Abstract
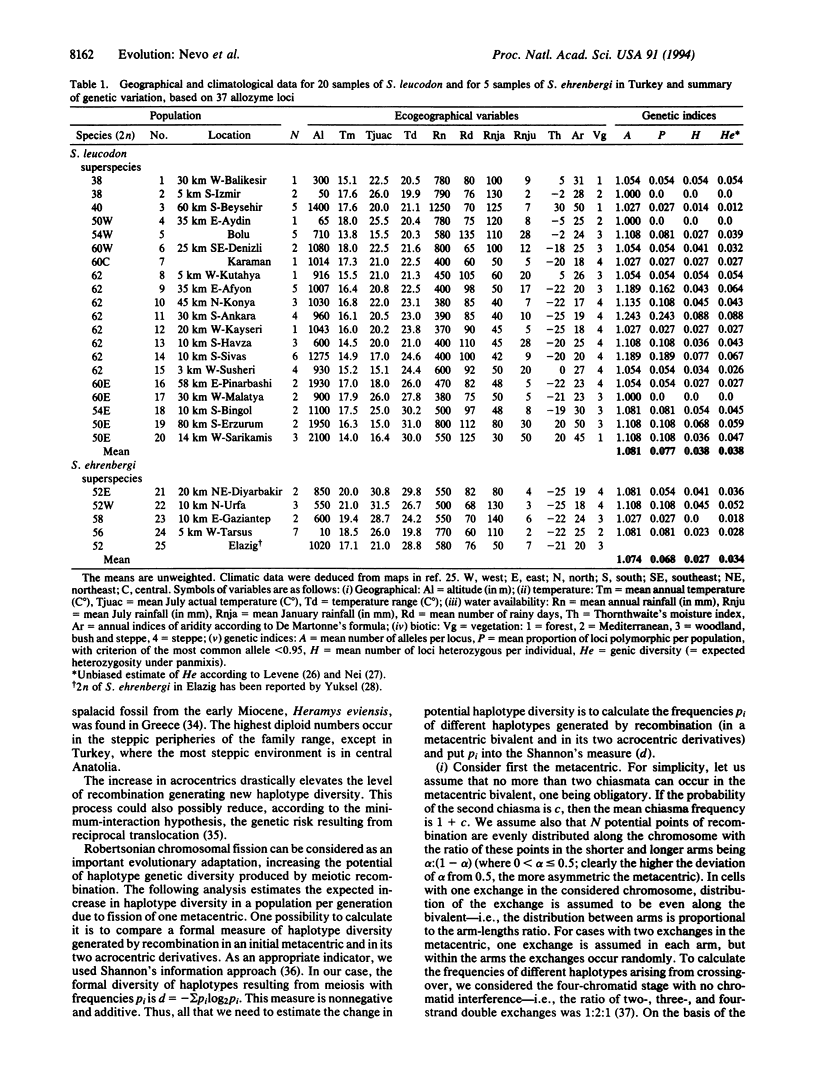
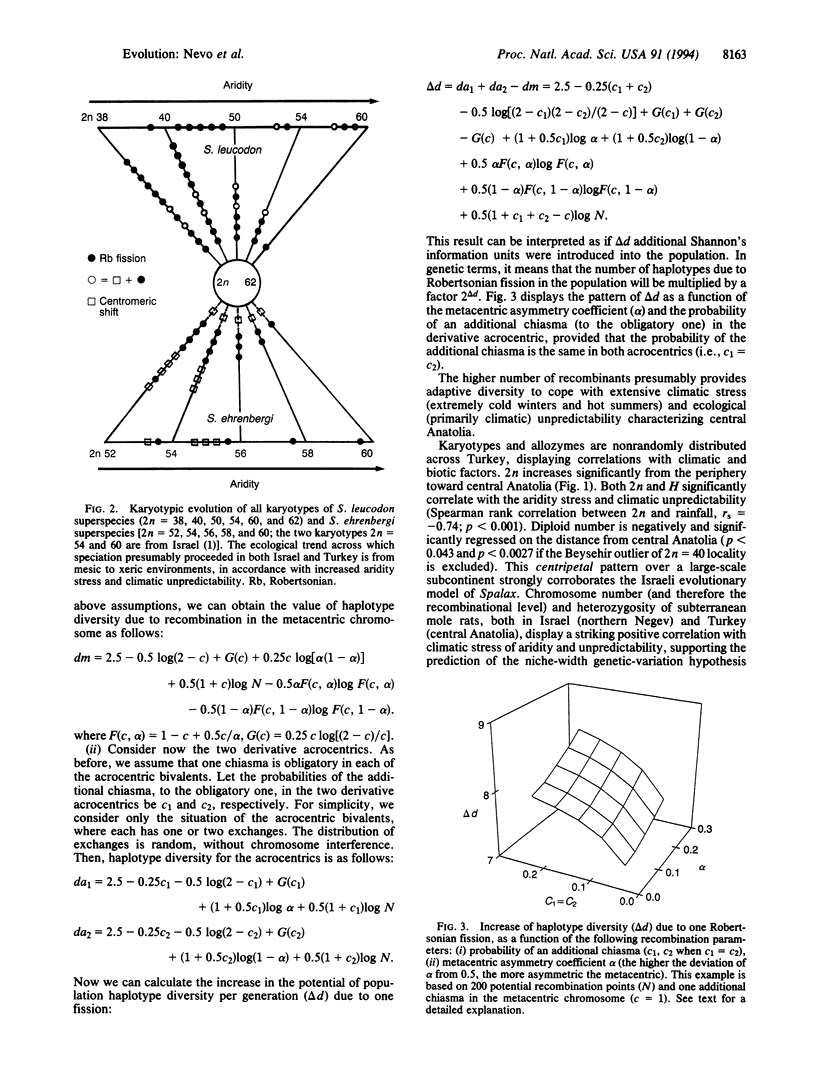
The evolutionary forces causing chromosomal speciation and adaptation are still enigmatic. Here we tested the Israel evolutionary model of positive association of diploid chromosome number (2n) and genetic diversity with aridity stress in subterranean mole rats, on a 30-times-larger scale in Asia Minor. We analyzed both karyotype and allozyme diversity across Turkey, based on 37 allozymic loci in 20 localities of the Spalax leucodon and 4 localities of the Spalax ehrenbergi superspecies. We found extensive chromosomal speciation in S. leucodon (2n = 38, 40, 50, 54, 60, and 62) and in S. ehrenbergi (2n = 52, 56, and 58), presumably representing from 14 to > 20 additional biological species. Genetic diversity indices were low, but, like the chromosome number (2n), positively correlated with aridity stress, increasing centripetally from the periphery toward geologically young, arid, and climatically unpredictable central Anatolia. Nei's genetic distance D across all populations averaged 0.174 (range 0.002-0.422), supporting, combined with 2n and ecogeography, the biological species status of most tested populations. Chromosome evolution is the basis of speciation and adaptation in Spalax; it provides both postmating reproductive isolation, as well as higher levels of recombination with increased 2n. A mathematical model shows that a Robertsonian fission of a single metacentric considerably increases haplotype diversity. This haplotype diversity may contribute to population adaptation to climatic stress and ecological unpredictability in space and time. The increase in diversity corroborates the nichewidth genetic-variation hypothesis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnason U The role of chromosomal rearrangement in mammalian speciation with special reference to Cetacea and Pinnipedia. Hereditas. 1972;70(1):113–118. [PubMed] [Google Scholar]
- Bengtsson B. O. Rates of karyotype evolution in placental mammals. Hereditas. 1980;92(1):37–47. doi: 10.1111/j.1601-5223.1980.tb01676.x. [DOI] [PubMed] [Google Scholar]
- Capanna E. Robertsonian numerical variation in animal speciation: Mus musculus, an emblematic model. Prog Clin Biol Res. 1982;96:155–177. [PubMed] [Google Scholar]
- Lande R. The fixation of chromosomal rearrangements in a subdivided population with local extinction and colonization. Heredity (Edinb) 1985 Jun;54(Pt 3):323–332. doi: 10.1038/hdy.1985.43. [DOI] [PubMed] [Google Scholar]
- May R. M. How many species are there on Earth? Science. 1988 Sep 16;241(4872):1441–1449. doi: 10.1126/science.241.4872.1441. [DOI] [PubMed] [Google Scholar]
- Nei M. Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics. 1978 Jul;89(3):583–590. doi: 10.1093/genetics/89.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevo E., Filippucci M. G., Beiles A. Genetic diversity and its ecological correlates in nature: comparisons between subterranean, fossorial, and aboveground small mammals. Prog Clin Biol Res. 1990;335:347–366. [PubMed] [Google Scholar]
- Sarich V. M. Rates, sample sizes, and the neutrality hypothesis for electrophoresis in evolutionary studies. Nature. 1977 Jan 6;265(5589):24–28. doi: 10.1038/265024a0. [DOI] [PubMed] [Google Scholar]
- Wahrman J., Goitein R., Nevo E. Mole rat Spalax: evolutionary significance of chromosome variation. Science. 1969 Apr 4;164(3875):82–84. doi: 10.1126/science.164.3875.82. [DOI] [PubMed] [Google Scholar]
- Wilson A. C., Sarich V. M., Maxson L. R. The importance of gene rearrangement in evolution: evidence from studies on rates of chromosomal, protein, and anatomical evolution. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3028–3030. doi: 10.1073/pnas.71.8.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]


