Abstract
The diseases caused by dermatophytes are common among several other infections which cause serious threat to human health. It is evident that enzyme squalene epoxidase is responsible for prolonged dermatophyte infection and it is appealing to note that this enzyme is also responsible for fatty acid synthesis in these groups of fungi. In the present study, terbinafine drug which targets enzyme squalene epoxidase has been explored to design its various novel analogues. The present study suggests that many more prominent drug analogues could be constituted which may be crucial towards designing new drug candidates. In the present study, we have designed a series of such analogues viz. [(2E)-6,6-dimethylhept-2-en-4-yn-1-yl](methyl)(naphthalen-1-ylmethyl)amine, N-[8-({[(2E)-6,6-dimethylhept-2-en-4-yn-1-yl](methyl)amino}methyl)naphthalen-1-yl]-2-(sulfoamino) acetamide, {[4-(dihydroxyamino)-8-({[(2E)-6,6-dimethylhept-2-en-4-yn-1-yl](methyl)amino}methyl)naphthalen-1-yl]sulfanyl}methanol and (R)-{[4-({[(2E,6R)-6,7-dimethyloct-2-en-4-yn-1-yl](methyl)amino}methyl)-5-[(hydroxysulfamoyl)amino]naphthalen-1-yl]amino}sulfinic acid. Moreover, further by molecular docking approach the binding between enzyme and designed analogues was further analysed. The present preliminary report suggested a considerably good docking interaction score of −338.75 kcal/mol between terbinafine and squalene epoxidase from Trichophyton rubrum. This preliminary study implies that few designed candidate ligands can be effectual towards the activity of this enzyme and can play crucial role in pathogenesis control of T. rubrum.
Keywords: Dermatophytes, Molecular docking, Squalene epoxidase, Trichophyton rubrum
Introduction
Trichophyton rubrum is a major dermatophyte causing several skin diseases which infects superficial layers of skin feeding on keratinous regions [1]. There are several reports about some commercially available drugs to control the infection caused by this fungus. In a recent report it is described that marine microorganisms can be crucial source for such drugs [2]. The major concern about drug action against this fungi is changes in target site that are caused by mutations in active site of amino acid sequence corresponding to the protein which is responsible for interaction of the target with this drug [3].
Squalene epoxidase is a prominent enzyme produced by T. rubrum, it is responsible for the fatty acid synthesis in dermatophyxtes and plays key role in sterol biosynthesis [4]. Moreover, it is evidenced that Squalene epoxidase leads to synthesis of ergosterol following a cascade of biosynthetic pathways which are essential to maintain integrity and functionality of fungal cells. Due to this property, it acts as major target for the drugs which are designed to inhibit dermatophytosis [5]. terbinafine, chemically written as N,6,6-trimethyl-N-(naphthalen-1-ylmethyl)hept-2-en-4-yn-1-amine is commercially used drug for treating dermatophytosis [6]. It inhibits activity of the enzyme squalene epoxidase and thus pausing ergosterol synthesis which leads to the death of fungi. It is obvious that the drugs available to inhibit dermatophytes are not being efficient in long run, so there is necessity to design drugs that can encumber the activity of dermatophytes [7]. A report on associated employ of terbinafine and tramadol illustrated that it reduces μ-opioid receptor-related analgesia and augmented risk of serotonergic effects and it should be avoided [8].
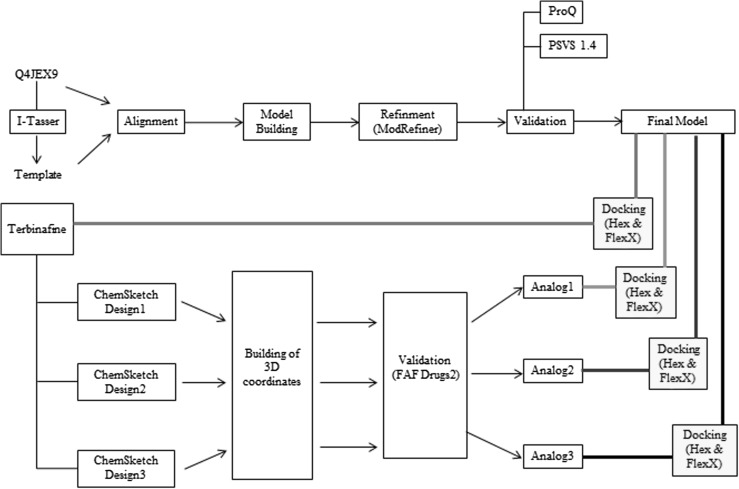
We understand that there are several studies in which antifungal analogs were designed to improve their inhibitory action against dermatophytes which targets the function of squalene epoxidase. In 1999 and 2008, Gokhale et al. and Kharkar et al. carried out comparative molecular field analysis (CoMFA) of fungal inhibitors that act against squalene epoxidase of Candida albicans, Aspergillus fumigatus, and Trichophyton mentagrophyte, and simultaneously designed few compounds that show Minimum Inhibitory Concentration (MIC) with less toxicity [9, 10]. In 2008–2009, three different studies by Francis et al., Che et al., and Wang et al. [1, 11, 12] respectively were conducted and in these studies speculating design and synthesis of antifungal agents was suggested. However, Che et al. [11] designed and synthesized a series of azoles drugs against C. albicans whereas Francis et al. [12] modified the structure of 1-benzylamino-2-phenyl-3-(1H-1,2,4-triazol-1-yl)propan-2-ols to act better against C. albicans [11]. Furthermore, Wang et al., designed, synthesized 2-(2,4-Difluorophenyl)-3-(methyl-(3-phenoxyalkyl)amino)-1-(1H-1,2,4-triazol-1-yl)propan-2-ols against C. albicans [1]. In 2014, computational docking study of cytochrome P450 14alpha-demethylase (CYP51) in C. albicans was carried out with a series of fluconazole analogs [13] which resulted in synthesis of few analogs that were proposed to be better inhibitors than fluconazole. Though structure of Squalene epoxidase enzyme shares similarity with other fungi and mammals its structure in T. rubrum is different as a stretch of 32–34 residues that are present in Sachharomyces cerevisiae, C. albicans and Neospora are not present in T. rubrum [14–16]. Though studies of anti fungals against fungal squalene epoxidase were presented till now, insight of the structure and binding of squalene epoxidase in T. rubrum with its inhibitors is not illustrated yet. In this study, details about the structure and residues involved in binding of squalene epoxidase of T. rubrum are provided. Figure 1 shows the schematic representation of the work flow presented in this study and Fig. 2 provides phylogenetic tree and multiple sequence alignment of active sites of squalene epoxidase among T. rubrum and 15 other fungi. Further, there are few studies have also reported about the perceptive enzyme substrate interactions which are also helpful in understanding the role of enzymes [17, 18].
Fig. 1.
Schematic representation of the work flow presented in this study
Fig. 2.
Multiple sequence alignment to locate the conserved sequence in the enzyme
Despite the fact that few studies proposed the activity of terbinafine against squalene epoxidase, the 3D structure and binding activities of squalene epoxidase were not elaborated. Furthermore, failure of long lasting inhibition of this enzyme by antifungals has raised the need for new drugs which can bind more firmly with this prominent enzyme. In our study we presented a tertiary model of squalene epoxidase enzyme from T. rubrum and tried to study its interaction with terbinafine like compounds. In this process, following in-silico methods, we designed few analogues of terbinafine that can bind well with squalene epoxidase better than terbinafine. These designed analogues were also tested for drug-like properties. Therefore, the present work might be helpful in further developing drug candidates that can inhibit the squalene epoxidase activity under in vitro and in vivo environments.
Materials and Methods
Protein Structure Prediction
Amino acid sequence of squalene epoxidase of T. rubrum (Q4JEX9) was obtained from UniProt Protein Database (http://www.uniprot.org/uniprot/) and this was used as a target to generate 3D structure for this enzyme [19]. In addition, an automated protein modelling server I-Tasser which automatically searches for best templates to build an efficient protein model was preferred for the present study [20]. Further, the protein model was refined using ModRefiner and was appraised for its quality using Protein Quality Predictor (ProQ) [21]. The overall evaluation of different parameters of the generated model was completed using Protein Structure Validation Server (PSVS 1.4) [22].
Ligand Preparation
Structure of drug terbinafine (DB00857) is obtained from DrugBank (http://drugbank.ca/) [23], an open drug database. ChemSketch package (ACD/ChemSketch Freeware, version 11.00) from ACD/Labs (http://www.acdlabs.com/home/) software is used to view and modify the structure of terbinafine. Marvin Sketch 5.10.3 tool of ChemAxon software (http://www.chemaxon.com) is also used for the same purpose. Analogs designed by modifying the structure of terbinafine and named as Analog 1,2 and so on. Building of 3D coordinates and optimization of these designed analogues was done using Avagadro software [24]. Physico-Chemical and ADMET properties of these designed structures are estimated by FAF Drugs2 of Mobyle (http://bioserv.rpbs.univ-paris-diderot.fr/FAF-Drugs) [25].
Molecular Docking
The evaluation of interactions between squalene epoxidase with terbinafine and designed analogs, docking was performed using Hex6.3 software [26] and FlexX software [27]. In addition to this, the improved docking strategy by using the concept of ‘designed analogues ligands’ was taken into consideration. After this, the binding competence of the commercially available terbinafine analogues was compared to these designed analogues. This was achieved by using Hex6.3 and FlexX softwares. The commercially available terbinafine analogues are represented in Table 1.
Table 1.
The names and docking results of the analogs compared to that of terbinafine using Hex and FlexX
| Name | IUPAC name | Hex score | FlexX score | Interacting residues (predicted by FlexX) |
|---|---|---|---|---|
| Original | [(2E)-6,6-dimethylhept-2-en-4-yn-1-yl](methyl)(naphthalen-1-ylmethyl)amine | −338.75 | −7.6749 | 25, 27, 31, 63, 156 |
| Analog1 | N-[8-({[(2E)-6,6-dimethylhept-2-en-4-yn-1-yl](methyl)amino}methyl)naphthalen-1-yl]-2-(sulfoamino)acetamide | −400.79 | −12.9380 | 27, 31, 63 |
| Analog2 | {[4-(dihydroxyamino)-8-({[(2E)-6,6-dimethylhept-2-en-4-yn-1-yl](methyl)amino}methyl)naphthalen-1-yl]sulfanyl}methanol | −376.16 | −13.7826 | 25, 27, 30, 31, 62, 63, 64, 155 |
| Analog3 | (R)-{[4-({[(2E,6R)-6,7-dimethyloct-2-en-4-yn-1-yl](methyl)amino}methyl)-5-[(hydroxysulfamoyl)amino]naphthalen-1-yl]amino}sulfinic acid | −369.98 | −16.1865 | 25, 27, 29, 31, 63, 64 |
Results and Discussion
Tertiary model for squalene epoxidase protein built by I-Tasser server and refined by Mod-Refiner server showed 90.0 % of residues in the most favoured regions, 6.6 % of residues in additionally allowed regions, 1.5 % of residues in generously allowed regions, 2.0 % of residues in disallowed region, LGscore of 3.932 and MaxSub of 0.264. LGscore and MaxSub are two parameters used by ProQ to determine quality of the proteins, where LGscore is negative log of a P value ranging between 1.5 for good models and five for very good protein models. MaxSub ranges between zero for significant models and one for very significant models. These results showed that the model generated is of better quality. 3D structure of enzyme squalene epoxidase generated by I-Tasser server is represented in Fig. 3.
Fig. 3.
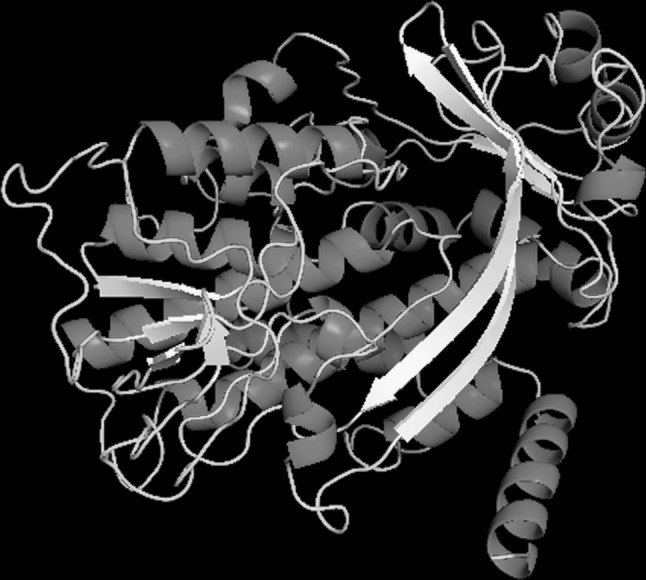
The 3D model of squalene epoxidase generated by ITasser
Ligands designed by ChemSketch and Marvin Sketch were checked for their drug likeliness taking its properties into consideration as per the Lipinski’s rule of five. Formula Weight, logP value, Number of H-Bonds and Number of H-Acceptors of the designed analogs were calculated using online tool FAF Drugs2 of Mobyle. Scores of these five properties for drug likeliness test are exhibited in Table 1. ADME-Tox properties (Adsorption, Distribution, Metabolism, Excretion and Toxicity) of the drug candidates have been analyzed based on the five parameters namely molecular weight, number of hydrogen bond donors, number of hydrogen bond acceptors, logP and number of flexible bonds in the compound. Acceptance level of the drug candidate is measured on the basis of its permeability and also ratio of octanol solubility to water solubility referred as liphophilicity. More the molecular weight less would be the permeability of the compound. Same as the prior, more the number of h-bond donors or acceptors less would be the permeability. Therefore, accepted level of drug likeliness of the compounds were considered as molecular weight <500, number of hydrogen bond donors less than 5, number of hydrogen bond acceptors <10 and logP value <5. 10 or <10 flexible bonds or rotatable bonds in a compound indicate good oral bio-availability [28]. As all the results are satisfying the basic rules for drug likeliness, these compounds were considered as ligands to study their interaction with the enzyme squalene epoxidase.
Docking
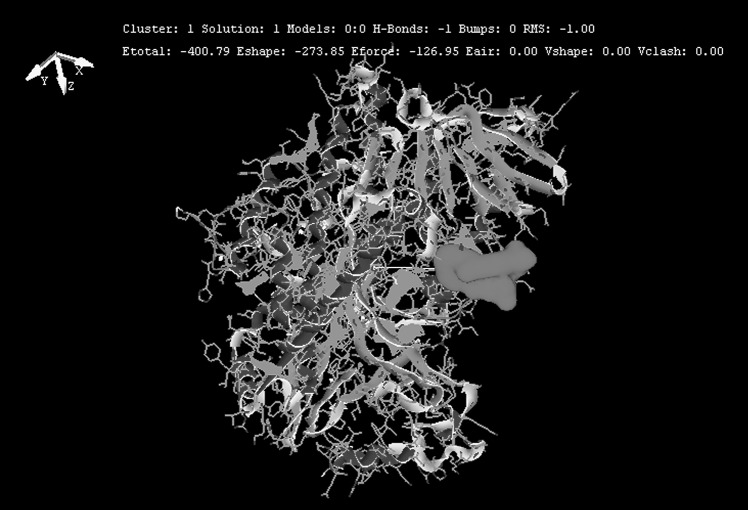
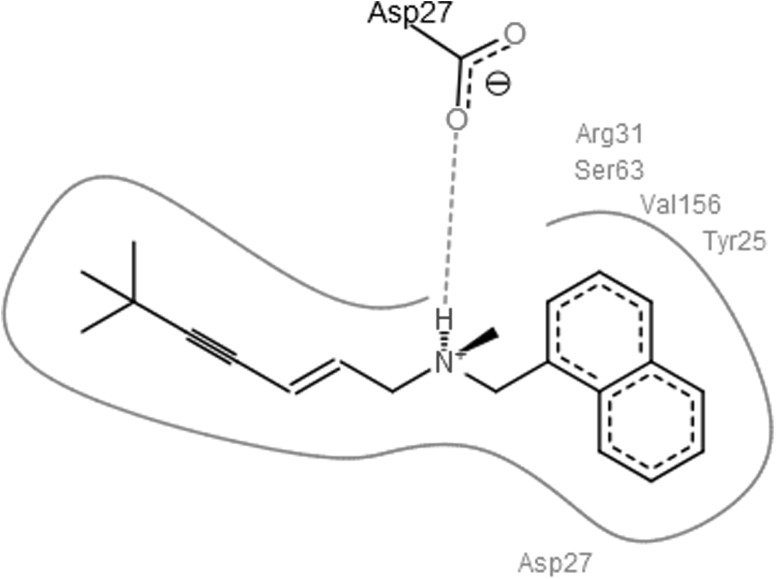
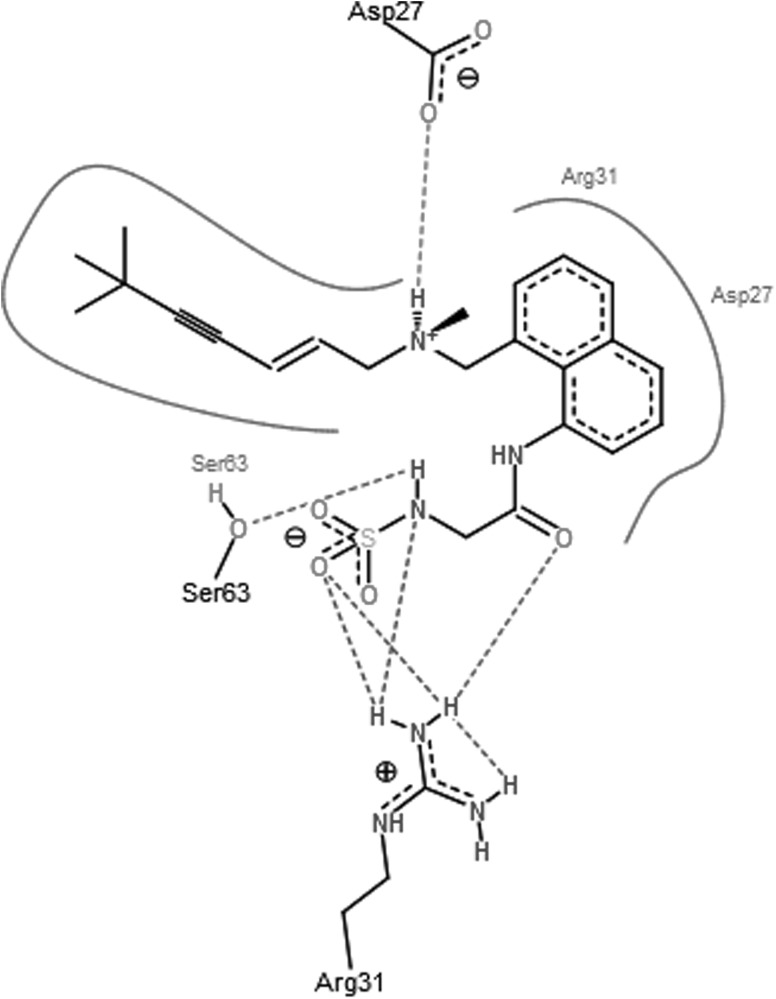
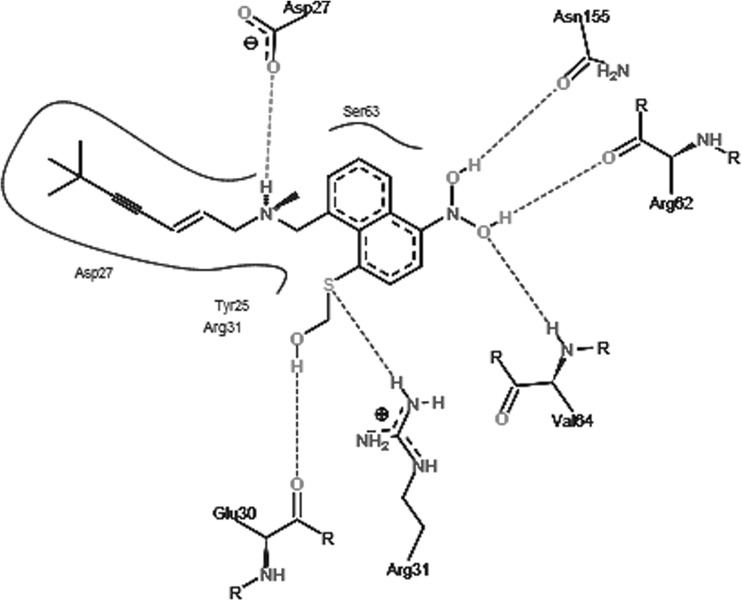
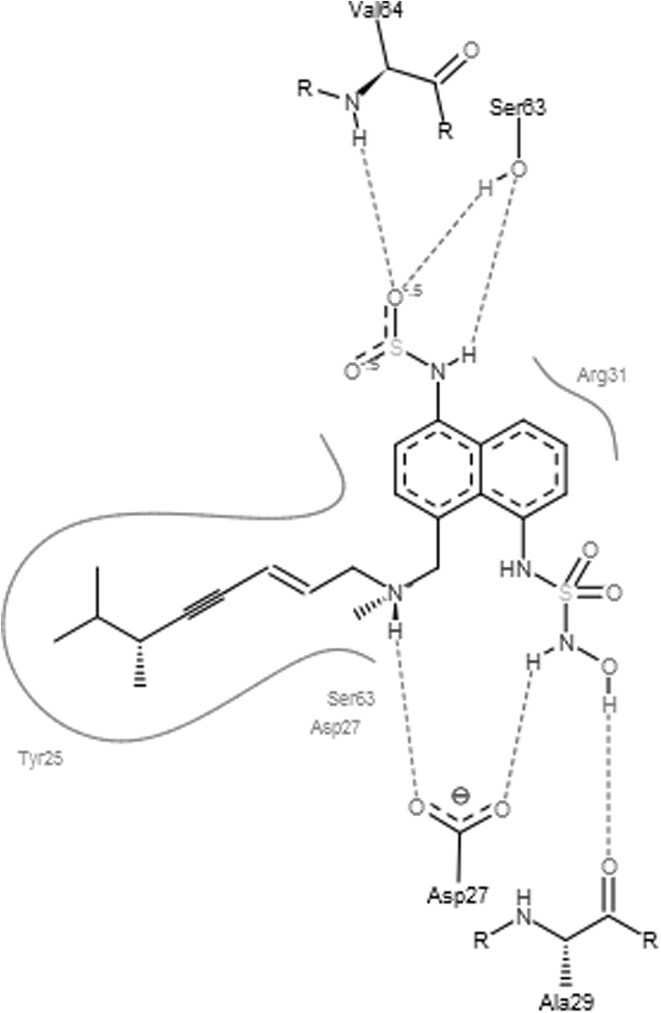
Docking scores of original drug compound terbinafine with squalene epoxidase was compared with docking scores of the designed analogues. Docking scores of these analogues and original drug after interaction with squalene epoxidase are shown in Table 2 along with the residues that are predicted to be involved in the binding. From Hex docking scores it is found that E-Total (kcal mol) of Analog1 (−400.79), shown in Fig. 4, Analog2 (−376.16) and Analog3 (−369.98) were better than that of terbinafine (−338.75). FlexX results were also found to be supporting the docking results obtained by Hex as the FlexX E-Scores (kcal/mol) of Analog1 (−12.9380), Analog2 (−13.7826) and Analog3 (−16.1865) are better than that of the original drug (−7.6749). Figures 5, 6, 7 and 8 obtained from the FlexX docking results of squalene epoxidase with terbinafine analogs 1, 2 and 3 respectively, showed that residues 25, 27, 31 and 63 of squalene epoxidase were involved in interacting strongly with the ligands, whereas residues 29, 30, 31, 62, 64, 155 and 156 also contribute in docking.
Table 2.
The demonstration of Lipinski’s rule of 5 for the Analogs
| Name | Mol. wt. | No. of H-bond donors | No. of H-bond acceptors | logP | No. of flexible bonds | State based on ADME-Tox |
|---|---|---|---|---|---|---|
| Terbinafine | 301.36 | 1 | 5 | 3.5 | 5 | Accepted |
| Analog1 | 443.56 | 3 | 7 | 1.13 | 8 | Accepted |
| Analog2 | 400.53 | 3 | 5 | 4.65 | 7 | Accepted |
| Analog3 | 494.63 | 5 | 9 | 3.55 | 10 | Accepted |
Fig. 4.
Docking of the Analog1 with squalene epoxidase using Hex
Fig. 5.
Best pose of terbinafine generated by FlexX, with the interacting aminoacids
Fig. 6.
Best pose of the Analog1 generated by FlexX, with the interacting aminoacids
Fig. 7.
Best pose of the Analog2 generated by FlexX, with the interacting aminoacids
Fig. 8.
Best pose of the Analog3 generated by FlexX, with the interacting aminoacids
From FlexX results it was shown that residues Asp at 27th position along with Arg at 31st, Ser at 63rd, Val at 156th and Tyr at 25th positions are responsible for the strong binding of squalene epoxidase with terbinafine. Residues Asp at 27th position along with Arg at 31st and Ser at 63rd position of squalene epoxidase favoured binding with analog 1 whereas residues Asp at 27th, Arg at 31st, Ser at 63rd positions favoured binding with analog2 and residues Asp at 27th, Arg at 31st, Ser at 63rd, Tyr at 25th position along with Ala at 29th, Val at 64th positions favoured binding with analog3. From all the above observations, it can be concluded that the residues Asp27, Arg31, Ser63, Tyr25, Ala29 and Val64 residues play an important role while determining the bonding of squalene epoxidase with terbinafine and its analogs.
Speculation from docking results indicate the presence of another methyl group and sulphur functional groups namely sulfoamino, sulfanyl and hydroxysulfamoyl in the ligands which was absent in the native and was cause of efficient binding with the enzyme. These additional functional groups were also checked for their acceptance levels, by inspecting their presence and function among already existing drugs. Presence of methyl group is known to influence the drug-like properties of small molecules. Sulphur functional groups have property of easily penetrating into the skin and are known to treat adverse skin conditions. Derivatives of sulfoamino, sulfanyl and hydroxysulfamoyl with sulfinic acid are present in the analogs 1,2 and 3 respectively which were missing in the original terbinafine molecule. Sulfoamino is used among several drugs namely Fondaparinux, which is an antithrombotic drug. Derivatives of sulfanyl are used in the designing antifungal drugs following mechanism of Michael addition between α,β-unsaturated carbonyl compounds and mercaptans. In 1978, Steven et al. [29] used hydroxysulfamoyl derivatives as a prominent sulphur analog of l-asparagine to act against l-asparagine synthetase. These structural analyses of the designed analogs support our proposal to use these analogs for further experimentation in designing inhibitors of squalene epoxidase.
Structural analysis of squalene epoxidase, structural as well as functional insight into the designed analogs provides several clues regarding interaction of T. rubrum squalene epoxidase with its inhibitors. Taken together our study of structure based drug design against squalene epoxidase might be considered while designing novel dermatophyte inhibitors in an in-vivo system.
Conclusion
It is evident that, in silico studies provides insights in drug designing and this leads to analysis of the compounds behaviour. The structural modification of commercially available terbinafine may be a useful tool towards its effective binding to the respective re-designed analogues. We understood that interaction of the squalene epoxidase with above designed analogs are showing effective binding energy values which shows a prominent clue improved efficiency of terbinafine. This illustrate that different analogs of the present drug could open the avenues to search for the better compound which might work more effectively than the present drug. Furthermore, drug likeliness tests scores for these restructured also add more authenticity to the work related to the analogs designing. Thus, the present preliminary report also provides a scope to improve the efficient binding between terbinafine analogues and squalene epoxidase from a dermatophyte T. rubrum.
Acknowledgments
We hereby acknowledge BTIS-Sub DIC facility at BIT Mesra through DBT, GOI and the Department of Agriculture, Government of Jharkhand for financial support for our department towards infrastructure facilities.
Footnotes
The authors Sudha Karumuri and Puneet Kumar Singh should be regarded as the joint first authors.
References
- 1.Wang W, Chunquan S, Xiaoying C, Haitao J, Zhenyuan M, Jianzhong Y, Wannian Z. Design, synthesis, and antifungal activity of novel conformationally restricted triazole derivatives. Arch Pharm Chem Life Sci. 2009;342:732–739. doi: 10.1002/ardp.200900103. [DOI] [PubMed] [Google Scholar]
- 2.Cheung RC, Wong JH, Pan WL, Chan YS, Yin CM, Dan XL, Wang HX, Fang EF, Lam SK, Ngai PH, Xia LX, Liu F, Ye XY, Zhang GQ, Liu QH, Sha O, Lin P, Ki C, Bekhit AA, Ael-D Bekhit, Wan DC, Ye XJ, Xia J, Ng TB. Antifungal and antiviral products of marine organisms. Appl Microbiol Biotechnol. 2014;98:3475–3494. doi: 10.1007/s00253-014-5575-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Regina L, Sandra F, Vlasta K, Natascha S, Eva P, Kathrin W, Mojca L, Karina L, Christoph R, Ivan H, Friederike T. Molecular mechanism of terbinafine resistance in Saccharomyces cerevisiae. Antimicrob Agents Chemother. 2003;47:3890–3900. doi: 10.1128/AAC.47.12.3890-3900.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Favre B, Ryder NS. Characterization of squalene epoxidase activity from the dermatophyte Trichophyton rubrum and its inhibition by terbinafine and other antimycotic agents. Antimicrob Agents Chemother. 1996;40:443–447. doi: 10.1128/aac.40.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marcin N, Marcin H, Lucjan SW, Piotr S, Dariusz MP, Michal L, Krzysztof G, Leszek R. Detailed mechanism of squalene epoxidase inhibition by terbinafine. J Chem Inf Model. 2011;51:455–462. doi: 10.1021/ci100403b. [DOI] [PubMed] [Google Scholar]
- 6.Petranyi G, Meingassner JG, Mieth H. Antifungal activity of the allylamine derivative terbinafine in vitro. Antimicrob Agents Chemother. 1987;31:1365–1368. doi: 10.1128/AAC.31.9.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Ludmila MB, Patrícia CS, Talles PP, Milene AR, Patrícia SC, Danielle GS, Daniel AS. IFN-γ impairs Trichophyton rubrum proliferation in a murine model of dermatophytosis through the production of IL-1β and reactive oxygen species. Med Mycol. 2013;52:293–302. doi: 10.1093/mmy/myt011. [DOI] [PubMed] [Google Scholar]
- 8.Tuukka S, Teijo IS, Nora MH, Janne TB, Pertti JN, Mika S, Klaus TO, Kari L. Effects of terbinafine and itraconazole on the pharmacokinetics of orally administered tramadol. Eur J Clin Pharmacol. 2015 doi: 10.1007/s00228-014-1799-2. [DOI] [PubMed] [Google Scholar]
- 9.Bhakti P, Meena AK, Pissurlenkar R, Mamata J, Evans CC, Sudha S. Probing molecular level interaction of antifungal drugs with model membranes by molecular modeling, multinuclear NMR and DSC methods. Int J Cur Pharm Res. 2010;2:47–56. [Google Scholar]
- 10.Prashant SK, Meenakshi ND, Vithal MK. Design, synthesis, antifungal activity, and ADME prediction of functional analogues of terbinafine. Med Chem Res. 2008;18:421–432. [Google Scholar]
- 11.Che X, Sheng C, Wang W, Cao Y, Xu Y, Ji H, Dong G, Miao Z, Yao J, Zhang W. New azoles with potent antifungal activity: design, synthesis and molecular docking. Eur J Med Chem. 2009;44:4218–4226. doi: 10.1016/j.ejmech.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 12.Francis G, Remi G, Cedric L, Fabrice P, Carine P, Marc LB, Patrice LP. Synthesis and structure–activity relationships of 2-phenyl-1-[(pyridinyland piperidinylmethyl)amino]-3-(1H-1,2,4-triazol-1-yl)propan-2-ols as antifungal agents. Bioorg Med Chem Lett. 2009;19:301–304. doi: 10.1016/j.bmcl.2008.11.101. [DOI] [PubMed] [Google Scholar]
- 13.Nalu TAP, Fernanda CAM, Antonio R, Nilce MMR. Dermatophytes: host-pathogen interaction and antifungal resistance. An Bras Dermatol. 2010;85:657–667. doi: 10.1590/S0365-05962010000500009. [DOI] [PubMed] [Google Scholar]
- 14.Yan Z, Shichong Y, Renwu L, Qingjie Z, Xiang L, Maocheng W, Ting H, Xiaoxun C, Honggang Hu, Qiuye W. Synthesis, antifungal activities and molecular docking studies of novel 2-(2,4-difluorophenyl)-2-hydroxy-3-(1H-1,2,4-triazol-1-yl)propyl dithiocarbamates. Eur J Med Chem. 2014;74:366–374. doi: 10.1016/j.ejmech.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 15.Nalu TAP, Pablo RS, Juliana PF, Henrique CSS, Fernanda GP, Fernanda CAM, Diana EG, Fernando S, Rodrigo AC, Mendelson M, Jeny RCS, Roseli AF, Antonio R, Nilce MMR. Transcriptional profiling reveals the expression of novel genes in response to various stimuli in the human dermatophyte Trichophyton rubrum. BMC Microbiol. 2010;10:39. doi: 10.1186/1471-2180-10-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anke B, Ekaterina S, Gernot G, Christoph H, Susann S, Peter S, Andrew H, Marius F, Andreas P, Karol S, Marc F, Ivo P, Steffen P, Marco G, Robert W, Wenjun L, Olaf K, Volker S, Christian H, Bernhard H, Theodore CW, Matthias P, Reinhard G, Joseph H, Johannes W, Peter FZ, Michel M, Axel AB. Comparative and functional genomics provide insights into the pathogenicity of dermatophytic fungi. Genom Biol. 2011;12:R7. doi: 10.1186/gb-2011-12-1-r7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karthik MVK, Satya Deepak MVKN, Shukla P. Explication of interactions between HMGCR isoform 2 and various statins through In silico modeling and docking. Comp Biol Med. 2012;42:156–163. doi: 10.1016/j.compbiomed.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Singh PK, Shukla P. Molecular modeling and docking of microbial inulinases towards perceptive enzyme-substrate interactions. Ind J Microbiol. 2012;52:373–380. doi: 10.1007/s12088-012-0248-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Osborne CS, Leitner I, Favre B, Ryder NS. Amino acid substitution in Trichophyton rubrum squalene epoxidase associated with resistance to terbinafine. Antimicrob Agents Chemother. 2005;49:2840–2844. doi: 10.1128/AAC.49.7.2840-2844.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang Z. I-TASSER server for protein 3D structure prediction. BMC Bioinformatics. 2008;9:40. doi: 10.1186/1471-2105-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong X, Yang Z. Improving the physical realism and structural accuracy of protein models by a two-step atomic-level energy minimization. Biophys J. 2011;101:2525–2534. doi: 10.1016/j.bpj.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhattacharya A, Tejero R, Montelione GT. Evaluating protein structures determined by structural genomics consortia. Proteins. 2007;66:778–795. doi: 10.1002/prot.21165. [DOI] [PubMed] [Google Scholar]
- 23.Wishart DS, Knox C, Guo AC, Cheng D, Shrivastava S, Tzur D, Gautam B, Hassanali M. DrugBank: a knowledgebase for drugs, drug actions and drug targets. Nucl Acids Res. 2008;36:D901–D906. doi: 10.1093/nar/gkm958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marcus DH, Donald EC, David CL, Tim V, Eva Z, Geoffrey RH. Avogadro: an advanced semantic chemical editor, visualization, and analysis platform. J Cheminformatics. 2012;4:17. doi: 10.1186/1758-2946-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lagorce D, Maupetit J, Baell J, Sperandio O, Tuffery P, Miteva MA, Galons H, Villoutreix BO. The FAF-Drugs2 server: a multistep engine to prepare electronic chemical compound collections. Bioinformatics. 2011;27:2018–2020. doi: 10.1093/bioinformatics/btr333. [DOI] [PubMed] [Google Scholar]
- 26.Macindoe G, Mavridis L, Venkatraman V, Devignes MD, Ritchie DW. HexServer: an FFT-based protein docking server powered by graphics processors. Nucl Acids Res. 2010;38:W445–W449. doi: 10.1093/nar/gkq311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dessalew N, Bharatam PV. 3D-QSAR and molecular docking study on bisarylmaleimide series as glycogen synthase kinase 3, cyclin dependent kinase 2 and cyclin dependent kinase 4 inhibitors: an insight into the criteria for selectivity. Eur J Med Chem. 2007;42:1014–1027. doi: 10.1016/j.ejmech.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 28.Veber DF, Johnson SR, Cheng HY, Smith BR, Ward KW, Kopple KD. Molecular properties that influence the oral bioavailability of drug candidates. J Med Chem. 2002;45:2615–2623. doi: 10.1021/jm020017n. [DOI] [PubMed] [Google Scholar]
- 29.Steven B, Gilbert JB, Michael M. Potential inhibitors of l-asparagine biosynthesis. 4. Substituted sulfonamide and sulfonylhydrazide analogs of l-asparagine. J Med Chem. 1978;978:45–49. doi: 10.1021/jm00199a008. [DOI] [PubMed] [Google Scholar]