Abstract
Pathomorphological alterations of chicken coccidiosis in Jammu division were undertaken in both organized and backyard chickens during the year 2010–2011. A total 240 intestines were examined from both organized farms and backyard chickens for histopathological studies. Out of 240 samples processed, 48 samples were found to be positive for coccidiosis with a prevalence of 20 %. Coccidiosis was initially diagnosed on the basis of faecal examination and confirmed by the presence of sporulated oocysts and pathomorphological alterations in intestines. Eimeria species were identified by morphometry. Five Eimeria species identified were Eimeria tenella, E. necatrix, E. maxima, E. acervulina and E. mitis. Histopathological lesions revealed loss of epithelial tissue, congestion of blood vessels which indicate disruption followed by haemorrhage, severe muscular oedema and necrosis of submucosa of intestine and caecum. There was loss of intestinal villi, disruption of caecal mucosa and clusters of oocysts seen. There was massive infiltration by heterophils and mononuclear cells. Several merozoites, schizonts and microgametes were found in the epithelial cells.
Keywords: Chickens, Coccidiosis, Pathomorphological alterations
Introduction
Coccidiosis is caused by species of intracellular protozoan parasites belonging to the genus Eimeria (Phylum Apicomplexa). It is one of the most economically important diseases in poultry in modern poultry production. India is one of the world’s largest and fastest growing poultry industries, ranking third in hen egg production and sixth in broiler meat production. According to Ministry of Food Processing Industries, about 70 % of poultry is in the organized sector and 30 % is in the unorganized sector. Broiler production grew at an annual percentage growth rate of 8.35 % from 2001 (1.25 million metric tons) to 2010 (2.65 million metric tons). Per capita consumption has grown from 1.22 kg in 2001 to 2.26 kg in 2010. India’s egg production is anticipated to reach 61.5 billion eggs, up to 68 % from 36.6 billion in 2001 (Basic Animal Husbandry Statistics (2010). The Jammu and Kashmir state is no more exception to this as the poultry population has increased from 2.039 million in 2003 to 3.48 million in 2007 (Livestock census 2007). But the tremendous growth of poultry industry in India is hampered by various factors and prevalence of various diseases in poultry are of main concern. Among the various diseases, protozoan parasites of the genus Eimeria, which resides and multiplies in intestinal mucosa causing coccidiosis (Hadipour et al. 2011) characterized by dysentery, enteritis, emaciation, drooping wings, poor growth, low production (Rehman et al. 2010; Awais et al. 2012) with high rate of mortality and morbidity (Shirzad et al. 2011). Mortality and economic losses especially in case of outbreaks are frequent (Williams 1999: Morris and Gasser 2006) and it causes high mortality in young chicks because most of the Eimeria spp affects birds between the age of 3 and 18 weeks (Nematollahi et al. 2009; Toulah, 2007).
Avian coccidiosis is classified into caecal and intestinal forms. Caecal coccidiosis is an acute disease characterised by diarrhoea and massive caecal haemorrhages. Caecal coccidiosis may produce bloody droppings and anemia (Lillehoj and Trout 1993; Whitmarsh 1997). E. necatrix has been reported as the most common pathogenic species causing intestinal coccidiosis in domestic poultry among all the Eimerian species (Johnson 1930). In this form, lesions are distributed throughout the length of the intestine, predominantly in the middle portion of the small intestine. These intestinal lesions of variable extent and severity reduce the absorptive function of mucosa and thus leading to weight loss, diarrhoea, poor feed conversion and higher mortality of the affected flocks and therefore it is recognized as the parasitic disease that has the greatest economic impact on the commercial poultry industry. Due to higher stocking densities and intensive husbandry practices, its incidence is being increased in poultry (Nnadi and George 2010).
Eimeria tenella and E. necatrix are the most pathogenic species. E. acervulina, E. maxima and E. mivati are common and slightly to moderately pathogenic; E. brunetti is uncommon but pathogenic when it does occur. E. mitis, E. praecox and E. hagani are relatively non-pathogenic species (Soulsby 1982; Dalloul and Lillehoj 2006). In view of the lack of authentic information available regarding the pathogenic impact of Eimeria sp. affecting poultry in Jammu region, the present study was undertaken.
Materials and methods
A total of 240 intestines were collected from organized farms and backyard poultry (unorganized) of Jammu. After detail gross examination of intestines, morbid tissues from the intestine and caeca with lesions were collected and fixed in 10 % Neutral buffer formalin. The particulars like age, farm management practices were recorded. The age groups of studied flocks of birds were categorized into difference of 15 days.
Wet smear method
Deep scrapings from duodenum, jejunum, ileum and caecum were taken and wet smears were prepared for microscopic examination of coccidial oocyst (Fleck and Moody 1993).
Impression smear cytological examination
Impression smears were made from duodenum, jejunum, ileum and caecum and stained with Giemsa stain (Osorio et al. 2010).
Morphometry of oocyst
Identification of Eimeria species in chickens was done on the basis of Oocyst morphology viz., size, shape, colour, appearance of the wall, presence or absence of micropyle and micropylar cap and its shape and sporulation time as described by (Lima 1979; Soulsby 1982; Levine 1985; Smith and Sherman 1994; Bhatia et al. 2004). Morphometry of sporulated Oocyst was studied under high power objective with an eyepiece scale calibrated by means of a stage micrometer. A total of 20 oocysts in each species were measured and mean values were arrived.
Gross and histopathomorphological examination of intestinal lesions
For histopathological examination, representative tissue samples of intestines were collected in 10 % Neutral buffer formalin and then processed for paraffin embedding employing alcohol as dehydrating agent and xylene as clearing agent. The sections were cut at 4–5 μm thickness and stained by routine Harris haematoxylin and eosin method (Luna 1968).
Results
In Floatation and Wet smear examination of positive faecal samples, oocysts were seen while as in Impression smear examination, Eimerian oocysts and schizonts were observed.
Pathomorphological alterations
The observation of the present study with respect to the, gross pathology and histopathological changes included the following:
Gross observations
The post-mortem examination revealed lesions of intestinal and caecal forms of coccidiosis. While looking grossly, extremely balooned intestine and caeca (Fig. 1) and haemorrhages in intestinal mucosa (Fig. 2) were seen. Watery ingesta mixed with mucus and blood was observed. Haemorrhagic enteritis was seen in whole portion of intestine (Fig. 3). Liver was found to be pale (Fig. 4).
Fig. 1.

Balooned intestine and caeca
Fig. 2.
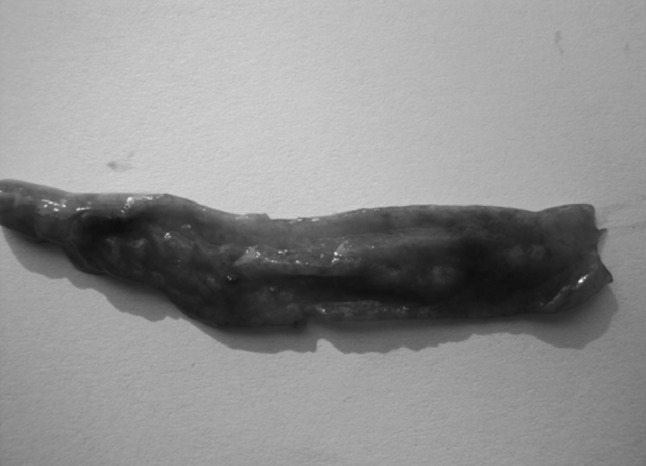
Haemorrhages in intestinal mucosa
Fig. 3.
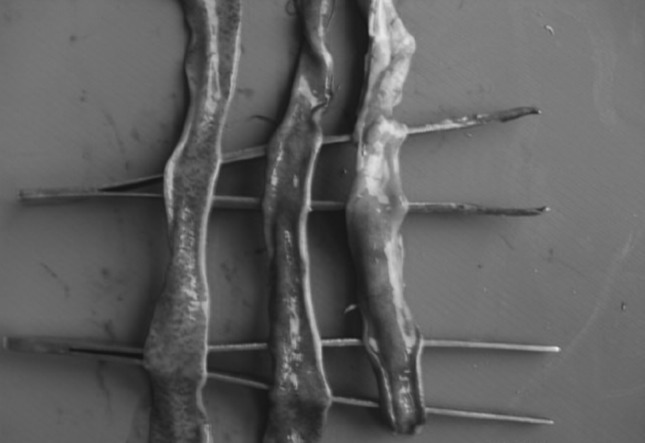
Haemorrhagic enteritis
Fig. 4.
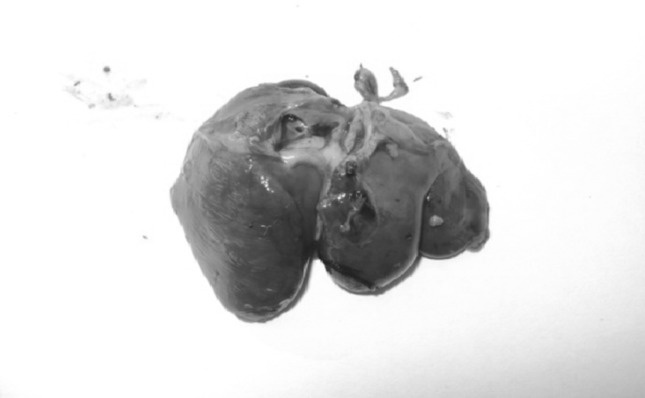
Liver was pale
Histopathological changes
Histopathological studies revealed presence of developing and developed oocysts in the epithelial cells of sub-mucosal glands of caecum (arrow) with massive infiltration of heterophils and mononuclear cells (Fig. 5) when compared to normal caecum. Presence of developing meronts (arrow) and schizonts (arrowhead) in lamina propria of villi with desquamation of enterocytes, MNC infiltration and fibrosis in muscularis mucosa was also observed (Fig. 6). Massive congestion of sub-mucosal blood vessels, fibrosis and oedema with degeneration of sub-mucosal glandular epithelium harbouring coccidian life-cycle stages and also shedding of oocysts in the glandular lumen (arrow) was observed (Fig. 7).
Fig. 5.
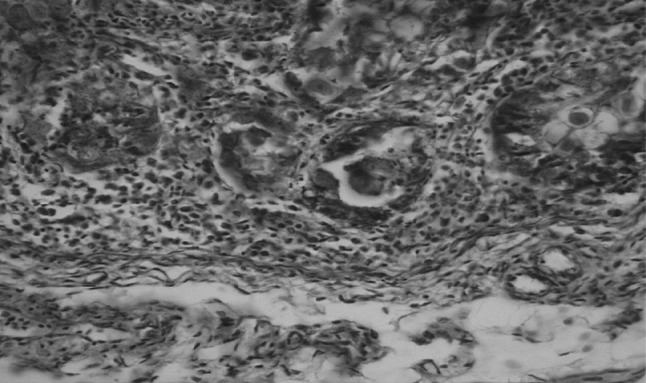
Caecum showing presence of developing and developed oocysts in the epithelial cells of sub-mucosal glands (arrow) with massive infiltration by heterophils and mononuclear cells. H&E ×400
Fig. 6.
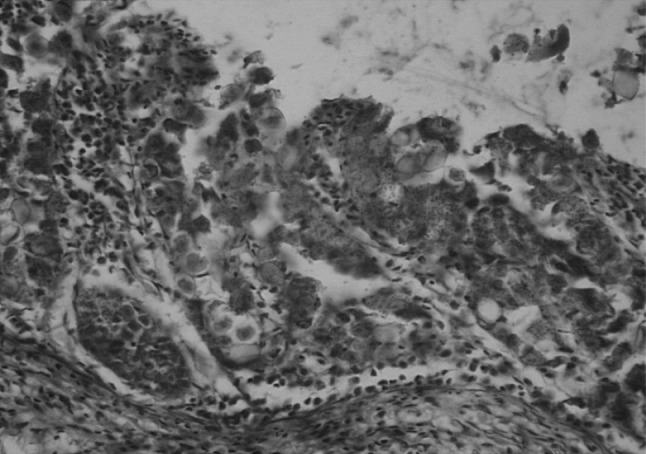
Intestine showing presence of developing meronts (arrow) and schizonts (arrowhead) in lamina propria of villi with desquamation of enterocytes, MNC infiltration and fibrosis in muscularis mucosa. H&E ×400
Fig. 7.
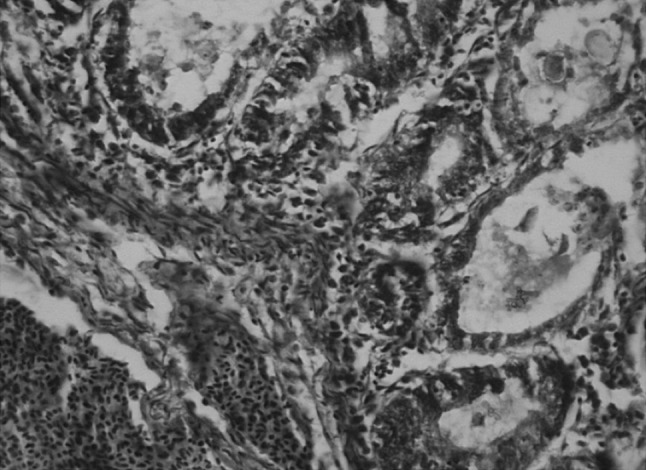
Massive congestion of sub-mucosal blood vessels, fibrosis and oedema with degeneration of sub-mucosal glandular epithelium harbouring coccidian life-cycle stages and also shedding of oocysts in the glandular lumen (arrow)
Intestinal epithelium showed desquamation and presence of oocysts in the lamina propria when compared to normal intestinal epithelium. Intestine showed desquamation and sloughing of enterocytes leading to necrosis of intestinal villi with presence of oocysts in lamina propria and MNC infiltration. Intestinal villi showed fusion, blunting and desquamation of enterocyte with heterophilic infiltration and presence of oocysts in lamina propria. The sub-mucosal glandular epithelium was impacted with shed coccidian oocysts and necrotic epithelium debris (arrow) with MNC infiltration and fibrous connective tissue proliferation when compared to normal caecum. Shedding of oocysts in intestinal lumen mixed with blood stained contents were also observed. Epithelial cells of caecal sub-mucosal glands showed degeneration with coccidial life-stage i.e. microgamete (arrow) and shedding of oocysts in glandular lumen (arrow) with massive MNC infiltration and fibrosis.
Discussion and conclusion
Coccidiosis was determined through demonstration of post-mortem changes recorded in dead birds. The post-mortem revealed extremely ballooned intestine and haemorrhages were seen while looking grossly without opening the gut. Intestine was often found oedematous, thickened, showing necrosis and sloughing of intestinal epithelium and friable. The findings thus recorded coincide with those of Tyzzer (1929), Johnson (1930); Davis (1963).
The caecum was enlarged with clotted blood, haemorrhagic spots on caecal wall, inflammation, necrotic patches and dilation of caecum with consolidation of caecal contents were observed in almost all the cases. On opening the caeca, the bloody mass, a characteristic of caecal coccidiosis, was found in some cases. The change in color from red to mottled reddish or milky white due to formation of oocysts were observed in some cases. These findings however, correspondingly are related to those recorded by Raillet and Lucet (1891), Fantham (1910), Tyzzer (1929) and Long et al. (1975). They also observed enlargement of caecum with clotted blood, haemorrhages throughout caecal mucosa and change in coloration from reddish to milky white.
Histopathological changes
Histopathological lesions revealed loss of epithelial tissue, congestion of blood vessels which indicate disruption followed by haemorrhage, severe muscular oedema, necrosis of submucosa, loss of villi, disruption of caecal mucosa, clusters of oocysts and marked haemorrhage and necrosis of caecal mucosa. There was massive infiltration by heterophils and mononuclear cells. These findings were similar to those of Rasheda and Bano (1985). They also observed oedema and erosion of the sub-mucosa, glandular tissue cells infiltration and villous atrophy. Several merozoites, schizonts and microgametes were found in the epithelial cells. Similar changes were also observed by Soomro et al. (2001) and Sood et al. (2009).
Based on the above findings, conclusions can be elucidated from the present as, Gross observations included extremely ballooned intestine and caeca, haemorrhages in intestinal mucosa, watery ingesta mixed with mucus and blood and haemorrhagic enteritis was seen in whole portion of intestine. Histopathological examination revealed various changes in the tissue sections of caeca and small intestine. There was presence of coccidial oocysts in the lamina propria of intestine and in the epithelial cells of sub-mucosal glands of caecum with massive infiltration by heterophils and mononuclear cells. Intestine showed desquamation and sloughing of enterocytes leading to necrosis of intestinal villi. Massive congestion of sub-mucosal blood vessels, fibrosis and oedema with degeneration of sub-mucosal glandular epithelium was observed. Various stages of schizogony and gametogony were found in the tissue sections of the intestine and caecum.
Acknowledgments
The authors thanks to the Dean, FVSc and A.H, R.S. Pura Jammu for providing necessary facilities at time of research.
References
- Awais MM, Akhtar M, Iqbal Z, Muhammad F, Anwar MI. Seasonal prevalence of coccidiosis in industrial broiler chickens in Faisalabad, Punjab, Pakistan. Trop Anim Health Prod. 2012;44(2):323–328. doi: 10.1007/s11250-011-0024-x. [DOI] [PubMed] [Google Scholar]
- Basic animal husbandry statistics (2010) Government of India, Ministry of Agriculture, Department of Animal Husbandry, Dairying and Fisheries, New Delhi
- Bhatia BB, Pathak KML, Banerjee DP. A text book of veterinary parasitology. 2. New Delhi: Kalyani; 2004. pp. 337–343. [Google Scholar]
- Dalloul RA, Lillehoj HS. Poultry coccidiosis. Recent advancements in control measures and vaccine development. Expert Revise Vaccines. 2006;5(3):143–162. doi: 10.1586/14760584.5.1.143. [DOI] [PubMed] [Google Scholar]
- Davis SFM. Eimeria brunette and additional cause of intestinal coccidiosis in poultry domestic fowl. Brazilian Vet Rec. 1963;75:1–4. [Google Scholar]
- Fantham HB. The morphology and life-history of Eimeria (coccidium) avium: a sporozoön causing a fatal disease among young grouse. Proceed Zoolo Socie of London. 1910;3:672–691. doi: 10.1111/j.1096-3642.1910.tb01910.x. [DOI] [Google Scholar]
- Fleck SL, Moody AH. Diagnostic technique in medical parasitology, 11th Edition. Cambridge: Cambridge University Press; 1993. pp. 10–14. [Google Scholar]
- Hadipour MM, Olyaie A, Naderi M, Azad F, Nekouie O. Prevalence of Eimeria species in scavenging native chickens of Shiraz Iran. African J Micro Res. 2011;5:3296–3299. [Google Scholar]
- Johnson WT. A Study on Eimeria necatrix. Agricul Experi Station Tech Bull. 1930;538:30–33. [Google Scholar]
- Levine ND. Veterinary Protozoology. Ames: Iowa State University Press; 1985. p. 414. [Google Scholar]
- Lillehoj HS, Trout JM. Coccidia: a review of recent advances on immunity and vaccine development. Avian Pathol. 1993;22:3–21. doi: 10.1080/03079459308418897. [DOI] [PubMed] [Google Scholar]
- Lima JD. Eimeria caprina sp. from the domestic goat (Capra hircus) from the United States of America. J Parasitol. 1979;65(6):902–903. doi: 10.2307/3280246. [DOI] [PubMed] [Google Scholar]
- Livestock census (2007) Ministry of Agriculture and Dairing, Government of India
- Long PL, Tompkins RV, Millard BJ. Coccodiosis in broilers: evaluation of infection by the examination of broiler house litter for oocysts. Avian Pathol. 1975;4(4):287–294. doi: 10.1080/03079457509353877. [DOI] [PubMed] [Google Scholar]
- Luna LG (1968) Manual of histological staining methods of the armed registry of pathology.3rd edition. Mc Graw Hill, New York, P 36–95
- Morris GM, Gasser RB. Biotechnological advances in the diagnosis of avian coccidiosis and the analysis of genetic variation in Eimeria. Biotech Advan. 2006;24:590–603. doi: 10.1016/j.biotechadv.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Nematollahi A, Moghaddam GH, Pourabad RF. Prevalence of Eimeria species among broiler chicks in Tabriz (Northwest of Iran) Mun Ent Zool. 2009;4:53–58. [Google Scholar]
- Nnadi PA, George SO. A cross-sectional survey on parasites of chickens in selected villages in the subhumid zones of south-eastern Nigeria. J Parasitol Res. 2010;141:1–6. doi: 10.1155/2010/141824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio C, Clark S, Martin M, Barnes HJ. Cytological diagnosis of coccidial infections in turkeys. Alpharma Anim Heal. 2010;33:3. [Google Scholar]
- Raillet F, Lucet M. An account of coccidiosis in the domestic fowl. Vet Med. 1891;2:661–663. [Google Scholar]
- Rasheda M, Bano L. Histopathology of coccidiosis by Eimeria garnhami in coturnix coturnix of N.W.F.P. Pak Vet J. 1985;1:27–29. [Google Scholar]
- Rehman TU, Khan MN, Sajid MS, Abbas RZ, Arshad M, Iqbal Z, Iqbal A. Epidemiology of Eimeria and associated risk factors in cattle of district Toba Tek Singh, Pakistan. Parasitol Res. 2010;108:1171–1177. doi: 10.1007/s00436-010-2159-5. [DOI] [PubMed] [Google Scholar]
- Shirzad MR, Seifi S, Gheisari HR, Hachesoo BA, Habibi H, Bujmehrani H. Prevalence and risk factors for subclinical coccidiosis in broiler chicken farms in Mazandaran province, Iran. Trop Anim Health Prod. 2011;43:1601–1604. doi: 10.1007/s11250-011-9876-3. [DOI] [PubMed] [Google Scholar]
- Smith MC, Sherman DM. Goat medicine, 2nd Ed. Philadelphia: Wiley–Blackwell; 1994. pp. 429–431. [Google Scholar]
- Sood S, Yadav A, Vohra S, Katoch R, Ahmad BD, Borkatari S (2009) Prevalence of coccidiosis in poultry birds in R.S.Pura region, Jammu. Vet Prac 10(1):69–70
- Soomro NM, Rind R, Arijo AG, Soomro SA. Clinical, gross and histopathological studies of coccidial infection in chicken. Intern J Agri and Biol. 2001;3(4):426–427. [Google Scholar]
- Soulsby EJL. Helminths, arthropods & protozoa of domesticated animals. 8. London: English Language Book Society and Baillere Tindal; 1982. p. 809. [Google Scholar]
- Toulah FH. Prevalence and comparative morphological studies of four Eimeria sp. of sheep in Jeddah area, Saudi Arabia. J Biol Sci. 2007;7:413–416. doi: 10.3923/jbs.2007.413.416. [DOI] [Google Scholar]
- Tyzzer EE. Coccidiosis in gallinaceous birds. Am J Hyg. 1929;10:269. [Google Scholar]
- Whitmarsh S (1997) Protozoan Poultry diseases. poultry science homepage, College of Agricultural and Life Sciences, Mississippi State University. http://www.misstate.edu/dept/poultry/disproto.htm
- Williams RB. A compartmentalised model for the estimation of the cost of coccidiosis to the world’s chicken production industry. Intern J of Parasitol. 1999;29:1209–1229. doi: 10.1016/S0020-7519(99)00086-7. [DOI] [PubMed] [Google Scholar]


