygen activation step indicate the possibility of metabolic switching if one of the reactive C-H sites (16α-, 17-, 21-) is deuterated.
5.3. Inversion of configuration at C-16
Experiments with CYP17A1 and [16α-2H]-progesterone (Fig. 3A, 19) showed that the 16β-hydrogen atom is also accessible for abstraction (Fig. 8, pathway 3d). If the 16α-hydrogen of this substrate is always abstracted, the 16OHP product of these incubations should lose all deuterium enrichment. In contrast, mass spectrometry demonstrated retention of the deuterium in 33–40% of the 16OHP product (Fig. 8, 8B) [20]. This type of switching at the same methylene site does not always occur for P450 enzymes. In the case of CYP3A4-catalyzed testosterone 6β-hydroxylation, incubations with [6β-2H]-testosterone substrate afforded only unenriched 6β-hydroxytestosterone, and no [6α-2H]-6β-hydroxytestosterone was detected [32].
Thus, human CYP17A1 hydroxylates the 16α-, 17-, and 21-positions of progesterone and also abstracts the 16β-hydrogen atom. In contrast, we could only demonstrate 17-hydroxylation of pregnenolone when [17-2H]-pregnenolone was used as the substrate. This strict regioselectivity might be due to the hydrogen-bonding located between the 3β-hydroxy group of the steroid ring and asparagine-202, as observed in the CYP17A1 crystal structure [6].
6. CYP17A1-catalyzed carbon-carbon bond cleavage reactions
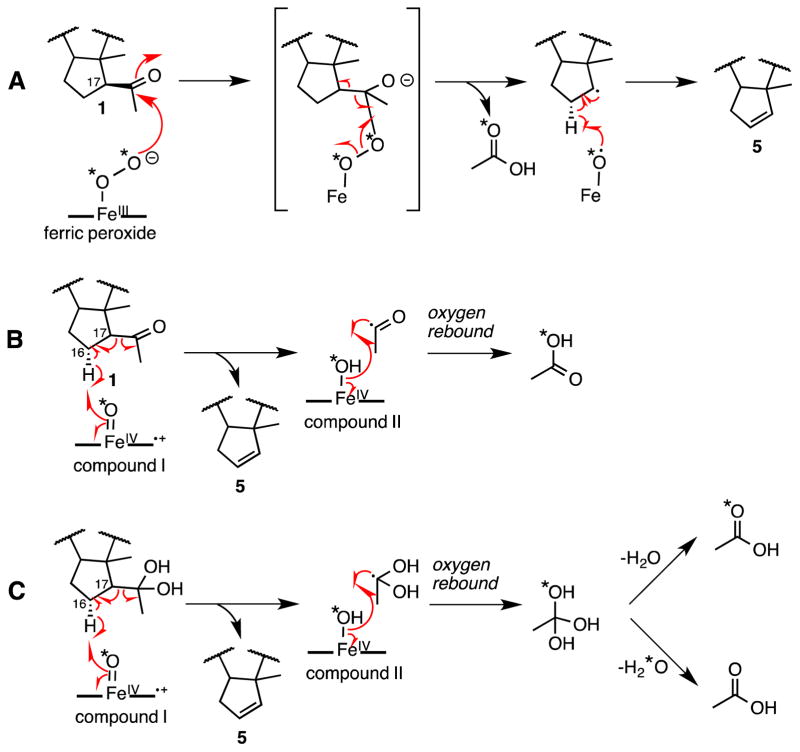
CYP17A1 inhibitors are used to treat androgen dependent cancers, because the 17,20-lyase activity is required for all androgen biosynthesis. Although all 17-hydroxysteroid intermediates are substrates for the 17,20-lyase reaction, the preferred substrates are 17Preg and 17-hydroxyallopregnanolone (5α-pregnan-3α,17-diol-20-one) over 17OHP and its 5α-reduced homolog. The 17,20-lyase reaction, as well as other P450-catalyzed C-C bond cleavage reactions, has been studied intensively, yet the mechanism of this reaction remains controversial. Akhtar and colleagues proposed a mechanism equivalent to an enzymatic Baeyer-Villiger reaction using a ferric peroxide intermediate as a nucleophile onto the C20-carbonyl of 17Preg (Figure 9A) [33, 34]. Evidence in favor of this mechanism derived from studies with the incubation of [16α,17,21,21,21-2H5]-pregnenolone with pig microsomes, where an 18O atom from molecular oxygen (18O2) was incorporated into the acetic acid product, which was derivatized as benzyl acetate and detected by gas-chromatography mass spectrometry [33]. The fact that an 18O-atom was incorporated into the acyl cleavage product, however, does not rule out a mechanism that includes compound I (Fig. 9B). In these studies, the desaturase products were also observed (Fig. 10A).
Figure 9.
Different plausible mechanisms for C17-C20 bond cleavage for CYP17A1 of 17-hydroxypregnenolone (2) to form DHEA (3). (A): Ferric peroxide mechanism. (B–D): Compound I mechanism. “*O” = “18O”
Figure 10.
Mechanism for andiene (5) synthesis from C21-steroid precursor (1) as proposed by Akhtar et al. from deuterated substrate [33] (A) and compound I proposals (B) and (C). “*O” = “18O”
These data were, however, derived from low-resolution mass spectrometry, with resolution (ΔM/M) of 1,000 full-width at half maximum (FWHM) to analyze the cleaved products. These experiments were reproduced with the use of purified human enzyme [35] and a 17-carboxaldehyde analog of pregnenolone; however, low-resolution mass spectrometry was again used to analyze the incubation products [34, 35]. Particularly for the experiments using a mixture of deuterated 17-carboxaldehydes, low-resolution mass spectrometry cannot reliably distinguish endogenous formic acid from the non-18O-incorporated formic acid product of the enzyme reaction: the [13C]-benzyl formate isotopomer (m/z 137.0552, background source) from the [2H]-benzyl formate (m/z 137.0582, incubation source) due to the small mass difference (17 ppm) [35]. Moreover, if there is any other compound with a similar mass that co-elutes with the target mass range (i.e. within 50 ppm), then low-resolution mass spectrometry would not be able to distinguish the impurity from the target mass. Consequently, product percentages derived from these studies should be interpreted with caution. High-resolution mass spectrometry, which is available today, can provide a resolving power of >100,000 FWHM [36, 37] and reliably distinguish these compounds. Alternatively, fragmentation data of the isolated benzyl formate masses, using tandem mass spectrometry, can verify the identities of the observed molecular ions. On the other hand, retention of deuterium and incorporation of 18O in the benzyl acetate derived from the natural substrate pregnenolone was nicely shown in spectra from the published work [33].
In contrast, hydrogen peroxide does not support enzyme-mediated conversion of 17Preg to DHEA, which would exclude a ferric-peroxide mediated mechanism if the ferric peroxide is formed under the experimental conditions [38]. Radical-based mechanisms remain plausible as well. Currently, no definitive evidence is available to refute either proposal.
When the enzyme was first purified from porcine testis, the 17,20-lyase activity was lost disproportionate to the 17-hydroxylase activity [39]. Addition of b5 to the reconstituted assay restored most of the 17,20-lyase activity [34, 40]. Recent descriptions of undervirilized males with low testosterone production from b5 deficiency have confirmed the critical importance of b5 for enhancing the 17,20-lyase activity of CYP17A1 [41, 42]. The action of b5 appears to be an allosteric function rather than electron transfer [43, 44] and involves CYP17A1 residues R347 and R358 [16] as well as b5 residues E48 and E49 [45, 46].
In the presence of b5, porcine CYP17A1 catalyzes two other C-C bond cleavage reactions with pregnenolone (not 17Preg) substrate. The first is andien-β-synthase activity, yielding androsta-5,16-dien-3β-ol (Fig. 10, 5 from 1) [35], which is a precursor to an active pheromone in boar taint. The second trace activity is formation of androsta-5-en-3β,17α-diol (Fig. 11, 4), which was first observed from crude microsomal enzyme sources [33, 47–50], and later confirmed with purified recombinant human CYP17A1 from both pregnenolone and its 17-carboxaldehyde analog in the presence of b5 [35].
Figure 11.
Mechanistic proposals for the formation of androsta-5-en-3β,17α-diol (4) from pregnenolone (1) as shown by Akhtar et al. [33, 35] (A and B). (C) is an alternative compound I mechanism from the gem-diol intermediate of pregnenolone. Radical intermediate 1-r explains the 17α-stereochemistry of compound 4. “*O” = “18O”
Moreover, in the Swinney study, 17-O-acetyltestosterone (Fig. 1, 11) product was observed upon incubation of purified CYP17A1 from neonatal pig testes with progesterone [51]. A Baeyer-Villiger mechanism was proposed for the formation of this product. The assignment of 17-O-acetyltestosterone as product (17β-hydroxy, 11) was based on its comigration with authentic standard by HPLC and GC-MS. Curiously, this reaction yields the opposite stereochemistry (17α-hydroxy) when the pregnenolone substrate is used. Furthermore, the acetyl group appears to be retained in the C-C bond cleavage product with progesterone substrate [51] but is lost as acetic acid [35] for pregnenolone substrate. The reasons for the differences in stereochemistry at C17 in the 17-hydroxy androgen products and the fates of the acetate moieties derived from C20–C21 in these reactions are not clear. One possible explanation is the existence of a 17-radical intermediate in the case of the pregnenolone substrate (Figure 11, 1-r), which undergoes inversion of configuration and oxygen rebound on the α-face of the steroid, yielding the 17α-hydroxyandrogen (4). While the 17α-hydroxyandrogens are not androgen receptor agonists, these alternative 19-carbon steroid compounds might serve as biomarkers of health and disease.
The microsomal b5 (type A form) stimulates both andien-β-synthase and 17,20-lyase activities [52], while a second form of b5, the type B form located on the outer mitochondrial membrane, was shown to only stimulate the 17,20-lyase activity to form DHEA. The presence of cytochrome b5 reductase promoted the andien-β-synthase activity of both porcine and bovine CYP17A1 [52, 53]. Human CYP17A1 catalyzes the same andien-β-synthase activity with b5 using either pregnenolone (1) or allopregnanolone (15) as substrate [14], but the functions of these products in human physiology, if any, remain obscure.
7. Conclusions and future directions
Evidence derived from experiments using site-directed mutagenesis, substrate analogs, structural biology, and isotopic labeling have begun to yield the biophysical principles that yield the catalytic promiscuity of this versatile and physiologically critical enzyme. Human CYP17A1 is known to catalyze at least 13 different reactions with endogenous substrate, and even some minor activities are physiologically important. The physiologic functions of human CYP17A1 in the adrenal and gonads demands a high degree of promiscuity, to both synthesize cortisol via the Δ4-pathway and to synthesize sex steroids via the Δ5-pathway. The activities in the backdoor pathway and other minor reactions add to the intrigue, leading to androgen and mineralocorticoid synthesis in disease states, pheromone production, and salvage pathways.
The study of additional mutations to alter the enzyme activity of CYP17A1 holds the potential for additional discovery. For example, if the alanine-105 interaction with leucine-209 is crucial for regiochemistry, then substitution of other bulky amino acids at position 105 or smaller residues at 209 might increase progesterone 21-hydroxylase activity further. If small molecules could be found to allosterically force the enzyme to favor these conformations leading to DOC formation, then this strategy could coopt a minor activity to treat the mineralocorticoid deficiency of 21-hydroxylase deficiency. Additionally, since P450 enzymes are found in all kingdoms of life and are studied in other research areas (i.e. biotechnology, drug metabolism and pharmacokinetics, reaction development, etc.), knowledge in the reactivity of CYP17A1 can benefit other fields of research. In conclusion, the KIE and related studies with CYP17A1 have revealed that this enzyme is not meant to have strict fidelity. The promiscuity of biological catalysts as illustrated with CYP17A1 depicts the complexity of the big picture and suggests that there may be hidden features that still need to be discovered, even for systems that might seem to be fully characterized.
Highlights.
CYP17A1 catalyzes at least 13 different reactions with endogenous steroids
Cytochrome b5 stimulates 17,20-lyase activity and enables andiene-β-synthase activity
Metabolic switching occurs with deuterium labeled-progesterone substrates
Minor products can have physiologic functions
Acknowledgments
We thank all of our coworkers and collaborators who contributed to the studies described herein. This work was supported by grant R01GM086596 to RJA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Guengerich FP. Common and uncommon cytochrome P450 reactions related to metabolism and chemical toxicology. Chem Res Toxicol. 2001;14:611–650. doi: 10.1021/tx0002583. [DOI] [PubMed] [Google Scholar]
- 2.Krest CM, Onderko EL, Yosca TH, Calixto JC, Karp RF, Livada J, Rittle J, Green MT. Reactive intermediates in cytochrome P450 catalysis. J Biol Chem. 2013;288:17074– 17081. doi: 10.1074/jbc.R113.473108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rittle J, Green MT. Cytochrome P450 compound I: capture, characterization, and C-H bond activation kinetics. Science. 2010;330:933–937. doi: 10.1126/science.1193478. [DOI] [PubMed] [Google Scholar]
- 4.Hasseman CA, Kurumbail RG, Boddupalli SS, Peterson JA, Deisenhofer J. Structure and function of cytochrome P450: a comparative analysis of three crystal structures. Structure. 1995;3:41–62. doi: 10.1016/s0969-2126(01)00134-4. [DOI] [PubMed] [Google Scholar]
- 5.Hanukoglu I. Steroidogenic enzymes: structure, function, and role in regulation of steroid hormone biosynthesis. J Steroid Biochem Mol Biol. 1992;43:779–804. doi: 10.1016/0960-0760(92)90307-5. [DOI] [PubMed] [Google Scholar]
- 6.Devore NM, Scott EE. Structures of cytochrome P450 17A1 with prostate cancer drugs abiraterone and TOK-001. Nature. 2012;482:116–119. doi: 10.1038/nature10743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akhtar M, Wright JN, Lee-Robichaud P. A review of mechanistic studies on aromatase (CYP19) and 17α-hydroxylase-17,20-lyase (CYP17) J Steroid Biochem Mol Biol. 2011;125:2–12. doi: 10.1016/j.jsbmb.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Fan YS, Sasi R, Lee C, Winter JS, Waterman MR, Lin CC. Localization of the human CYP17 gene (cytochrome P450(17α)) to 10q24.3 by fluorescence in situ hybridization and simultaneous chromosome banding. Genomics. 1992;14:1110–1111. doi: 10.1016/s0888-7543(05)80140-5. [DOI] [PubMed] [Google Scholar]
- 9.Missaghian E, Kempna P, Dick B, Hirsch A, Alikhani-Koupaei R, Jegou B, Mullis PE, Frey BM, Fluck CE. Role of DNA methylation in the tissue-specific expression of the CYP17A1 gene for steroidogenesis in rodents. J Endocrinol. 2009;202:99–109. doi: 10.1677/JOE-08-0353. [DOI] [PubMed] [Google Scholar]
- 10.Auchus RJ, Gupta MK. Towards a unifying mechanism for CYP17 mutations that cause isolated 17,20-lyase deficiency. Endocrin Res. 2002;28:443–447. doi: 10.1081/erc-120016821. [DOI] [PubMed] [Google Scholar]
- 11.Azad AA, Eigl BJ, Leibowitz-Amit R, Lester R, Kollmannsberger C, Murray N, Clayton R, Heng DY, Joshua AM, Chi KN. Outcomes with abiraterone acetate in metastatic castration-resistant prostate cancer patients who have poor performance status. Eur Urol. 2014 doi: 10.1016/j.eururo.2014.01.030. In press. [DOI] [PubMed] [Google Scholar]
- 12.Dreicer R, Maclean D, Suri A, Stadler WM, Shevrin D, Hart L, Macvicar GR, Hamid O, Hainsworth J, Gross ME, Shi Y, Webb IJ, Agus DB. Phase I/II trial of orteronel (TAK-700)-an investigational 17,20-lyase inhibitor-in patients with metastatic castration-resistant prostate cancer. Clin Cancer Res. 2014;20:1335–1344. doi: 10.1158/1078-0432.CCR-13-2436. [DOI] [PubMed] [Google Scholar]
- 13.Hoy SM. Abiraterone acetate: a review of its use in patients with metastatic castration-resistant prostate cancer. Drugs. 2013;73:2077–2091. doi: 10.1007/s40265-013-0150-z. [DOI] [PubMed] [Google Scholar]
- 14.Gupta MK, Guryev OL, Auchus RJ. 5α-reduced C21 steroids are substrates for human cytochrome P450c17. Arch Biochem Biophys. 2003;418:151–160. doi: 10.1016/j.abb.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Storbeck KH, Swart P, Africander D, Conradie R, Luow R, Swart AC. 16α-hydroxyprogesterone: origin, biosynthesis and receptor interaction. Mol Cell Endocrinol. 2011;336:92–101. doi: 10.1016/j.mce.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 16.Geller DH, Auchus RJ, Miller WL. P450c17 mutations R347H and R358Q selectively disrupt 17,20-lyase activity by disrupting interactions with P450 oxidoreductase and cytochrome b5. Mol Endocrinol. 1999;13:167–175. doi: 10.1210/mend.13.1.0219. [DOI] [PubMed] [Google Scholar]
- 17.Sherbet DP, Tiosano D, Kwist KM, Hochberg Z, Auchus RJ. CYP17 mutation E305G causes isolated 17,20-lyase deficiency by selectively altering substrate binding. J Biol Chem. 2003;49:48563–48569. doi: 10.1074/jbc.M307586200. [DOI] [PubMed] [Google Scholar]
- 18.Wang YH, Tee MK, Miller WL. P450c17: single step purification and phosphorylation of serine 258 by protein kinase A. Endocrinology. 2010;151:1677–1684. doi: 10.1210/en.2009-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parikh A, Gillam EMJ, Guengerich FP. Drug metabolism by Escherichia coli expressing human cytochromes P450. Nature Biotechnology. 1997;15:784–788. doi: 10.1038/nbt0897-784. [DOI] [PubMed] [Google Scholar]
- 20.Yoshimoto FK, Zhou Y, Peng HM, Stidd D, Yoshimoto JA, Sharma KK, Matthew S, Auchus RJ. Minor activities and transition state properties of the human steroid hydroxylases cytochromes P450c17 and P450c21, from reactions observed with deuterium-labeled substrates. Biochemistry. 2012;51:7064–7077. doi: 10.1021/bi300895w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frei RW. High-performance liquid chromatography in clinical and biological chemistry. Proc Anlayt Div Chem Soc. 1979;16:289–307. [Google Scholar]
- 22.Soucy P, Lacoste L, Luu-The V. Assessment of porcine and human 16-ene-synthase, a third activity of P450c17, in the formation of an androstenol precursor. Role of recombinant cytochrome b5 and P450 reductase. Eur J Biochem. 2003;270:1349–1355. doi: 10.1046/j.1432-1033.2003.03508.x. [DOI] [PubMed] [Google Scholar]
- 23.Guengerich FP. Kinetic deuterium isotope effects in cytochrome P450 oxidation reactions. J Labelled Comp Radiopharm. 2013;56:428–431. doi: 10.1002/jlcr.3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miwa GT, Lu AY. Kinetic isotope effects and ‘metabolic switching’ in cytochrome P450-catalyzed reactions. Bioessays. 1987;7:215–219. doi: 10.1002/bies.950070506. [DOI] [PubMed] [Google Scholar]
- 25.Westheimer F. The magnitude of the primary kinetic isotope effect for compounds of hydrogen and deuterium. Chem Rev. 1965;61:265–273. [Google Scholar]
- 26.Sheng X, Zhang H, Hollenberg PF, Newcomb M. Kinetic isotope effects in hydroxylation reactions effected by cytochrome P450 compounds I implicate multiple electrophilic oxidants for P450-catalyzed oxidations. Biochemistry. 2009;48:1620–1627. doi: 10.1021/bi802279d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones JP, Korzekwa KR, Rettie AE, Trager WF. Isotopically sensitive branching and its effect on the observed intramolecular isotope effects in cytochrome P450-catalyzed reactions – a new method for the estimation of intrinsic isotope effects. J Am Chem Soc. 1986;108:7074–7078. [Google Scholar]
- 28.Korzekwa KR, Trager WF, Gillette JR. Theory for the observed isotope effects from enzymatic systems that form multiple products via branched reaction pathways – cytochrome P450. Biochemistry. 1989;28:9012–9018. doi: 10.1021/bi00449a009. [DOI] [PubMed] [Google Scholar]
- 29.Gillette JR, Darbyshire JF, Sugiyama K. Theory for the observed isotope effects on the formation of multiple products by different kinetic mechanisms of cytochrome P450 enzymes. Biochemistry. 1994;33:2927–2937. doi: 10.1021/bi00176a024. [DOI] [PubMed] [Google Scholar]
- 30.Meyer MP, Klinman JP. Investigating inner-sphere reorganization via secondary kinetic isotope effects in the C-H cleavage reaction catalyzed by soybean lipoxygenase: tunneling in the substrate backbone as well as the transferred hydrogen. J Am Chem Soc. 2011;133:430–439. doi: 10.1021/ja1050742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swart AC, Storbeck KH, Swart P. A single amino acid residue, Ala 105, confers 16α-hydroxylase activity to human cytochrome P450 17α-hydroxylase/17,20-lyase. J Steroid Biochem Mol Biol. 2010;119:112–120. doi: 10.1016/j.jsbmb.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 32.Krauser JA, Guengerich FP. Cytochrome P450 3A4-catalyzed testosterone 6β-hydroxylation stereochemistry, kinetic deueterium isotope effects, and rate-limiting steps. J Biol Chem. 2005;280:19496–19506. doi: 10.1074/jbc.M501854200. [DOI] [PubMed] [Google Scholar]
- 33.Akhtar M, Corina D, Miller S, Shyadehi AZ, Wright JN. Mechanism of the acyl-carbon cleavage and related reactions catalyzed by multifunctional P450s: studies on cytochrome P45017α. Biochemistry. 1994;33:4410–4418. doi: 10.1021/bi00180a039. [DOI] [PubMed] [Google Scholar]
- 34.Lee-Robichaud P, Wright JN, Akhtar ME, Akhtar M. Modulation of the activity of human 17α-hydroxylase-17,20-lyase (CYP17) by cytochrome b5: endocrinological and mechanistic implications. Biochem J. 1995;308:901–908. doi: 10.1042/bj3080901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee-Robichaud P, Shyadehi AZ, Wright JN, Akhtar ME, Akhtar M. Mechanistic kinship between hydroxylation and desaturation reactions: acyl-carbon bond cleavage promoted by pig and human CYP17 (P45017α; 17α-hydroxylase-17,20-lyase) Biochemistry. 1995;34:14104–14113. doi: 10.1021/bi00043a015. [DOI] [PubMed] [Google Scholar]
- 36.Kalli A, Smith GT, Sweredoski MJ, Hess S. Evaluation and optimization of mass spectrometric settings during data-dependent acquisition mode: focus on LTQ-orbitrap mass analyzers. J Proteome Res. 2013;12:3071–3086. doi: 10.1021/pr3011588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scigelova M, Hornshaw M, Giannakopulos A, Makarov A. Fourier Transform Mass Spectrometry. Mol Cell Proteomics. 2011;10:M111.009431. doi: 10.1074/mcp.M111.009431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Auchus RJ, Miller WL. Molecular modeling of human P450c17 (17α-hydroxylase/17,20-yase): insights into reaction mechanisms and effects of mutations. Mol Endocrinol. 1999;7:1169–1182. doi: 10.1210/mend.13.7.0326. [DOI] [PubMed] [Google Scholar]
- 39.Nakajin S, Shinoda M, Haniu M, Shively JE, Hall PF. C21 steroid side chain cleavage enzyme from porcine adrenal microsomes. Purification and characterization of the 17α-hydroxylase/C17,20-lyase cytochrome P450. J Biol Chem. 1984;259:3971–3976. [PubMed] [Google Scholar]
- 40.Onoda M, Hall PF. Cytochrome b5 stimulates purified testicular microsomal cytochrome P450 (C21 side-chain cleavage) Biochem Biophys Res Commun. 1982;108:454–460. doi: 10.1016/0006-291x(82)90850-6. [DOI] [PubMed] [Google Scholar]
- 41.Idkowiak J, Randell T, Dhir V, Patel P, Shackleton CH, Taylor NF, Krone N, Arlt W. A missense mutation in the human cytochrome b5 gene causes 46 X,Y disorder of sex development due to true isolated 17,20-lyase deficiency. J Clin Endocrinol Metab. 2012;97:E465–E475. doi: 10.1210/jc.2011-2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kok RC, Timmerman MA, Wolffenbuttel KP, Drop SL, Jong FHd. Isolated 17,20-lyase deficiency due to the cytochrome b5 mutation W27X. J Clin Endocrinol Metab. 2010;95:994–999. doi: 10.1210/jc.2008-1745. [DOI] [PubMed] [Google Scholar]
- 43.Auchus RJ, Lee TC, Miller WL. Cytochrome b5 augments the 17,20-lyase activity of human P450c17 without direct electron transfer. J Biol Chem. 1998;273:3158–3165. doi: 10.1074/jbc.273.6.3158. [DOI] [PubMed] [Google Scholar]
- 44.Lee-Robichaud P, Kaderbhai MA, Kaderbhai N, Wright JN, Akhtar M. Interaction of human CYP17 (P-45017α, 17α-hydroxylase-17,20-lyase) with cytochrome b5: importance of the orientation of the hydrophobic domain of cytochrome b5. Biochem J. 1997;321:857–863. doi: 10.1042/bj3210857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Estrada DF, Laurence JS, Scott EE. Substrate-modulated cytochrome P450 17A1 and cytochrome b5 interactions revealed by NMR. J Biol Chem. 2013;288:17008–17018. doi: 10.1074/jbc.M113.468926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Naffin-Olivos JL, Auchus RJ. Human cytochrome b5 requires E48 and E49 to stimulate the 17,20-lyase activity of cytochrome P450c17. Biochemistry. 2006;45:755–762. doi: 10.1021/bi051623y. [DOI] [PubMed] [Google Scholar]
- 47.Kohara H. Formation of 5,16-androstadien-3β-ol from pregnenolone in human testicular microsomes. Steroids. 1988;52:295–309. doi: 10.1016/0039-128x(88)90010-4. [DOI] [PubMed] [Google Scholar]
- 48.Miller SL, Wright JN, Corina DL, Akhtar M. Mechanistic studies on pregnene side-chain cleavage enzyme (17α-hydroxylase-17,20-lyase) using 18O. J Chem Soc Chem Commun. 1991;3:157–159. [Google Scholar]
- 49.Shimizu K. Formation of 5-[17β-2H]-androstene-3β,17α-diol from 3β-hydroxy-5-[17,21,21,21-2H]-pregnen-20-one by the microsomal fraction of boar testis. J Biol Chem. 1978;253:4237–4241. [PubMed] [Google Scholar]
- 50.Shimizu K. Metabolism of 17-[2H]-pregnenolone into 5-[17β-2H, 17α-18O]-androstene-3β,17α-diol and other products by incubation with the microsomal fraction of boar testis under 18O2 atmosphere. Biochem Biophys Acta - Lipids and Lipid Metabolism. 1979;575:37–45. [PubMed] [Google Scholar]
- 51.Mak AY, Swinney DC. 17-O-Acetyltestosterone formation from progesterone in microsomes from pig testes: evidence for the Baeyer-Villiger rearrangement in androgen formation catalyzed by CYP17. J Am Chem Soc. 1992;114:8309–8310. [Google Scholar]
- 52.Billen MJ, Squires EJ. The role of porcine cytochrome b5A and cytochrome b5B in the regulation of cytochrome P450 17A1 activities. J Steroid Biochem Mol Biol. 2009;113:98–104. doi: 10.1016/j.jsbmb.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 53.Meadus WJ, Mason JI, Squires EJ. Cytochrome P450c17 from porcine and bovine adrenal catalyses the formation of 5,16-androstadien-3β-ol from pregnenolone in the presence of cytochrome b5. J Steroid Biochem Mol Biol. 1993;46:565–572. doi: 10.1016/0960-0760(93)90183-w. [DOI] [PubMed] [Google Scholar]