Abstract
The activated transcription factor NF-κB shuttles between the cytoplasm and the nucleus resulting in the oscillation of nuclear NF-κB (NF-κBn). The oscillation pattern of NF-κBn is implicated in the regulation of gene expression profiles. Using computational models, we previously reported that spatial parameters, such as the diffusion coefficient, nuclear to cytoplasmic volume ratio, transport through the nuclear envelope, and the loci of translation of IκB protein, modified the oscillation pattern of NF-κBn. In a subsequent report, we elucidated the importance of the “reset” of NF-κBn (returning of NF-κB to the original level) and of a “reservoir” of IκB in the cytoplasm. When the diffusion coefficient of IκB was large, IκB stored at a distant location from the nucleus diffused back to the nucleus and “reset” NF-κBn. Herein, we report mechanisms that regulate the persistency and frequency of NF-κBn oscillation by nuclear transport. Among the four parameters of nuclear transport tested in our spatio-temporal computational model, the export of IκB mRNA from the nucleus regulated the persistency of oscillation. The import of IκB to the nucleus regulated the frequency of oscillation. The remaining two parameters, import and export of NF-κB to and from the nucleus, had virtually no effect on the persistency or frequency. Our analyses revealed that lesser export of IκB mRNA allowed NF-κBn to transcript greater amounts of IκB mRNA, which was retained in the nucleus, and was subsequently exported to the cytoplasm, where large amounts of IκB were synthesized to “reset” NF-κBn and drove the persistent oscillation. On the other hand, import of greater amounts of IκB led to an increase in the influx and the efflux of NF-κB to and from the nucleus, resulting in an increase in the oscillation frequency. Our study revealed the importance of nuclear transport in regulating the oscillation pattern of NF-κBn.
Introduction
NF-κB is a transcription factor that regulates the expression profiles of a vast number of genes. In the classical pathway of NF-κB activation, extracellular stimuli such as TNFα lead to the phosphorylation and proteasomal degradation of IκB, a negative regulator of NF-κB, which retains it in the cytoplasm in the resting state. NF-κB thus liberated from inhibition translocates to the nucleus, leading to the expression of genes, including IκB. Newly synthesized IκB protein binds to NF-κB in the nucleus causing an export of nuclear NF-κB (NF-κBn), leading to the raise of the cytoplasmic concentration of NF-κB again. If the NF-κB activating stimulus remains, IκB in the IκB:NF-κB complex is degraded, and the liberated NF-κB translocates to the nucleus again. Thus, the oscillation of NF-κB emerges [1–6].
The biological functions of NF-κB oscillation and its mechanisms have been investigated by multiple research groups leading to the discovery of several sensitive parameters affecting of NF-κB oscillation [7–14], the importance of the transport of proteins, including NF-κB [15], importance of negative feedback and inhibitor proteins [16–19], the effects of stimulation pattern on gene expression profiles [5,20] and stimulus specificity of gene expression [21]. These analyses revealed the critical parameters that regulate the activity of NF-κB, including negative feedback loops, rate constants, concentrations of molecules, and patterns of stimulation. Previously, we constructed a three-dimensional (3D) spatio-temporal model of NF-κB oscillation, and reported the importance of spatial parameters in the regulation of the NF-κBn oscillation pattern [22]. We found several factors, such as the diffusion coefficient, nuclear transport, nuclear to cytoplasmic volume (N/C) ratio, and loci of protein synthesis, to regulate the oscillation pattern of NF-κBn. In a subsequent report, we showed why the diffusion coefficient regulated the oscillation pattern [23]. We revealed that the “reset” of NF-κBn was critical for the sustained oscillation of NF-κBn, and that a distant location in the cytoplasm acted as a “reservoir” for newly synthesized IκB, which subsequently caused the “reset” of NF-κBn. A larger diffusion coefficient of IκB helped to store a greater amount of IκB in the cytoplasm, which could then diffuse back to the nucleus to sufficiently “reset” NF-κBn. Furthermore, we identified a possible change in the effective value of the diffusion coefficient caused by a change in the crowdedness of organelles, which was observed in hypoxic cells [24].
Herein, we report how and why nuclear transport regulates the oscillation pattern of NF-κBn. Several reports are available regarding the effect of nuclear transport on the activity of NF-κB in the nucleus. An earlier study showed that an siRNA-induced knock-down of nucleoporin Nup88, which is one of the constituent proteins of the nuclear pore complex (NPC) at the cytoplasmic face, prevented the nuclear accumulation of NF-κB and reduced the expression of a reporter gene [25]. Senescence has been shown to alter the expression of nucleoporins (nups) and to decrease the number of NPCs, thus impairing the nuclear translocation of NF-κB [26]. In addition, leukemogenic Nup98 fusion proteins have been reported to cause aberrant localization of CRM1, which is required for the export of NF-κBn, and the nuclear accumulation of NF-κB [27]. This was correlated with the enhanced transcription activity of NF-κBn. Constitutively active NF-κB was found in malignant melanoma cells, and knock-down of the Nup88 gene reduced the nuclear accumulation of NF-κB in these cells [25]. Aberrant localization of CRM1 caused by over expression of Nup88 and Nup214 has also been reported [28]. Although all these reports strongly suggested the importance of nuclear transport in the regulation of NF-κB, it is possible that the reduced or increased localization of NF-κB in the nucleus resulting due to the reduced or increased nuclear transport could be a simple causal effect, while there may exist a much more complicated mechanisms for the regulation of NF-κBn by nuclear transport. To elucidate the effect of alterations in parameters affecting the nuclear transport on the oscillation pattern of NF-κB, we conducted spatio-temporal simulations.
We investigated four pathways of nuclear transport in our spatio-temporal model: NF-κB import to the nucleus, its export from the nucleus, export of IκB mRNA (mRNAIκB) from the nucleus, and the import of newly synthesized IκB to the nucleus. We performed simulations by modifying each one of these parameters, and found that these four nuclear transport pathways altered the oscillation pattern of NF-κBn differently. While alteration in NF-κB nuclear import and its export from the nucleus resulted in no appreciable change in the oscillation pattern, alteration in the nuclear export of mRNAIκB and the import of IκB altered the persistency and the frequency of oscillation, respectively. Furthermore, reduction but not enhancement of the nuclear export of mRNAIκB increased the persistency of the oscillation, which was an unexpected result.
Results
Export of mRNAIκB from and import of IκB to the nucleus independently regulate persistency and frequency of NF-κB oscillation
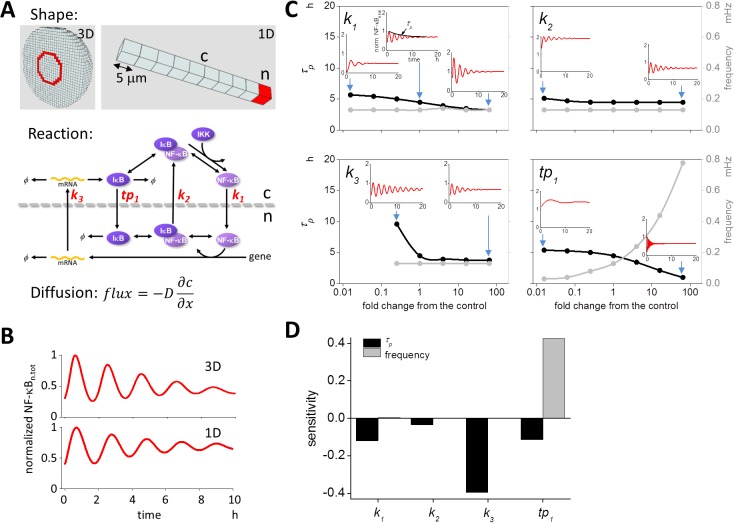
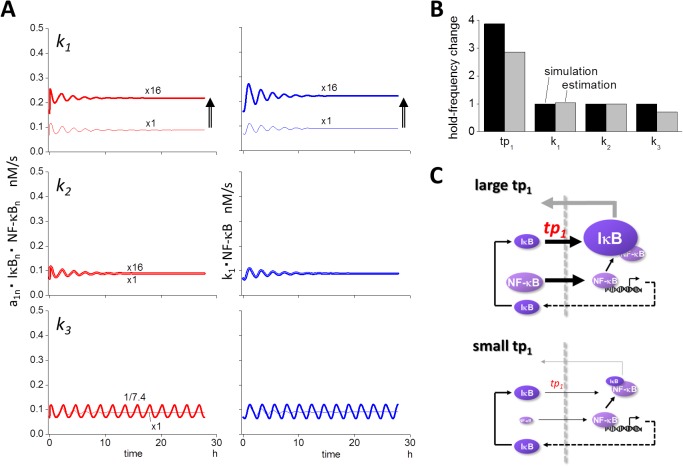
To examine the effect of the individual components in the nuclear transport, we employed a simple spatio-temporal model of NF-κB oscillation designed to elucidate the underlying mechanisms more clearly. For this purpose, the 3D model used originally was reduced to a 1D model, as previously described (top panels of Fig 1A [23]), which included a nuclear and a nuclear membrane compartment as shown in red. Reaction schemes and rate constants were identical to those preciously shown (bottom panel of Fig 1A and S1 Fig [22,23]). We referred to the rate constants of the four pathways of nuclear transport as k 1 (NF-κB import to the nucleus), k 2 (NF-κB export from the nucleus), k 3 (mRNAIκB export from the nucleus), and tp 1 (IκB import to the nucleus). We examined the changes in the oscillation pattern induced by individual changes in these rate constants. Diffusion was simulated by Fick’s equation as shown in our previous report (Fig 1A).
Fig 1. Effects of nuclear transport on the oscillation pattern of NF-κB.
(A) 3D and 1D spatio-temporal models of NF-κB oscillation. The 3D shape was identical to the one used in a previous report (top left panel, [22,23]). A spherical model cell was divided into compartments enabling reaction-diffusion computational simulation. Red compartments indicates the nuclear membrane. A simplified 1D shape was employed for detail analyses (top right panel). There was a single nuclear membrane and nuclear compartment (indicated in red). Reaction schemes shown in the middle panel are identical to those presented in a previous report [23]. The effects of four kinetic rate constants (shown in red in the middle panel) were investigated. Diffusion was calculated using Fick’s equation. D and ∂c / ∂x represent the diffusion coefficient and spatial derivative of the concentration, respectively. (B) Simulated oscillation pattern of NF-κBn.tot. We obtained the same oscillation pattern from both 3D and 1D models similar to prior analyses. All parameters for the simulation were the same as previously described [23]. (C) Parameter values were changed within a range of 1/64-fold decrease to 64-fold increase of control (×1) to investigate the subsequent modification in the oscillation pattern, as characterized by τ p (a measure of persistency; black lines) and frequency (gray lines). Insets show oscillations at parameter values indicated by blue arrows. The rate constants k 1 (NF-κB import to the nucleus), k 2 (NF-κB export from the nucleus), k 3 (export of mRNAIκB from the nucleus), and tp 1 (the import of newly synthesized IκB to the nucleus) were investigated. (D) Sensitivity analyses of nuclear transport. Sensitivities over the entire range of each parameter were averaged. It is clear that k 3 affected τ p, and tp 1 affected the frequency.
First, we confirmed the oscillation of NF-κBn.tot, which was the combined concentration of NF-κBn and the IκBn:NF-κBn complex as a measure of the fluorescence intensity of NF-κB in the nucleus. By using the previously set 1D parameters [23], we obtained oscillation of NF-κBn.tot similar to those obtained in the 3D model (Fig 1B).
Next, we examined the change in the oscillation pattern of NF-κBn.tot in the 1D model induced by changing the values of k 1, k 2, k 3, and tp 1. Decay time constant τ p for the envelope of the peaks of oscillation waveform of NF-κBn.tot, which was a measure of the persistency of the oscillation, and the frequency were employed as parameters characterizing the oscillation pattern. Changing k 1 values within a range from 1/64-fold to 64-fold of control slightly decreased τ p (black line in the top left panel of Fig 1C). There was virtually no change in the frequency (gray line). Insets indicate the oscillation of NF-κBn.tot at values of k 1 indicated by blue arrows. Changing k 2 caused almost no change in τ p and frequency (top right panel of Fig 1C). Thus, the import and export of NF-κB to and from the nucleus had little or no effect on the persistency and frequency of NF-κB oscillation. In contrast, modification of k 3 or tp 1 resulted in a large change in the persistency and/or frequency of oscillation. Persistency was increased by a reduction in k 3 without any appreciable change in the frequency (bottom left panel of Fig 1C). Changing the value of tp 1 significantly modified the frequency, and this was accompanied by a change in persistency (bottom right panel of Fig 1C). Increase in tp 1 dramatically increased the frequency of oscillation. Our 3D model provides almost identical results (S2 Fig).
These changes are clearly demonstrated by the sensitivity analysis (Fig 1D). We averaged sensitivities obtained over the entire range of parameter values. It was clear that k 3 and tp 1 independently regulated persistency and frequency, while k 1 and k 2 had only a marginal effect on these characterizing parameters. Therefore, we focused on k 3 and tp 1 in the following analyses.
Changing k 3 alters oscillation from dampened to inflating mode
We found that modifying k 3 changed τ p, but the change was limited to a certain range of k 3 (Fig 1C). To investigate this further, we ran simulations at wider range of k 3 (from 10−2 to 103-fold change compared to the control as shown in Fig 2). We found that τ p gradually increased with further decrease in k 3. At k 3 of 0.1357-fold of the control, the oscillation was still dampened, but seemed likely to persist for a considerably long time (bottom left panel in Fig 2). Further decrease in k 3 resulted in a greater increase in τ p, which is shown by black circles in the middle panel of Fig 2. If we reduced k 3 to 0.0833-fold of the control, however, the mode of the oscillation changed from dampened to inflating (top left panel of Fig 2). If we increased k 3 from this point, τ p was increased, shown by white circles in the middle panel of Fig 2. Thus, there was a dramatic change in the mode from dampened to inflating oscillation at k 3 around 0.1354-fold of the control. At a k 3 value smaller than 0.0833-fold of the control, we could not obtain a stable initial condition; that is the oscillation of NF-κBn never stopped during simulations aimed at generating stable initial conditions without an NF-κB-activating stimulus.
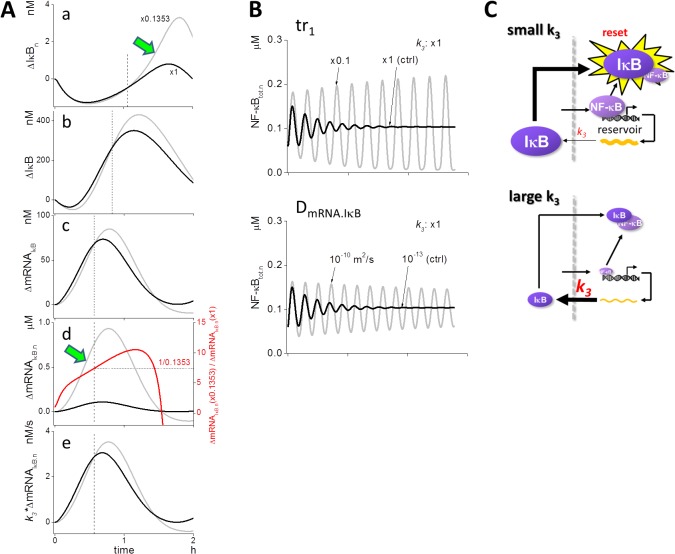
Fig 2. Decrease in k 3 results in a change in the oscillation from dampened to inflating mode.
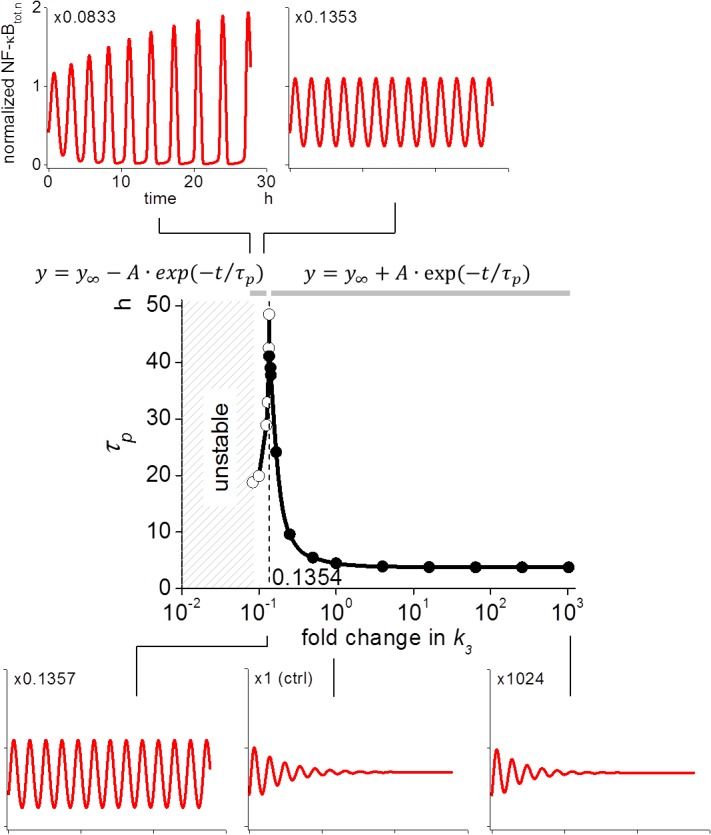
Reduction of k 3 to 0.0833-fold of the control changed the oscillation from a dampened to an inflating mode (top left panel). Decrease in k 3 increased τ p to a virtually persistent oscillation at k 3 ~0.1354-fold of control (bottom panels and black circles in the middle panel) while increases in k 3 from 0.0833-fold of the control resulted in the increase of τ p (white circles in the middle panel). Equations shown at the top of the middle panel were used for approximating τ p for inflating (left equation) and dampened (right one) oscillation by fitting the envelope of the peaks to these equations.
These results were unexpected, because we simply expected that any change in k 3 would result in a monotonic change in the persistency. It was also a surprise to find that a smaller k 3 resulted in persistent oscillation, because smaller k 3 was expected to result in less de novo synthesis of IκB, leading to an incomplete “reset” of NF-κBn ultimately resulting in a dampened oscillation [23]. However, our simulation showed the opposite results. Therefore, we tried to elucidate this mechanism further.
Persistent and inflating oscillation is caused by an accumulation of mRNAIκB in the nucleus
To elucidate the reason for the change in the oscillation mode at smaller k 3, we first investigated NF-κBn concentration at the nucleus (c0 in S3 Fig) to see whether “reset” of NF-κBn was observed during sustained oscillation as previously reported [23]. There was sufficient “reset” in the case of sustained oscillation, because the amount of NF-κBn at the troughs was smaller than the initial value during sustained oscillation (gray line in the middle panel of S3 Fig), while it was higher than the initial value under conditions of dampened oscillation (black line). These results were similar to those observed following a change in the diffusion coefficient of IκB in our previous report [23]. Next we investigated the IκB concentration at the most distant cytoplasmic compartment from the nucleus (c9 in S3 Fig) to see whether the mechanism for the sustained oscillation was identical to that previously reported for a larger diffusion coefficient [23]. We found that there was no appreciable change in the IκB concentration at c9. This result differed greatly from that observed in the diffusion coefficient experiment, where a considerable amount of IκB was stored at c9 in response to a large flux of IκB due to a large diffusion coefficient, and c9 acted as a “reservoir” for IκB [23]. Thus the mechanism for sustained and/or inflating oscillation of NF-κBn at a small k 3 value was different from that for the diffusion coefficient.
To explore the specific mechanism responsible for the effects of k 3, we investigated time courses of related species. As the “reset” of NF-κBn is also important for the oscillation (middle panel of S3 Fig) as demonstrated by diffusion coefficient studies, we first carefully investigated nuclear IκB (IκBn), because sufficient IκBn led to the “reset” of NF-κBn [23]. If we compared the increase in IκBn from its initial level (ΔIκBn in panel a of Fig 3A), there was virtually no difference in ΔIκBn between 0.1353-fold k 3 (gray line) and the control (black line) at the beginning of the oscillation. However, it increased steeply at 0.1353-fold k 3 (green arrow) to a value greater than control. The dashed vertical line indicates the time at which the gray and black lines crossed-over. It seemed that the delayed steep increase in ΔIκBn at 0.1353-fold k 3 was the reason for the sustained oscillation.
Fig 3. A mechanism that changes the persistency of NF-κBn oscillation.
(A) Time courses of concentration change relative to the initial value, for control (black lines) and 0.1353-fold decrease in k 3 (gray lines). ΔIκBn increased steeply after the crossover between k 3 at the control and 0.1353-hold decrease (green arrow in panel a). Crossover time point is indicated by a dashed line. ΔIκB was smaller under the condition of 0.1353-fold decreased k 3 compared to control and became larger at an earlier time point than ΔIκBn (panel b). Reduced k 3 resulted in ΔmRNAIκB levels greater than control at a much earlier time point (panel c). The level of ΔmRNAIκB.n was larger in decreased k 3 from the start of oscillation and displayed a steep increase (green arrow in panel d). If we calculate the ratio of mRNAIκB.n level at 0.1353-fold k 3 to the control (red line), it reached 1/0.1353 at the time point of the crossover for mRNA (panel d). The mRNAIκB flux out of the nucleus (= k 3*ΔmRNAIκB.n) crossed-over at the same time point for both (panel e). (B) Dampened oscillation under control conditions was rescued and converted to sustained oscillation by a reduction or increase in the translation rate of IκB (tr 1) or D mRNAIκB. (C) Model for the sustained oscillation driven by a small k 3. The mRNAIκB flux out of the nucleus is reduced by a small amount at reduced k 3, allowing NF-κBn to sustain mRNAIκB transcription. mRNAIκBn was stored in the nucleoplasmic space due to a small k 3. Thus nucleoplasmic space acted as a “reservoir” for mRNAIκBn. Subsequently, accumulated mRNAIκBn were exported to the cytoplasm, where large amount of IκB was newly synthesized leading to the “reset” of NF-κBn. This resulted in the sustained oscillation of NF-κBn.
We investigated the time courses of the upstream species next. The increase in cytoplasmic IκB from its initial level (ΔIκB) also resembled the delayed increase at 0.1353-fold k 3, when compared to control (panel b of Fig 3A). The same was also observed for cytoplasmic ΔmRNAIκB (panel c of Fig 3A). The crossover points shifted to earlier time points as we investigated the upstream species. If we compared these with the nuclear ΔmRNAIκB.n, however, the increase was much more steep at 0.1353-fold k 3 (gray line and green arrow in panel d of Fig 3A) than in control (black line), and there was no crossover at a time earlier than that observed for cytoplasmic ΔmRNAIκB. It is important to note that when we calculated the ratio of the increase in ΔmRNAIκB.n for both the cases, it was 1/0.1353 at the same time point as the crossover observed in conjunction with the increase of cytoplasmic ΔmRNAIκB (red curve and dashed line). We then calculated the flux of ΔmRNAIκB from the nucleus by multiplying k 3 with ΔmNRAIκB.n (panel e of Fig 3A). Fluxes at 0.1353-fold k 3 and the control crossed over at the same time point as the crossover observed when the ratio of the increase in ΔmRNAIκB.n was 1/0.1353. Thus, the delayed increase in IκB (panel b of Fig 3A) was caused by the sustained transcription of mRNAIκB (panel d of Fig 3A), which was caused by the small k 3.
We were surprised to find that a smaller k 3 value resulted in the sustained oscillation. However, the mechanism thus revealed was reasonable to account for the change in the oscillation mode by k 3. If this proposed mechanism holds true, a lower rate of de novo synthesis of IκB would also result in sustained transcription of mRNAIκB leading to the sustained oscillation of NF-κBn. This was true when tr 1, which was the translation rate of IκB (S1 Fig), was decreased to one-tenth to control; dampened oscillation at control was rescued to sustained and inflating oscillation (top panel of Fig 3B). If D mRNA.IκB, the diffusion coefficient of IκB mRNA, was larger, mRNAIκB would diffuse to distant locations within the cytoplasm, and the de novo synthesis of IκB should be reduced, leading to the sustained transcription of mRNAIκB and a sustained oscillation. This hypothesis also held true, as shown in the bottom panel of Fig 3B. In both the cases, we observed delayed increases in IκBn and mRNAIκB.n, as in the case of decreased tr 1, and NF-κBn level at troughs was lower than that of the control (S4 Fig). Thus, all three of the parameters tested, k 3, tr 1, and D mRNA.IκB contributed to the sustained oscillation by the same mechanism.
In summary, when k 3 was small, the mRNAIκB flux out of the nucleus was decreased leading to less de novo synthesis of IκB and a sustained transcription of mRNAIκB that was stored in the nucleus. This then led to the “reset” of NF-κB by an increase in de novo synthesis of IκB at subsequent time points, as well as to the sustained oscillation of NF-κB. Thus, a small k 3 resulted in the use of nucleoplasmic space as a “reservoir” for mRNAIκB (Fig 3C). Larger k 3 enabled greater mRNAIκB flux out of the nucleus, and early synthesis of IκB. This then prohibited sustained transcription of mRNAIκB by NF-κBn, leading to the dampened oscillation.
Finally, we compared the persistency of the observed oscillation following changes in k 1, k 2, and tp 1, since these parameters did not appear to have a major effect on persistency (Fig 1C). As shown in S5 Fig, neither a “reset” of NF-κBn nor a steep increase in mRNAIκB.n was observed after the start of the oscillation. In particular, no change in the time course of NF-κBn and mRNAIκB.n was induced following a change in k 2. Thus, the change in the persistency was specific to the nuclear transport induced by k 3 in our model.
Modification of tp 1 causes a large change in the oscillation frequency
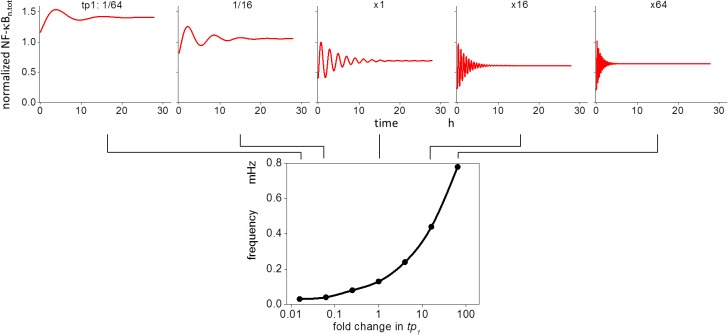
Our earlier results demonstrated that changes in tp 1 affected the oscillation frequency (Fig 1C). To investigate the effect further, we modified the tp 1 values, and the changes in the oscillation patterns for various tp 1 values are shown in Fig 4. Oscillation frequency increased with an increase in tp 1, while the average level of NF-κBn.tot was decreased. A greater than 20-fold increase in the frequency resulted from a change in tp 1, ranging from a 1/64-fold decrease to a 64-fold increase from the control. We then explored the reason for the change in frequency by tp 1.
Fig 4. Modification of tp 1 changes the oscillation frequency of NF-κBn.
Increasing tp 1 increased the oscillation frequency, while the average level of NF-κBn was decreased at the same time. A greater than 20-fold change in the frequency was observed.
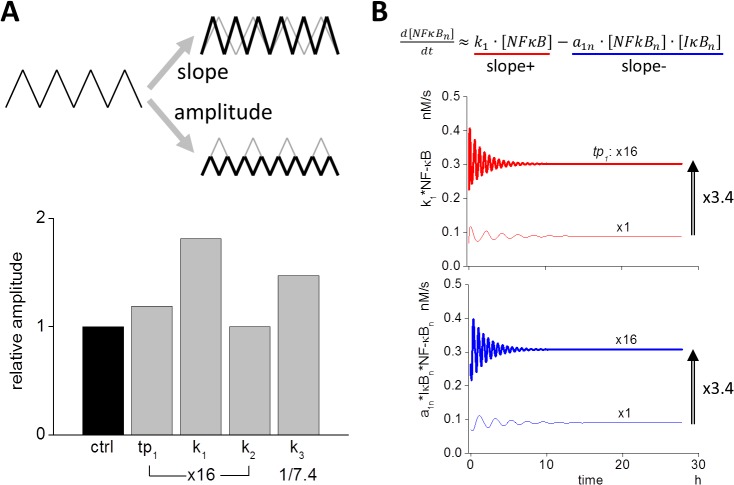
Increase in the change in slope of NF-κBn contributes to increased oscillation frequency
There are two ways to change the frequency of NF-κBn oscillation, namely, the slope and the amplitude (upper panel of Fig 5A). If the slope increases without a change in amplitude, oscillation frequency increases. If the amplitude decreases without a change in the slope, the oscillation frequency increases as well. Therefore, we first compared the amplitude that resulted from a change in the four parameters tp 1, k 1, k 2, and k 3 (lower panel of Fig 5 and S6 Fig). While an increase in amplitude was observed following an increase in k 1 or a decrease in k 3, respectively, virtually no change in amplitude was observed following a change in tp 1 and k 2. Therefore, a reduction in the amplitude caused by a change in tp 1 was not the reason for the increased oscillation frequency.
Fig 5. Slopes and amplitude of oscillation waveform of NF-κBn determine frequency.
(A) If increasing (slope+) and/or decreasing slopes (slope-) are steeper without a corresponding change in amplitude, oscillation frequency increases. Conversely, if the amplitude decreases without changing slopes, the frequency also increases (upper panel). The change in amplitude resulting from the modifications in each of the four parameters are shown (bottom panel). While a 16-fold increase in tp 1 resulted in higher frequency, the change in the amplitude was small. While there was virtually no change in the frequency in response to modifications of k 1 and k 3, an increase in k 1 or a decrease in k 3 resulted in the increase in the amplitude. (B) Slope+ and slope- were approximated by the inward flux of k 1*NF-κB and the flux of complex formation a 1n*NF-κBn*IκBn (see main text). When tp 1 was increased 16-fold, both inward and outward fluxes were increased (lower two panels).
Next we investigated the slope of NF-κBn. Rising (slope+) and falling (slope-) slopes were analyzed independently (S7A Fig). Slope+ and slope- were direct consequences of the inward and outward fluxes of NF-κB and IκBn:NF-κBn, respectively, which were calculated by k 1*NF-κB and k 2*IκBn:NF-κBn. If we compared NF-κBn.tot, which was the summation of IκBn:NF-κBn and NF-κBn with NF-κBn alone, there was only a small difference between the two (S7B Fig). In addition, d 1n*NF-κBn:IκBn was negligible in comparison to a 1n*NF-κBn*IκBn (Materials and Methods). We then approximated the change in NF-κBn.tot by using NF-κBn, which was further approximated by Eq 1 (Materials and Methods). Thus we employed k 1*NF-κB and a 1n*NF-κBn*IκBn as slope+ and slope-, respectively (Fig 5B).
Following the 16-fold increase in tp 1, an increase of 3.40-fold was observed both in k 1*NF-κB and a 1n*NF-κBn*IκBn, indicating that both slope+ and slope- became steeper with the same magnitude (Fig 5B). If we compared slope+ and slope- for the 16-fold increase in k 1, increases to the same degree were observed for both slopes. However, no change in these slopes were obtained from the 16-fold increase in k 2 and the 1/7.4-fold decrease in k 3 (Fig 6A). These are summarized in S8 Fig, together with the change in the slopes as designated by tp 1.
Fig 6. Estimated changes in the frequency resulting from changes in slopes and amplitude agree with simulations.
(A) Slope+ and slope- were larger given a 16-fold increase in k 1, while there was virtually no change in the slopes following a 16-fold increase or a 1/7.4-fold decrease in k 2 or k 3. (B) The change in the oscillation frequency was estimated by the change in slopes and amplitude (see main text). There was a reasonable agreement between simulation (black bars) and estimation (gray bars). (C) Summary of the mechanism of changes in the oscillation frequency generated by modifications of tp 1. Increasing tp 1 relocated NF-κB to the cytoplasm resulting in an increase in its inward flux to the nucleus. In addition, a larger tp 1 value directly resulted in an increase in the inward flux of IκB to the nucleus, resulting in a larger amount of IκBn and leading to an increase in the outward flux of NF-κBn.tot.
Since we hypothesized that the oscillation frequency could be calculated by using slopes and amplitude by a simplified oscillating waveform with triangle wave, we estimated oscillation frequency by Eq 2 (Materials and Methods, S9 Fig). Estimated frequencies for tp 1, k 1, k 2, and k 3 agreed reasonably with frequencies obtained by simulations (Fig 6B). With a large k 1, both the amplitude and the slopes were increased to almost the same extent (S9 Fig) and no change in the frequency was observed. Modification of tp 1, however, resulted in an appreciable change only in the slopes but not in the amplitude. Therefore, it is clear that the frequency was altered by a change in tp 1.
Under conditions of high tp 1 and k 1, NF-κB flux into the nucleus was increased (Figs 5B and 6A). Increase in the flux was obviously caused by the increase in k 1. However, why did high tp 1 also increase flux? As shown in S10 Fig, larger tp 1 caused greater IκB flux into the nucleus leading to an increase in IκBn. This then led to increased IκBn:NF-κBn flux out of the nucleus which subsequently reduced NF-κBn and increased NF-κB in the cytoplasm. Thus, the preferred storage site of NF-κB was relocated to the cytoplasm. In summary, slope+ and slope- were increased by an increase in cytoplasmic NF-κB and increase in the IκB flux into the nucleus, which was the direct consequence of the increase in k 1 and tp 1, respectively. This was the major mechanism that drove higher oscillation frequency of NF-κBn.tot (Fig 6C). Under conditions of a smaller tp 1, both the average level of cytoplasmic NF-κB and the inward flux of IκB toward the nucleus were small resulting in a lower frequency. Thus, tp 1 regulated the oscillation frequency by two different mechanisms for slope+ and slope-.
Discussion
We have been investigating the mechanisms that change the oscillation patterns of NF-κB following modification of spatial parameters ([22,23] and this report). Over the course of these analyses we found that the “reset” of NF-κBn was important for the sustained oscillation, and larger D IκB helped to “reset” NF-κBn by storing newly synthesized IκB at a cytoplasmic space distant from the nucleus, which acted as a “reservoir” [23]. Here we report that the efflux of mRNAIκB and influx of IκB from and to the nucleus independently regulate the persistency and frequency of oscillation (Fig 1C and 1D). In addition, the reduction in the rate of translation and increase in D mRNA.IκB increase the persistency of oscillation. Increase in D IκB rescued the heavily-dampened oscillation as shown in the previous report [23]. Using these analyses we developed a model to explain the regulation of the oscillation pattern by spatial parameters, as summarized in Fig 7. The model indicates that distinct spatial parameters regulate the persistency and frequency of NF-κBn oscillation. D mRNA.IκB and D IκB and the rate of mRNAIκB efflux are spatial parameters that regulate the persistency, which are shown in green arrows, and the rate of the influx of IκB is a spatial parameter that regulates the frequency of the oscillation, which is shown in a brown arrow. Transcription of mRNAIκB and the translation of IκB are non-spatial parameters regulating the persistency of oscillation. There is virtually no effect by any other nuclear membrane transport mechanisms on the persistency and the frequency, which is shown in black arrows. In summary, our results suggest that the mechanisms regulating IκB are responsible for the regulation of the oscillation pattern.
Fig 7. Mechanisms regulating the oscillation pattern of nuclear NF-κB.
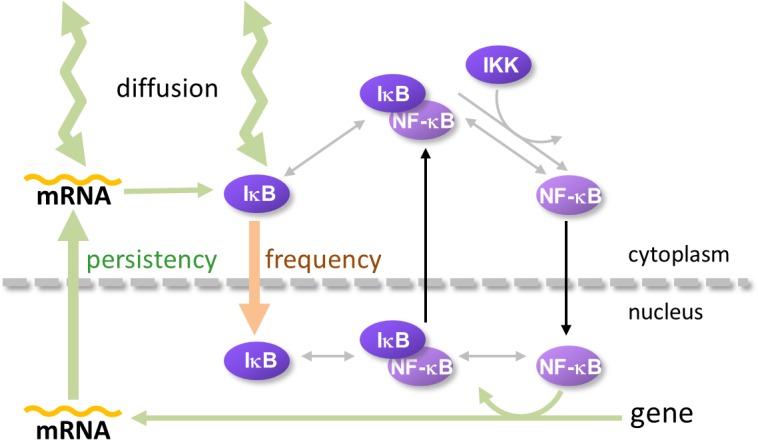
The present study together with our previous one [23], showed distinct spatial parameters regulating the persistency and frequency of NF-κBn oscillation. D mRNAIκB and D IκB (thick zigzag light green arrows) and the rate of mRNAIκB efflux (thick light green arrow) are spatial parameters that regulate the persistency of the oscillation. The rate of the influx of IκB (light brown arrow) is a spatial parameter that regulates frequency. In addition, transcription of mRNAIκB and translation of IκB are non-spatial parameters regulating persistency (thin light green arrows). There was virtually no effect on persistency and the frequency by other nuclear membrane transport mechanisms (black arrows).
In our studies, the rate of transcription was calculated by the equation shown at the top of S11 Fig, which was identical to that used in a previous report [22,23]. If we changed the “n” from the control value of 2 to 1 or 3 at the 0.1353-fold reduced k 3 condition, the sustained oscillation was modified to a dampened or an inflating oscillation, respectively (bottom panels in S11 Fig). Thus “n", the parameter describing how many NF-κB molecules bound to the κB site of DNA were required for transcription, seems to regulate persistency.
We observed an inflating oscillation of NF-κB in the 1D model following a low flux of mRNAIκB nuclear export (Fig 2). The same inflating oscillation was observed in our 3D model when IκB kinase (IKK) degradation was ignored (S12 Fig). Thus, the inflating oscillation was not unique to the 1D model, but could also be observed in the 3D model provided that the effects of IKK degradation were negligible.
Together with our previous results [23], our current study demonstrates that the frequency and persistency of NF-κB oscillation can be regulated by its inhibitor molecule IκB and mRNAIκB. There was only a marginal effect by parameters directly regulating NF-κB, such as its rate of influx and efflux. These results might seem to be counterintuitive, as rates of the influx and efflux of NF-κB were expected to directly regulate NF-κB oscillation. However, our analyses revealed that IκB predominantly regulated the oscillation pattern. This further suggests that currently unknown mechanisms regulating the concentration and/or dynamics of IκB might also regulate frequency and/or persistency.
In the present study, we have analyzed effects of fluxes through the nuclear envelope (NE) by changing the corresponding rate constants (Fig 1C). There are two ways to alter the flux through NE: by changing the density/number of NPCs or by altering the rate of the flux through a single NPC. Senescence has been shown to decrease the number of NPCs [26], which suggests that it could decrease the outward flux of IκB mRNA and inward flux of IκB protein, together with other proteins and mRNAs. Since the inward and outward fluxes of NF-κB have virtually no effect on the oscillation pattern (Fig 1C), senescence could potentially lead to a persistent and low-frequency oscillation of NF-κB. A previous study has shown that the leukemogenic Nup98 fusion proteins caused an aberrant localization of the CRM1 protein [27]. Although the mechanism of nuclear export of IκB mRNA is largely unknown, CRM1 and NXF1 are possibly involved in this process, as shown in other mRNA export studies [29,30], and the export of IκB mRNA could be retarded in cells expressing leukemogenic Nup98 fusion proteins leading to the persistent oscillation of NF-κB. Thus, the nuclear transport could play an important role in some diseases and in aging of the cell.
It is important to address possible experimental procedures to alter the nuclear transport of IκB mRNA and IκB protein selectively. Although mechanisms of nuclear import of IκB are not well known [31], it was reported that the nuclear import of IκBα is temperature and ATP dependent and is blocked by a dominant-negative mutant of importin β [32]. Thus, the efficacy of IκBα import could be regulated by the expression level of a dominant-negative mutant of importin β. The export of mRNA and protein complex (mRNP) is not simple, but is composed of many steps, including the proper assembly of a mRNP, and its targeting and docking to NPC [29,30]. If the binding of CRM1 or NXF1 to IκB mRNP is blocked partially, its export could be selectively impaired leading to the persistent oscillation of NF-κB. In fact, it is reported that the export of mRNPs is influenced by elements within the mRNA, which is responsible for the binding of CRM1 or NXF1 to mRNA [29]. If the element in IκB mRNA responsible for this process is mutated, its export could be retarded leading to the persistent oscillation of NF-κB. The diffusion coefficient is inherent to the molecular species. However, its effective value can be modified by a change in the effective size. If IκB mRNP is enlarged by the binding of non-functional proteins, the effective value of D mRNA.IκB will be reduced because of hindered diffusion. This could lead to the dampened oscillation of NF-κB.
In conclusion, our investigations on the possible regulatory mechanisms of NF-κBn oscillation revealed that the export of IκB mRNA from the nucleus and the import of IκB to the nucleus are important in regulating the persistency and frequency of the oscillation. A decrease in the export of IκB mRNA facilitated an enhanced transcription by NF-κBn, which was retained in the nucleus, to be subsequently exported to the cytoplasm to “reset” NF-κBn and to sustain the persistency of the oscillation. Conversely, an increase in the influx of IκB led to increases in the influx and efflux of NF-κB resulting in the higher oscillation frequency. These results provide a better understanding of the mechanism of NF-κBn oscillation and the importance of the nuclear transport, indicating its relevance in the understanding of disease mechanisms.
Materials and Methods
Computational model
We constructed spatio-temporal 3D and 1D computational models of NF-κB oscillation as previously described [23]. We used the same chemical reaction model as in the previous report (S1 Fig, [23]). Briefly, the model comprised the formation of IKK:IκBα:NF-κB complex, the degradation of IκBα and the subsequent nuclear transportation of NF-κB, NF-κB transcription of IκBα mRNA, IκBα protein synthesis, and the nuclear export of the IκBα:NF-κB complex. We employed a simplified chemical reaction model excluding A20 and CYLD as our model was intended to extract phenomena and mechanisms for the regulation of the NF-κB oscillation pattern by nuclear transport.
The 3D spherical cell model with a diameter of 50 μm was divided into small cubic compartments (total 62,417) of identical size enabling reaction-diffusion simulations (top left panel of Fig 1A). We used Fick’s equation for simulating diffusion, which was combined with differential equations for the chemical reactions. The central 8.3% of the compartments was assigned as the nucleus. In the 1D model, which was used for the analysis of the effect of nuclear transport, there were 10 cubic compartments with an edge length of 5 μm per cube, and the rightmost red compartment was assigned as the nucleus and nuclear membrane compartment (top right panel of Fig 1A). Reaction schemes shown in S1 Fig were embedded in the corresponding regions of the cytoplasm, nuclear membrane, and nucleus of the 3D and 1D models.
We employed the 1D model for the efficiency of analyses, because there were only 1/6241.7th compartments in the 1D model compared to the 3D model. All models were constructed using A-Cell software [33,34]. Models and all parameters used in the present study can be downloaded from http://dx.doi.org/10.6084/m9.figshare.1417973. Kinetic parameters used in our simulation were the same as in the previous report [23].
Simulations
Simulation programs in c language were automatically generated by A-Cell. We used the parallelized version by openMP for a multi-core CPU. Simulations were run on a Linux computer equipped with an Intel compiler. Every time we changed parameters for nuclear transport, we first acquired an equilibrium forcing IKK = 0, which ensured a resting state. Thereafter a simulation of NF-κB oscillations was run by setting concentrations acquired by equilibration. Simulated concentrations of nuclear NF-κB were plotted as values normalized to the maximum at the control condition, unless otherwise noted.
Analyses
Frequency of NF-κBn oscillation was analyzed by FFT (Fast Fourier Transform) using Origin8.5J by OriginLab Corp. The time constant of dampened or inflating oscillation was fitted to equations shown in Fig 2. Time series data of peak amplitude were extracted and fitted to either equation by using the curve-fit package of Origin 8.5J (inset in the top left panel of Fig 1C showing τ p).
Calculation of slopes in the oscillation waveform and estimation of oscillation frequency
The slope of the oscillation was defined as the rate of the increase or decrease in the total concentration of nuclear NF-κB (NF-κBn.tot), which was the summation of NF-κB and its complex IκB:NF-κB in the nucleus. Thus, the rate change in NF-κBn.tot was calculated by
Subscription n indicates species in the nucleus. Since there was only a small difference between NF-κBn.tot and NF-κBn (S7B Fig), we reduced the equation as follows:
If we compared the 2nd and the 3rd terms, the 3rd term was negligibly small (9.04 × 10−11 v.s. 1.59 × 10−12 M/s). Therefore, we further simplified the equation as follows:
| Eq.1) |
The 1st and 2nd terms on the right hand side were the positive (slope+) and negative (slope-) slopes, respectively (Fig 5B).
If we simplified the oscillation waveform of NF-κB by triangular wave (top panel of Fig 5A), the change in the oscillation frequency was proportional and inversely proportional to the average slope of slope+ and slope- and amplitude. Therefore, we calculated the estimated fold change in the frequency by`
| Eq.2) |
Supporting Information
The scheme is identical to that used in a previous report [23]. The diffusion process both for mRNAIκB (t.IκB) and protein IκB are explicitly shown by zigzag lines with bidirectional arrowheads, since these played an important role in the persistency of NF-κBn oscillation (see text). Other species diffused with diffusion coefficients of 10−11 m2/s.
(TIF)
Simulation results of τ p (black asterisks) and frequency (red asterisks) at selected value of each parameter in the 3D model are shown together with those used in the 1D simulations (black and gray circles). Although there were discrepancies between 1D and 3D simulations in k 3 at 0.25-fold decrease and tp 1 at 16-fold increase, the overall propensity of the change agreed between the 3D and 1D simulations.
(TIF)
At c0 (nuclear compartment), the NF-κBn concentration at the troughs was larger for the dampened oscillation and smaller for the sustained oscillation at control value of k 3 and 0.1353-fold of the control, respectively (middle panel), similar to the case of diffusion coefficient. However, there was no appreciable difference in the average level of IκB at c9 (most distant cytoplasmic compartment) in both oscillations (bottom panel), which was different from the case of diffusion coefficient.
(TIF)
We found the same steep increases in IκBn and mRNAIκB.n (green arrows) caused by the decreasing or increasing tr 1 (A) or D mRNA.IκB (B) at the control level of k 3. The levels of NF-κBn were lower at troughs than initial levels indicating a sufficient “reset” under these conditions, which led to persistent oscillation.
(TIF)
Neither “reset” nor a steep increase in mRNAIkB.n was observed following changes in these parameters. The time course of NF-kB and mRNAIkBn overlapped almost completely after a reduction of k 2 to 1/16 (middle panels).
(TIF)
There was virtually no change in the amplitude following changes in tp 1 and k 2, and there was a small change in the amplitude following changes in k 1 and k 3. Thin black and thick gray lines indicate the NF-κBn.tot oscillation under control conditions and at the 16-fold increase (tp 1, k 1, and k 2) or 1/7.4-fold decrease (k 3).
(TIF)
(A) If we approximated the oscillation by a triangular waveform, two slopes (slope+ and slope-) regulated the frequency. Slope+ and slope- were directly calculated by the inward and outward fluxes of NF-κB (k 1*NF-κB) and IκBn:NF-κBn complex (k 2*IκBn:NF-κBn). (B) There was almost no difference between NF-κBn.tot and NF-κBn indicating that we could perform the analyses using NF-κBn instead of NF-κBn.tot.
(TIF)
To generate slope+ and slope-, we needed to know the concentrations of NF-κB (top), NF-κBn (middle), and IκBn (bottom). We used average concentrations at equilibrium. Estimated slopes relative to the control values are listed. While a 3.40-fold increase in slope+ and slope- resulted from a 16-fold increase in tp 1, virtually no change occurred following a 16-fold increase in k 2 and a 0.1353-fold decrease in k 3. Increase in k 1 generated marginal changes.
(TIF)
Red and blue lines are slope+ and slope- under control (thin lines) and changed conditions (thick lines) designated in each panel. Estimated changes in the frequency (est.freq.) were calculated by the change in the amplitude and the average slope by Eq 2. Only change in tp 1 resulted in an appreciable change in the frequency.
(TIF)
1) Larger tp 1 increased the inward flux of IκB resulting in the reduction of cytoplasmic IκB. 2) This increased IκBn led to the reduction of NF-κBn due to the increase in the efflux of NF-κBn. 3) Because of the increase in the NF-κBn efflux, the cytoplasmic NF-κB increased. Thus, the equilibrium changed to a state of greater cytoplasmic NF-κB.
(TIF)
Transcription of mRNAIκB was calculated by the equation shown above. There were two parameters controlling the transcription, tr 2 and n. Among them, n described the nonlinearity of the transcription in relation to the concentration of NF-κBn. n = 2 at the control condition, assuming that the binding of two NF-κB molecules to the κB site of genes was required for their regulation. If n was set to 1 or 3, the sustained oscillation at 0.1353-fold decreased k 3 resulted in a dampened or an inflating oscillation (left and right panels, respectively).
(TIF)
When the rate of IκB kinase (IKK) degradation was set to 0 as in the 1D model, inflating oscillation was observed in the 3D model as well.
(TIF)
Data Availability
All relevant data are within the paper and its Supporting Information files. In addition, A-Cell model files are available at http://dx.doi.org/10.6084/m9.figshare.1417973.
Funding Statement
This work was supported by a Grant-in-Aid for Scientific Research on Innovative Areas from the Ministry of Education, Culture, Sports, Science, and Technology of Japan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Zhou A, Scoggin S, Gaynor RB, Williams NS (2003) Identification of NF-kappa B-regulated genes induced by TNFalpha utilizing expression profiling and RNA interference. Oncogene 22: 2054–2064. [DOI] [PubMed] [Google Scholar]
- 2. Bakkar N, Guttridge DC (2010) NF-kappaB signaling: a tale of two pathways in skeletal myogenesis. Physiol Rev 90: 495–511. 10.1152/physrev.00040.2009 [DOI] [PubMed] [Google Scholar]
- 3. Yamazaki K, Gohda J, Kanayama A, Miyamoto Y, Sakurai H, Ymamamoto M, et al. (2009) Two mechanistically and temporally distinct NF-kappaB activation pathways in IL-1 signaling. SciSignal 2: ra66 10.1126/scisignal.2000387 [DOI] [PubMed] [Google Scholar]
- 4. Hoffmann A, Levchenko A, Scott ML, Baltimore D (2002) The IkappaB-NF-kappaB signaling module: temporal control and selective gene activation. Science 298: 1241–1245. [DOI] [PubMed] [Google Scholar]
- 5. Ashall L, Horton CA, Nelson DE, Paszek P, Harper CV, Sillitoe K, et al. (2009) Pulsatile stimulation determines timing and specificity of NF-kappaB-dependent transcription. Science 324: 242–246. 10.1126/science.1164860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sung MH, Salvatore L, De Lorenzi R, Indrawan A, Pasparakis M, Hager GL, et al. (2009) Sustained oscillations of NF-kappaB produce distinct genome scanning and gene expression profiles. PLoS One 4: e7163 10.1371/journal.pone.0007163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cucurull-Sanchez L, Spink KG, Moschos SA (2012) Relevance of systems pharmacology in drug discovery. Drug Discov Today 17: 665–670. 10.1016/j.drudis.2012.01.015 [DOI] [PubMed] [Google Scholar]
- 8. Wang Y, Paszek P, Horton CA, Kell DB, White MR, Broomhead DS, et al. (2011) Interactions among oscillatory pathways in NF-kappa B signaling. BMC Syst Biol 5: 23 10.1186/1752-0509-5-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Joo J, Plimpton S, Martin S, Swiler L, Faulon JL (2007) Sensitivity analysis of a computational model of the IKK NF-kappaB IkappaBalpha A20 signal transduction network. Ann N Y Acad Sci 1115: 221–239. [DOI] [PubMed] [Google Scholar]
- 10. Park SG, Lee T, Kang HY, Park K, Cho KH, and Jung G. (2006) The influence of the signal dynamics of activated form of IKK on NF-kappaB and anti-apoptotic gene expressions: a systems biology approach. FEBS Lett 580: 822–830. [DOI] [PubMed] [Google Scholar]
- 11. Kearns JD, Basak S, Werner SL, Huang CS, Hoffmann A (2006) IkappaBepsilon provides negative feedback to control NF-kappaB oscillations, signaling dynamics, and inflammatory gene expression. J Cell Biol 173: 659–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cheong R, Bergmann A, Werner SL, Regal J, Hoffmann A, Levchenko A (2006) Transient IkappaB kinase activity mediates temporal NF-kappaB dynamics in response to a wide range of tumor necrosis factor-alpha doses. J Biol Chem 281: 2945–2950. [DOI] [PubMed] [Google Scholar]
- 13. Cho KH, Shin SY, Lee HW, Wolkenhauer O (2003) Investigations into the analysis and modeling of the TNF alpha-mediated NF-kappa B-signaling pathway. Genome Res 13: 2413–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cho K, Shin S, Kolch W, Wolkenhauer O (2003) Experimental Design in Systems Biology, Based on Parameter Sensitivity Analysis Using a Monte Carlo Method: A Case Study for the TNFα-Mediated NF-κB Signal Transduction Pathway. Simulation 79: 726–739. [Google Scholar]
- 15. Terry AJ, Chaplain MA (2011) Spatio-temporal modelling of the NF-kappaB intracellular signalling pathway: the roles of diffusion, active transport, and cell geometry. J Theor Biol 290: 7–26. 10.1016/j.jtbi.2011.08.036 [DOI] [PubMed] [Google Scholar]
- 16. Shih VF, Kearns JD, Basak S, Savinova OV, Ghosh G, Hoffmann A (2009) Kinetic control of negative feedback regulators of NF-kappaB/RelA determines their pathogen- and cytokine-receptor signaling specificity. Proc Natl Acad Sci U S A 106: 9619–9624. 10.1073/pnas.0812367106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim D, Kolch W, Cho KH (2009) Multiple roles of the NF-kappaB signaling pathway regulated by coupled negative feedback circuits. FASEB J 23: 2796–2802. 10.1096/fj.09-130369 [DOI] [PubMed] [Google Scholar]
- 18. Werner SL, Kearns JD, Zadorozhnaya V, Lynch C, O'Dea E, Boldin MP, et al. (2008) Encoding NF-kappaB temporal control in response to TNF: distinct roles for the negative regulators IkappaBalpha and A20. Genes Dev 22: 2093–2101. 10.1101/gad.1680708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nikolov S, Vera J, Rath O, Kolch W, Wolkenhauer O (2009) Role of inhibitory proteins as modulators of oscillations in NF kappa B signalling. Iet Systems Biology 3: 59–76. 10.1049/iet-syb.2008.0105 [DOI] [PubMed] [Google Scholar]
- 20. Nelson DE, Ihekwaba AE, Elliott M, Johnson JR, Gibney CA, Foreman BE, et al. (2004) Oscillations in NF-kappaB signaling control the dynamics of gene expression. Science 306: 704–708. [DOI] [PubMed] [Google Scholar]
- 21. Werner SL, Barken D, Hoffmann A (2005) Stimulus specificity of gene expression programs determined by temporal control of IKK activity. Science 309: 1857–1861. [DOI] [PubMed] [Google Scholar]
- 22. Ohshima D, Inoue J, Ichikawa K (2012) Roles of spatial parameters on the oscillation of nuclear NF-kappaB: computer simulations of a 3D spherical cell. PLoS One 7: e46911 10.1371/journal.pone.0046911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ohshima D, Ichikawa K (2014) Regulation of Nuclear NF-kappa B Oscillation by a Diffusion Coefficient and Its Biological Implications. Plos One 9: e109895 10.1371/journal.pone.0109895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Al-Mehdi AB, Pastukh VM, Swiger BM, Reed DJ, Patel MR, Bardwell GC, et al. (2012) Perinuclear mitochondrial clustering creates an oxidant-rich nuclear domain required for hypoxia-induced transcription. Sci Signal 5: ra47 10.1126/scisignal.2002712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Takahashi N, van Kilsdonk JW, Ostendorf B, Smeets R, Bruggeman SW, Alonso A, et al. (2008) Tumor marker nucleoporin 88 kDa regulates nucleocytoplasmic transport of NF-kappaB. Biochem Biophys Res Commun 374: 424–430. 10.1016/j.bbrc.2008.06.128 [DOI] [PubMed] [Google Scholar]
- 26. Kim SY, Ryu SJ, Ahn HJ, Choi HR, Kang HT, Park SC (2010) Senescence-related functional nuclear barrier by down-regulation of nucleo-cytoplasmic trafficking gene expression. Biochem Biophys Res Commun 391: 28–32. 10.1016/j.bbrc.2009.10.154 [DOI] [PubMed] [Google Scholar]
- 27. Takeda A, Sarma NJ, Abdul-Nabi AM, Yaseen NR (2010) Inhibition of CRM1-mediated nuclear export of transcription factors by leukemogenic NUP98 fusion proteins. J Biol Chem 285: 16248–16257. 10.1074/jbc.M109.048785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xylourgidis N, Roth P, Sabri N, Tsarouhas V, Samakovlis C (2006) The nucleoporin Nup214 sequesters CRM1 at the nuclear rim and modulates NFkappaB activation in Drosophila. J Cell Sci 119: 4409–4419. [DOI] [PubMed] [Google Scholar]
- 29. Bjork P, Wieslander L (2014) Mechanisms of mRNA export. Semin Cell Dev Biol 32: 47–54. 10.1016/j.semcdb.2014.04.027 [DOI] [PubMed] [Google Scholar]
- 30. Natalizio BJ, Wente SR (2013) Postage for the messenger: designating routes for nuclear mRNA export. Trends in Cell Biology 23: 365–373. 10.1016/j.tcb.2013.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lu M, Zak J, Chen S, Sanchez-Pulido L, Severson DT, Endicott J. et al. (2014) A code for RanGDP binding in ankyrin repeats defines a nuclear import pathway. Cell 157: 1130–1145. 10.1016/j.cell.2014.05.006 [DOI] [PubMed] [Google Scholar]
- 32. Sachdev S, Bagchi S, Zhang DD, Mings AC, Hannink M (2000) Nuclear import of IkappaBalpha is accomplished by a ran-independent transport pathway. Mol Cell Biol 20: 1571–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ichikawa K (2001) A-Cell: graphical user interface for the construction of biochemical reaction models. Bioinformatics 17: 483–484. [DOI] [PubMed] [Google Scholar]
- 34. Ichikawa K (2005) A modeling environment with three-dimensional morphology, A-Cell-3D, and Ca2+ dynamics in a spine. Neuroinformatics 3: 49–64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The scheme is identical to that used in a previous report [23]. The diffusion process both for mRNAIκB (t.IκB) and protein IκB are explicitly shown by zigzag lines with bidirectional arrowheads, since these played an important role in the persistency of NF-κBn oscillation (see text). Other species diffused with diffusion coefficients of 10−11 m2/s.
(TIF)
Simulation results of τ p (black asterisks) and frequency (red asterisks) at selected value of each parameter in the 3D model are shown together with those used in the 1D simulations (black and gray circles). Although there were discrepancies between 1D and 3D simulations in k 3 at 0.25-fold decrease and tp 1 at 16-fold increase, the overall propensity of the change agreed between the 3D and 1D simulations.
(TIF)
At c0 (nuclear compartment), the NF-κBn concentration at the troughs was larger for the dampened oscillation and smaller for the sustained oscillation at control value of k 3 and 0.1353-fold of the control, respectively (middle panel), similar to the case of diffusion coefficient. However, there was no appreciable difference in the average level of IκB at c9 (most distant cytoplasmic compartment) in both oscillations (bottom panel), which was different from the case of diffusion coefficient.
(TIF)
We found the same steep increases in IκBn and mRNAIκB.n (green arrows) caused by the decreasing or increasing tr 1 (A) or D mRNA.IκB (B) at the control level of k 3. The levels of NF-κBn were lower at troughs than initial levels indicating a sufficient “reset” under these conditions, which led to persistent oscillation.
(TIF)
Neither “reset” nor a steep increase in mRNAIkB.n was observed following changes in these parameters. The time course of NF-kB and mRNAIkBn overlapped almost completely after a reduction of k 2 to 1/16 (middle panels).
(TIF)
There was virtually no change in the amplitude following changes in tp 1 and k 2, and there was a small change in the amplitude following changes in k 1 and k 3. Thin black and thick gray lines indicate the NF-κBn.tot oscillation under control conditions and at the 16-fold increase (tp 1, k 1, and k 2) or 1/7.4-fold decrease (k 3).
(TIF)
(A) If we approximated the oscillation by a triangular waveform, two slopes (slope+ and slope-) regulated the frequency. Slope+ and slope- were directly calculated by the inward and outward fluxes of NF-κB (k 1*NF-κB) and IκBn:NF-κBn complex (k 2*IκBn:NF-κBn). (B) There was almost no difference between NF-κBn.tot and NF-κBn indicating that we could perform the analyses using NF-κBn instead of NF-κBn.tot.
(TIF)
To generate slope+ and slope-, we needed to know the concentrations of NF-κB (top), NF-κBn (middle), and IκBn (bottom). We used average concentrations at equilibrium. Estimated slopes relative to the control values are listed. While a 3.40-fold increase in slope+ and slope- resulted from a 16-fold increase in tp 1, virtually no change occurred following a 16-fold increase in k 2 and a 0.1353-fold decrease in k 3. Increase in k 1 generated marginal changes.
(TIF)
Red and blue lines are slope+ and slope- under control (thin lines) and changed conditions (thick lines) designated in each panel. Estimated changes in the frequency (est.freq.) were calculated by the change in the amplitude and the average slope by Eq 2. Only change in tp 1 resulted in an appreciable change in the frequency.
(TIF)
1) Larger tp 1 increased the inward flux of IκB resulting in the reduction of cytoplasmic IκB. 2) This increased IκBn led to the reduction of NF-κBn due to the increase in the efflux of NF-κBn. 3) Because of the increase in the NF-κBn efflux, the cytoplasmic NF-κB increased. Thus, the equilibrium changed to a state of greater cytoplasmic NF-κB.
(TIF)
Transcription of mRNAIκB was calculated by the equation shown above. There were two parameters controlling the transcription, tr 2 and n. Among them, n described the nonlinearity of the transcription in relation to the concentration of NF-κBn. n = 2 at the control condition, assuming that the binding of two NF-κB molecules to the κB site of genes was required for their regulation. If n was set to 1 or 3, the sustained oscillation at 0.1353-fold decreased k 3 resulted in a dampened or an inflating oscillation (left and right panels, respectively).
(TIF)
When the rate of IκB kinase (IKK) degradation was set to 0 as in the 1D model, inflating oscillation was observed in the 3D model as well.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files. In addition, A-Cell model files are available at http://dx.doi.org/10.6084/m9.figshare.1417973.