Abstract
This work investigates the effect of direct-current electric field on the extracellular enzymatic activity, concentration and other experimental parameters of laccase from Trametes versicolor. The results showed that laccase could significantly contribute to the change of pH at the end of graphite electrode. In addition, it increased the electrical conductivity of the water. In the experiment, the optimum pH and catalytic pH range for laccase activity were 3.0 and pH 2.5–4.0. The application of 6 V direct current showed significant effects on the laccase enzyme activity. The activity of laccase was enhanced in the anodic region, but at the same time was strongly inhibited at the cathode. The electric charge characteristics of laccase were changed when exposed to electric field, and some laccases molecules moved to the anode, which produced a slight migration phenomenon. This study is the basis of combination of laccase and electrical technology, at the same time, providing a new direction of enhancing laccase activity. Compared to immobilization, using electric field is simple, no chemical additives, and great potential.
Keywords: Direct-current electric field, Laccase, Enzymatic activity, Concentration
Introduction
Laccase is a Cu-contained polyphenol oxidase (p-diphenol oxidase EC. 1. 10. 3. 2), which uses molecular oxygen to catalyze and oxidize many organic pollutants. It has been widely applied in treating organic pollutants or repairing contaminated soils because its only byproduct is water [1, 2].
The applied electric field can improve the degradation rate and increase the treatment efficiency during the degradation process of organic pollutants. In recent years, the electrokinetic remediation technology has been used to put the electrode in the polluted soil and set up direct current on the electrode to form the applied electrode field. The next step in the process is when the pollutants in the soil move directionally in the electric field and accumulate in some specific region. Hence, it can be concluded that the pollutants in the soil and groundwater can be removed [3, 4]. Laccase is widely used in many different ways, such as delignification, dye bleaching, bioremediation, biosensors, biofuel cells and so on. And the research on laccase degrading organic compounds is increasing, because of its spectral characteristic of the substrate degradation and special catalytic property [5]. Although the effect of the applied electric field on the growth and metabolism of white rot fungi, as well as enzymatic activity of laccase, were studied [6], the change of the law of extracellular laccase with no cell structure is rarely reported.
The extracellular laccase readily loses much of its enzymatic activity in the water. To overcome this problem and improve the laccase application, immobilization of the enzyme has been demonstrated [7]. But immobilization often has complex operation conditions, high cost. A new direction of enhancing laccase activity is significant. The effect of the applied electric field on laccase is an innovative attempt. This work reports the effect of direct current electric field on the catalytic activity of extracellular laccase and the concentration of laccase. In addition, explores other related parameters that will be investigated in the hopes of providing a corresponding base to remediation technology of electric field strengthened soil and underground water. The studied laccase belongs to white rot fungi, which has strong degradation ability of polycyclic aromatic hydrocarbons, so it will potentially have a promising application towards the future.
Materials and Methods
Laccase
Laccase from Trametes versicolor (T. versicolor) was purchased from Sigma (St. Louis, MO, USA).
Main Instruments and Materials
UV–Vis spectrophotometer UV2550, Shimadzu, Japan; DC regulated power supply, Gwinstek, China; Standard pH meter PB-10, Sartorius, Germany; Pen typed electrical conductivity meter TM-03, Shanghai INESA Scientific Instrument Co. Ltd, China.
2,2′-azino-bis-(3-ethylbenzothiazo line-6-sulfonicacid (ABTS) were purchased from Sigma (St. Louis, MO, USA); Bovine serum albumin (BSA) were purchased from Genview (China); Coomassie brilliant blue G-250 were purchased from Beijing Jingguo Changsheng Biotechnology (China); other regents were all analytically pure.
Electric Field Experiments
All the experiments are carried out at room temperature and pressure. Figure 1 shows the schematic view of the apparatus used for electric field experiment. The volume of square groove is around 100 ml with the graphite electrode immersed in the solution and placed at the end of the groove. The voltage output by DC regulated power supply to form uniform direct current electric field.
Fig. 1.
Schematic view of the electric field apparatus
Enzyme Activity Detection
Laccases dissolving in pure water become enzyme solutions. Enzyme solutions were taken from cathodic region, anodic region, and the middle region of the apparatus at 2, 5, 10, 23, 30 and 48 h of the start of direct current electric field. The results were compared with enzyme solution not subjected to direct current electric field.
Assay method used 2,2′-azino-bis-(3-ethylbenzothiazo line-6-sulfonicacid (ABTS) as substrate; 1 ml appropriately diluted enzyme solution(enzyme solution is 0.1 U/ml) was added to 1.5 ml of 0.1 mol/l acetate buffer (pH 4.5) and 0.5 ml of 0.5 mmol/l ABTS at room temperature; increased in absorbance at 420 nm immediately after 3 min (molar absorptivity ε = 36000 l/mol cm) was measured; and 1 μmol substrate converted in 1 min was defined as 1 enzyme activity unit (U).
Enzyme Concentration Detection
Samples were taken periodically as in enzyme activity detection to measure the concentration of enzyme solution with the applied direct current electric field. The results were compared with the samples at the same time without electric field.
Determination method of enzyme concentration was: 1 ml enzyme solution was mixed with 5 ml coomassie brilliant blue G-250 reagent and absorbance measured at 595 nm. 1 ml water and 5 ml coomassie brilliant blue G-250 was taken as comparison. The protein standard curve was used to get enzyme concentration using Bradford method [8]. Bovine serum albumin was used for standard protein solution and measured by coomassie brilliant blue G-250 reagent. Standard curve equation: y = 0.0083x + 0.0048, R2 = 0.9981, where y is absorbance of protein at 595 nm, and x is the protein concentration (μg/ml), and R is correlation coefficient.
Optimum pH
At room temperature, a certain molar concentration of laccase was added in buffers with different pH (pH 2.0–6.0, 0.1 mol/l HAc–NaAc) to measure the optimum pH for enzyme activity [9].
Water Correlation Coefficient Measurement
With the applied direct current electric field, cathodic region, anodic region, and the middle region of the apparatus were monitored periodically. The changes of pH and electrical conductivity were measured directly by pH meter and electrical conductivity meter, respectively.
Results and Discussion
The Effect of Direct Current Electric Field on Pure Water Environment
The application of external electric field can change the physical and chemical properties of pure water environment [10]. Hence, the pH and electrical conductivity of each region were monitored by applying direct current electric field of 6 V direct voltage (Fig. 2).
Fig. 2.

Effect of electric field on pH and electrical conductivity of pure water environment. Squares, triangle and circle represent the environment in cathodic region, anodic region, and the middle region, respectively. Inverted triangle represents the environment without electric field. Every value represents the mean of in duplicate measurements
pH in cathodic region increases continually, while pH in anodic region decrease, and pH in middle region does not change. It is attributed to the application of current that is too small to reach hydrolysis of current voltage: however, pure water can produce ionic reaction owing to the existence of weak current. That may lead to the increase of hydrogen ion concentration in anodic region and the increase of hydroxyl ion concentration in cathodic region. Therefore, the anodic region is acidic and the cathodic region is alkaline. The electrical conductivity represents the ability of conduction current in water environment, and the continuous increase of ion concentration in water under the effect of electric field leads to conductivity increased with time [10, 11].
The Effect of Direct Current Electric Field on Water Contained Laccase
Water environment that contained laccase causes the changes of pH and electrical conductivity are slightly different from pure water, hence, direct current electric field of 6 V voltage was applied on water contained laccase and the changes of pH and electrical conductivity were monitor in each region at different time (Fig. 3).
Fig. 3.

Effect of Laccase on DC electric field mediated changes in pH and electrical conductivity. Squares, triangle and circle represent the environment in cathodic region, anodic region, and the middle region, respectively. Every value represents the mean of in duplicate measurements
In both experiments, to see the effect of laccase on change in pH and conductivity. Figures 2 and 3 initial pH (at time 0 h) are different (6.55 and 4.86 respectively), and initial electrical conductivity (at time 0 h) are also different (2 and 46 µs/cm respectively). And the changes of pH and conductivity are always greater in water contained laccase than pure water. This indicates that laccase can accelerate electrode reaction with the effect of electric filed because laccase is a covellite oxidase. In addition, it belongs to the family of multicopper oxidases [12].
The distribution of laccase molecular internal amino acids is composed of about 500 amino acid single polypeptide [13], including all kinds of acid or alkalized amino acid side chains. There are many alkalized amino groups or acid carboxyl groups. The changes of pH in water environment are accelerated because the basic unit amino acid can produce carboxyl ionization or amino protonation, and the ionization of laccase with different pH is also different. It don’t increase the concentration of conducting materials and also leads to different conduction effects when exposed to the electric field owing to self-ionization characteristic of laccase.
The Optimum pH for Enzyme Activity
ABTS measured catalytic activity of laccase as substrate in different pH. The enzyme activity at pH = 3.0 was taken as comparison to calculate the relative enzymatic activity of laccase in different pH (Fig. 4).
Fig. 4.
Catalytic activity of laccases in different pH. The effect of pH is determined using buffer solutions (HAc–NaAc) to obtain the desired pH in the standard assay method. The maximum activities of laccase is shown as 100 % in the figure. Every value represents the mean of triplicate measurements that varied from the mean by no more than 8 %
Laccase often have high activity in acid conditions [9]. As seen in Fig. 4, the Optimum pH for enzyme activity is pH = 3. when the pH = 6, the activity decreases by 80 %. If 70 % of best activity is used as critical value to determine the pH range, the suitable pH range for laccase catalysis is 2.5–4.0. This results demonstrate that the active center of laccase has best catalytic efficiency in acid pH conditions, which is significant in practical application of laccase reaction system.
The Effect of Electric Field on the Concentration of Laccase
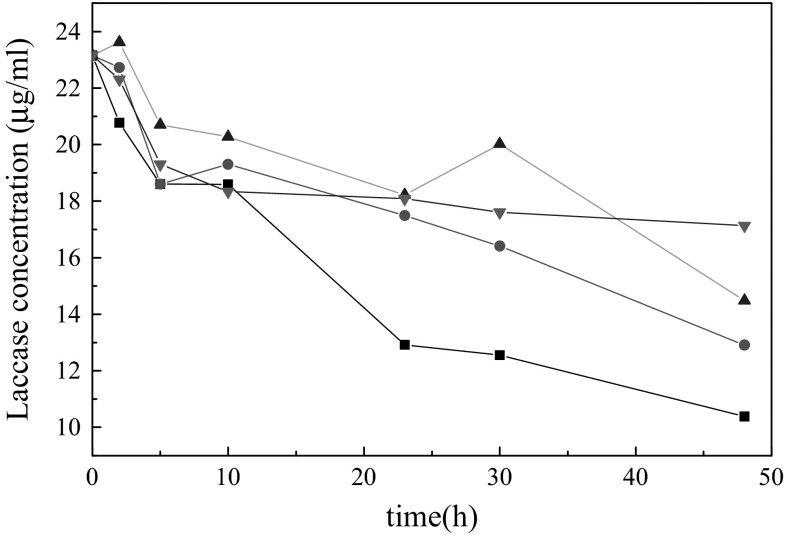
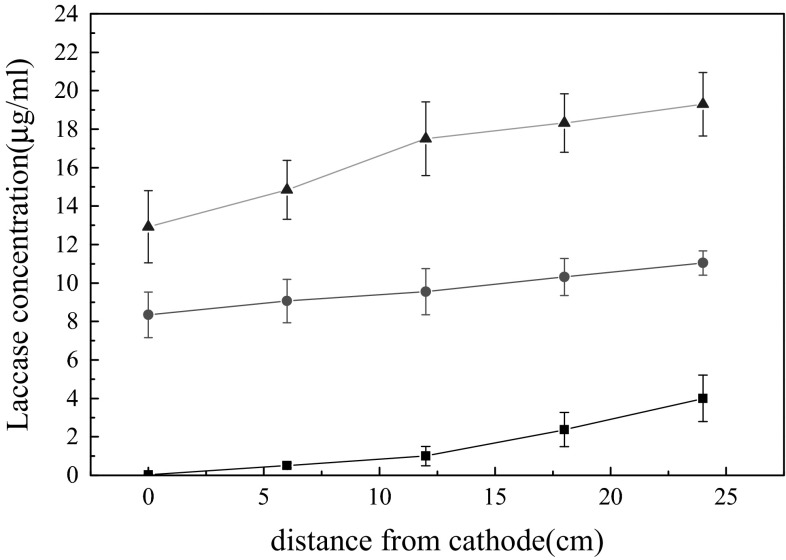
From the experiment in this work, laccase from T. versicolor belongs to extracellular protein and it usually exists as monomer protein [14]. The concentration of protein in the system can be considered as the concentration of laccase. The change of laccase concentration in each region was measured with different time periods by applying direct current electric field of 6 V voltage (Fig. 5). At the same time, the change of laccase concentration was also measured with different concentrations of laccase subjected to 23 h direct current electric field (Fig. 6).
Fig. 5.
Effect of electric field on the concentration of 4 U/ml laccases. Squares, triangle and circle represent the environment in cathodic region, anodic region, and the middle region, respectively. Inverted triangle represent the environment without electric field. Every value represents the mean of in duplicate measurements
Fig. 6.
Effect of electric field on the different concentration of laccases. Squares, circle and triangle represent 1 U/ml laccases, 2 U/ml laccases, and 4 U/ml laccases, respectively. The initial concentration of 1, 2 and 4 U/ml laccases is 5.10, 12.04, and 23.16 µg/ml, respectively. Every value represents the mean of triplicate measurements that varied from the mean by no more than 5 %
The results of Fig. 5 demonstrate that the enzyme concentration decreases slowly without electric field. Laccase concentration is best under the anode conditions. The anode environment can decelerate the degeneration of enzyme protein. The main reason is that laccase in water causes the degeneration of enzyme protein. Many free radicals form in water with the formed electromagnetic force [15], at the same time, pH are changed in different region with the application of electric field (Fig. 2), and laccase exists as protein [14], these can affect the spatial conformation of laccase. Regarding conformational changes of laccase, these are highly dependent on the state of oxidation of the copper atoms in active center [16]. So the enzyme activity in anode will be best. It will be proved in the following work.
Compared with the initial concentration, low concentrations of laccase subjected to electric field is easier to decrease enzyme protein (Fig. 6). Low concentration of laccase have more opportunity by oxidation in water environment. So accumulation of laccase can be used to improve the stability of laccase.
Laccase has amphoteric electro-dissociation and isoelectric point phenomenon in water environment. As shown in Figs. 5 and 6, the concentration of laccase increases from cathode to anode by applying electric field. Moreover, the ionization degree of the carboxylic groups and the extent of amino protonation are different in different pH environment.
In recent year, research on the characteristics of laccase shows that the isoelectric point of laccase is usually an acid range of pH 3–5 [17, 18]. It has been reported that the isoelectric point of laccase from T. versicolor is 3.07–3.27 [19], which is measured by isoelectric focusing electrophoresis. Combining the effects of direct current electric field and pH, cathode is observably alkaline, and laccase is negatively charged and moves to anode. However, as the direct current voltage is small, the motion of laccase is not as observable with the applied electric field. The research for this characteristic of laccase can be applied to the improvement of stability and environmental pollutants degradation.
The Effect of Electric Field on Enzyme Activity of Laccase
The enzymatic activity at time 0 h was taken as comparison to calculate the relative enzyme activity of laccase in different time. Under the effect of direct current fields of 6 V voltage, samples were taken periodically in order to evaluate changes of enzyme activity in each region of enzyme solution (Figs. 7 and 8).
Fig. 7.
Effect of electric field on catalytic activity of 0.1 U/mL laccases. Circle, inverted triangle and triangle represent the environment in cathodic region, anodic region, and the middle region, respectively. Squares represents the environment without electric field. The maximum activities of laccase is shown as 100 % in the figure. Every value represents the mean of in duplicate measurements
Fig. 8.
Effect of electric field on catalytic activity of 4 U/ml laccases. Circle, inverted triangle and triangle represent the environment in cathodic region, anodic region, and the middle region, respectively. Squares represents the environment without electric field. The maximum activities of laccase is shown as 100 % in the figure. Every value represents the mean of in duplicate measurements
The results are shown in Figs. 7 and 8 which shows that, no matter how the laccase concentration in water environment changes, the applied direct current electric field will produce similar trend of changes on the catalytic activity of laccase. The enzyme activities in anodic and middle regions slightly increase in initial time, and then gradually decreased in each region after the application of electric field. In comparison, the enzyme activity of anodic region is high, while that of cathodic region is observably inhibited. The enzyme activity of anodic region with the applied electric field is best in comparison with other environments. The result is demonstrated as described previously. All enzyme activities from different concentrations of laccase with no applied electric field are observed to slowly decrease.
There are many physical and chemical as well as self-structure variation factors affecting the enzyme activity of laccase under the effect of electric field. All other factors may not have single action but also synergistic effect on the enzyme activity, where pH in the region is the main environment factor. Under the effect of electric field, pH in both cathodic and anodic regions changes greatly (Fig. 3). One of the changes is that the acid condition of anodic region is the most suitable pH range (Fig. 4). It can affect the dissociated state of catalytic groups at active site of laccase. The recognition ability of laccase on substrates has room to improve; therefore, enzyme activity of anodic region is considered the best.
However, with the applied electric field, automatic oxidization of air, the concentration of oxygen in water, the formation of free radicals [15] and the motion of charged groups of laccase in the electric field can also affect the enzyme activity. The factors affecting enzyme activity under the effect of electric field needs to be investigated systematically and more deeply.
Conclusions
Laccases have been important, industrially relevant enzymes that are used for diverse applications [20, 21]. But the losing activity is still a serious problem. This study is to provide a new attempt to extend practical applications of enzyme. Compared with immobilized laccase, the combination of laccase and electrical field show better consideration to practical environment changes. It has mild and easy preparing conditions, as well as no chemical additives. The activity and laccase concentration in anodic region is considered the best. Laccase has its own unique response in an electric field. Therefore, the optimum conditions of enzyme activity in the electric field are the focus of future study. Laccase and electrokinetic remediation can have enormous potential to achieve optimal efficiency of laccase.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 41201306).
Conflict of interest
None.
References
- 1.Kolb M, Sieber V, Amann M, Faulstich M, Schieder D. Removal of monomer delignification products by laccase from Trametes versicolor. Bioresour Technol. 2012;104:298–304. doi: 10.1016/j.biortech.2011.11.080. [DOI] [PubMed] [Google Scholar]
- 2.Sheikhi F, Ardakani MR, Enayatizamir N, Rodriguez-Couto S. The determination of assay for laccase of Bacillus subtilis WPI with two classes of chemical compounds as substrates. Indian J Microbiol. 2012;52:701–707. doi: 10.1007/s12088-012-0298-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gomes HI, Dias-Ferreira C, Ribeiro AB. Electrokinetic remediation of organochlorines in soil: enhancement techniques and integration with other remediation technologies. Chemosphere. 2012;87:1077–1090. doi: 10.1016/j.chemosphere.2012.02.037. [DOI] [PubMed] [Google Scholar]
- 4.Reddy KR, Darko-Kagya K, Al-Hamdan AZ. Electrokinetic remediation of pentachlorophenol contaminated clay soil. Water Air Soil Pollut. 2011;221:35–44. doi: 10.1007/s11270-011-0767-z. [DOI] [Google Scholar]
- 5.Sharma KK, Kuhad RC. Laccase: enzyme revisited and function redefined. Indian J Microbiol. 2008;48:309–316. doi: 10.1007/s12088-008-0028-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.She P, Song B, Xing XH, Mark VL, Liu Z. Electrolytic stimulation of bacteria enterobacter dissolvens by a direct current. Biochem Eng J. 2006;28:23–29. doi: 10.1016/j.bej.2005.08.033. [DOI] [Google Scholar]
- 7.Patel SKS, Kalia VC, Choi JH, Haw JR, Kim IW, Lee JK. Immobilization of laccase on SiO2 nanocarriers improves its stability and reusability. J Microbiol Biotechnol. 2014;24:639–647. doi: 10.4014/jmb.1401.01025. [DOI] [PubMed] [Google Scholar]
- 8.Carlsson N, Borde A, Wölfel S, Akerman B, Larsson A. Quantification of protein concentration by the Bradford method in the presence of pharmaceutical polymers. Anal Biochem. 2010;411:116–121. doi: 10.1016/j.ab.2010.12.026. [DOI] [PubMed] [Google Scholar]
- 9.Hu M, Zhou X, Shi Y, Lin J, Irfan M, Tao Y. Essential role of the N-and C-terminals of laccase from pleurotus florida on the laccase activity and stability. Appl Biochem Biotechnol. 2014;174:2007–2017. doi: 10.1007/s12010-014-1147-0. [DOI] [PubMed] [Google Scholar]
- 10.Zhang JP, Zhao L, Tan X. Structural change of water clusters and the corresponding biological effects. Chemistry. 2004;4:278–283. [Google Scholar]
- 11.Chen B, Hu XH, Li JH, Liu SH. Mechanism of increasing of conductivity of water processed by electro-magnetic field. Biomagnetism. 2003;3:69–72. [Google Scholar]
- 12.Strong PJ, Claus H. Laccase: a review of its past and its future in bioremediation. Crit Rev Environ Sci Technol. 2011;41:373–434. doi: 10.1080/10643380902945706. [DOI] [Google Scholar]
- 13.Yaropolov AI, Skorobogat’ko OV, Vartanov SS, Varfolomeyev SD. Laccase. Appl Biochem Biotechnol. 1994;49:257–280. doi: 10.1007/BF02783061. [DOI] [Google Scholar]
- 14.Zhang GQ, Tian T, Liu YP, Wang HX, Chen QJ. A laccase with anti-proliferative activity against tumor cells from a white root fungus Abortiporus biennis. Process Biochem. 2011;46:2336–2340. doi: 10.1016/j.procbio.2011.09.020. [DOI] [Google Scholar]
- 15.Miao J, Fu DX, Lu XJ. Degradation of phenol in wastewater by ultrasound/titanium-iron double-anodes electric catalysisoxidation system. J China Coal Soc. 2005;30:652–655. [Google Scholar]
- 16.Ivanka S, Albert K, Veselin S. Properties of crude laccase from Trametes versicolor produced by solid-substrate fermentation. Adv Biosci Biotechnol. 2010;1:208–215. doi: 10.4236/abb.2010.13029. [DOI] [Google Scholar]
- 17.Baldrian P. Purification and characterization of laccase from the white-rot fungus Daedalea quercina and decolorization of synthetic dyes by the enzyme. Appl Microbiol Biotechnol. 2004;63:560–563. doi: 10.1007/s00253-003-1434-0. [DOI] [PubMed] [Google Scholar]
- 18.Mikolasch A, Schauer F. Fungal laccases as tools for the synthesis of new hybrid molecules and biomaterials. Appl Microbiol Biotechnol. 2009;82:605–624. doi: 10.1007/s00253-009-1869-z. [DOI] [PubMed] [Google Scholar]
- 19.Cassland P, Jonsson LJ. Characterization of a gene encoding Trametes versicolor laccase A and improved heterologous expression in Saccharomyces cerevisiae by decreased cultivation temperature. Appl Microbiol Biotechnol. 1999;52:393–400. doi: 10.1007/s002530051537. [DOI] [PubMed] [Google Scholar]
- 20.Dhiman SS, Garg G, Sharma J, Kalia VC, Kang YC, Lee JK. Reduction in acute ecotoxicity of paper mill effluent by sequential application of xylanase and laccase. PLoS One. 2014;9:e102581. doi: 10.1371/journal.pone.0102581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh G, Kaur K, Puri S, Sharma P. Critical factors affecting laccase-mediated biobleaching of pulp in paper industry. Appl Microbiol Biotechnol. 2015;99:155–164. doi: 10.1007/s00253-014-6219-0. [DOI] [PubMed] [Google Scholar]