Abstract
Hydatidosis is caused by a tapeworm which infects humans by the larval stage. In humans, the disease is so serious that it requires surgery for treatment. Documents show that there have been many efforts in finding new scolicidal agents for reducing the rate of the infection. The objective of this study was determination of the scolicidal effect of two fungal chitosan types, produced from Penicillium spp. and commercially chitosan (CC) on Echinococcus granulosus protoscolex. Protoscolices were aseptically aspirated from sheep livers hydatid cysts. Four concentrations (50, 100, 200, 400 μg/ml) of each type of prepared chitosan were used for 10, 30, 60 and 180 min. Viability of protoscolices was detected by 0.1 % eosin staining. Fungal chitosan which was the most bioactive type with higher degree of deacetylation showed stronger scolicidal activity in vitro (P < 0.05). Fungal chitosan could be recommended, as good as CC for hydatid cysts control and is a noble alternative for synthetic and chemical scolicidal.
Keywords: Chitosan, Protoscolices, Echinococcus granulosus, Penicillium
Introduction
Chitosan with poly-N-acetylglucosamine sequences is deacetylated derivative of chitin obtained from outer skeleton of crabs, shrimp, lobster, cuticles of insects and cell wall of fungi. Chitosan as a cationic natural polysaccharide and biodegradable biopolymer demonstrated to be biocompatible with no toxicity (Nishimura et al. 1984). Chitosan has been widely uses in pharmaceutical formulation, food engineering, biotechnology, agriculture industry and cosmetics. Due to its unique properties the considerable antimicrobial, antiviral and antifungal activities of chitosan receives such a great attention in biomedical field (Chatelet et al. 2001; Niederhofer and Muller 2004; Nwe et al. 2001; Shahidi et al. 2005). The ratio of 2-acetamido-2-deoxy-d-glucopyranose to 2-amino-2-deoxy-d-glucopyranose structural units is one of the important parameter in chitosan, named the degree of deacetylation (DD), has effect on the solubility, intrinsic, extrinsic properties and anti-parasitic activities (Khan et al. 2002). Echinococcosis is a zoonotic parasitic disease which caused by Echinococcus granulosus (E.g). The mature stage of E.g is found in the small intestine of dog as final host, human, cattle, and sheep as intermediate hosts are infected by ingesting the eggs. The released embryo crosses the intestinal wall to become located in the liver, lungs, or any other organ where the larval form of the parasite develops. To complete the parasite’s life cycle, dog acquires the parasite during the consumption of fertile hydatid cysts (Larrieu et al. 2001). Echinococcosis in humans and animals is an economic and public health concern in many parts of the world. Cystic hydatid disease affects mainly the liver (50–70 % of all cysts) but can also develop in lung (20–30 %) and, less frequently, in spleen, kidney, bone, brain, and other organs (Ammann and Eckert 1996). Avoiding spillage of the cyst contents and the use of effective scolicidal agents are essential to reduce the recurrence rate (Topcu et al. 2009). Currently, many scolicidal agents, which have some complications of their own, have been used for inactivation of the cyst content (McManus et al. 2003). Recently, a wide variety of researchers have targeted bacteria and fungi rather than parasites as target organisms for chitosan (Li et al. 2008; Tayel et al. 2010a; Tikhonov et al. 2006; Tokura et al. 1997; Xu et al. 2007). A piece of information is available on the antiparasitic effects of chitosan obtained from fungi. Penicillium genus isolated from agricultural soils in Iran and can be easily cultivated in low cost of culture media. The objective of this study is comparing the scolicidal effect of chitosan isolated from cell wall of Penicillium waksmanii and Penicillium citrinum with available commercial chitosan by in vitro. In this experiment, effect of fungal chitosan (FC) and commercially chitosan (CC) concentrations, exposure time and DD on viability of protoscolices of E.g was evaluated.
Materials and methods
Collection of protoscolices
The E.g protoscolices were collected from the livers of infected sheep slaughtered at Sari slaughterhouse, Mazandaran Province, in northern Iran. The hydatid fluid was transferred into test tube under sterilized condition and left to set for 30 min. The supernatant was removed and the remains protoscolices were washed with normal saline.
Extraction of chitosan
Low molecular weight commercial chitosan was purchased from Fluka (CC). Two Penicillium species, P. waksmanii and P. citrinum from the department of mycology at Mazandaran Agriculture University, were used as a source of fungal chitosan (FC). The fungal chitin was deacetylated by a modified method of (Rane and Hoover 1993). After cultivation of fungal mycelia, the dry biomass were suspended with NaOH solution (1 molar) and autoclaved at 121 °C for 20 min. The Alkaline undissolved fractions were collected by centrifugation at 12,000 rpm for 20 min, washed with distilled water and recentrifuged to obtained pH 7. The precipitation was further extracted using 2 % acetic acid at 95 °C for 8 h. The insoluble particles suspension was centrifuged, then the supernatant solution was neutralized with NaOH (2 M), the solution centrifuged and the precipitated chitosan was washed with distilled water. DD of FC and CC was measured by FT-IR spectroscopy (Perkin Elmer Pergamon 781, from 4,000 to 450) and the calculation proposed as DD = 100−[(ACO/AOH) × 100/1.33] (Miya et al. 1980). To preparation of chitosan solutions, each prepared chitosan sample from Penicillium species and CC was dissolved in 1 % (w/v) acetic acid solution and then neutralized the solution by 1 molar NaOH (medium).
Scolicidal assay
Four concentrations of the chitosan solution (50, 100, 200, and 400 μg/ml) were prepared. Then 10,000 washed protoscolices was added in batches into 2 ml of each chitosan solutions and gently mixed. The mixed solution was incubated at 37 °C for 10, 30, 60 and 180 min. Two milliliters of 0.1 % eosin stain was then added to their remaining settled protoscolices and mixed gently. The upper portion of the solution was discarded after 15 min of incubation. The pellet of protoscolices was examined under a light microscope. The percentages of dead protoscolices were determined by counting a minimum of 250 (usually more than 500) protoscolices. The Control experiment was samples containing medium without chitosan.
Viability
The viability of the protoscolices was detected by 0.1 % eosin as vital dye under a light microscope. The protoscolices with no absorbed dye were considered potentially viable and otherwise, they were recorded as dead (Figs. 1, 2).
Fig. 1.

Live colorless evaginated protoscolices (×20)
Fig. 2.
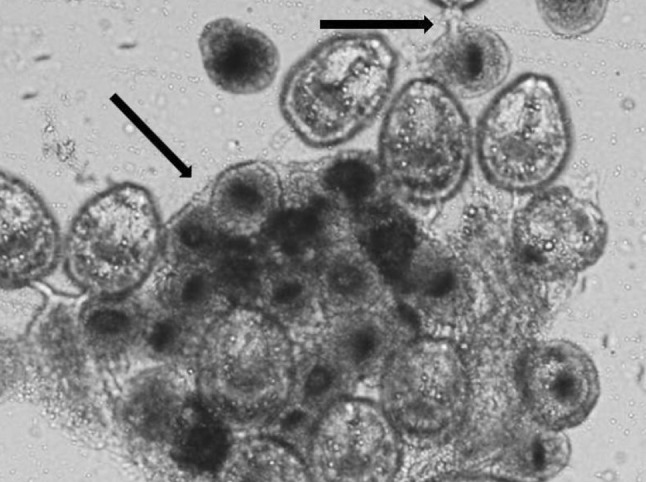
Dead invaginated colored protoscolices (×40)
Statistical analysis
Triplicate trials were performed for each experiment. Statistical calculations were carried out by Kruskal-wallis single-direction analysis, using the SPSS software package. P-values of <0.05 were considered significant.
Results
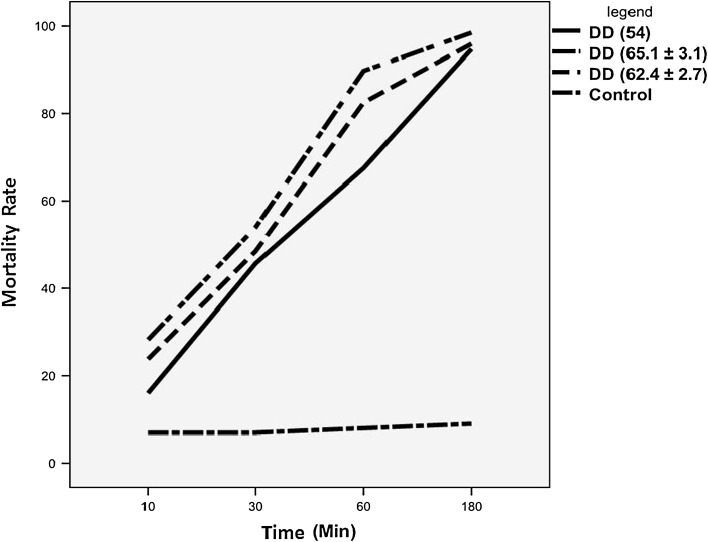
In the present study, the DD and scolicidal activity for the prepared chitosan from P. waksmanii, P. citrinum and commercial one are shown in Tables 1, 2, 3 and 4. The results indicate that there were significant difference between FC, CC and control group at 10, 30, 60, 180 min of exposure times with P value = 0.044, 0.006, 0.001 and 0.0001, respectively. As represented in Table 2, P. waksmanii with 65.1 % of DD showed high scolicidal activity at the concentration of 50, 100, 200 and 400 μg/ml with 95, 99, 100, 100 mortality rate after 180 min, respectively. The percentage reduction of protoscolices was observed in all tested concentration of chitosan with mortality rate range between 89 and 100 % which was represented significant difference with control group (P value = 0.024, 0.015, 0.010 and 0.003) respectively, Although chitosan failed to inhibit the growth of protoscolices completely, in the less concentration than 400 μg/ml. As illustrated in Fig. 3, with the increase of chitosan deacetylation degree, the antiparasitic activity was increased sharply. It seems that higher concentrations, DD and exposure time were more effective in scolicidality. The results of our study indicated that FC and CC represented high scolicidal activity in vitro.
Table 1.
Degree of deacetylation (%) of commercially and fungal chitosan
| Chitosan source | Degree of deacetylation (%) |
|---|---|
| Commercially | 54 |
| P. waksmanii | 65.1 ± 3.1 |
| P. citrinum | 62.4 ± 2.7 |
Table 2.
Scolicidal effect of different concentrations of prepared chitosan from P. waksmanii on the mortality rate of protoscolices of hydatid cyst at the following various exposure times
| Exposure time/concentration | 50 | 100 | 200 | 400 | P value |
|---|---|---|---|---|---|
| 10 | 19 | 26 | 27 | 41 | 0.019 |
| 30 | 41 | 47 | 59 | 69 | 0.005 |
| 60 | 78 | 86 | 96 | 99 | 0.0001 |
| 180 | 95 | 99 | 100 | 100 | 0.0001 |
| P value | 0.015 | 0.007 | 0.006 | 0.002 | – |
Table 3.
Scolicidal effect of different concentrations of prepared chitosan from P. citrinum on the mortality rate of protoscolices of hydatid cyst at the following various exposure times
| Exposure time/concentration | 50 | 100 | 200 | 400 | P value |
|---|---|---|---|---|---|
| 10 | 17 | 19 | 21 | 38 | 0.041 |
| 30 | 34 | 41 | 57 | 61 | 0.007 |
| 60 | 69 | 78 | 87 | 96 | 0.001 |
| 180 | 91 | 94 | 98 | 100 | 0.0001 |
| P value | 0.019 | 0.015 | 0.011 | 0.002 | – |
Table 4.
Scolicidal effect of different concentrations of commercially chitosan on the mortality rate of protoscolices of hydatid cyst at the following various exposure times
| Exposure time/concentration | 50 | 100 | 200 | 400 | P value | Control group |
|---|---|---|---|---|---|---|
| 10 | 8 | 11 | 16 | 29 | 0.148 | 7 |
| 30 | 33 | 39 | 51 | 59 | 0.007 | 7 |
| 60 | 60 | 63 | 69 | 78 | 0.001 | 8 |
| 180 | 89 | 93 | 97 | 100 | 0.0001 | 9 |
| P value | 0.065 | 0.038 | 0.018 | 0.005 | – | – |
Fig. 3.
Effect of degree of deacetylation (DD) on the mortality rate of protoscolices
Discussion
The investigation on the antimicrobial activity of chitosan is a hot topic, but there is a few attention on antiparasitic effect of chitosan (Tayel et al. 2010b; Tokura et al. 1997; Xu et al. 2007). It has been discussed that the antimicrobial activity of chitosan influenced by its molecular weight, DD, pH of chitosan solution and the target organism (Tokura et al. 1997). Most of the researchers found that with increasing DD the chitosan activity against fungi and bacteria increased. As reported above, the physicochemical properties of produced fungal chitosan obviously affected their antiparasitic activities. The increase of DD up to 65.1 % achieved higher scolicidal activity in reduction the number of living cysts (Tayel et al. 2010a; Xu et al. 2007). We found that, DD as a main factor shows significant correlation to fungal chitosan inhibitory effect, similar to the result of Ikinci et al. (2002), Tayel et al. (2010b), Tipparat and Oraphan (2008), highest degraded chitosan serve a better inhibitory effect on Escherichia coli, Staphylococcus aurous, Candida albicans, Saccharomyces unisporus and Saccharomyces Bayanus, Porphyromonas gingivalis. Chitosan has a cationic nature, increasing DD would result greater existence of −NH3+ group, in which amino group interact with a negative charged microbial plasma membrane, promote the leakage of intracellular constituent and disrupture of cell membrane (Guo et al. 2008). Another possible mechanism proposed that the high concentration of chitosan, enable it to make a film which chelate nutrient material and causing the biomaterial inaccessible for survival indirectly (Li et al. 2008). Several studies demonstrated that considering fungi as a source of chitosan over the other traditional sources has beneficial advantages including easy access, low cost culture nutrient and processing, independence of seasonal varieties (Tayel et al. 2010a; Tajdini et al. 2010). Tajdini et al. (2010) investigated on isolated chitosan from Rhizomucor miehei and Mucor racemosus and their potential against six bacteria strains and two fungal strains and yeast. They reported fungal chitosan showed higher antibacterial activity against gram negative bacteria rather than shrimp chitosan at 0.05–0.2 % concentration. As reported by Roller and Covill (1999), Seyfarth et al. (2008) Candida spp. showed more sensitivity to fungal chitosan. However, Liu et al. (2004), No et al. (2006), Je and Kim et al. (2006) published that gram positive bacteria were more sensitive to crustacean chitosan rather than fungal chitosan. This might be attributed to impurities in fungal chitosan causes lower antimicrobial effect. Tayel et al. (2010b) reported fungal chitosan obtained from Mucor rouxii strains were more effective on stopped the growth of the yeasts. As reported in our result, exposure time could be an important parameter in blocked the viability of potoscolices. Similarly reported by Gómez-Rivas et al. (2004), Tajdini et al. (2010) and Tavassoli et al. (2012) prolonging the exposure time could improve the minimum inhibitory concentration value. The data indicate that scolicidal effect of FC and CC increased with increase of concentration. Similar reported by Jeihanipour et al. (2007) much higher concentration is need to reduce the viability of bacteria strains. Various studies have evaluated the effectiveness of synthetic scolicidal agents and herbal medicines for inactivation of protoscolices of hydatid cyst. Many of these scolicidal agents may causes origin undesirable complications that limit their use (Moazeni and Alipour-Chaharmahali 2011; Moazeni and Roozitalab 2012; Sadjjadi et al. 2008). Various investigations proposed the use of albendazol, mebendazol, isoquinolinec, benzimidazole, carbamate and praziquantel as safe scolicidal agent, however the length of treatment and drug resistance limit their uses (Chinnery and Morris 1986; Morris et al. 1987). Taylor et al. (1990) suggesting praziquantel to be a most active protoscolicidal agent, while achieved complete growth inhibition of protoscolices with 250 μg/ml albendazole sulphoxide, needing up to 31 days to reach a comparable effect. To administer of chitosan in combination to other agents might reduce the drug resistant, complications, and obtained much shorter periods of treatments. The high number of published studies discusses chitosan’s potential to be used as a safe material and promising exepient for the pharmaceutical industry for medication applications (Singh et al. 2011).
Conclusion
According to the results of our study, fungal and commercially chitosan with concentration in 400 μg/ml killed all protoscolices of hydatid cyst at the end of 180 min. The highest effect was gained by higher DD, concentration and prolonging of exposure time. Our data suggest that fungal chitosan can be a promising source of potential antiprotoscolices. Although, we here demonstrate the in vitro parasiticidal activity of chitosan was satisfactory, in vivo efficacy of chitosan solutions, its derivatives and nanoparticles remain to be more investigated. Fungal chitosan seems to be economical for reduced the cost of medication because of the easy access to native chitosan and its availability.
Acknowledgments
The authors would like to appreciate Dr. Mohammad Ali Ebrahimzadeh for his precious directions. This work was supported by a Grant from Student Research Committee of Mazandaran University of Medical Sciences, Sari, Iran.
References
- Ammann RW, Eckert JC. Echinococcus. Gastroenterol Clin North Am. 1996;25:655–689. doi: 10.1016/S0889-8553(05)70268-5. [DOI] [PubMed] [Google Scholar]
- Chatelet C, Damour O, Domard A. Influence of the degree of acetylation on some biological properties of chitosan film. Biomaterials. 2001;22:261–268. doi: 10.1016/S0142-9612(00)00183-6. [DOI] [PubMed] [Google Scholar]
- Chinnery JB, Morris DL. Effect of albendazole sulphoxide on viability of hydatid protoscoleces in vitro. Trans R Soc Trop Med Hyg. 1986;80:815–817. doi: 10.1016/0035-9203(86)90392-5. [DOI] [PubMed] [Google Scholar]
- Gómez-Rivas L, Escudero-Abarca BI, Aguilar-Uscanga MG, Hayward-Jones PM, Mendoza P, et al. Selective antimicrobial action of chitosan against spoilage yeasts in mixed culture fermentations. J Ind Microbiol Biotechnol. 2004;31(1):16–22. doi: 10.1007/s10295-004-0112-2. [DOI] [PubMed] [Google Scholar]
- Guo Z, Xing R, Liu S, Zhong Z, Ji X, et al. The influence of molecular weight of quaternized chitosan on antifungal activity. Carbohydr Polym. 2008;71(4):694–697. doi: 10.1016/j.carbpol.2007.06.027. [DOI] [Google Scholar]
- İkinci G, Şenel S, Akıncıbay H, Kaş S, Erciş S, et al. Effect of chitosan on a periodontal pathogen Porphyromonas gingivalis. Int J Pharm. 2002;235:121–127. doi: 10.1016/S0378-5173(01)00974-7. [DOI] [PubMed] [Google Scholar]
- Je JY, Kim SK. Chitosan derivatives killed bacteria by disrupting the outer and inner membrane. J Agric Food Chem. 2006;54:6629–6633. doi: 10.1021/jf061310p. [DOI] [PubMed] [Google Scholar]
- Jeihanipour A, Karimi K, Taherzadeh MJ. Antimicrobial properties of fungal chitosan. Res J Biol Sci. 2007;2(3):239–243. [Google Scholar]
- Khan TA, Peh KK, Chang HS. Reporting degree of deacetylation values of chitosan: the influence of analytical methods. J Pharm Pharm Sci. 2002;5:205–212. [PubMed] [Google Scholar]
- Larrieu E, Costa MT, Cantoni G. Ovine Echinococcus granulosus transmission dynamics in the province of Rio Negro, Argentina, 1980–1999. Vet Parasitol. 2001;98:263–272. doi: 10.1016/S0304-4017(01)00442-3. [DOI] [PubMed] [Google Scholar]
- Li X-F, Feng X-Q, Yang S, Wang T-P, Su Z-X. Effects of molecular weight and concentration of chitosan on antifungal activity against Aspergillus niger. Iran Polym J. 2008;17(11):843–852. [Google Scholar]
- Liu H, Du Y, Wang X, Sun L. Chitosan kills bacteria through cell membrane damage. Int J Food Microbiol. 2004;95:147–155. doi: 10.1016/j.ijfoodmicro.2004.01.022. [DOI] [PubMed] [Google Scholar]
- McManus DP, Zhang W, Li J. Echinococcosis. Lancet. 2003;362:1295–1304. doi: 10.1016/S0140-6736(03)14573-4. [DOI] [PubMed] [Google Scholar]
- Miya M, Iwamoto R, Yoshikawa S, Mima S. IR spectroscopic determination of CONH content in highly deacetylated chitosan. Int J Biol Macromol. 1980;2:323–325. doi: 10.1016/0141-8130(80)90056-2. [DOI] [Google Scholar]
- Moazeni M, Alipour-Chaharmahali MR. Echinococcus granulosus: in vitro effectiveness of warm water on protoscolices. Exp Parasitol. 2011;127(1):14–17. doi: 10.1016/j.exppara.2010.06.021. [DOI] [PubMed] [Google Scholar]
- Moazeni M, Roozitalab A. High scolicidal effect of Zatariamultiflora on protoccoleces of hydatid cyst: an in vitro study. Comp Clin Pathol. 2012;21:99–104. doi: 10.1007/s00580-010-1069-3. [DOI] [Google Scholar]
- Morris DL, Chinnery JB, Ubhi C. A comparison of the effects of albendazole, its sulphone metabolite and mebendazole on viability of Echinococcus granulosus protoscoleces in an in vitro culture system. Trans R Soc Trop Med Hyg. 1987;81:804–806. doi: 10.1016/0035-9203(87)90038-1. [DOI] [PubMed] [Google Scholar]
- Niederhofer A, Muller BW. A method for direct preparation of chitosan with low molecular weight from fungi. Eur J Pharm Biopharm. 2004;57:101–105. doi: 10.1016/S0939-6411(03)00189-9. [DOI] [PubMed] [Google Scholar]
- Nishimura K, Nishimura S, Nishi N, Saiki I, Tokura S, et al. Immunological activity of chitin and its derivatives. Vaccine. 1984;2:93–99. doi: 10.1016/S0264-410X(98)90039-1. [DOI] [PubMed] [Google Scholar]
- No HK, Kim SH, Lee SH, Park NY, Prinyawiwatkul W. Stability and Antibacterial activity of chitosan solutions affected by storage temperature and time. Carbohydr Polym. 2006;65:174–178. doi: 10.1016/j.carbpol.2005.12.036. [DOI] [Google Scholar]
- Nwe N, Chandrkrachang S, Stevens WF, Maw T, Tan TK, et al. Production of fungal chitosan by solid state and submerged fermentation. Carbohydr Polym. 2001;49:235–237. doi: 10.1016/S0144-8617(01)00355-1. [DOI] [Google Scholar]
- Rane KD, Hoover DG. An evaluation of alkali and acid treatments for chitosan extraction from fungi. Process Biochem. 1993;28:115–118. doi: 10.1016/0032-9592(93)80016-A. [DOI] [Google Scholar]
- Roller S, Covill N. The antifungal properties of chitosan in laboratory media and apple juice. Int J Food Microbiol. 1999;47:67–77. doi: 10.1016/S0168-1605(99)00006-9. [DOI] [PubMed] [Google Scholar]
- Sadjjadi SM, Zoharizadeh MR, Panjeshahin MR. In vitro screening of different Allium sativum extracts on hydatid cysts protoscoleces. J Invest Surg. 2008;21:318–322. doi: 10.1080/08941930802348261. [DOI] [PubMed] [Google Scholar]
- Seyfarth F, Schliemann S, Elsner P, Hipler UC. Antifungal effect of high- and low-molecular-weight chitosan hydrochloride, carboxymethyl chitosan, chitosan oligosaccharide and N-acetyl-d-glucosamine against Candida albicans, Candida krusei and Candida glabrata. Int J Pharm. 2008;353:139–148. doi: 10.1016/j.ijpharm.2007.11.029. [DOI] [PubMed] [Google Scholar]
- Shahidi F, Arachchi JKV, You JJ. Food applications of chitin and chitosan. Trends Food Sci Technol. 2005;10:37–51. doi: 10.1016/S0924-2244(99)00017-5. [DOI] [Google Scholar]
- Singh MK, Prajapati SK, Mahor A, Rajput N, Singh R. Chitosan: a novel exepient in pharmaceutical formulation. Int J Pharm Sci Res. 2011;2(9):2266–2277. [Google Scholar]
- Tajdini F, Amini MA, Nafissi-Varcheh N, Faramarzi MA. Production, physiochemical and antimicrobial properties of fungal chitosan from Rhizomucor miehei and Mucor racemosus. Int J Biol Macromol. 2010;47:180–183. doi: 10.1016/j.ijbiomac.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Tavassoli M, Imani A, Tajik H, Moradi M, Pourseyed SH. Novel in vitro efficiency of chitosan biomolecule against Trichomonas gallinae. Iran J Parasitol. 2012;7:92–96. [PMC free article] [PubMed] [Google Scholar]
- Tayel AA, Moussa S, el-Tras WF, Knittel D, Opwis K. Anticandidal action of fungal chitosan against Candida albicans. Int J Biol Macromol. 2010;47:454–457. doi: 10.1016/j.ijbiomac.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Tayel AA, Moussa S, Opwis K, Knittel D, Schollmeyer E. Inhibition of microbial pathogens by fungal chitosan. Int J Biol Macromol. 2010;47:10–14. doi: 10.1016/j.ijbiomac.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Taylor DH, Morris DL, Richards KS. Chemotherapy of Echinococcus granulosus: determination of minimum effective length of albendazole therapy in vitro protoscolex culture. HPB Surg. 1990;2:159–164. doi: 10.1155/1990/67385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikhonov VE, Stepnova EA, Babak VG, Yamskov IA, Palma-Guerrero J, et al. Bactericidal and antifungal activities of a low molecular weight chitosan and its N-/2(3)-(dodec-2-enyl)succinoyl/-derivatives. Carbohydr Polym. 2006;64:66–72. doi: 10.1016/j.carbpol.2005.10.021. [DOI] [Google Scholar]
- Tipparat H, Oraphan R. Effect of deacetylation conditions on antimicrobial activity of chitosans prepared from carapace of black tiger shrimp (Penaeusmonodon) J Sci Technol. 2008;30:1–9. [Google Scholar]
- Tokura S, Ueno K, Miyazaki S, Nishi N. Molecular weight dependent antimicrobial activity by chitosan. Macromol Symp. 1997;120:1–9. doi: 10.1002/masy.19971200103. [DOI] [Google Scholar]
- Topcu O, Sumer Z, Tuncer E. Efficacy of chlorhexidine gluconate during surgery for hydatid cyst. World J Surg. 2009;33:1274–1280. doi: 10.1007/s00268-009-9971-z. [DOI] [PubMed] [Google Scholar]
- Xu J, Zhao X, Han X, Du Y. Antifungal activity of oligochitosan against Phytophthora capsici and other pathogenic fungi in vitro. Pest Biochem Phys. 2007;87(3):220–228. doi: 10.1016/j.pestbp.2006.07.013. [DOI] [Google Scholar]