Abstract
Regular physical exercise has been shown to be one of the most important lifestyle influences on improving functional performance, decreasing morbidity and all causes of mortality among older people. However, it is known that acute physical exercise may induce an increase in oxidative stress and oxidative damage in several structures, including DNA. Considering this, the purpose of this study was to identify the effects of 16 weeks of combined physical exercise in DNA damage and repair capacity in lymphocytes. In addition, we aimed to investigate the role of oxidative stress involved in those changes. Fifty-seven healthy men (40 to 74 years) were enrolled in this study. The sample was divided into two groups: the experimental group (EG), composed of 31 individuals, submitted to 16 weeks of combined physical exercise training; and the control group (CG), composed of 26 individuals, who did not undergo any specifically orientated physical activity. We observed an improvement of overall physical performance in the EG, after the physical exercise training. A significant decrease in DNA strand breaks and FPG-sensitive sites was found after the physical exercise training, with no significant changes in 8-oxoguanine DNA glycosylase enzyme activity. An increase was observed in antioxidant activity, and a decrease was found in lipid peroxidation levels after physical exercise training. These results suggest that physical exercise training induces protective effects against DNA damage in lymphocytes possibly related to the increase in antioxidant capacity.
Keywords: Physical exercise training, DNA damage, FPG-sensitive sites, DNA repair, Antioxidant capacity, MDA
Introduction
It is well known that physical exercise induces organism changes that may vary between damage, improved function, or even no changes, depending on workload adjustment to each individual level of fitness. Related with that, clinical and epidemiological studies suggest that beneficial effects of regular physical exercise may depend on the intensity or the amount of work performed during physical exercise (Kemi et al. 2005; Matsuo et al. 2014; ACSM. American College of Sports Medicine position stand 2009). Although several studies confirm the benefits of regular physical exercise, it has also been shown that above certain intensity and duration, acute physical exercise may induce an increase in the production of reactive oxygen species (ROS). It is well known that ROS could react with several different organic structures, such as lipids, proteins, enzymes, nucleic acids, and other molecules, causing dangerous changes and cell dysfunction (Beckman and Ames 1998; Ortenblad et al. 1997). The possible injuries caused, directly or indirectly, by ROS on DNA are predominantly single strand breaks and modified bases (Lombard et al. 2005). 8-Oxoguanine (8-oxoG) has been considered as one of the most important damages found (Wilson et al. 2003). The 8-oxoG originates from the reaction of the hydroxyl radical (OH•) with the guanine base. This is one of the most abundant and potentially mutagenic lesions, and therefore has been used as an oxidative stress biomarker (Kasai et al. 1986) that can be assessed by different techniques, namely the formamidopyrimidine DNA glycosylase (FPG) by the comet assay.
The increase in oxidative stress state induced by the physical exercise may vary with the characteristics of the exercise, such as intensity, time, and frequency (Fisher-Wellman and Bloomer 2009). It is widely accepted that oxidative stress results in molecular damage, some of which may accumulate with age, probably causing progressive physiological attrition increasing the susceptibility to disease and risk of death (Metcalf et al. 2002). However, oxidative stress is also responsible for the stimulation of several cell signaling pathways, which may result in cell adaptation leading to an improved resistance to stress. Related to that, the effects of regular physical exercise in the oxidant/antioxidant state have been studied, and an up-regulation of the related mechanisms by the elevation of transcriptional factors of several antioxidant enzymes, has been found in some tissues (Siu et al. 2011; Radak et al. 2001).
Most of the studies concerned with physical exercise and related oxidative stress only considered aerobic exercise, which is far from the guidelines proposed by the ACSM for health benefits (Chodzko-Zajko et al. 2009; Garber et al. 2011). These guidelines state that the exercise prescription for older adults should include aerobic exercise, muscle-strengthening exercises, and flexibility exercises. Aerobic exercise improves muscle mitochondria density, as well as bioenergetics capacity, leading to a more efficient energetic state of the cell (Trost et al. 2005). Moreover, some studies confirmed a decrease in cell ROS production of different tissues (Trost et al. 2005; Alessio 1993). However, only a few studies (Parise et al. 2005; Vincent et al. 2006) described changes in oxidative stress-related variables with strength training.
Although different perspectives and mechanisms have been proposed to explain the occurrence of DNA mutations, it is possible that oxidative stress plays an important role in exercise-related DNA damage (Shigenaga et al. 1994; Mergener et al. 2009). It is widely described that chronic exercise reduces oxidative stress and damage, both by decreasing ROS production and increasing antioxidant capacity, and improves mitochondria efficiency in several organs and systems (Ascensao et al. 2003). Considering this, it is expected that exercise may decrease nuclear DNA damage, reducing the risk of developing cell mutations associated to several diseases. Although physical fitness has already been inversely related with DNA damage and mitochondria ROS production, revealing a positive effect on mitochondria function (Mota et al. 2010), it is not possible to conclude that exercise is responsible for these changes since no physical exercise training was implemented. It is likely that aerobic fitness is related to higher antioxidant capacity, reduced DNA damage, or with increased DNA repair capacity, which would favor a lower nuclear DNA damage. In fact, it seems reasonable to suppose that a higher repair rate will lead to a decrease in the level of damage and considering the oxidative stress mechanisms, the more ROS is produced, the more repair should be induced, and consequently, the level of damage might be kept constant (Collins and Gaivao 2007). Concerning the role of physical exercise in the DNA repair mechanisms, very little has yet been studied. Considering that chronic exercise increases antioxidant capacity, it seems likely to expect a reduction of DNA damage resulting from increase in both repair and antioxidant capacity (Collins and Gaivao 2007).
This study aimed to identify the effects of 16 weeks of combined physical exercise training on human lymphocytes DNA damage and DNA repair, considering physical fitness improvement. In addition, we aimed to investigate the role of oxidative stress in such changes.
Methods
Sample
Fifty-seven healthy Caucasian men, between 40 and 74 years old, were randomly divided into two groups: the experimental group (EG), composed of 31 individuals, submitted to a training of 16 weeks of combined physical exercise; and the control group (CG), composed of 26 individuals, who did not undergo any specifically orientated physical activity. Efforts were made to recruit subjects in order to form comparable groups. Before inclusion in the study, all candidates were thoroughly screened by a physician and subjected to an individual interview (face-to-face questionnaire) to address lifestyle habits. Inclusion criteria were men aged over 40 years, without any health condition that could interfere with performance in the cardiorespiratory exercise test. Exclusion criteria included metallic prosthesis implants, artificial pacemakers, walking only with assistance, and metabolic or endocrine disorders known to affect the musculoskeletal system. The experimental procedures were approved following the Helsinki Declaration (UNESCO. Universal declaration on bioethics and human rights 2006) and have been performed with the approval of the Ethics Committee of Research Centre in Sports, Health and Human Development, of the University of Trás-os-Montes and Alto Douro (reference number 052012). Written informed consent was obtained from each participant for the permission to use their information for the present report.
Testing procedures
The tests were applied to both groups before and after the experimental period (pre- and post-training). Considering that physical fitness tests were used as an indicator and control of the physical exercise training, only the experimental group was subjected to those tests. Testing (pre and post) were supervised by the same researchers. Each subject was familiarized with all physical testing procedures, preceded by a warm-up. Verbal encouragement was given throughout the voluntary test and biofeedback provided in order to maximize motivation.
Anthropometric measure
Height (cm) and total body weight (kg) were measured according to the international standards for anthropometric assessment. To evaluate height (cm), a stadiometer (Cabral, model 14) with a scale range of 0.10 cm was used and body mass (kg) was measured to the nearest 0.1 kg using a digital scale (Philips, type HF 351/00). Subjects were measured while wearing shorts and t-shirts (shoes and socks were removed). Abdominal circumference (AC) was used taking the umbilicus point as a reference.
Physical exercise training
The subjects followed the exercise training over 16 weeks, with three sessions per week, of 60–75 min, on non-consecutive days. Each session was divided into three components: 25–30 min of aerobic exercise, 30–35 min of strength exercise, and 5–10 min of stretching and cool down. The aerobic exercises were walking, running, biking, rowing, and elliptical. The intensity of the aerobic component was progressive between 55 and 75 % of the heart rate reserve (HRR), and the subjects were controlled by a cardiac monitor polar S625X. The strength exercises were bench press, leg press, leg curl, leg extension, latissimus, abdominals, and arm flexion. The intensity of the strength component was between 65 and 75 % of the one-repetition maximum (1RM). Two sets of 10–15 repetitions in the first 4 weeks, and three sets of 10–15 repetitions in the following weeks. A 5–10-min stretching and cooling-down was included at the end of the exercise session.
Physical testing
Strength tests
Bench press, squat jump, and leg extension
Maximal strength was assessed using one-repetition maximum (Izquierdo et al. 2005). Specific warm-up was allowed consisting of one set of five repetitions at 40–50 % of the perceived maximum. Thereafter, four to five separate attempts were performed until the subject was unable to perform the exercise to the required position. The last acceptable extension with the highest possible load was determined as 1RM.
Power tests
Vertical jump
A trigonometric carpet (Ergojump Digitime 1000; Digitest, Jyvaskyla, Finland) was used to assess maximum height in counter-movement jump (CMJ). Each subject started from an erect standing position, and the end of the concentric phase corresponded to a full leg extension (180°). The test was performed three times, each separated by a 2-min rest period. The average maximum height of three trials was adopted.
Functional tasks
The 8-ft up-and-go test, chair stand test, and 6-min walking test (6MWT) were performed according to Rikli and Jones (1999).
8-ft up-and-go test
This test enables dynamic balance assessment, represented by the total time required for the subjects to rise from a seated position, walk 8 ft (2.44 m), turn and return to a seated position (back straight, feet flat on the floor, and hands on thighs). Each participant was allowed to practice it once, followed by two test trials.
Chair stand test
From the sitting position, the subject stood up to full height, then completely back down; this cycle was being repeated as quickly as possible for 30 s. When the subject was in the process of completing a full stand from the sitting position when the time elapsed, the final stand was counted in the total.
Six-minute walking test
The 6MWT is a practical simple test that measures the distance that a person can quickly walk on a flat hard surface in a period of 6 min. It evaluates the global and integrated responses of all systems involved during exercise, including the pulmonary and cardiovascular systems, systemic and peripheral circulation, neuromuscular system, and muscle metabolism (Rikli and Jones 1999). It does not provide specific information on the function of each of the different organs and systems involved in exercise or the mechanism of exercise limitation. The self-paced 6MWT assesses the submaximal level of functional capacity. Most patients do not achieve maximal exercise capacity during the 6MWT; instead, they choose their own intensity of exercise and are allowed to stop and rest during the test. However, because most activities of daily living are performed at submaximal levels of exertion, the 6MWT may better reflect the functional exercise level for daily physical activities.
Blood sample collection
A venous blood sample (9 mL) was taken from each subject, between the hours of 8:30 and 10:00 a.m. in fasting conditions, and collected in EDTA test tubes to prevent coagulation. Blood samples were collected and processed in the following hour and half.
Plasma separation and lymphocytes isolation for comet assay
Although the following protocol isolates peripheral blood mononuclear cells, as most of the cells are lymphocytes, we have considered lymphocytes. The blood sample (≈9 mL) was mixed with 9 mL of cold phosphate-buffered saline solution (PBS). After gentle mixing, the sample was layered onto 18 mL of Lymphoprep in a 50-mL conical plastic tube and centrifuged for 20 min at 700×g. Then, about 6 mL of the plasma band was collected into a 2-mL Eppendorf tube and frozen for later analysis. Also, about 5 mL of the band containing lymphocytes was removed and transferred to a plastic centrifuge tube containing 10 mL of cold PBS; after mixing, the sample was centrifuged 10 min at 2000×g. The pellet (lymphocytes from band) was resuspended into 10 mL of cold PBS and divided into two tubes, 125 μL to the DNA damage protocol and the remaining (≈10 mL) to the repair assay protocol.
Total protein determination
Total protein concentration in plasma was spectrophotometrically estimated according to biuret method, using serum albumin as standard (Gornall et al. 1949).
DNA damage estimation with the comet assay
DNA strand breaks (DNA SBs) and oxidative DNA damage (FPG-sensitive sites) were measured using the comet assay, single cell gel electrophoresis (SCGE). Comet assay is a simple and sensitive method for quantitatively measuring DNA breakage in individual cells. Cells with increased DNA damage display increased DNA migration from the nucleus toward the anode. The migration is observed by fluorescence microscopy after staining with a fluorescent dye (ethidium bromide), and the intensity of the comet tail reflects the number of DNA breaks.
The 100 μL of the sample were centrifuged at 200×g for 3 min at 4 °C. The supernatant was then removed as much as possible using a pipette, and the cells in the pellet were resuspended in 280 μL of 1 % (w/v) low melting point agarose (Gibco) in PBS at 37 °C. The estimated final concentration of agarose was about 0.75 % (w/v).
The cells suspended in low melting point agarose were transferred as two 70 μL drops to microscope slides, pre-coated with 1 % (w/v) normal melting point agarose. Each drop on a slide was covered with an 18 × 18-mm cover slip, and left at 4 °C for 5 min to form the gel. Two slides (with two gels on each) were prepared per subject, one to measure basal DNA strand breaks and the other for incubation with formamidopyrimidine DNA glycosylase (FPG) which, during incubation after lysis, creates a strand break at 8-oxoguanine and other altered purines. Cover slips were then removed and the slides were placed in a vertical Coplin-staining jar with 1 mL Triton X-100 to 100 mL of lysis solution (2.5 M NaCl, 0.1 M EDTA, 10 mM Tris, pH 10, 4 °C), and kept in the dark for 1–4 h. This removes membranes and soluble cell components, including histones, leaving DNA as a “nucleoid”. The slides were then washed for three times with enzyme reaction buffer (buffer F) (40 mM HEPES, 0.1 M KCl, 0.5 mM EDTA, 0.2 mg/mL BSA, pH 8.0 with KOH, 4 °C) for 5 min each. Excess liquid was dabbed off with absorbent paper. Then 50 μL of FPG solution (diluted crude extract from over-producing bacteria, provided by A. R. Collins), or buffer alone as a control, was placed over each gel on one slide, and covered with a 22 × 22-mm cover slip. Slides were placed into a moist box (to prevent desiccation), and incubated at 37 °C for 30 min. After this incubation, the slides were placed (without cover slips) in a horizontal electrophoresis tank, and immersed in electrophoresis solution (0.3 M NaOH and 1 mM EDTA) for 40 min at 4 °C. Then electrophoresis was performed for 30 min at 25 V (constant voltage setting). The slides were then washed three times with neutralizing buffer (0.4 M Tris, pH 7.5 with concentrated HCl) in a staining jar for 5 min at 4 °C. The slides were then stored at room temperature. Slides were stained with ethidium bromide (20 μg/mL) immediately before visualization of comet DNA using a Nikon Eclipse E400. DNA damage estimation was evaluated by the image analysis software Comet IV (Perceptive Instruments, Ltd.) (Fig. 1). DNA damage scoring was performed based on percent tail intensity (%TI) of DNA. The Olive tail moment and tail length have been also used in different studies, however, it seems that %TI DNA is strongly recommended as the parameter of choice since it covers the widest range of damage; it is linearly related to break frequency and has standard units of measurement (%) (Collins et al. 2008). Regarding FPG-sensitive sites, the DNA damage score observed in the absence of FPG was subtracted from the score with FPG to give the net FPG-sensitive sites as a measure of oxidative damage.
Fig. 1.
Fluorescent micrograph of comet assay from lymphocytes stained with ethidium bromide. (a) Low DNA damage. (b) High DNA damage
Assessment of repair capacity with comet assay
Extract preparation
Activity of 8-oxoguanine DNA glycosylase (OGG1), which is the primary enzyme responsible for the excision of 8-oxoguanine (8-oxoG), was studied in extracts obtained from the lymphocytes of each subject.
After resuspending, the sample (PBS plus lymphocytes) was centrifuged 10 min at 700×g. The pellet was mixed with 10 mL of 3 times diluted extraction buffer (buffer A) (45 mM Hepes, 0.4 M KCl, 1 mM EDTA, 0.1 mM dithiothreitol, 10 % (v/v) glycerol, pH 7.8), and cells were counted. Then, the sample was centrifuged 5 min at 700×g. A volume of 20 μL of buffer A was added per 10^6 cells and frozen by dropping into liquid nitrogen. The sample was then stored at –80 °C. On the day of the assay, the sample was thawed, and 12 μL of Triton solution (buffer A with 1 % of Triton X-100) was added for each 50 μL of sample. Then, the sample was centrifuged (∼15,000×g) for 5 min at 4 °C to remove nuclei and cell debris. The supernatant was mixed with 4 volumes of buffer F. A control solution, similar to the extract but with no cells, was prepared with 50 μL buffer A plus 12 μL Triton solution and 200 μL buffer F.
Substrate nucleoid preparation and treatment with paraquat
We used human colon adenocarcinoma-derived cell line, Caco-2 (CLS, Eppelheim, Germany), grown at 37 °C under 5 % CO2 environment in DMEM (Dulbeco’s modified Eagle’s medium, Gibco) supplemented with 2 mM of l-glutamine (Gibco, Life Technologies), 10 % (v/v) of fetal bovine serum (FBS, Gibco, Life Technologies), and antibiotics (200 U/mL of penicillin and 200 μg/mL of streptomycin (Gibco, Life Technologies)). To avoid any bacterial or viral contamination, a laminar flow cabinet class II was used to manipulate the cells (Faster, BH-En 2004).
To induce oxidative DNA damage, subconfluent cells were submitted to paraquat treatment (8 μM) for 24 h at 37 °C.
Extract reaction
After 24 h treatment with paraquat, trypsin solution (trypsin-EDTA 0.05 % (v/v), Gibco) was added, after removal of paraquat solution and cell washing with versene solution (Gibco). Then, cells were collected into 1.5 mL microcentrifuge tubes and centrifuged for 5 min at low speed (mini-bench centrifuge) to remove trypsin. Trypsinized cells were embedded in low melting agarose (1 % w/v), and after this step, cells were treated as previously described for lymphocytes assay. The reaction step was the same as described for the FPG incubation but with 45 μL of extract per gel instead of FPG enzyme. A 45-μL extract (or “control solution”) was added to each gel and covered with glass coverslip. The slides were incubated for 30 min at 37 °C. Repair rates were calculated according to Gaivão et al. (Gaivao et al. 2009) represented by the following formula:
OGG1 activity was expressed in %TI, where TI extract represents the extract incubated with exposed substrate (paraquat/extract), control 1 represents the reaction buffer incubated with non-exposed untreated substrate, i.e., background control (non-paraquat/buffer), and control 2 represents the reaction buffer incubated with exposed substrate, i.e., treatment control (paraquat/buffer). Substrates were also incubated with FPG enzyme solution as a control of the damage induced by paraquat treatment.
Lipid peroxidation
Malondialdehyde (MDA) assay. Nonspecific lipid peroxidation level in plasma was measured by determining the levels of lipid peroxides as the amount of thiobarbituric acid reactive substances (TBARS) formed, according to Wills (Wills ED Evaluation of lipid peroxidation in lipids and biological membranes Biochemical Toxicology A practical Approach ed Oxford IRL1987 2999) with some modifications. Plasma samples of 100 μL were taken and mixed with 200 μL trichloroacetic acid (10 %) and centrifuged at ∼15,000×g for 1 min. Then, 200 μL of supernatant were taken and mixed with 200 μL of thiobarbituric acid (TBA) reagent (1 % thiobarbituric acid). The mixture was heated at 80–90 °C for 10 min, and cooled down at room temperature for 20 min. Lipid peroxidation was estimated by the appearance of MDA which were quantified spectrophotometrically by reading the absorbance at 535 nm. The amount of MDA formed was calculated using molar extinction coefficient (ɛ) of 1.56 × 105 M–1 cm–1, and the results was expressed as MDA concentration (nmol mg–1 of protein).
Total antioxidant capacity (TAC)
The total antioxidant capacity (TAC) in plasma was determined using the ABTS radical-scavenging activity measured by a procedure previously reported (Ozgen et al. 2006), with slight modifications. A solution was prepared with ABTS•+ (7 mM) and potassium persulfate (140 mM) in 5 mL of distilled water. The solution was held at room temperature, in the dark, for 12–16 h before use. The ABTS•+ solution was diluted in acetate buffer (100 mM, pH 4.5), in order to obtain an absorbance of 0.7 at 734 nm. Fresh ABTS•+ solution was prepared for each analysis. To obtain Trolox equivalent, a standard solution was prepared at 0 (control), 1.25, 2.50, 5.00, 7.50, 10.00, 15.00, and 20.00 μM. To measure the antioxidant capacity of the samples, three different sample volumes were used. The samples antioxidant capacity was expressed in terms of Trolox equivalent activity.
Physical activity
Inventory physical activity questionnaire (IPAQ) (Craig et al. 2003) was used for the evaluation of the total weekly physical activity. IPAQ assesses physical activity undertaken across a comprehensive set of domains including leisure time physical activity, domestic and gardening (yard) activities, work-related physical activity, and transport-related physical activity. The specific types of activity that were assessed are walking, moderate-intensity activities, and vigorous-intensity activities.
Perceived stress scale (PSS)
All participants filled out the questionnaire in its 10-item form. For the specific analysis, the questionnaire version described, translated, and validated for the Portuguese population was used (Trigo et al. 2010).
Statistical analysis
Data distribution was tested with the Kolmogorov-Smirnov test and with the analysis of box plots. To compare the results within each group between pre- and post-training, a paired sample t test was used for normal distribution variables, and a Wilcoxon signed ranks test was used for non-normal distribution variables. To compare the variables between groups (comparison between the variation pre- and post-training), a Mann-Whitney test was used for non-normal distribution variables. To test the possible association between variables, a Spearman correlation test was performed. The level of significance was set at 0.05. Data analysis was performed using the software Statistical Program for Social Sciences—SPSS version 20.0.
Results
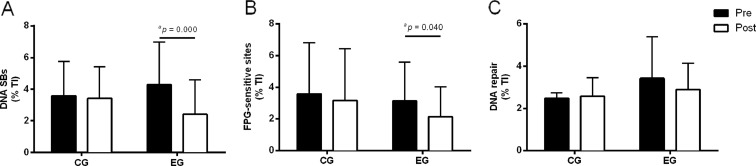
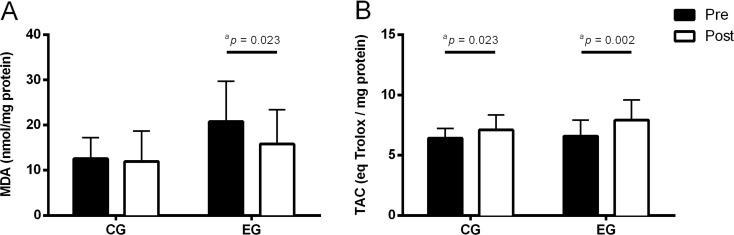
The results showed significant differences between groups in age; the experimental group was older than the control group (Table 1, Figs. 2 and 3). The comparison between groups revealed significant differences in the average age (t = −2.608, p = 0.012) with an effect size of −0.290 (95 % IC 0.503–0.045). Regarding differences between pre- and post-training, significant increases were observed in weight, BMI, and TAC in the control group. In the experimental group, a significant decrease in the abdominal circumference was observed, with no significant changes in weight and BMI. A significant increase in physical activity and TAC was observed. A significant decrease was observed in DNA SBs, FPG-sensitive sites, and MDA levels, with a slight decrease in OGG1 repair activity.
Table 1.
Anthropometric, lifestyle, DNA and oxidative stress variables of the subjects in the control group (CG) and experimental group (EG) pre- and post-training
| Variables | CG | EG | ||||||
|---|---|---|---|---|---|---|---|---|
| Pre | Post | P | Diff | Pre | Post | p | Diff | |
| Age | 52.4 ± 9.1 | 58.4 ± 10.2 | ||||||
| Weight (kg) | 80.6 ± 16.5 | 81.7 ± 17.8 | .040 | 1.1 | 80.9 ± 13.0 | 81.0 ± 12.7 | .724 | 0.1 |
| BMI | 28.0 ± 4.3 | 28.4 ± 4.7 | .040 | 0.4 | 28.5 ± 3.6 | 28.6 ± 3.5 | .724 | 0.1 |
| Abdominal circumference (cm) | 104.2 ± 11.6 | 104.7 ± 11.4 | .332 | 0.4 | 101.5 ± 9.9 | 98.8 ± 9.3 | .000 | −2.7# |
| Physical activity METs (MET-min/week) | 1056 ± 950 | 1099 ± 926 | .063 | 43 | 801 ± 644 | 2507 ± 751 | .000 | 1706# |
| PSS | 15.8 ± 1.4 | 14.5 ± 3.5 | .170 | −1.3 | 13.6 ± 5.3 | 14.5 ± 5.6 | .453 | 0.9 |
Data presented are mean ± SD
p – p values related to the statistical differences within group between pre-training and post-training evaluations, analysed by Wilcoxon test, Diff difference between moments within groups (post-training values minus pre-training values)
#p < 0.05 statistical differences between different groups analysed by Mann-Whitney test
Fig. 2.
DNA damage and repair in lymphocytes, measured with the comet assay in control group (CG) and in experimental group (EG), pre-training and post-training. a DNA strand breaks. b FPG-sensitive sites. c DNA repair capacity (OGG1). Mean values are shown with SD. aSignificant differences, p values within groups were analysed by related variables Wilcoxon test
Fig. 3.
Biomarkers of oxidative stress (MDA) and of antioxidant capacity (TAC) in control group (CG) and in experimental group (EG), pre-training and post-training. a Lipoperoxidation (MDA). b Total antioxidant capacity (TAC). Mean values are shown with SD. aSignificant differences, p values within groups were analysed by related variables Wilcoxon test
Considering the comparison of the difference (diff) within groups between moments (post-training minus pre-training), our results showed significant changes in physical activity (higher increase in the EG than in the CG) (z = −6.523, p = 0.000), abdominal circumference (reduction in the EG and increase in the CG) (z = −2.517, p = 0.011), and in DNA SBs (higher decrease in EG compared to CG) (z = −2.917, p = 0.004).
Table 2 shows the physical changes between pre-training and post-training in the EG. Considering the physical assessment; the results showed an overall significant improvement of the variables studied.
Table 2.
Physical fitness assessments, pre-training and post-training of the experimental group
| Variable | Pre | Post | p | % |
|---|---|---|---|---|
| Bench press (kg) | 35.6 ± 8.6 | 44.4 ± 8.8 | .000 | 24.72 |
| CMJ (cm) | 20.8 ± 4.9 | 23.9 ± 5.0 | .000 | 14.9 |
| Leg extension (kg) | 66.0 ± 13.87 | 88.3 ± 22.2 | .001 | 33.3 |
| 8-ft up-and-go test (s) | 4.35 ± 0.59 | 4.01 ± 0.67 | .022 | −7.82 |
| Chair stand test (repetitions) | 21.87 ± 3.95 | 28.48 ± 5.20 | .000 | 30.2 |
| 6MWT (m) | 658.5 ± 61.9 | 684.9 ± 68.5 | .002 | 4.01 |
Data presented are mean ± SD; p – p value (pre vs. post) statistical differences within experimetal group between pre-training and post-training evaluations (pre and post), p values were analysed by paired-samples t test, % = percentage change between pre and post-training
The correlation test between variables revealed negative associations between DNA SBs and physical activity METs (rho = −0.324, p = 0.019) and aerobic fitness (rho = −0.462, p = 0.035), and positive with FPG-sensitive sites (rho = 0.342, p = 0.012).
Discussion
The health-related benefits of regular physical exercise are widely described in the literature. Some of the main mechanisms are related to the prevention of DNA damage involved in several generative diseases and in the aging process. Considering that a period of 16 weeks of combined physical exercise training should improve physical fitness, confirmed in this study, we aimed to identify its effects on human lymphocyte DNA damage and DNA repair. In addition, we aimed to investigate the role of oxidative stress in those changes.
Our main findings showed that DNA damage (DNA SBs) and oxidative DNA damage (FPG-sensitive sites) decreased after 16 weeks of combined physical exercise training, with no significant changes in the DNA repair activity (OGG1 activity enzyme). In this experimental research, the training protocol followed the guidelines recommended by the American College of Sports Medicine (Chodzko-Zajko et al. 2009; Garber et al. 2011). The health benefits of combined training have been studied, and a combination of aerobic training and strength training has been recommended as an effective strategy to improve overall functional capacity and to promote health (Donnelly et al. 2009), which was adopted in our study. Considering the older subjects of our sample, recent studies on elderly individuals (≥65 years old) have demonstrated similar improvements in cardiorespiratory fitness and muscular strength after a single-mode and a combined training (Cadore et al. 2012; Izquierdo et al. 2004; Takeshima et al. 2004). Our results showed that the physical exercise training applied produced significant effects on the cardiorespiratory, strength and functional capacity of the subjects. Also, a body composition change was observed, namely a significant decrease in abdominal perimeter. Although no change has been observed in the average weight of the sample, a decrease in abdominal perimeter was likely related to the loss of fat mass which may be considered to be a healthy effect of the exercise training. In sum, our exercise program induced a significant increase in the subjects’ fitness.
The stress caused by regular physical exercise, might be responsible for several local and systemic modifications leading to the organism’s adaptation. Our results showed that after the physical exercise training, SBs, and FPG-sensitive sites decreased significantly. Two main hypotheses could be advanced to explain these results: a decrease in ROS production and/or an increase in antioxidant protection. Although lifestyle factors have been considered as important modulators of DNA damage (Huang et al. 2013; Fenech and Bonassi 2011; Wilson et al. 2008), the main differences between groups should be considered as a consequence of the physical exercise training. Indeed, our results reinforce the association between higher daily physical activity and higher aerobic fitness with lower DNA strand breaks. So, it seems that exercise may cause a set of adaptations which protect the integrity of DNA. Some studies have investigated the mechanisms underlying the benefits of regular physical exercise (Radak et al. 2001; Mergener et al. 2009; Fogarty et al. 2011). Some of these mechanisms may be related to the lower production of oxidants, up-regulation of antioxidant capacity, or even higher DNA repair activity. Several studies have shown that oxidative stress decreases after a period of physical exercise in different animal tissues and cells (Nakamoto et al. 2007; Lambertucci et al. 2006). It seems that low-to-moderate ROS generation induced by regular physical exercise could be beneficial, since it induces an up-regulation of some antioxidant enzymes. In our study, the antioxidant capacity, evaluated by TAC, increased after the training period. Although not statistically different, it seems that a trend of a greater increase in TAC was observed in the experimental group (23 % increase) compared to the control group (11 % increase). This increased antioxidant activity in the control group may be partly explained by seasonal lifestyle variations related to seasonal food intake, such as different fruits and vegetables; though, it is likely that the higher trend to TAC increase in the experimental group was a consequence of lifestyle and exercise training. Considering this, the significantly lower DNA damage observed post-training in the experimental group may be partly related to the increased antioxidant activity. A study by Siu et al. (Siu et al. 2011) found an increased resistance of lymphocytes to oxidant-induced DNA damage in lymphocytes from rats, after 20 weeks of physical exercise training. According to this, as expected, a decrease in MDA levels in the experimental group after 16 weeks of exercise training was observed.
The DNA damage also depends on the DNA repair, which could reverse the damage. In this study, we analyzed a base excision repair mechanism by the OGG1. This enzyme has a primary role of recognizing oxidized bases, namely 8-oxoG, the most common lesion for assessing oxidative DNA damage (Halliwell 2000). As far as DNA repair activity is concerned, we expected that a possible reduction in FPG-sensitive sites due to exercise could be related to the increased activity in OGG1. However, our results showed a non-significant decreasing trend in the OGG1 activity in the experimental group in post-training. Regarding the specific literature, a few studies have been made in humans. Huang et al. (Huang et al. 2014) observed no changes in plasma 8-OHdG and a significant increase in plasma OGG1 level after 12 weeks of Tai-Chi exercise practice of light intensity (54.3–57.1 % of maximum HR). These results are somewhat confusing since OGG1 is a DNA nuclease located in cell nuclei, and the analyses were performed in plasma. In our opinion, there is a lack of information in the methodology described in the previous study which compromises the interpretation and the comparisons of the results with our results. On the other hand, some research (Siu et al. 2011) in animals found an increase in the resistance of induced DNA damage in lymphocytes from rats, after 8 and 20 weeks of physical exercise training. These results were attributed to the exercise-induced elevated expressions of antioxidant enzymes (mitochondrial superoxide dismutase, and catalase) and DNA repair enzymes (APEX1 nuclease; protein kinase, DNA activated, catalytic polypeptide, Prkdc; and O-6-methylguanine-DNA methyltransferase) which were found after the training period. However, regarding the OGG1 expression, no significant change was observed. In another study carried out by Nakamoto et al. (Nakamoto et al. 2007), aged rats (21 months old) subjected to 2 months of regular treadmill running evidenced a significant decrease in 8-oxodG content, both in nuclear and mitochondrial DNA of the liver, which was accompanied by an up-regulation of the OGG1 activity in the nucleus, but not in mitochondria. This study showed that OGG1 was differently influenced when considering the mitochondria or nucleus. Also, Radak et al. (Radak et al. 2007) found that 8 weeks of treadmill exercise training resulted in increased OGG1 activity in the nuclei of red fibers, and decreased activity in nuclei of white fibers, and in the mitochondria of both red and white fibers in rats. These findings strongly indicate that the regulation of OGG1 in nucleus and in mitochondria, as well as in different tissues, could be different as a result of exercise training workloads. Considering all of this research, it is worth highlighting that results are somewhat dependent on the tissue/sample, organelles, and method used. Moreover, when analyzing the results, other variables should be taken in consideration that may be related to the dynamics of DNA damage accumulation, namely the protection to DNA (i.e., antioxidant enzymes and histones) and DNA repair capacity. Even if only considering the DNA repair capacity, several mechanisms exist which might explain why in some circumstances OGG1 increases, and in others, shows no changes or even decreases, possibly compensated by other repair mechanisms or protection mechanisms.
Considering our results, it appears that in some way, exercise contradicts age-related DNA damage. The decrease in oxidative damage associated with exercise training could be explained by an increase in antioxidant and metabolic efficiency, which possibly prevent the stimulation of DNA repair enzyme activity. These results enhance the importance of regular exercise in the prevention of DNA damage accumulation which has been related to aging (Nalapareddy et al. 2008) and some age-related diseases including: cardiovascular disease (Collins et al. 1998), diabetes mellitus (Hannon-Fletcher et al. 2000), cataracts (Jiang et al. 2013; Su et al. 2013), and several cancers (Malins et al. 2001; Akcay et al. 2003; Peddireddy et al. 2012). In addition, it should be noted that DNA damage depends on a complex system which is not thoroughly understood. Besides, other repair systems, such as uracil DNA glycosylase and single strand break repair mechanisms, might contribute to comprehension of this matter. The data in this study clearly highlight the need for further examination to clarify the role of this (OGG1) and other DNA repair mechanisms in the aging process.
Conclusion
Our study showed that 16 weeks of combined physical exercise training increased physical fitness and reduced DNA damage (SBs and FPG-sensitive sites) in lymphocytes and MDA levels, with an increase in total antioxidant capacity.
Acknowledgments
Funding
This work was supported by the Foundation of Science and Technology (FCT) for the research grant SFRH/BD/66438/2009 to JS and for the project entitled Physical exercise role on humans’ lymphocytes DNA damage reduction: possible influence of oxidative stress and DNA repair capacity PTDC/DES/121575/2010. We also would like to acknowledge FCT and FEDER/COMPETE under the references PEst-C/AGR/UI4033/2014.
References
- ACSM. American College of Sports Medicine position stand Progression models in resistance training for healthy adults. Med Sci Sports Exerc. 2009;41(3):687–708. doi: 10.1249/MSS.0b013e3181915670. [DOI] [PubMed] [Google Scholar]
- Akcay T, Saygili I, Andican G, Yalcin V. Increased formation of 8-hydroxy-2′-deoxyguanosine in peripheral blood leukocytes in bladder cancer. Urol Int. 2003;71(3):271–274. doi: 10.1159/000072677. [DOI] [PubMed] [Google Scholar]
- Alessio HM. Exercise-induced oxidative stress. Med Sci Sport Exer. 1993;25(2):218–224. doi: 10.1249/00005768-199302000-00010. [DOI] [PubMed] [Google Scholar]
- Ascensao A, Magalhaes J, Soares J, Oliveira J, Duarte JA. Exercise and cardiac oxidative stress. Rev Port Cardiol. 2003;22(5):651–678. [PubMed] [Google Scholar]
- Beckman KB, Ames BN. The free radical theory of aging matures. Physiol Rev. 1998;78(2):547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- Cadore EL, Izquierdo M, Alberton CL, et al. Strength prior to endurance intra-session exercise sequence optimizes neuromuscular and cardiovascular gains in elderly men. Exp Gerontol. 2012;47(2):164–169. doi: 10.1016/j.exger.2011.11.013. [DOI] [PubMed] [Google Scholar]
- Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, et al. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc. 2009;41(7):1510–1530. doi: 10.1249/MSS.0b013e3181a0c95c. [DOI] [PubMed] [Google Scholar]
- Collins AR, Gaivao I. DNA base excision repair as a biomarker in molecular epidemiology studies. Mol Asp Med. 2007;28(3–4):307–322. doi: 10.1016/j.mam.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Collins AR, Gedik CM, Olmedilla B, Southon S, Bellizzi M. Oxidative DNA damage measured in human lymphocytes: large differences between sexes and between countries, and correlations with heart disease mortality rates. FASEB J. 1998;12(13):1397–1400. [PubMed] [Google Scholar]
- Collins AR, Oscoz AA, Brunborg G, et al. The comet assay: topical issues. Mutagenesis. 2008;23(3):143–151. doi: 10.1093/mutage/gem051. [DOI] [PubMed] [Google Scholar]
- Craig CL, Marshall AL, Sjostrom M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK, American College of Sports Medicine Position Stand Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41(2):459–471. doi: 10.1249/MSS.0b013e3181949333. [DOI] [PubMed] [Google Scholar]
- Fenech M, Bonassi S. The effect of age, gender, diet and lifestyle on DNA damage measured using micronucleus frequency in human peripheral blood lymphocytes. Mutagenesis. 2011;26(1):43–49. doi: 10.1093/mutage/geq050. [DOI] [PubMed] [Google Scholar]
- Fisher-Wellman K, Bloomer RJ. Acute exercise and oxidative stress: a 30-year history. Dyn Med. 2009;8:1. doi: 10.1186/1476-5918-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty MC, Hughes CM, Burke G, et al. Exercise-induced lipid peroxidation: implications for deoxyribonucleic acid damage and systemic free radical generation. Environ Mol Mutagen. 2011;52(1):35–42. doi: 10.1002/em.20572. [DOI] [PubMed] [Google Scholar]
- Gaivao I, Piasek A, Brevik A, Shaposhnikov S, Collins AR. Comet assay-based methods for measuring DNA repair in vitro; estimates of inter- and intra-individual variation. Cell Biol Toxicol. 2009;25(1):45–52. doi: 10.1007/s10565-007-9047-5. [DOI] [PubMed] [Google Scholar]
- Garber CE, Blissmer B, Deschenes MR, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43(7):1334–1359. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- Gornall AG, Bardawill CJ, David MM. Determination of serum proteins by means of the biuret reaction. J Biol Chem. 1949;177(2):751–766. [PubMed] [Google Scholar]
- Halliwell B. Why and how should we measure oxidative DNA damage in nutritional studies? How far have we come? Am J Clin Nutr. 2000;72(5):1082–1087. doi: 10.1093/ajcn/72.5.1082. [DOI] [PubMed] [Google Scholar]
- Hannon-Fletcher MP, O’Kane MJ, Moles KW, Weatherup C, Barnett CR, Barnett YA. Levels of peripheral blood cell DNA damage in insulin dependent diabetes mellitus human subjects. Mutat Res. 2000;460(1):53–60. doi: 10.1016/S0921-8777(00)00013-6. [DOI] [PubMed] [Google Scholar]
- Huang Y, Zhang M, Zou H, et al. Genetic damage and lipid peroxidation in workers occupationally exposed to organic bentonite particles. Mutat Res. 2013;751(1):40–44. doi: 10.1016/j.mrgentox.2012.10.006. [DOI] [PubMed] [Google Scholar]
- Huang XY, Eungpinichpong W, Silsirivanit A, Nakmareong S, Wu XH. Tai chi improves oxidative stress response and DNA damage/repair in young sedentary females. J Phys Ther Sci. 2014;26(6):825–829. doi: 10.1589/jpts.26.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo M, Ibanez J, HA K, Kraemer WJ, Larrion JL, Gorostiaga EM. Once weekly combined resistance and cardiovascular training in healthy older men. Med Sci Sports Exerc. 2004;36(3):435–443. doi: 10.1249/01.MSS.0000117897.55226.9A. [DOI] [PubMed] [Google Scholar]
- Izquierdo M, Hakkinen K, Ibanez J, Kraemer WJ, Gorostiaga EM. Effects of combined resistance and cardiovascular training on strength, power, muscle cross-sectional area, and endurance markers in middle-aged men. Eur J Appl Physiol. 2005;94(1–2):70–75. doi: 10.1007/s00421-004-1280-5. [DOI] [PubMed] [Google Scholar]
- Jiang J, Zhou J, Yao Y, et al. Copy number variations of DNA repair genes and the age-related cataract: Jiangsu Eye Study. Invest Ophthalmol Vis Sci. 2013;54(2):932–938. doi: 10.1167/iovs.12-10948. [DOI] [PubMed] [Google Scholar]
- Kasai H, Crain PF, Kuchino Y, Nishimura S, Ootsuyama A, Tanooka H. Formation of 8-hydroxyguanine moiety in cellular DNA by agents producing oxygen radicals and evidence for its repair. Carcinogenesis. 1986;7(11):1849–1851. doi: 10.1093/carcin/7.11.1849. [DOI] [PubMed] [Google Scholar]
- Kemi OJ, Haram PM, Loennechen JP, et al. Moderate vs. high exercise intensity: differential effects on aerobic fitness, cardiomyocyte contractility, and endothelial function. Cardiovasc Res. 2005;67(1):161–172. doi: 10.1016/j.cardiores.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Lambertucci RH, Levada-Pires AC, Rossoni LV, Curi R, Pithon-Curi TC. Effects of aerobic exercise training on antioxidant enzyme activities and mRNA levels in soleus muscle from young and aged rats. Mech Ageing Dev. Dec 23 2006 [DOI] [PubMed]
- Lombard DB, Chua KF, Mostoslavsky R, Franco S, Gostissa M, Alt FW. DNA repair, genome stability, and aging. Cell. 2005;120(4):497–512. doi: 10.1016/j.cell.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Lopes C, Aro A, Azevedo A, Ramos E, Barros H. Intake and adipose tissue composition of fatty acids and risk of myocardial infarction in a male Portuguese community sample. J Am Diet Assoc. 2007;107(2):276–286. doi: 10.1016/j.jada.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Malins DC, Johnson PM, Wheeler TM, Barker EA, Polissar NL, Vinson MA. Age-related radical-induced DNA damage is linked to prostate cancer. Cancer Res. 2001;61(16):6025–6028. [PubMed] [Google Scholar]
- Matsuo T, Saotome K, Seino S, et al. Low-volume, high-intensity, aerobic interval exercise for sedentary adults: [Formula: see text]O, cardiac mass, and heart rate recovery. Eur J Appl Physiol. Jun 11 2014 [DOI] [PubMed]
- Mergener M, Martins MR, Antunes MV, et al. Oxidative stress and DNA damage in older adults that do exercises regularly. Clin Biochem. 2009;42(16–17):1648–1653. doi: 10.1016/j.clinbiochem.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Metcalf BS, Curnow JS, Evans C, Voss LD, Wilkin TJ. Technical reliability of the CSA activity monitor: the earlybird study. Med Sci Sports Exerc. 2002;34(9):1533–1537. doi: 10.1097/00005768-200209000-00022. [DOI] [PubMed] [Google Scholar]
- Mota MP, Peixoto FM, Soares JF, et al. Influence of aerobic fitness on age-related lymphocyte DNA damage in humans: relationship with mitochondria respiratory chain and hydrogen peroxide production. Age (Dordr) 2010;32(3):337–346. doi: 10.1007/s11357-010-9138-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamoto H, Kaneko T, Tahara S, et al. Regular exercise reduces 8-oxodG in the nuclear and mitochondrial DNA and modulates the DNA repair activity in the liver of old rats. Exp Gerontol. 2007;42(4):287–295. doi: 10.1016/j.exger.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Nalapareddy K, Jiang H, Guachalla Gutierrez LM, Rudolph KL. Determining the influence of telomere dysfunction and DNA damage on stem and progenitor cell aging: what markers can we use? Exp Gerontol. 2008;43(11):998–1004. doi: 10.1016/j.exger.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Ortenblad N, Madsen K, Djurhuus MS. Antioxidant status and lipid peroxidation after short-term maximal exercise in trained and untrained humans. Am J Physiol. 1997;272(4 Pt 2):R1258–R1263. doi: 10.1152/ajpregu.1997.272.4.R1258. [DOI] [PubMed] [Google Scholar]
- Ozgen M, Reese RN, Tulio AZ, Scheerens JC, Miller AR. Modified 2,2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) method to measure antioxidant capacity of selected small fruits and comparison to ferric reducing antioxidant power (FRAP) and 2,2′-diphenyl-1-picrylhydrazyl (DPPH) methods. J Agr Food Chem. 2006;54(4):1151–1157. doi: 10.1021/jf051960d. [DOI] [PubMed] [Google Scholar]
- Parise G, Brose AN, Tarnopolsky MA. Resistance exercise training decreases oxidative damage to DNA and increases cytochrome oxidase activity in older adults. Exp Gerontol. 2005;40(3):173–180. doi: 10.1016/j.exger.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Peddireddy V, Siva Prasad B, Gundimeda SD, Penagaluru PR, Mundluru HP. Assessment of 8-oxo-7, 8-dihydro-2′-deoxyguanosine and malondialdehyde levels as oxidative stress markers and antioxidant status in non-small cell lung cancer. Biomarkers. 2012;17(3):261–268. doi: 10.3109/1354750X.2012.664169. [DOI] [PubMed] [Google Scholar]
- Radak Z, Taylor AW, Ohno H, Goto S. Adaptation to exercise-induced oxidative stress: from muscle to brain. Exerc Immunol Rev. 2001;7:90–107. [PubMed] [Google Scholar]
- Radak Z, Kumagai S, Nakamoto H, Goto S. 8-Oxoguanosine and uracil repair of nuclear and mitochondrial DNA in red and white skeletal muscle of exercise-trained old rats. J Appl Physiol. 2007;102(4):1696–1701. doi: 10.1152/japplphysiol.01051.2006. [DOI] [PubMed] [Google Scholar]
- Rikli RE, Jones CJ. Development and validation of a functional fitness test for community-residing older adults. J Aging Phys Activ. 1999;7(2):129–161. [Google Scholar]
- Shigenaga MK, Hagen TM, Ames BN. Oxidative damage and mitochondrial decay in aging. Proc Natl Acad Sci U S A. 1994;91(23):10771–10778. doi: 10.1073/pnas.91.23.10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu PM, Pei XM, Teng BT, Benzie IF, Ying M, Wong SH. Habitual exercise increases resistance of lymphocytes to oxidant-induced DNA damage by upregulating expression of antioxidant and DNA repairing enzymes. Exp Physiol. 2011;96(9):889–906. doi: 10.1113/expphysiol.2011.058396. [DOI] [PubMed] [Google Scholar]
- Su S, Yao Y, Zhu R, et al. The associations between single nucleotide polymorphisms of DNA repair genes, DNA damage, and age-related cataract: Jiangsu Eye Study. Invest Ophthalmol Vis Sci. 2013;54(2):1201–1207. doi: 10.1167/iovs.12-10940. [DOI] [PubMed] [Google Scholar]
- Takeshima N, Rogers ME, Islam MM, Yamauchi T, Watanabe E, Okada A. Effect of concurrent aerobic and resistance circuit exercise training on fitness in older adults. Eur J Appl Physiol. 2004;93(1–2):173–182. doi: 10.1007/s00421-004-1193-3. [DOI] [PubMed] [Google Scholar]
- Trigo M, Canudo N, Branco F, Silva D. Estudo das propriedades psicométricas da perceived stress scale (PSS) na população portuguesa. Psychologica. 2010;53:353–378. doi: 10.14195/1647-8606_53_17. [DOI] [Google Scholar]
- Trost SG, McIver KL, Pate RR. Conducting accelerometer-based activity assessments in field-based research. Med Sci Sports Exerc. 2005;37(11):S531–S543. doi: 10.1249/01.mss.0000185657.86065.98. [DOI] [PubMed] [Google Scholar]
- UNESCO. Universal declaration on bioethics and human rights. 2006; http://unesdoc.unesco.org/images/0014/001461/146180E.pdf [PubMed]
- Vincent HK, Bourguignon C, Vincent KR. Resistance training lowers exercise-induced oxidative stress and homocysteine levels in overweight and obese older adults. Obesity. 2006;14(11):1921–1930. doi: 10.1038/oby.2006.224. [DOI] [PubMed] [Google Scholar]
- Willett WC. Invited commentary: comparison of food frequency questionnaires. Am J Epidemiol. 1998;148:1157–1159. doi: 10.1093/oxfordjournals.aje.a009600. [DOI] [PubMed] [Google Scholar]
- Wills ED. Evaluation of lipid peroxidation in lipids and biological membranes. Biochemical Toxicology: A practical Approach ed. Oxford: IRL1987
- Wilson DM, Sofinowski TM, McNeill DR. Repair mechanisms for oxidative DNA damage. Front Biosci. 2003;8:963–981. doi: 10.2741/1109. [DOI] [PubMed] [Google Scholar]
- Wilson DM, 3rd, Bohr VA, McKinnon PJ. DNA damage, DNA repair, ageing and age-related disease. Mech Ageing Dev. 2008;129(7–8):349–352. doi: 10.1016/j.mad.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]