Abstract
Low- and middle-income countries account for the majority of hypertension disease burden. However, little is known about the distribution of this illness within subpopulations of these countries, particularly among those who live in urban informal settlements. A cross-sectional hypertension survey was conducted in 2003 among 5649 adult residents of a slum settlement in the city of Salvador, Brazil. Hypertension was defined as either an elevated arterial systolic (≥140 mmHg) or diastolic (≥90 mmHg) blood pressure. Sex-specific multivariable models of systolic blood pressure were constructed to identify factors associated with elevated blood pressure. The prevalence of hypertension in the population 18 years and older was 21 % (1162/5649). Men had 1.2 times the risk of hypertension compared with women (95 % confidence intervals (CI), 1.05, 1.36). Increasing age and lack of any schooling, particularly for women, were also significantly associated with elevated blood pressure (p < 0.05). There was also a direct association between men who were black and an elevated blood pressure. Among those who were hypertensive, 65.5 % were aware of their condition, and only 36.3 % of those aware were actively using anti-hypertensive medications. Men were less likely to be aware of their diagnosis or to use medications (p < 0.01 for both) than women. The prevalence of hypertension in this slum community was lower than reported frequencies in the non-slum population of Brazil and Salvador, yet both disease awareness and treatment frequency were low. Further research on hypertension and other chronic non-communicable diseases in slum populations is urgently needed to guide prevention and treatment efforts in this growing population.
Keywords: Hypertension, Non-communicable disease, Urban slum, Prevalence, Brazil
Introduction
Hypertension is the single most modifiable risk factor for cardiovascular disease and a leading cause of death worldwide.1 It has been estimated to be responsible for approximately 9.4 of the 18.4 million cardiovascular disease deaths each year.2,3 Low- and middle-income countries accounted for 80 % of all cardiovascular disease in 2010,4 and the number of hypertensive individuals in these areas is projected to increase by 80 % in the next 20 years.5 Hypertension will play a major role in the ongoing cardiovascular disease epidemic, and early recognition and treatment of hypertension are essential to the prevention of cardiovascular disease and its sequelae.6,7
Although a number of studies have reported the prevalence of hypertension in low- and middle-income countries,2,8,9 few have explicitly studied hypertension within urban informal settlements or slums, which are communities with limited access to the formal healthcare system.10–19 In 2003, the United Nations Human Settlements Program (UN-Habitat) operationally defined slums as urban areas characterized by their poor sanitation infrastructure, limited access to safe water, poor structural housing quality, and overcrowding. In 2012, they estimated that 863 million people in developing regions lived in slums, making up 33 % of their urban populations,20 further estimating that this population will reach two billion by 2025.21 The burden of undiagnosed or untreated hypertension in these communities is virtually unknown until they present to the formal health sector with severe and financially costly end-stage complications such as stroke, myocardial infarction, or renal disease.22
Brazil is a paramount example of a middle-income country where slums comprise a large portion of its urban area.23–25 The proportion of the urban population in Brazil grew from 45 to 84 % between 1960 and 2010.26 This growth occurred during a major demographic transition as the proportion of the population older than 60 years doubled during this same time frame.23 The majority of these recent migrants have settled in urban slums.27 Salvador, the capital of the state of Bahia, is the Brazilian city with the highest proportion of slum residents; the 2010 Census reported that 33 % of Salvador’s 2.64 million residents lived in slums.26,28
We report the findings of a cross-sectional community-based hypertension survey of slum residents in Salvador, Brazil. The findings from this study provide an understanding of the distribution and risk factors of a major chronic disease in an under-studied population and are relevant to other populations residing in poor urban areas worldwide.
Methods
Study Population
A cross-sectional, household hypertension screening survey was conducted in 2003 in Pau da Lima, a community situated on the periphery of Salvador. This community was established as a squatter settlement in the 1970s and meets the UN’s and the Brazilian Census Bureau’s operational criteria for an informal settlement.21,26 In 2003, Salvador’s Oswaldo Cruz Foundation (FIOCRUZ) established a study site within this community for a cohort study of urban leptospirosis.29–31 A household census by FIOCRUZ at that time identified 14,867 residents in 3068 households within the 0.46 km2 study area.
During implementation of the leptospirosis study, community leaders and resident associations identified hypertension as a major health problem in the community and requested that FIOCRUZ screen for the disease. Between January 2003 and December 2004, the study team subsequently visited all households in the study area and enrolled all consenting residents aged 18 years and older.
The Ethics Committees of FIOCRUZ, University of California, Berkeley, and Weill Medical College of Cornell University approved this study.
Data Collection
Arterial blood pressure was measured in all consenting participants during the initial house visits. Measurements were performed by trained community health workers with an aneroid sphygmomanometer (BDTM Sphygmomanometer, Becton, Dickinson and Company, New Jersey, USA) and taken in a sitting position after 10 min of rest and without recent history of exercise, smoking, or alcohol use. A standardized questionnaire was administered during interviews to ascertain demographic characteristics. Participants self-reported their race, education, and employment status. All individuals with an elevated blood pressure were referred for evaluation at the district health post. Follow-up home visits began in May 2004 for individuals with an elevated blood pressure measurement during their initial visit. At this time, further information was collected on the participant’s current health status including comorbidities such as diabetes, coronary artery disease, or stroke. Participants were asked about current medical care and treatment for hypertension as denoted by use of medications within the previous 7 days. Medications and prescriptions were reviewed at the time of follow-up interview to verify the use of medications specific to hypertension and diabetes.
Analysis
Hypertension was defined as having a systolic (SBP) and/or diastolic (DBP) pressure ≥140 or ≥90 mmHg, respectively.32,33 Mean SBP and DBP were calculated within each stratum of selected demographic characteristics, and 95 % confidence intervals (CI) were calculated by bootstrapping estimates 1000 times with replacement. Chi-squared tests were used to assess for significant differences between men and women in the prevalence of hypertension.
The Stata software package v.12.1 (StataCorp LP, Texas, USA) was used for all analyses. Sex-specific ordinary least-squares regression models relating risk factors for systolic and diastolic blood pressure were created using a reverse stepwise process, maintaining variables in the final models with a p value < 0.20, in addition to factors that have been previously confirmed to influence blood pressure (race).
Results
Household Survey
Visits to all 3068 households in the study site identified 7380 present and eligible adults. Among these, 5649 subjects (76.5 %) consented to participate in the survey. A higher proportion of females than males chose to participate (58.3 % of 5649 participants were female versus 34.4 % of 1731 non-participants). Mean age for participants and non-participants was 34.0 years (standard deviation (SD), 12.8 years) and 33.1 years (SD, 12.2 years), respectively. The age distribution of participants reflected the young age structure of slum populations; 3988 (70.6 %) were between 18 and 39 years of age. Four thousand and seventy subjects (72.1 %) reported mixed or white ancestry, and 1576 (27.9 %) reported black ancestry. Only 1433 (25.4 %) had, at a minimum, attended the Brazilian equivalent of high school (Table 1). The average per capita monthly income among participants was US$56.46 and included those who were unemployed, students, women caring for children at home, and non-pensioned retirees. Among these 3068 households, 91 and 57 % had access to potable water and were served by closed sewage systems, respectively.
TABLE 1.
Demographic characteristics of a cross-sectional survey of residents in the community of Pau de Lima, Salvador, Brazil, 2003
| Total | Women | Men | p value | |
|---|---|---|---|---|
| n (% total) | n (% total) | n (% total) | ||
| 5649 | 3291 (58.3) | 2358 (41.7) | <0.01 | |
| Age (years) | ||||
| 18–29 | 2529 (44.8) | 1472 (44.7) | 1057 (44.8) | 0.549 |
| 30–39 | 1459 (25.8) | 847 (25.7) | 612 (26.0) | |
| 40–49 | 963 (17.0) | 545 (16.6) | 418 (17.7) | |
| 50–59 | 444 (7.9) | 268 (8.1) | 176 (7.5) | |
| 60–69 | 176 (3.1) | 109 (3.3) | 67 (2.8) | |
| >70 | 78 (1.4) | 50 (1.5) | 28 (1.2) | |
| Race | ||||
| Mixed/other | 4070 (72.1) | 2416 (73.5) | 1654 (70.1) | <0.01 |
| Black | 1576 (27.9) | 872 (26.5) | 704 (29.9) | |
| Education | ||||
| Never studied | 381 (6.8) | 237 (7.20) | 144 (6.11) | |
| Elementary (complete or incomplete) | 3833 (67.9) | 2167 (65.9) | 1666 (70.7) | 0.01 |
| Some high school or beyond | 1433 (25.4) | 886 (26.9) | 547 (23.2) | |
| Monthly per capita income (US$ Jan 2004) | ||||
| Missing/not reported | 2113 (37.4) | 1607 (48.8) | 506 (21.5) | <0.01 |
| 0.01–57.7 | 870 (15.4) | 606 (18.4) | 264 (11.2) | |
| 57.8–69.2 | 952 (16.9) | 615 (18.7) | 337 (14.3) | |
| 69.3–100.9 | 735 (13.0) | 259 (7.9) | 476 (20.2) | |
| ≥100.9 | 979 (17.3) | 204 (6.20) | 775 (32.9) | |
| Employment Status | ||||
| Employed | ||||
| Formal sector | 1240 (21.9) | 462 (14.0) | 778 (33.0) | <0.01 |
| Informal sector | 2072 (36.7) | 1035 (31.5) | 1037 (44.0) | |
| Othera | 2336 (41.4) | 1793 (54.5) | 543 (23.0) | |
p values are presented comparing the distributions of each demographic variable between men and women
aIncludes students, retirees, women working at home, and the unemployed
Summary of Blood Pressure Measurements in the Population
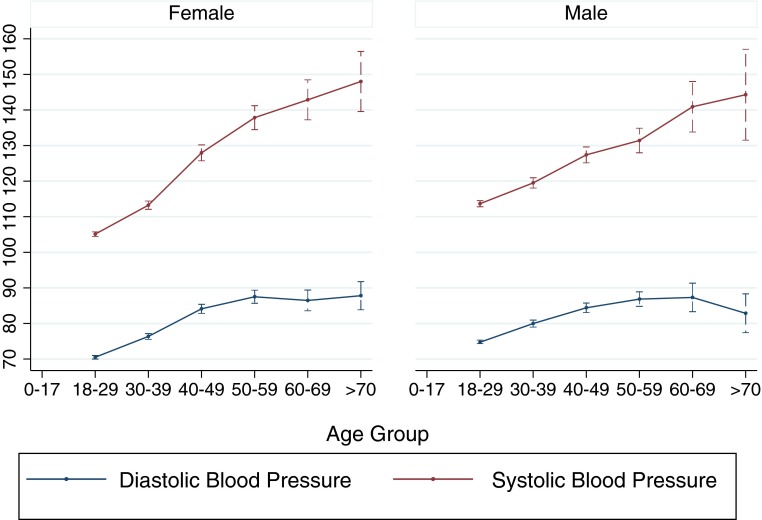
During the initial house visit 1192 (21 % of 5649) subjects had an elevated systolic or diastolic blood pressure. The follow-up visit (median interval between first and second visits of 299 days) was performed for 796 (66.8 % of 1192) subjects with an initial elevated blood pressure (Table 2). The mean systolic blood pressures among men and women were 120.1 and 115.6 mmHg, respectively, while the mean diastolic blood pressures were 79.2 and 76.4, respectively. Figure 1 shows age- and sex-specific systolic and diastolic blood pressures with standard deviation, indicating an age effect.
TABLE 2.
Systolic (SBP) and diastolic and blood pressure (DBP) in a cross-sectional survey of residents of the community of Pau de Lima, Salvador, Brazil, 2003
| Women | Men | p valuea | |||||
|---|---|---|---|---|---|---|---|
| SBP | DBP | SBP ≥ 140 or DBP ≥ 90 | SBP | DBP | SBP ≥ 140 or DBP ≥ 90 | ||
| Mean | Mean | Prevalence (95 % CI) | Mean | Mean | Prevalence (95 % CI) | ||
| Totalb | 115.6 | 76.4 | 19.8 (18.5–21.2) | 120.1 | 79.2 | 22.9 (21.1–24.5) | <0.01 |
| Age (years) | |||||||
| 18–29 | 105.1 | 70.5 | 4.1 (3.1–5.1) | 113.7 | 74.7 | 9.6 (7.8–11.3) | <0.01 |
| 30–39 | 113.2 | 76.4 | 16.1 (13.6–18.5) | 119.5 | 80.0 | 23.0 (19.7–26.4) | <0.01 |
| 40–49 | 128.0 | 84.1 | 40.0 (35.9–44.1) | 127.4 | 84.4 | 38.3 (33.8–42.8) | 0.59 |
| 50–59 | 137.8 | 87.5 | 53.7 (47.8–59.7) | 131.4 | 86.9 | 48.9 (41.5–56.3) | 0.32 |
| 60–69 | 142.8 | 86.5 | 56.9 (47.6–66.2) | 140.9 | 87.3 | 53.7 (42.0–65.4) | 0.68 |
| >70 | 148.0 | 87.8 | 66.0 (52.8–79.2) | 144.3 | 82.9 | 53.6 (35.8–71.3) | 0.28 |
| Race | |||||||
| Mixed/other | 114.8 | 76.0 | 19.0 (17.4–20.5) | 119.8 | 79.0 | 22.8 (20.7–24.9) | <0.01 |
| Black | 117.7 | 77.6 | 22.2 (19.5–25.0) | 120.6 | 79.6 | 23.0 (19.9–26.2) | 0.72 |
| Educational status | |||||||
| Never studied | 107.8 | 72.5 | 48.5 (42.3–54.7) | 116.9 | 84.9 | 45.8 (37.7–54.0) | <0.01 |
| Elementary (complete or incomplete) | 116.6 | 77.1 | 21.3 (19.6–23.1) | 120.1 | 79.2 | 23.0 (20.9–25.1) | 0.22 |
| Some high school or beyond | 134.8 | 84.9 | 8.6 (6.8–10.4) | 131.8 | 77.5 | 16.4 (13.4–19.5) | 0.61 |
| Monthly per capita income (US$ Jan 2004) | |||||||
| Missing/not reported | 113.6 | 75.1 | 17.1 (15.4–18.8) | 118.9 | 78.1 | 19.8 (16.4–23.2) | 0.17 |
| 0.01–57.7 | 114.2 | 76.2 | 17.5 (14.6–20.4) | 119.2 | 77.9 | 20.1 (15.3–24.9) | 0.37 |
| 57.8–69.2 | 121.7 | 79.5 | 28.8 (25.2–32.4) | 120.1 | 78.9 | 23.1 (18.6–27.7) | 0.06 |
| 69.3–100.9 | 115.3 | 77.1 | 20.5 (15.7–25.2) | 118.7 | 78.8 | 20.6 (16.9–24.3) | 0.97 |
| ≥100.9 | 116.8 | 77.4 | 20.6 (15.2–25.9) | 121.9 | 80.6 | 27.0 (24.0–30.2) | 0.06 |
| Employment status | |||||||
| Employed | |||||||
| Formal sector | 116.0 | 77.3 | 19.0 (15.5–22.6) | 120.0 | 79.5 | 24.0 (21.1–27.0) | 0.04 |
| Informal sector | 115.2 | 76.6 | 19.3 (17.0–21.7) | 119.1 | 78.8 | 21.3 (18.8–23.9) | 0.26 |
| Otherc | 115.7 | 76.1 | 20.4 (18.5–22.2) | 122.0 | 79.3 | 24.1 (20.5–27.7) | 0.06 |
a p values are presented for comparisons of the prevalence of elevated blood pressure between men and women
bMen were significantly more likely to have an SBP ≥ 140 or DBP ≥ 90 (p < 0.01)
cIncludes students, retirees, women working at home, and the unemployed
FIG. 1.
Age and sex-specific systolic and diastolic blood pressures with 95 % confidence intervals in a cross-sectional study of residents of the community of Pau da Lima, Salvador, Brazil, 2003.
Factors Associated with High Blood Pressure
Table 3 shows sex-specific multivariable linear models with factors associated with changes in blood pressure. Models were developed for both systolic and diastolic blood pressure, but results and models were nearly identical; therefore, only systolic blood pressure is presented. Increasing age and lack of a formal education were correlated with increased systolic blood pressure. Each year of age corresponded with increases in systolic blood pressure of 0.61 and 0.57 mmHg in men and women, respectively (p < 0.05). The lack of any schooling was correlated with increasing systolic blood pressures in both men and women after adjusting for the influence of other covariates (men, β = 3.58 [95 % CI, −0.03, 7.2]; women, β = 9.67 [95 % CI, 0.71, 18.64]). We did not identify significant associations (p < 0.05) between per capita monthly income or employment status and systolic blood pressure. Males who reported that they were black had increased systolic blood pressures albeit not statistically significant, which was not observed for black women (black men, β = 1.57 [95 % CI, −0.07, 3.22]; black women, β = 2.23 [95 % CI, −2.55, 7.00]).
TABLE 3.
Multivariate association between risk factors and systolic blood pressure in men and women in a cross-sectional study of residents of the community of Pau de Lima, Salvador, Brazil, 2003
| Women (R 2 = 0.12) | Men (R 2 = 0.15) | |||||
|---|---|---|---|---|---|---|
| β | 95 % CI | β | 95 % CI | |||
| Upper | Lower | Upper | Lower | |||
| Age (continuous, years) | 0.57 | 0.37 | 0.76 | 0.61 | 0.54 | 0.67 |
| Currently on anti-hypertensive medicationa | −0.73 | −1.47 | 0.01 | – | – | – |
| Education (reference high school or beyond) | ||||||
| Elementary (complete or incomplete) | 5.83 | −1.42 | 13.09 | −0.76 | −2.61 | 1.09 |
| Never studied | 9.67 | 0.71 | 18.64 | 3.58 | −0.03 | 7.2 |
| Race (reference not black) | 2.23 | −2.55 | 7.00 | 1.57 | −0.07 | 3.22 |
aMedication was not associated with systolic blood pressure in men (p > 0.20)
Medical Care and Complications
Women who used anti-hypertensive medication had a systolic blood pressure that was on average 0.73 mmHg lower than those that did not (95 % CI, 0.01, 1.47). Men who used anti-hypertensive medication did not experience a significant decrease in systolic blood pressure (p < 0.05). Among the 747 subjects with an elevated systolic or diastolic blood pressure and complete follow-up interview information, 275 (34.5 %) were not aware that they had an elevated blood pressure at the time of the survey (Table 4). Among those with an elevated blood pressure, 289 (36.3 %) were using an anti-hypertensive medication. Men were significantly less likely than women to be aware of their elevated blood pressure (51.3 % compared with 75.8 % among women, p < 0.01) and significantly less likely to be using anti-hypertensive medication if they were aware (19.3 versus 48.8 % for women, p < 0.01) (Table 4). Women had 3.0 times the odds (95 % CI, 1.26, 7.33) of having previously suffered a stroke compared to men (prevalence of 5.2 versus 1.8 %).
TABLE 4.
Medical care and medical history among follow-up of residents of the community of Pau de Lima, Salvador, Brazil, with elevated blood pressure in 2003
| Characteristic | Total (n = 796) | Women (n = 459) | Men (n = 337) | p value (men vs. women) |
|---|---|---|---|---|
| n (% of total responding) | ||||
| Aware of hypertension status | 521 (65.5) | 348 (75.8) | 173 (51.3) | <0.01 |
| Previously receiving treatment for hypertension (% aware) | 279 (53.6) | 210 (60.3) | 69 (39.9) | <0.01 |
| Currently on anti-hypertensive medication | 289 (36.3) | 224 (48.8) | 65 (19.3) | <0.01 |
| Past history | ||||
| Diabetes | 55 (6.9) | 38 (8.3) | 17 (5.0) | 0.09 |
| Myocardial infarction | 23 (2.9) | 14 (3.1) | 9 (2.7) | 0.51 |
| Stroke | 30 (3.8) | 24 (5.2) | 6 (1.8) | 0.05 |
Discussion
This survey assessed the prevalence of elevated blood pressure and its potential impact in a slum population in the largest city in northeastern Brazil. Our household survey of adults 18 years and older estimated that 21 % of residents had a high blood pressure in 2003. The results from this study suggest that the overall prevalence of hypertension was lower in this population than that which has been found in other temporally similar non-self-reported studies in the general Brazilian population.34–36 In the current study, among the population greater than 50 years old, prevalence surpassed 50 %. The only other study, to our knowledge, presenting age-specific estimates in the general population of Salvador reported a prevalence of approximately 60 % in those older than 50.35
The reported differences in hypertension prevalence are due, in part, to the differences in the age structures of study populations, varying survey methodologies, and differences in the way in which disease outcome was defined. In our study, 70 % of subjects were between 18 and 39 years of age, reflecting the younger age demographic of slums.37
A summary of 18 studies of the disease conducted in Brazil found a prevalence that ranged from 21 to 29 %.24 However, the majority of these studies came from non-slum populations in the more heavily industrialized southeast.14,38 The phone-based, self-reported Vigitel survey reported a prevalence of 21.7 % in Salvador in 2006. However, self-reported surveys rely on the assumption that a medical professional is regularly screening individuals for the disease.
In Salvador, a survey was conducted in 2002 that recruited individuals from shopping malls.36 This study found a hypertension prevalence of 34 %, but sampling from this population could be prone to a significant amount of selection bias. Those choosing to participate in a study in a shopping mall might be wealthier and more concerned about high blood pressure. A separate study in Salvador reported higher overall, age- and sex-specific rates of hypertension but sampled from a previous project assessing cardiovascular risk factors, possibly elevating estimates.35
The prevalence of hypertension in the Pau da Lima population in Salvador was lower than that previously reported in other Latin American countries, where the age-standardized prevalence in Mexico, Paraguay, and Venezuela has been reported to be between 30 and 42 %.39 A report by the WHO in 2013 indicated that Latin America had the lowest prevalence of hypertension and other cardiovascular diseases, while the African region had the highest rates.4
The disparity between the present study and other previous studies carried out in Salvador suggests heterogeneity in the prevalence of high blood pressure within similar geographic areas, reflecting the importance of studies that elucidate location-specific risk factors and disease burden. These findings further undermine the utility of broad country or even statewide surveys that assess disease burden. This is especially true in informal communities such as slums where the terrain, population, municipal services, and other important demographic and infrastructure characteristics vary dramatically.37
Our study found that any elementary education compared to none at all was enough to reduce blood pressure. This indicates that even within this slum community characterized by high levels of absolute poverty, differences in educational status influence blood pressure. This may reflect differences in awareness of the disease or the increased prevalence of factors such as poor diet and lack of exercise among members of this population with lower levels of education.40 However, the fit of the linear models was extremely poor in both women and men (R2 of 0.12 and 0.15) suggesting that there were other unmeasured factors contributing to these differences.
Interestingly, although males developed hypertension earlier and were better educated than women, they were still less likely than hypertensive women to be aware of their condition, receive medical care, or use anti-hypertensive medications. Studies have consistently shown that women are more aware and better treated for their hypertension, despite often reporting higher disease prevalence than men, particularly in older age groups.8,16,41,42 However, the factors affecting gender differences in prevalence, awareness, and treatment are generally poorly clarified.43 In Brazil, healthcare access should be universal and free through the national health plan called the Sistema Único de Saúd or SUS, and men should theoretically have the same level of access to primary care as women. However, the use of health services has been consistently shown to be lower in men than in women in Brazil and was affected by age, education, income, and type of employment (i.e., formal, informal).44–46 Sex-specific disparities in our study population, where the majority were between 18 and 39 years, may also have been related to targeted efforts by the Brazilian health sector to make health care available to women of childbearing age. Women of reproductive age have been shown to have a higher prevalence of access to health services, greater access to information, and may more easily perceive health risks. Men tend to seek medical assistance with more severe or morbid diseases.47
The role of gender in the development and control of hypertension is complex, but our study emphasizes the need for targeting young males in urban slums for the screening, prevention, and management of the disease. This is particularly important because almost 10 % of the male population under the age of 30 had an elevated blood pressure. Similarly, high rates of hypertension among men have been shown in military recruits in Brazil and are not uncommon in other countries.18,48 It is also worthy of note that compared to the general population studied in the Vigitel study, there was higher prevalence of elevated blood pressures in all age groups of men in this slum population—except for the oldest age group greater than 70 years where there was a small sample size.49 These findings may also reflect the prevalence of factors associated with lifestyle in slum populations, such as diet high in sodium and fats, tobacco use, alcohol consumption, central obesity, and physical activity, and are certainly worthy of further study.18,50,51
Despite the relatively high awareness of hypertension in this community, which is comparable to awareness in the USA,32 slum residents had low levels of care for their hypertension, including among those with severe hypertension (defined as a systolic and diastolic blood pressure greater than 180 and 110, respectively). Less than one in five men had previously received any care for their hypertension despite being aware of their disease status. Despite this, the population in Pau de Lima had much higher levels of awareness than in other reported studies carried out in other countries with similar economic indicators.40,41 In slums, disease awareness may not translate into effective care, particularly when local private pharmacies or informal health providers distribute it.
Cardiovascular and cerebrovascular diseases are the two principal causes of mortality worldwide.1 The increasing burden of these diseases in countries such as Brazil has been attributed to changing population demographics, urbanization, and lifestyle changes (i.e., dietary shifts away from traditional foods, decreasing physical activity, and increased tobacco use).52–54 In recent years, public health experts have called attention to the potential impact associated with non-communicable diseases in slums,55 and yet we know little about the burden of these chronic health conditions and their associated sequelae in this marginalized population until residents enter the formal health sector. This tends to occur after an individual is already suffering from costly end-stage disease. Slum residents experience restricted access to formal health care, and as a result, they tend to enter the formal health sector for treatment later than their non-slum urban counterparts.56 As medically certified information is available for less than 30 % of deaths per year worldwide, the cause of death would only be correctly ascertained for individuals who access the formal health sector.57 Reports such as the Global Burden of Disease Study underestimate the disease burden in these populations as they are underrepresented in medical records. Our findings suggest that slum populations are making a substantial, yet underreported, contribution to the ongoing global epidemic of non-communicable diseases. The development of slum-specific health policies is essential to meet the World Health Organization’s “25 by 25” goal, a 25 % reduction in non-communicable diseases among adults between 30 and 70 years of age by 2025.58
Limitations
This was a community-based screening survey in a cohort of nearly 15,000 residents in one slum community originally selected due to a high prevalence of leptospirosis. The characteristics of this community are consistent with those of similar poor urban communities in Salvador and Brazil; however, the selection of the community may affect the generalizability of the results. The majority (70 %) of those surveyed in this community-based study were between the ages of 18 and 39 years, which makes comparisons to studies in the general population difficult.
Although all personnel were trained in using the manual sphygmomanometer, technical skill and knowledge between users may have affected the accuracy of pressure measurements. Traditionally, two repeated blood pressure measurements on two occasions are used to diagnose hypertension; however, other studies in low- and middle-income regions report hypertension based on one measurement.59 The US National Health and Nutrition Examination Survey (NHANES) study also does not consistently use multiple measurements.60
The lack of information of patients taking medications on the first survey may underestimate the prevalence of hypertension in this community. Those with elevated blood pressure on the first survey were followed up with questions about their use of medications for hypertension and any cardiovascular complications. Loss to follow-up was 38.8 % (these medication and complications data were only available for 730 of the original 1192 individuals with an elevated systolic and/or diastolic blood pressure). Additionally, it is not possible to establish a causal or temporal link between cardiovascular outcomes and hypertension in this study.
Acknowledgments
Other members of the Pau da Lima Urban Health Team included Rosan Barbosa, Reinaldo Barreto, Jorge Costa, Maria Raimunda da Cruz, Ana Carla Duarte, Leila Gouveia, Analéa Lima, Simone Nascimento, Osmar Paixão, Amaro Silva, and Érika Sousa from the Oswaldo Cruz Foundation and the Residents’ Association of Pau da Lima, Salvador, Brazil. We would like to thank Claudio Pereira da Sá, Edilane Gouveia, and Marília Sá Carvalho for the assistance with the preliminary statistical analysis, and Salvatore Cala and Emanuel Costa for their support with data management and administration, as well as the Pau da Lima Health District and the Municipal Secretary of Health of Salvador for coordinating care for those enrolled in the study. Lastly, this work could not have been accomplished without the joint collaborative effort of the Residents’ Associations, leaders, and members of the Pau da Lima community.
Author Contributions
AK, RF, VC, and FS formulated the original idea and designed the study together with the resident associations of Pau da Lima. RF, SM, VC, AM, RR, and FS collected the data. MR and AK supervised the field team. AU and RS performed the data analysis. AU wrote the initial draft with RS. AK, LR, and GR critically reviewed the manuscript and helped to prepare the final version. All authors approved the final manuscript prior to submission.
Funding
This work was supported by the Brazilian National Research Council (grant 300.861/96-6), Oswaldo Cruz Foundation (grant 0250.250.102), and the US National Institutes of Health (grants R01 AI052473, U01 AI088752, D43 TW00919, R25 TW009338). A Unger was supported by a Fogarty International Center/Ellison Medical Foundation Fellowship in Global Health and Clinical Research (grant TW00018).
Footnotes
Ridalva D. M. Felzemburgh and Robert E. Snyder contributed equally to this work.
References
- 1.Murray CJL, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. 2013;380(9859):2197–2223. doi:10.1016/S0140-6736(12)61689-4. [DOI] [PubMed]
- 2.Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Fact sheet: the top ten causes of death. Fact sheet. 2008;(310). Available at: http://www.who.int/healthinfo/global_burden_disease/cod_2008_sources_methods.pdf. Accessed 23 Aug 2014.
- 4.World Health Organization. A global brief on hypertension: silent killer, global public health crisis. Geneva: WHO Press; 2013. Available at: http://www.who.int/cardiovascular_diseases/publications/global_brief_hypertension/en/.
- 5.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365(9455):217–223. doi:10.1016/S0140-6736(05)17741-1. [DOI] [PubMed]
- 6.Strong K, Mathers C, Leeder S, Beaglehole R. Preventing chronic diseases: how many lives can we save? Lancet. 2005;366(9496):1578–1582. doi:10.1016/S0140-6736(05)67341-2. [DOI] [PubMed]
- 7.Horton R. The neglected epidemic of chronic disease. Lancet. 2005;366(9496):1514. doi:10.1016/S0140-6736(05)67454-5. [DOI] [PubMed]
- 8.Kearney PM, Whelton M, Reynolds K, Whelton PK, He J. Worldwide prevalence of hypertension: a systematic review. J Hypertens. 2004;22(1):11–19. doi: 10.1097/00004872-200401000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Tailakh A, Evangelista LS, Mentes JC, Pike NA, Phillips LR, Morisky DE. Hypertension prevalence, awareness, and control in Arab countries: a systematic review. Nurs Health Sci. 2013;16(1):126–130. doi: 10.1111/nhs.12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sitthi-Amorn C, Chandraprasert S, Bunnag SC, Plengvidhya CS. The prevalence and risk factors of hypertension in Klong Toey slum and Klong Toey government apartment houses. Int J Epidemiol. 1989;18(1):89–94. doi: 10.1093/ije/18.1.89. [DOI] [PubMed] [Google Scholar]
- 11.Daniel OJ, Adejumo OA, Adejumo EN, Owolabi RS, Braimoh RW. Prevalence of hypertension among urban slum dwellers in Lagos, Nigeria. J Urban Health. 2013 doi: 10.1007/s11524-013-9795-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banerjee A, Nikumb VB, Thakur RP. Health problems among the elderly: a cross-sectional study. Ann Med Health Sci Res. 2013;3(1):19. doi: 10.4103/2141-9248.109466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anchala R, Kannuri NK, Pant H, et al. Hypertension in India. J Hypertens. 2014;32(6):1170–1177. doi: 10.1097/HJH.0000000000000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Picon RV, Fuchs FD, Moreira LB, Riegel G, Fuchs SC. Trends in prevalence of hypertension in Brazil: a systematic review with meta-analysis. Baradaran HR, ed. PLoS ONE. 2012;7(10):e48255. doi:10.1371/journal.pone.0048255.s004. [DOI] [PMC free article] [PubMed]
- 15.Ayah R, Joshi MD, Wanjiru R, et al. A population-based survey of prevalence of diabetes and correlates in an urban slum community in Nairobi, Kenya. BMC Public Health. 2013;13(1):371. doi: 10.1186/1471-2458-13-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van de Vijver SJM, Oti SO, Agyemang C, Gomez GB, Kyobutungi C. Prevalence, awareness, treatment and control of hypertension among slum dwellers in Nairobi, Kenya. J Hypertens. 2013;31(5):1018–1024. doi: 10.1097/HJH.0b013e32835e3a56. [DOI] [PubMed] [Google Scholar]
- 17.Marins VMR, Almeida RMVR, Pereira RA, Sichieri R. The association between socioeconomic indicators and cardiovascular disease risk factors in Rio de Janeiro, Brazil. J Biosoc Sci. 2006;39(02):221. doi: 10.1017/S0021932006001246. [DOI] [PubMed] [Google Scholar]
- 18.Joshi M, Ayah R, Njau E, et al. Prevalence of hypertension and associated cardiovascular risk factors in an urban slum in Nairobi, Kenya: a population-based survey. BMC Public Health. 2014;14(1):1177. doi: 10.1186/1471-2458-14-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ribeiro GS, Tartof SY, Oliveira DWS, et al. Surgery for valvular heart disease: a population-based study in a Brazilian urban center. Akhter SA, ed. PLoS ONE. 2012;7(5):e37855–7. doi:10.1371/journal.pone.0037855. [DOI] [PMC free article] [PubMed]
- 20.United Nations Human Settlement Programme (UN-Habitat). State of the world's cities 2012/2013. Nairobi, Kenya: UN-Habitat; 2012.
- 21.United Nations Human Settlement Programme (UN-Habitat). The challenge of slums: global report on human settlements 2003. London and Sterling, VA, USA: Earthscan Publications Ltd; 2003.
- 22.Riley LW, Ko AI, Unger A, Reis MG. Slum health: diseases of neglected populations. BMC Int Health Hum Rights. 2007;7:2. doi: 10.1186/1472-698X-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paim J, Travassos C, Almeida C, Bahia L, Macinko J. The Brazilian health system: history, advances, and challenges. Lancet. 2011;377(9779):1778–1797. doi:10.1016/S0140-6736(11)60054-8. [DOI] [PubMed]
- 24.Schmidt MI, Duncan BB, Menezes AM, et al. Chronic non-communicable diseases in Brazil: burden and current challenges. Lancet. 2011;377(9781):1949–1961. doi: 10.1016/S0140-6736(11)60135-9. [DOI] [PubMed] [Google Scholar]
- 25.Victora CG, Aquino EM, do Carmo Leal M, Monteiro CA, Barros FC, Szwarcwald CL. Maternal and child health in Brazil: progress and challenges. Lancet. 2011;377(9780):1863–1876. doi: 10.1016/S0140-6736(11)60138-4. [DOI] [PubMed] [Google Scholar]
- 26.Instituto Brasileiro de Geografia e Estatística (IBGE). Censo Demográfico: Aglomerados Subnormais Primeiros Resultados. Rio de Janeiro: IBGE; 2010. [PubMed]
- 27.Patel RB, Burke TF. Urbanization—an emerging humanitarian disaster. N Engl J Med. 2009;361(8):741–743. doi: 10.1056/NEJMp0810878. [DOI] [PubMed] [Google Scholar]
- 28.Hacker KP, Seto KC, Costa F, et al. Urban slum structure: integrating socioeconomic and land cover data to model slum evolution in Salvador, Brazil. Int J Health Geogr. 2013;12(1):45. doi: 10.1186/1476-072X-12-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reis RB, Ribeiro GS, Felzemburgh RDM, et al. Impact of environment and social gradient on Leptospira infection in urban slums. Gurtler RE, ed. PLoS Negl Trop Dis. 2008;2(4):e228. doi:10.1371/journal.pntd.0000228. [DOI] [PMC free article] [PubMed]
- 30.Shei A, Costa F, Reis MG, Ko AI. The impact of Brazil’s Bolsa Família conditional cash transfer program on children’s health care utilization and health outcomes. BMC Int Health Hum Rights. 2014;14(1):10. doi: 10.1186/1472-698X-14-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Felzemburgh RDM, Ribeiro GS, Costa F, et al. Prospective study of leptospirosis transmission in an urban slum community: role of poor environment in repeated exposures to the Leptospira agent. PLoS Negl Trop Dis. 2014;8(5):e2927. doi: 10.1371/journal.pntd.0002927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chobanian AVA, Bakris GLG, Black HRH, et al. Seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42(6):1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 33.James PA, Oparil S, Carter BL, et al. Evidence-based guideline for the management of high blood pressure in adults. JAMA. 2014 doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 34.Ministério da Saúde, Secretaria de Gestão Estratégica e Participativa, Secretaria de Vigilância em Saúde. Vigitel Brasil 2007: Vigilância De Fatores De Risco E Proteção Para Doenças Crônicas Por Inquérito Telefônico. Ministério da Saúde: Brasília; 2008.
- 35.Lessa I, Magalhães L, Araújo MJ, de Almeida Filho N, Aquino E, Oliveira MMC. Arterial hypertension in the adult population of Salvador (BA)–Brazil. Arq Bras Cardiol. 2006;87(6):747–756. doi: 10.1590/S0066-782X2006001900011. [DOI] [PubMed] [Google Scholar]
- 36.Guimarães AC. Hypertension in Brazil. J Hum Hypertens. 2002;16(Suppl 1):S7–S10. doi: 10.1038/sj.jhh.1001332. [DOI] [PubMed] [Google Scholar]
- 37.Snyder RE, Jaimes G, Riley LW, Faerstein E, Corburn J. A comparison of social and spatial determinants of health between formal and informal settlements in a large metropolitan setting in Brazil. J Urban Health. 2014;91(3):432–445. doi: 10.1007/s11524-013-9848-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lessa I. Doenças crônicas não-transmissíveis no Brasil: um desafio para a complexa tarefa da vigilância. Cien Saude Colet. 2004;9(4):931–943. doi: 10.1590/S1413-81232004000400014. [DOI] [Google Scholar]
- 39.Lanas F, Avezum A, Bautista LE, et al. Risk factors for acute myocardial infarction in Latin America: the INTERHEART Latin American study. Circulation. 2007;115(9):1067–1074. doi: 10.1161/CIRCULATIONAHA.106.633552. [DOI] [PubMed] [Google Scholar]
- 40.Bovet P, Ross AG, Gervasoni JP, et al. Distribution of blood pressure, body mass index and smoking habits in the urban population of Dar es Salaam, Tanzania, and associations with socioeconomic status. Int J Epidemiol. 2002;31(1):240–247. doi:10.1093/ije/31.1.240. [DOI] [PubMed]
- 41.Fuentes R, Ilmaniemi N, Laurikainen E, Tuomilehto J, Nissinen A. Hypertension in developing economies: a review of population-based studies carried out from 1980 to 1998. J Hypertens. 2000;18(5):521–529. doi: 10.1097/00004872-200018050-00003. [DOI] [PubMed] [Google Scholar]
- 42.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation. 2013;127(1):e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Doumas M, Papademetriou V, Faselis C, Kokkinos P. Gender differences in hypertension: myths and reality. Curr Hypertens Rep. 2013;15(4):321–330. doi: 10.1007/s11906-013-0359-y. [DOI] [PubMed] [Google Scholar]
- 44.de Moraes SA, Lopes DA, de Freitas ICM. Sex-specific differences in prevalence and in the factors associated to the search for health services in a population based epidemiological study. Rev Bras Epidemiol. 2014;17(2):323–340. doi: 10.1590/1809-4503201400020004ENG. [DOI] [PubMed] [Google Scholar]
- 45.de Oliveira Campos Miquilin I, Marin-Leon L, Monteiro MI, Correa Filho HR. Inequalities in health services access and use among formal, informal, and unemployed workers, based on data from the Brazilian National Household Sample Survey, 2008. Cad Saude Publica. 2013;29(7):1392–1406. doi: 10.1590/S0102-311X2013001100013. [DOI] [PubMed] [Google Scholar]
- 46.de Moreira JPL, de Moraes JR, Luiz RR. Use of medical consultations and the occurrence of systemic arterial hypertension in urban and rural areas of Brazil, according to PNAD data 2008. Cien Saude Colet. 2011;16(9):3781–3793. doi: 10.1590/S1413-81232011001000014. [DOI] [PubMed] [Google Scholar]
- 47.Mendoza-Sassi R, Béria JU, Barros AJD. Outpatient health service utilization and associated factors: a population-based study. Rev Saude Publica. 2003;37(3):372–378. doi: 10.1590/S0034-89102003000300017. [DOI] [PubMed] [Google Scholar]
- 48.Gao Y, Chen G, Tian H, et al. Prevalence of hypertension in China: a cross-sectional study. PLoS ONE. 2013 doi: 10.1371/journal.pone.0065938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ministério da Saúde, Secretaria de Gestão Estratégica e Participativa, Secretaria de Vigilância em Saúde. Vigitel Brasil 2012: Vigilância De Fatores De Risco E Proteção Para Doenças Crônicas Por Inquérito Telefônico. Ministério da Saúde: Brasília; 2013.
- 50.Heitzinger K, Montano SM, Hawes SE, Alarcón JO, Zunt JR. A community-based cluster randomized survey of noncommunicable disease and risk factors in a peri-urban shantytown in Lima, Peru. BMC Int Health Hum Rights. 2014;14(1):19. doi: 10.1186/1472-698X-14-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anand K, Shah B, Yadav K, et al. Are the urban poor vulnerable to non-communicable diseases? A survey of risk factors for non-communicable diseases in urban slums of Faridabad. Natl Med J India. 2007;20(3):115–120. [PubMed] [Google Scholar]
- 52.Mendis S, Chestnov O. The global burden of cardiovascular diseases: a challenge to improve. Curr Cardiol Rep. 2014;16(5):486. doi: 10.1007/s11886-014-0486-3. [DOI] [PubMed] [Google Scholar]
- 53.Tibazarwa KB, Damasceno AA. Hypertension in developing countries. Can J Cardiol. 2014;30(5):527–533. doi: 10.1016/j.cjca.2014.02.020. [DOI] [PubMed] [Google Scholar]
- 54.Yusuf S, Reddy S, Ounpuu S, Anand S. Global burden of cardiovascular diseases: part i: general considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation. 2001;104(22):2746–2753. doi: 10.1161/hc4601.099487. [DOI] [PubMed] [Google Scholar]
- 55.Sclar ED, Garau P, Carolini G. The 21st century health challenge of slums and cities. Lancet. 2005;365(9462):901. doi: 10.1016/S0140-6736(05)71049-7. [DOI] [PubMed] [Google Scholar]
- 56.Unger A, Riley LW. Slum health: from understanding to action. PLoS Med. 2007;4(10):1561–1566. doi: 10.1371/journal.pmed.0040295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kanungo S, Tsuzuki A, Deen JL, et al. Use of verbal autopsy to determine mortality patterns in an urban slum in Kolkata, India. Bull World Health Organ. 2010;88(9):667–674. doi: 10.2471/BLT.09.073742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hunter DJ, Reddy KS. Noncommunicable Dis. 2013;369(14):1336–1343. doi: 10.1056/NEJMra1109345. [DOI] [PubMed] [Google Scholar]
- 59.Awad M, Ruzza A, Mirocha J, et al. Prevalence of hypertension in the Gambia and Sierra Leone, western Africa: a cross-sectional study. Cardiovasc J Afr. 2014;25:1–10. doi: 10.5830/CVJA-2014-058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nwankwo T, Yoon SS, Burt V, Gu Q. Hypertension among adults in the United States: national health and nutrition examination Survey, 2011–2012. NCHS Data Brief. 2013;133:1–8. [PubMed] [Google Scholar]