Abstract
Theileria annulata, a protozoan parasite of cattle is causes tropical theileriosis. Polymerase chain reaction (PCR) was used to assess the presence and the frequency of T. annulata infection in blood samples obtained from carrier cattle in Kerman, Southeast of Iran. Blood samples were collected in citrate solution from 150 native cattle with mean age of 1 year which selected randomly. Primarily, a thin layer smear was prepared from their ear sublime vein blood and was fixed with methanol and stained with Giemsa dye. Blood smears were examined for the presence of parasites, and blood samples were analyzed by PCR. Piroplasmic forms of T. annulata were seen in 16 of 150 (10.66 %) by examination the blood smears with light microscope, whereas 68 of 150 (45.33 %) cattle were positive by PCR method. All animals that were positive by blood smears were also positive by PCR. Difference between these methods was significant (P < 0.05). Our results demonstrate that this PCR assay in diagnosing T. annulata parasites in carrier cattle is more sensitive than method of smear preparation and can be used in epidemiological studies.
Keywords: Theileria annulata, Cattle, PCR, Smear method
Introduction
Theileriosis, a tick-transmitted protozoan disease, is a major constraint for cattle production in the tropics and subtropics (Dehkordi et al. 2012). The disease is transmitted by ixodid ticks of the genus Hyalomma and affects cattle and water buffalo. The distribution of tropical theileriosis ranges from southern Europe and northern Africa to as far as China and an estimated 250 million domestic cattle are at risk from this disease and acts as a major constraint on livestock production and improvement in many developing countries (Slodki et al. 2011).
In Iran, the native cattle are more resistant to theileriosis and are affected by subclinical form of the theileriosis while the European cattle are very sensitive to disease and if they don’t treat effectively; their mortality rate will be between 40–60 % (Habibi 2012).
The treated cattle and native cattle are carriers for a long period and even until the end of life that within this period of time, only few number of erythrocytes are contaminated with the parasite which their observation and also demonstrating of their presence can be done hardly (Tavassoli et al. 2011). Such carriers are important contributers to the infection within Hyalomma ticks. Hence, detection of piroplasms in carrier animals is an important epidemiological parameter. Diagnosis of the disease is based on clinical findings and microscopic examination of blood and lymph node smears stained with Giemsa and detection of macroschizonts in acute cases.
Smear method is associated with technical problems and even wrong diagnosing and has low sensitivity in diagnosing carrier cattle (Nayel et al. 2012).
In addition, serological tests such as the complement fixation test (CFT), indirect immunofluorescent assay (IFA), and enzyme linked immunosorbent assay (ELISA) can be used to detect circulating antibodies by using either piroplasms or cultured macroschizonts as the antigen (Omer et al. 2011). However, cross-reactivity with antibodies directed against other Theileria species limits the specificity of the IFA (Burridge et al. 1974, Hoghooghi-Rad et al. 2011). Moreover, antibodies tend to disappear in long-term carriers, whereas Theileria piroplasms persist. Therefore, animals with a negative serological test can still infect ticks. Recently, diagnostic methods like PCR have been developed for the rapid and accurate detection of Theileria spp. (Zaeemi et al. 2011, Ghaemi et al. 2012).
This study was performed to assess the presence and the frequency of T. annulata infection in blood samples obtained from carrier cattle in Kerman, Southeast of Iran using PCR for amplification of T. annulata DNA from blood samples in compare with smear method.
Primers were derived from the gene encoding the 30 kDa major T. annulata merozoite surface antigen (Shiels et al. 1995). The same primers had been previously used to amplify T. annulata DNA from blood samples obtained from carrier cattle (Khattak et al. 2012).
Materials and methods
Smear preparation
Primarily, a thin layer smear was prepared from ear sublime vein blood from 150 native cattle randomly selected and was fixed with methanol and stained with Giemsa dye. Also, 9 mL blood samples were obtained from their jugular vein in tubes containing 1 mL of 0.1 M (3.2 %) buffered citrate solution. Giemsa-stained blood smears were examined for the presence of parasites; At least 50 microscopical areas were carefully examined for Theileria piroplasms under the oil immersion lens. The presence of even a single piroplasm was considered positive. Then blood samples were analyzed by PCR.
DNA isolation, PCR amplification, and sequencing
Theileriaannulata piroplasm DNA was purified from bovine blood with approximately 25 % parasitemia. Genomic DNA extracted with a Genomic DNA extraction kit (AccuPrep, BIONEER). Aliquots of extracted DNA were kept at 20 °C.
PCR was performed using one set of primers (Table 1) (N516 GTAACCTTTAAAAACGT 234–250, T. annulata specific and N517 GTTACGAACATGGGTTT 954–938, T. annulata specific) in a final reaction volume of 100 μl containing 50 mM KCl, 10 mM Tris–HCl (pH 8.3), 1.5 mM MgCl2, 0.1 % Triton X-100, 200 μM deoxynucleoside triphosphate, 2.5 U of Taq polymerase (Biozyme, England), 20 pmol of primers and 5 μl of template DNA. The reactions were performed in an automatic DNA thermal cycler (Biorad, USA) for 35 cycles. Each cycle consisted of a denaturing step of 1 min at 94 °C, an annealing step of 1 min at 55 °C or 1 min at and an extension step of 1 min at 72 °C.
Table 1.
Oligonucleotide primers used in the PCR
| Primer | Sequence | Position | Amplified DNA fragment (bp) |
|---|---|---|---|
| N516 | GTAACCTTTAAAAACGT | 234–250 | 721 |
| N517 | GTTACGAACATGGGTTT | 954–938 |
Results
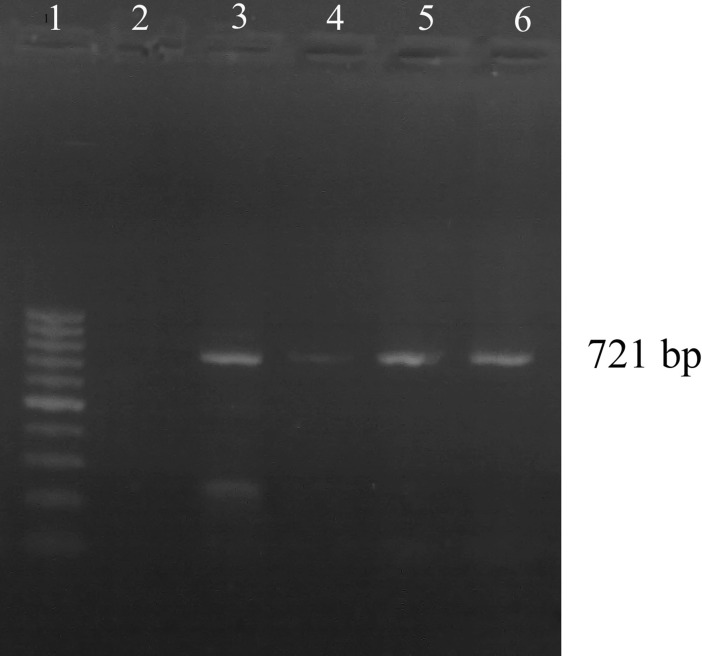
Out of 150 smears examined microscopically, 16(10.66 %) were positive for Piroplasmic forms of T. annulata, whereas 68 of 150(45.33 %) cattle were positive by PCR method. The 721 bp fragment was generated in all samples that were positive by blood smears (Fig. 1).
Fig. 1.
Detection of Theileria annulata DNA in native cattle. The amplified 721-bp product was subjected to electrophoresis in 1.5 % agarose gel and stained with ethidium bromide. Lane 1 100-bp ladder. Lane 2 negative control, Lane 3 PCR positive control of T. annulata. Lanes 4, 5 and 6 positive samples
Also, a comparison of results from PCR, and Giemsa-stained blood smears for detecting T. annulata was performed. Difference between these methods was significant (P < 0.05).
Discussion
Tick-transmitted diseases such as theileriosis are economically important globally (Uilenberg 1981). Carrier animals have an important role in the transmission of the infection by ticks. The diagnosis of piroplasm infections is based on clinical findings and microscopic examination of Giemsa-stained blood smears, but the low sensitivity of this method does not permit its use in epidemiological investigations.
However, this method is not sensitive enough or sufficiently specific to detect chronic carriers, particularly when mixed infections occur. Serological tests are frequently used for diagnosis of latent infections. Furthermore, false positive and negative results are commonly observed in serological tests. Therefore, development of a highly specific and sensitive method for the diagnosis of theileriosis infections is required. Recently, molecular techniques have become the preferred methods for diagnosis of babesiosis and theileriosis, because these techniques are more sensitive and specific than other conventional methods (Alhassan et al. 2005, Altay et al. 2005, Nagore et al. 2004).
Nevertheless, it is difficult and time consuming to identify piroplasmic forms within the erythrocytes from carrier animals. Various methods have been used to identify these parasites (Jongejan and Uilenberg 1994, Uilenberg 1981).
With the availability of sequenced parasite genes and PCR, it is possible to detect parasites within samples of blood (Bishop et al. 1995, Shahnawaz et al. 2011). Amplification of parasite DNA is far more sensitive than parasite detection by light microscopy.
The native carrier cattle are the major agent of spreading the infection and have the most important role in alternation of parasite life cycle between cows and ticks.
In this study, we used a PCR assay for detection of T. annulata in blood samples from carrier cattle in Iran. Piroplasms were detected in only 16 of 150 animals by microscopic examination; whereas 68 animals were positive by PCR.
Our results demonstrate that this PCR assay detects T. annulata parasites at low parasitemias in carrier cattle.
These results suggest that cattle could be subclinical carriers of T. annulata. A study should be carried out to determine whether these parasites could serve as a source of infection to disease-transmitting ticks.
The prevalence of T. annulata is widespread in cattle of Kerman, Iran. Urgent measures such as anti-Theileria vaccines, chemotherapy, chemoprophylaxis and vector control should therefore be taken for prevention of theileriosis.
However, screening and detection methods for whole herds prior to treatment are time-consuming. Therefore, prevention and management of this disease using insecticides and preimmunization with infected ticks on the grazing ranch is very important.
Acknowledgments
This work was supported by Shahid Bahonar University of Kerman, Iran(Grant number:1390.6.19). We thank Dr. Namavari for his critical comments on the manuscript.
References
- Alhassan A, Pumidonming W, Okamura M, Hirita H, Battsetseg B, Fujisaki F. Development of a single-round and multiplex PCR method for the simultaneous detection of Babesia caballi and Babesia equi in horse blood. Vet Parasitol. 2005;129:43–49. doi: 10.1016/j.vetpar.2004.12.018. [DOI] [PubMed] [Google Scholar]
- Altay K, Dumanli N, Holman PJ, Aktas M. Detection of Theileria ovis infected sheep by nested PCR. Vet Parasitol. 2005;127:99–104. doi: 10.1016/j.vetpar.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Bishop R, Allsopp B, Spooner P, Sohanpal B, Morzaria S, Gob-right E. Theileria: improved species discrimination using oligonucleotides derived from large subunit ribosomal RNA sequences. Exp Parasitol. 1995;80:107–115. doi: 10.1006/expr.1995.1012. [DOI] [PubMed] [Google Scholar]
- Burridge MJ, Brown CGD, Kimber CD. Theileria annulata: cross reactions between a cell culture schizont antigen and antigens of East African Theileria species in the indirect fluorescent antibody test. Exp Parasitol. 1974;35:374–380. doi: 10.1016/0014-4894(74)90043-5. [DOI] [PubMed] [Google Scholar]
- Dehkordi F, Safarpoor P, Parsaei S, Saberian S, Moshkelani P, Hajshafiei S. Prevalence study of Theileria annulata by comparison of four diagnostic techniques in southwest Iran. Bulg J Vet Med. 2012;15:123–130. [Google Scholar]
- Ghaemi P, Hoghooghi-Rad N, Shayan P, Eckert B. Detection of Theileria orientalis in Iran by semi-nested PCR. Parasitol Res. 2012;110:527–531. doi: 10.1007/s00436-011-2517-y. [DOI] [PubMed] [Google Scholar]
- Habibi G. Phylogenetic analysis of Theileria annulata infected cell Line S15 Iran vaccine strain. Iranian J Parasitol. 2012;7:73–81. [PMC free article] [PubMed] [Google Scholar]
- Hoghooghi-Rad N, Ghaemi P, Shayan P, Eckert B, Sadr-Shirazi N. Detection of native carrier cattle infected with Theileria annulata by semi-nested PCR and smear method in Golestan Province of Iran. World Appl Sci J. 2011;12:317–323. [Google Scholar]
- Jongejan F, Uilenberg G. Ticks and control methods. Rev Sci Tech Off Int Epiz. 1994;13:1201–1226. doi: 10.20506/rst.13.4.818. [DOI] [PubMed] [Google Scholar]
- Khattak RM, Rabib M, Khan Z, Ishaq M, Hameed H, Taqddus A. A comparison of two different techniques for the detection of blood parasite, Theileria annulata, in cattle from two districts in Khyber Pukhtoon Khwa Province (Pakistan) Parasite. 2012;19:91–95. doi: 10.1051/parasite/2012191091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagore D, Garcı′a-Sanmartı′n J, Garcı′a-Pe′rez AL, Juste RA, Hurtado A. Identification, genetic diversity and prevalence of Theileria and Babesia species in a sheep population from Nortern Spain. Int J Parasitol. 2004;34:1059–1067. doi: 10.1016/j.ijpara.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Nayel M, El-Dakhly KM, Aboulaila M, Elsify A, Hassan H, Ibrahim E. The use of different diagnostic tools for Babesia and Theileria parasites in cattle in Menofia. Egypt Parasitol Res. 2012;111(3):1019–1024. doi: 10.1007/s00436-012-2926-6. [DOI] [PubMed] [Google Scholar]
- Omer OH, Mahmoud O, Al-Sadrani A. Evaluation of the acridine orange fluorescence technique and the indirect fluorescent antibody as diagnostic tests for tropical theileriosis. Vet World. 2011;4:341–344. doi: 10.5455/vetworld.2011.341-344. [DOI] [Google Scholar]
- Shahnawaz S, Ali M, Aslam MA, Fatima R, Chaudhry ZI, Hassan MU. A study on the prevalence of a tick-transmitted pathogen, Theileria annulata, and hematological profile of cattle from Southern Punjab (Pakistan) Parasitol Res. 2011;109:1155–1160. doi: 10.1007/s00436-011-2360-1. [DOI] [PubMed] [Google Scholar]
- Shiels BR, d’Oliveira C, McKellar S, Ben-Miled L, Kawazu S, Hide G. Selection of diversity at putative glycosylation sites in the immunodominant merozoite/piroplasm surface antigen of Theileria. Parasites Mol Biochem Parasitol. 1995;72:149–162. doi: 10.1016/0166-6851(95)00074-B. [DOI] [PubMed] [Google Scholar]
- Slodki J, Jasik KP, Kepa M, Idzik D, Wojtyczka R. Tick-transmitted disease caused by apicomplexa. Acta Protozool. 2011;50:155–161. [Google Scholar]
- Tavassoli M, Tabatabaei M, Nejad B, Tabatabaei M, Najafabadi A, Pourseyed S. Detection of Theileria annulata by the PCR-RFLP in ticks (Acari, Ixodidae) collected from cattle in West and North-West Iran. Acta Parasitologica. 2011;56:8–13. doi: 10.2478/s11686-011-0001-6. [DOI] [Google Scholar]
- Uilenberg, G, 1981. Theilerial species of domestic livestock. Advances in the control of theileriosis. In A. D. Irvin, M. P. Cunningham, and A. S. Young (ed.);.Martinus Nijhoff, The Hague, The Netherlands. 4–37
- Zaeemi M, Haddadzadeh H, Khazraiinia P, Kazemi B, Bandehpour M. Identification of different Theileria species (Theileria lestoquardi, Theileria ovis, and Theileria annulata) in naturally infected sheep using nested PCR–RFLP. Parasitol Res. 2011;108:837–843. doi: 10.1007/s00436-010-2119-0. [DOI] [PubMed] [Google Scholar]