Abstract
Binge eating disorder is characterized by excessive consumption of highly palatable food within short periods of time accompanied by loss of control over eating. Extensive evidence provides support for the consideration of binge eating disorder as an addiction-like disorder. In this study, we wanted to determine whether rats undergoing an operant binge-like eating procedure could develop maladaptive forms of conditioned feeding behaviors. For this purpose, we trained male rats to self-administer either a sugary, highly palatable diet (Palatable rats) or a chow diet (Chow rats) for 1 hour a day. Following escalation and stabilization of palatable food intake, we tested Chow and Palatable rats in i) a conditioned place preference test, ii) a second-order schedule of reinforcement, and iii) a cue-induced suppression of feeding test. In the conditioned place preference task, Palatable rats spent significantly more time in the compartment that was previously paired with the palatable food, compared to Chow controls. Furthermore, in the second order schedule of reinforcement task, Palatable rats exhibited active lever responding 4- to 6-fold higher than Chow control rats. Finally, in the cue-induced suppression of feeding test, while Chow control subjects reduced responding by 32% in the presence of the conditioned punishment, Palatable rats persevered in responding despite the aversive cue. These results further characterize this animal model of binge-like eating and provide additional evidence for the addictive properties of highly palatable food.
Keywords: binge eating disorder, conditioned place preference, food seeking, conditioned punishment, compulsive eating, loss of control
Introduction
Binge eating disorder affects more than 10 million people in the United States. It is characterized by excessive consumption of highly palatable foods within short periods of time, accompanied by loss of control over eating (American Psychiatric Association, 2013). An increasing number of behavioral and neurobiological findings provide strong support for the consideration of binge eating disorder as an addiction-like disorder (Colantuoni et al., 2002; Corwin & Buda-Levin, 2004; Cottone, Sabino, Steardo, & Zorrilla, 2008; Micioni Di Bonaventura et al., 2014).
Animal models continue to play a major role in understanding the neurobiological substrates underlying psychiatric diseases and they provide predictive information on potential targets for pharmacological treatment. We have recently developed an operant rat model of binge-like eating which is based on 1h/day limited access to a highly palatable sucrose diet (Blasio, Steardo, Sabino, & Cottone, 2013; Cottone et al., 2012; Velazquez-Sanchez et al., 2014). Under these limited access conditions, rats escalate responding for the palatable diet up to 4 times compared to control rats responding for a standard chow diet. Binge eating rats exhibit a heightened motivation to obtain the sugary food in a progressive ratio schedule of reinforcement (Blasio et al., 2013; Velazquez-Sanchez et al., 2014) and display loss of control over food (compulsive eating) shown by consumption that is resistant to disruption by aversive conditions of highly palatable food (Cottone et al., 2012; Velazquez-Sanchez et al., 2014). In addition, we have recently shown, using an approach similar to the one used to prove cocaine addiction-like behavior in the rat (Belin, Mar, Dalley, Robbins, & Everitt, 2008; Deroche-Gamonet, Belin, & Piazza, 2004), that not all rats exposed to the highly palatable food respond in the same way so that only some develop a phenotype that is suggestive of addiction-like behavior (Velazquez-Sanchez et al., 2014). This phenotype represents a highly translational aspect of the model as it mimics what is observed in Western societies where although palatable foods are omnipresent, only some individuals develop pathological overeating.
A critically important aspect in the study of addictive disorders is represented by the development of maladaptive conditioned behaviors. Indeed, stimuli conditioned to alcohol, drugs of abuse, or food exert a powerful control over behavior and can elicit craving and precipitate relapse/binge (Everitt & Robbins, 2000; Robinson, Yager, Cogan, & Saunders, 2014). On the other hand, aversive environmental stimuli which signal punishment and readily suppress appetitive behavior (i.e. conditioned suppression (Bouton & Bolles, 1980)) become ineffective in addicted individuals proving the compulsive nature of the disorders (Johnson & Kenny, 2010; Vanderschuren & Everitt, 2004).
Therefore, in this study we wanted to determine whether rats undergoing the operant binge-like eating procedure could develop maladaptive forms of conditioned feeding behaviors. For this purpose, we tested palatable eating rats and chow responding control rats in: (a) a conditioned place preference (CPP) test, a task used to measure the rewarding properties of stimuli associated with food (Tzschentke, 2007); (b) a second-order schedule of reinforcement, in which food-seeking responding is maintained by the presentation of conditioned stimuli (Everitt & Robbins, 2000) and, finally, (c) a cue-induced suppression of feeding test, which is used to measure the perseverance of instrumental responding in the presence of conditioned aversive stimuli (Belin et al., 2008; Deroche-Gamonet et al., 2004; Johnson & Kenny, 2010).
Materials and Methods
Subjects
Male Wistar rats, 45 days old upon arrival (Charles River, Wilmington, MA) in 3 different cohorts, were used as subjects. Procedures adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the Principles of Laboratory Animal Care, and were approved by Boston University Medical Campus Institutional Animal Care and Use Committee. No experimental procedures involved food or water restriction/deprivation.
Apparatus for self-administration procedures
The individual operant test chambers (30×24×29 cm) (Med Associates Inc., St. Albans, VT) had a grid floor and were located in ventilated and sound-attenuating enclosures (66×56×36 cm) (Blasio et al., 2013; Cottone et al., 2012; Velazquez-Sanchez et al., 2014). Food reinforcers were delivered by a pellet dispenser; water reinforcers were delivered by a solenoid into a liquid cup nosepoke receptacle. Two retractable levers were placed on the wall opposite to the pellet and water dispensers. 28-V stimulus cue-lights were located above each lever and above the food magazine.
Binge-like eating procedure in ad libitum-fed rats
For the operant binge-like eating procedure, all rats were individually placed in operant chambers and allowed to self-administer 45-mg chow pellets, which were identical in composition to the diet the rats received in their home-cages as 5g extruded pellets (5TUM diet formulated as 4–5g extruded pellets, 65.5% [kcal] carbohydrate, 10.4% fat, 24.1% protein, metabolizable energy 330 cal/100 g; TestDiet, Richmond, IN), for 1h sessions, during which every nosepoke resulted in the delivery of one pellet. Levers were retracted during the entire session in this procedure. After reaching a stable responding for standard chow, rats were assigned to either a “Chow” control group, which continued to receive the same 45-mg chow pellets offered during the training phase, or a “Palatable” group, which instead received 45-mg chocolate-flavored, high sucrose (50% kcal) pellets, similar in macronutrient composition and energy density to the chow 45-mg food (chocolate-flavored formula 5TUL: 66.7% [kcal] carbohydrate, 12.7% fat, 20.6% protein, metabolizable energy 344 cal/100 g; TestDiet, Richmond, IN). Rats underwent daily 1-h sessions under a Fixed Ratio 1 (FR1) schedule of reinforcement for at least 12 days, to allow escalation of the intake of the palatable diet. The different testing procedures were performed in Palatable rats which fully escalated and stabilized their intake and the Chow control counterpart as previously shown (Blasio et al., 2013; Cottone et al., 2012; Velazquez-Sanchez et al., 2014).
Conditioned place preference test
The conditioned place preference (CPP) apparatus (Med Associates, Georgia, VT) consisted of two equal sized outer chambers (27.5×20.6×21.5 cm), differing in color and floor texture, connected by a smaller center chamber (11.9×20.6×21.5 cm) and separated by automatic guillotine doors. Time spent in each chamber was measured with IR photobeam detectors. The CPP procedure consisted of three different phases: preconditioning, conditioning, and post-conditioning. During the pre-conditioning, rats were allowed to freely explore the apparatus for 15 minutes. Subjects that did not show a reliable baseline preference for either one of the outer chambers (<20-sec difference between the time spent in the two) were excluded from the experiment. Rats were confined on alternate days either to their initial preferred chamber with an empty food bowl or in their initial non-preferred chamber with a bowl of their respective food (either chow [Chow group] or palatable [Palatable group]) (Wang et al., 2014). These conditioning sessions were performed daily, lasted for 25 min, and continued for 16 days (eight pairings each). 24 hours after the last conditioning session, rats were tested following the same procedure as the preconditioning test (post-conditioning phase). The time spent in each chamber was recorded and CPP was determine by calculating a CPP score (time in paired chamber – time in unpaired chamber (Lax, George, Ignatz, & Kolber, 2014; Wang et al., 2014; Xue et al., 2014)).
Second order schedule of reinforcement
Food-seeking behavior under a second-order schedule of reinforcement is a procedure in which responding is maintained by the contingent presentation of food-paired stimuli that serve as conditioned reinforcers of instrumental behavior (Everitt & Robbins, 2000; Giuliano, Robbins, Nathan, Bullmore, & Everitt, 2012). In the second-order schedule of reinforcement (FI5(FR10:S)), every 10th active lever press (Fixed Ratio 10, FR10) resulted in a brief illumination of lights above both the active lever and the food magazine for 1 s. Responses on the inactive lever had no programmed consequences but were recorded to assess discriminative responding and general levels of motor activity. Following the tenth active lever press after a Fixed Interval of 5 min (FI5 min) had elapsed, 20 pellets (45-mg chow pellets for Chow rats or 45-mg sucrose pellets for Palatable rats) were delivered in the food magazine, both the active and inactive levers retracted, and the lights above both the active lever and the food magazine were presented during a 20-sec time out. During the FI interval, rats that pressed the active lever did not receive any pellets. After the time out, the lights above both the active lever and the food magazine turned off and the two levers were again extended into the chamber. The second-order schedule of reinforcement session lasted 40 min.
Cue-induced suppression of feeding
The conditioned suppression of feeding task was modified from (Johnson & Kenny, 2010). Following stabilization of intake in the binge-like eating procedure, the operant sessions were shortened to 30-min until a new stable baseline of responding was established. Under these conditions, Palatable rats still showed a more than 2-fold increase in intake compared with Chow rats (Figure 2C). The procedure consisted of three 30 min phases: pre-conditioning, conditioning, and post-conditioning. During the 30-min preconditioning session, rats were allowed to nose poke to obtain food pellets (45-mg chow pellets for Chow rats or 45-mg sugar pellets for Palatable rats); tone was activated for 10 min, turned off for 10 min, and then turned back on for 10 min. Levers were retracted during the entire session in this procedure. Pellet delivery was paired with a light-cue located above the nosepoke hole. Four daily 30 min conditioning sessions were performed in different self-administration boxes where no pellet dispensers were available. During the session, the same tone was activated for 10 min, turned off for 10 min, and then turned back on for 10 min. Footshocks were paired with the presentation of the cue tone (0.5 mA for 1.0 sec; 10 stimulations with 1-min intervals). During the 30-min postconditioning session, rats were allowed to nose poke to obtain food pellets, and the tone was activated for 10 min, turned off for 10 min, and then turned back on for 10 min. No foot shock was delivered. Food responses were recorded in the pre- and post-conditioning sessions as the main dependent variables.
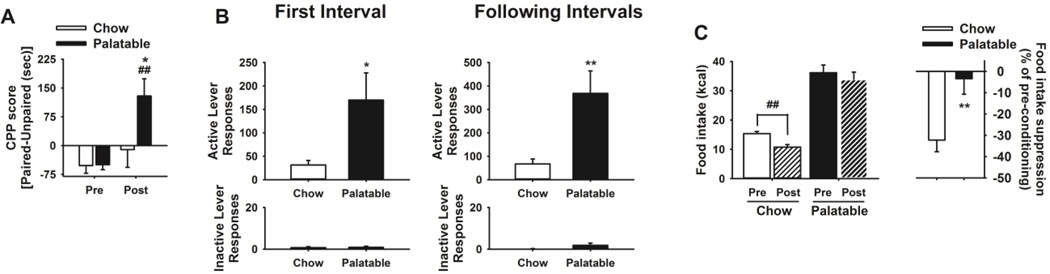
Figure 2.
Palatable rats show palatable food-induced conditioned place preference, high levels of food seeking behavior as well as resistance to conditioned suppression of feeding. (A) Palatable animals showed enhanced conditioned food reward compared to Chow control animals when tested in a conditioned place preference paradigm in which the food was located in the least preferred compartment. (B) Under a second order schedule of reinforcement, Palatable rats show a 4 to 6-fold increase in food seeking responding (both during the first and the following intervals) compared to control Chow rats. No effect was observed during the first or during the following trials in the number of inactive lever responses. (C) Intermittent access to highly palatable food induces resistance to cue-induced suppression of feeding behavior. (Left) Palatable rats showed resistance to the suppression of feeding behavior by a cue previously associated with a foot shock. (Right) The presentation of an aversive cue decreased the intake of control Chow rats by ~32%. Conversely, Palatable rats’ intake was resistant to the aversive properties of the cue (~3% decrease). Data show M±SEM. *p≤0.05 **p≤0.01, ***p≤0.001 Chow vs Palatable. ##p≤0.01, Post vs Pre.
Statistical analyses
Data were analyzed by simple or factorial ANOVAs. Pairwise post-hoc comparisons were made using the Fisher’s LSD test; Student-t test was used to compare two groups. Statistical significance level was set at α≤0.05.
Results
Development of an Operant Model of Binge-Like Eating in Rats
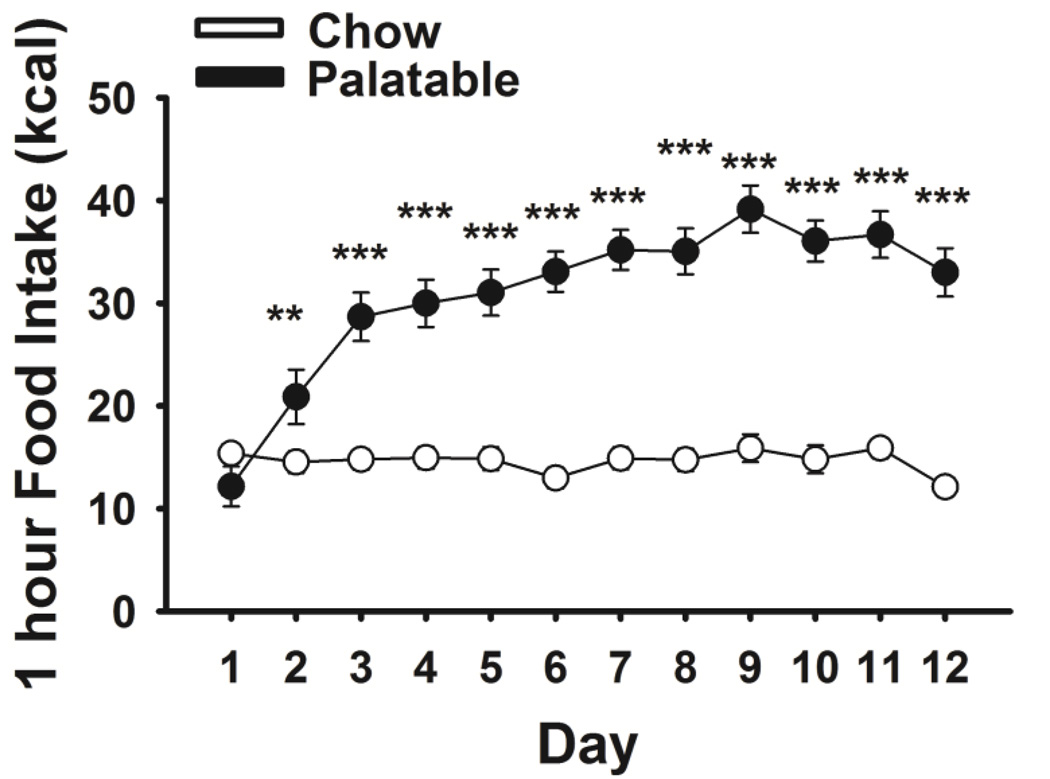
Rats (n=59) allowed to self-administer the highly palatable diet for 1 hr per day rapidly increased their intake compared to the control group which had access to the chow diet (Food: F(1,57)=67.68, p≤0.001, Food*Day: F(11,627)=23.86, p≤0.001, Fig 1). As we previously showed (Cottone et al., 2012; Velazquez-Sanchez et al., 2014), body weights did not differ between Palatable fed rats and Chow fed rats (not shown).
Figure 1.
Effects of daily 1-h self-administration of a highly palatable diet on food intake in male Wistar rats. Data show M±SEM. **p≤0.01, ***p≤0.001 Chow vs Palatable.
Palatable rats show conditioned place preference for palatable food
In the CPP experiment (n = 8), the analysis of the CPP scores indicated that Palatable rats spent significantly more time in the initially nonpreferred compartment (biased procedure), which was paired with the palatable food, when compared with either their score in the preconditioning phase or the Chow control group (Food: F(1,6)=7.87, p≤0.05; Day: F(1,6)=6.08, p≤0.05; Food*Day: F(1,6)=5.70, p≤0.05, Figure 2A). No effect was observed in Chow rats.
Palatable rats show heightened seeking behavior for palatable food
In the second-order schedule of reinforcement task (n = 14), Palatable rats performed a higher number of responses on the active lever compared with the Chow group in both the first interval and the remaining intervals (Food: F(1,12)=8.99, p≤0.02). No difference in the number of inactive lever responses was observed (Food: F(1,12)=2.40, n.s.).
Palatable rats show resistance to conditioned punishment
In the conditioned suppression of feeding test (n=37), when exposed to the tone which was previously paired with the foot shock, Chow control animals significantly decreased their intake compared to their intake during the pre-conditioning phase (i.e. when the tone was still a neutral stimulus). Conversely, the conditioned punishment did not exert any effect on responding for the sucrose diet in Palatable rats (Food: F(1,35)=102.86, p≤0.001; Day: F(1,35)=4.00, p≤0.05, Figure 2C). Specifically, while control Chow rats reduce their intake of the standard chow diet, Palatable rats reduced the intake of the Palatable diet by only 3% therefore showing resistance to the aversive properties of the cue (t(35) = 3.13, p ≤ 0.005).
Discussion
Rats undergoing an operant limited access to a sucrose diet dramatically escalate responding and show excessive food intake in a short period of time, heightened motivation for food and loss of control over intake (Blasio et al., 2013; Cottone et al., 2012; Velazquez-Sanchez et al., 2014). In this paper, we found that Palatable rats showed increased conditioned place preference, heightened seeking behavior and developed inflexible responding for palatable food, compared to Chow control rats. The results of this study further characterize the phenotype of Palatable rats providing evidence that bingeing rats show multiple maladaptive forms of conditioned feeding behavior.
We show that in Palatable eating rats, tactile and visual cues associated with the palatable diet can acquire rewarding properties as measured in a conditioned place preference paradigm. In the same conditions, cues associated with the regular chow diet fail to elicit place preference. Conditioned place preference investigates the effects of rewards on the salience of contextual stimuli in which they are experienced; therefore, contextual neutral stimuli, through an associative learning process, acquire rewarding properties that are expressed in the absence of the reward. Hence, conditioned place preference measures an associative conditioned process that is fundamentally distinct from drug/food self-administration and it has been extensively used to study the salience of drugs/food associated contextual cues that set the stage for behaviors to be engendered (Bardo & Bevins, 2000). A limitation of the biased procedure is that the results could be potentially confounded by a reduced aversion for the non-preferred compartment. Nevertheless, both drugs and natural rewards generally have similar effects in both biased and unbiased procedures (Tzschentke, 2007). It is relevant to mention that it is unlikely that the CPP results are due to differences in locomotor activity, as we previously showed that Chow and Palatable rats do not differ in this measure (Cottone et al., 2012).
In this study, Palatable animals trained in a second order schedule of reinforcement for palatable food showed a very high rate of responding, exhibiting a ~4 to ~6 fold increase in seeking behavior compared to control chow responding rats. The higher rate of responding was evident in both the first interval, before any intake of food had occurred, as well as in the following intervals. The second order schedule of reinforcement is a widely used task to measure seeking behavior for food and drugs of abuse in which responding is maintained not only by the primary reinforcer but also by contingent presentation of food-paired stimuli that serve as conditioned reinforcers of instrumental behavior (Everitt & Robbins, 2000; Giuliano et al., 2012).
Therefore, both the CPP for food and the food seeking behavior tasks are highly relevant in the investigation of the mechanisms underlying maladaptive behaviors driven by stimuli associated with palatable food. Indeed, it is well established that palatable food-associated environmental cues exert a powerful control over feeding behavior in people and have the power to override energy-homeostasis signals and trigger binge eating episodes (Ng & Davis, 2013).
We show here that Palatable rats develop inflexible responding for the highly palatable, sucrose diet measured as resistance to the behavioral suppressing properties of a conditioned aversive stimulus. The persistence of instrumental behavior despite the presence of conditioned aversive stimuli is one of the strategies used to operationalize the construct of loss of control in addictive disorders as it depicts the compulsive nature of the disease (Johnson & Kenny, 2010; Vanderschuren & Everitt, 2004). Previous findings have shown that rats with a history of cocaine exposure became inflexible and resistant to conditioned punishment (Vanderschuren & Everitt, 2004). Similarly, intake of rats that had a daily extended access to a palatable, cafeteria diet became insensitive to the anorexic effects of a cue predicting punishment (Johnson & Kenny, 2010). Our results show that inflexible responding occurs in rats with limited operant access to a highly palatable diet.
Similar to what was observed in animal models of drug addiction, we show that the history of escalation with palatable food reward is important to develop the behavioral profile observed in the Palatable animals. However, we cannot completely rule out the possibility that the palatable diet itself supports stronger food seeking across the different behavioral tests. Further experiments will be needed to address this possibility.
Unlike previous reports (Delamater, Sclafani, & Bodnar, 2000; Giuliano et al., 2012), in the present study, food restriction or deprivation was not used; this feature minimizes any possible confounding factor that might be driven by a negative energy balance, and it also allows us to dissociate the mechanisms underlying behaviors triggered by the rewarding properties of the palatable diet from behaviors induced by purely homeostatic needs. An operant model of binge-like eating in non-deprived subjects, therefore, better models what occurs in Western societies where palatable food is omnipresent and overeating is triggered by environmental cues (Wardle, 1990).
In summary, in this study we continued the characterization of a novel operant model of binge-like eating induced by providing limited access to a highly palatable, sucrose diet. This animal model is “multisymptomatic” (Belin & Deroche-Gamonet, 2012) as Palatable rats show multiple excessive food-related behaviors mimicking the different aspects of the symptomatology observed in subjects affected by binge eating disorder. Our results further characterize this animal model and provide a better understanding of the potential addictive properties of highly palatable food.
Acknowledgements
We are very grateful to Dr. Barry J Everitt and Dr. Chiara Giuliano for helpful discussion. This publication was made possible by grant numbers DA030425, MH091945, and MH093650 from the National Institute on Drug Abuse (NIDA) and the National Institute of Mental Health (NIMH), by the Peter Paul Career Development Professorship (P.C.), the McManus Charitable Trust (V.S.), and Boston University's Undergraduate Research Opportunities Program (UROP). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest
The authors have no conflict of interest.
References
- APA. Diagnostic and Statistical Manual of Mental Disorders. 5 th ed. American Psychiatric Association; 2013. [Google Scholar]
- Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl) 2000;153(1):31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Belin D, Deroche-Gamonet V. Responses to novelty and vulnerability to cocaine addiction: contribution of a multi-symptomatic animal model. Cold Spring Harb Perspect Med. 2012;2(11) doi: 10.1101/cshperspect.a011940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320(5881):1352–1355. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasio A, Steardo L, Sabino V, Cottone P. Opioid system in the medial prefrontal cortex mediates binge-like eating. Addict Biol. 2013 doi: 10.1111/adb.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME, Bolles RC. Conditioned Fear Assessed by Freezing and by the Suppression of 3 Different Baselines. Animal Learning & Behavior. 1980;8(3):429–434. [Google Scholar]
- Colantuoni C, Rada P, McCarthy J, Patten C, Avena NM, Chadeayne A, Hoebel BG. Evidence that intermittent, excessive sugar intake causes endogenous opioid dependence. Obes.Res. 2002;10(6):478–488. doi: 10.1038/oby.2002.66. [DOI] [PubMed] [Google Scholar]
- Corwin RL, Buda-Levin A. Behavioral models of binge-type eating. Physiol Behav. 2004;82(1):123–130. doi: 10.1016/j.physbeh.2004.04.036. [DOI] [PubMed] [Google Scholar]
- Cottone P, Sabino V, Steardo L, Zorrilla EP. Opioid-dependent anticipatory negative contrast and binge-like eating in rats with limited access to highly preferred food. Neuropsychopharmacology. 2008;33(3):524–535. doi: 10.1038/sj.npp.1301430. [DOI] [PubMed] [Google Scholar]
- Cottone P, Wang X, Park JW, Valenza M, Blasio A, Kwak J, Sabino V. Antagonism of sigma-1 receptors blocks compulsive-like eating. Neuropsychopharmacology. 2012;37(12):2593–2604. doi: 10.1038/npp.2012.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delamater AR, Sclafani A, Bodnar RJ. Pharmacology of sucrose-reinforced place-preference conditioning: effects of naltrexone. Pharmacol Biochem Behav. 2000;65(4):697–704. doi: 10.1016/s0091-3057(99)00251-8. [DOI] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305(5686):1014–1017. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Second-order schedules of drug reinforcement in rats and monkeys: measurement of reinforcing efficacy and drug-seeking behaviour. Psychopharmacology (Berl) 2000;153(1):17–30. doi: 10.1007/s002130000566. [DOI] [PubMed] [Google Scholar]
- Giuliano C, Robbins TW, Nathan PJ, Bullmore ET, Everitt BJ. Inhibition of opioid transmission at the mu-opioid receptor prevents both food seeking and binge-like eating. Neuropsychopharmacology. 2012;37(12):2643–2652. doi: 10.1038/npp.2012.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci. 2010;13(5):635–641. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lax NC, George DC, Ignatz C, Kolber BJ. The mGluR5 antagonist fenobam induces analgesic conditioned place preference in mice with spared nerve injury. PLoS One. 2014;9(7):e103524. doi: 10.1371/journal.pone.0103524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micioni Di Bonaventura MV, Ciccocioppo R, Romano A, Bossert JM, Rice KC, Ubaldi M, Cifani C. Role of bed nucleus of the stria terminalis corticotrophin-releasing factor receptors in frustration stress-induced binge-like palatable food consumption in female rats with a history of food restriction. J Neurosci. 2014;34(34):11316–11324. doi: 10.1523/JNEUROSCI.1854-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng L, Davis C. Cravings and food consumption in Binge Eating Disorder. Eat Behav. 2013;14(4):472–475. doi: 10.1016/j.eatbeh.2013.08.011. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Yager LM, Cogan ES, Saunders BT. On the motivational properties of reward cues: Individual differences. Neuropharmacology. 2014;76(Pt B):450–459. doi: 10.1016/j.neuropharm.2013.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol. 2007;12(3–4):227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Everitt BJ. Drug seeking becomes compulsive after prolonged cocaine self-administration. Science. 2004;305(5686):1017–1019. doi: 10.1126/science.1098975. [DOI] [PubMed] [Google Scholar]
- Velazquez-Sanchez C, Ferragud A, Moore CF, Everitt BJ, Sabino V, Cottone P. High trait impulsivity predicts food addiction-like behavior in the rat. Neuropsychopharmacology. 2014;39(10):2463–2472. doi: 10.1038/npp.2014.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Su P, Zhang Y, Lu J, Xing B, Kang K, Wang Y. Protein kinase D1-dependent phosphorylation of dopamine D1 receptor regulates cocaine-induced behavioral responses. Neuropsychopharmacology. 2014;39(5):1290–1301. doi: 10.1038/npp.2013.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle J. Conditioning processes and cue exposure in the modification of excessive eating. Addict Behav. 1990;15(4):387–393. doi: 10.1016/0306-4603(90)90047-2. [DOI] [PubMed] [Google Scholar]
- Xue YX, Xue LF, Liu JF, He J, Deng JH, Sun SC, Lu L. Depletion of perineuronal nets in the amygdala to enhance the erasure of drug memories. J Neurosci. 2014;34(19):6647–6658. doi: 10.1523/JNEUROSCI.5390-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]