The complement system is a major component of the innate immune system and it provides a powerful and effective mechanism to protect the host from pathogens. It was first described in the late nineteenth century as a heat-labile component of serum that “complemented” the effects of antibodies in the lysis of bacteria and red blood cells1-4. The term complement was coined by Paul Ehrlich in 18995,6. We now know that the complement system is made up of some 50 serum and membrane proteins with tightly regulated proteolytic activation cascades that culminate in the production of effector molecules with multiple biological functions4-9. It has long been known that complement provides host surveillance and protection from microbes, but it is now clear that complement also plays important and diverse roles in several other physiological and homeostatic functions, such as the clearance of dead and dying cells, developmental and regenerative processes, and the modulation of humoral and cell-mediated immune responses10. Furthermore, disruption of the balance between complement activation and complement regulation is involved in the pathogenesis of several diseases and disease states, ranging from traumatic injury and ischemia-related conditions, to autoimmune disease, to alloreactivity and transplant rejection. Complement is also implicated in tumor immune surveillance, and recently tumor promoting functions of complement have also been described11,12.
Soluble complement proteins are synthesized primarily by hepatocytes, although significant amounts are also synthesized by monocytes, macrophages, and some epithelial cells in the gastrointestinal and urinary tracts5. Activation of complement is normally achieved via three different pathways: the classical, alternative and lectin. Each of these pathways is initiated by different stimuli (Table 1), but all lead to the cleavage and activation of the central complement protein C3, with the subsequent cleavage of C5 and generation of biological effector molecules. In addition to the three well-defined pathways of activation, there are also bypass mechanisms of activation, such as the direct proteolytic cleavage of C513.
Table 1.
Activation of the Complement System
| Pathway | Trigger for Activation |
|---|---|
| Classical | Antibodies bound to bacteria, fungi, viruses or tumor cells |
| Immune Complexes | |
| Apoptotic Cells | |
| C-reactive Protein | |
| Activated Factor XII | |
| Alternative | Continuous hydrolysis of complement protein C3 |
| Lectin | Microbes with terminal mannose groups |
|
Other Activators of
Complement |
Thrombin |
| Kallikrein |
ACTIVATION OF THE COMPLEMENT SYSTEM
The Classical Pathway
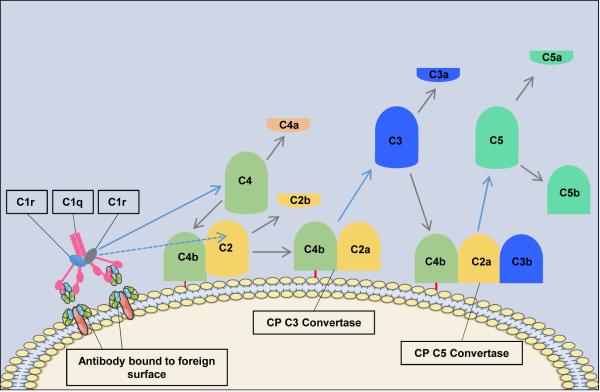
The classical pathway (CP) is triggered by antibody-antigen immune complexes via C1q recognition of Fc domains in conformationally-altered IgM or clustered IgG (Figure 1). The interaction of C1q with Fc causes a conformational change within the C1q molecule and the subsequent cleavage and activation of the associated C1r and C1s serine proteases. Activated C1s then cleaves C4 and C2 into two large active fragments (C4b and C2a) and two small soluble inactive fragments (C4a and C2b). Cleavage of C4 exposes a reactive thioester within the C4b fragment, which results in covalent attachment of C4b to the activating surface. The binding of C2 to C4b and the subsequent cleavage of C2 result in the covalently attached classical pathway C3 convertase, C4bC2a (note that there is discrepancy in the literature in the designation of C2a vs. C2b). This complex cleaves C3 into C3b (large) and C3a (small). Similar to C4b, C3b contains a reactive thioester that can become covalently bound to the activating surface, and which can initiate activation of the alternative pathway (see below). If C3b binds to the C4bC2a complex, it forms to classical pathway C5 convertase (C4bC2aC3b) that cleaves C5 into C5b and C5a, with initiation of the terminal complement pathway (below). Both C3a and C5a are known as anaphylatoxins and are powerful biological effector molecules with diverse functions, including inflammation, modulation of adaptive immunity and repair and regenerative processes.
Figure 1. The Classical Pathway of Complement Activation.
The classical pathway is initiated by the binding of the C1 complex (C1q, C1r and C1s) to bound antibody. C1r activates C1s which first cleaves C4 and then cleaves C2 leading to the formation of the classical pathway C3 convertase (C4bC2a). The C3 convertase subsequently cleaves C3 leading to the formation of the C5 convertase (C4bC2aC3b) and the release of the anaphylatoxin C3a. The C5 convertase then cleaves C5 into C5a and C5b. C5a is the most powerful of the anaphylatoxins and C5b is the first component of the terminal pathway of complement.
Not all antibody isotypes bind C1q and activate complement. IgM is very effective at activating complement, but some subclasses of IgG are poor activators (human IgG4 and mouse IgG1). Activation of the CP can also occur independently of antibodies, and C1q can bind directly to certain microbial epitopes or epitopes exposed on apoptotic and necrotic cells. C1q can also bind to cell surfaces via pattern recognition molecules such as C-reactive protein (CRP), an acute-phase protein that binds to the surface of pathogens or injured cells and activates the classical pathway14-16. This process tags surfaces with complement proteins to facilitate their removal by phagocytes. An interaction can also occur between the coagulation cascade and the complement system, as activated factor XII (FXIIa) is able to interact with C1 and activate the classical pathway17,18.
The Lectin Pathway
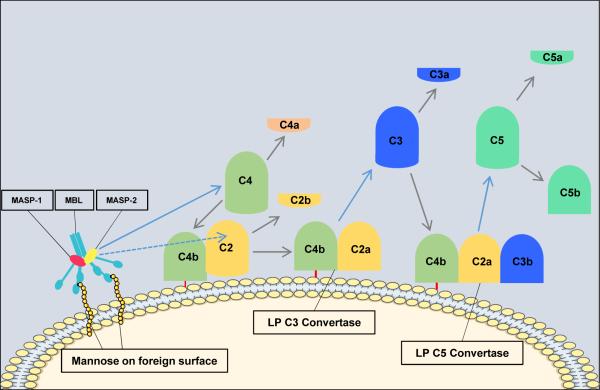
Activation of the lectin pathway results in formation of the same C3 convertase as is generated in the classical pathway (C4bC2a). The catalytic activation sequences of the lectin pathway resemble those of the classical pathway, but with the utilization of different recognition molecules and different associated serine proteases (Figure 2). In the lectin pathway, mannose-binding lectin (MBL) or ficolins recognize patterns of carbohydrates such as N-acetylglucosamine or mannose, but not sialic acid or galactose6, which provides selectivity for bacterial, viral, fungal and parasitic cell surfaces19,20. Mannose binding lectin is structurally and functionally similar to C1, and the binding of MBL (or ficolin) to a carbohydrate ligand activates MBL-associated serine proteases (MASP-1, MASP-2 and MASP-3), which generate C4bC2a in a manner analogous to that of the C1 complex.
Figure 2. The Lectin Pathway of Complement Activation.
The steps in components and reaction in the lectin pathway are very similar to those in the classical pathway. The only differences are the initiation steps. The lectin pathway is initiated by binding of the complex of mannose-binding lectin (MBL) and the serine proteases mannose-binding lectin associated proteases 1 and 2 (MASP-1 and MASP-2) to mannose groups on the surface of invading pathogens. Next, MASP-1 activates MASP-2 which acts like C1s in the classical pathway and lead to the formation of the C3 convertase. The remaining steps are the same as in the classical pathway.
The Alternative Pathway
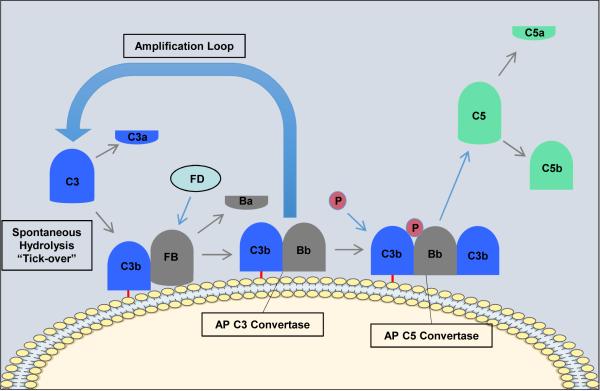
In contrast to the CP and LP, the alternative pathway (AP) is constitutively active and depends on the slow spontaneous hydrolysis of C3, which exposes a binding site for the AP protein, factor B (fB), and subsequent generation of the AP C3 convertase (C3bBb) (Figure 3). This “tick-over” process provides a primed and rapid response mechanism for the deposition of complement on foreign surfaces. Activation and amplification of the alternative pathway depends on the surface to which hydrolyzed C3 becomes covalently attached. Activating surfaces, such as the surface of a microbe, do not have the means to regulate further C3 activation and amplification of the cascade, whereas host cell membranes effectively inhibit further activation. For example, most mammalian cells (host cells) have high levels of sialic acid, and sialic acid favors the binding of factor H to the spontaneously deposited C3b on the cell membrane. Factor H acts as cofactor for the serine esterase Factor I, that cleaves and inactivates C3b rendering it unable participate further in complement activation4,21. On the other hand, microbial surfaces such as bacterial cell walls, viral envelopes or yeast cell walls have little to no sialic acid. Thus microbial and foreign surfaces favor the binding of factor B, not factor H, to the deposited C3b. Binding of factor B to C3b allows for the interaction of factor D with factor B. Factor D cleaves a small fragment from factor B (Ba) resulting in the formation of the AP C3 convertase, C3bBb22. The AP C3 convertase is stabilized by properdin which extends the half-life of the convertase23. The AP C3 convertase subsequently cleaves more C3 molecules leading to the rapid generation of C3b, and this amplification loop also amplifies the generation of C3b produced via the CP and LP. The binding and cleavage of an additional C3 molecule to C3bBb forms the AP C5 convertase, C3bBbC3b, with the same function as the CP C5 convertase. The AP can also be activated by the binding of properdin to molecular patterns found on pathogens or injured cells and the subsequent recruitment of fluid phase C3b24.
Figure 3. The Alternative Pathway of Complement Activation.
The alternative pathway is initiated by the spontaneous hydrolysis of C3 and the deposition of C3b on the surface of activating surfaces (non-host surfaces) and the release of C3a. Factor B (FB) binds to C3b and is subsequently cleaved by factor D (FD) leading to the formation of the alternative pathway C3 convertase (C3bBb). Properdin (P, green) then binds to the convertase to stabilize it. The C3 convertase cleaves C3 releasing more C3a and resulting in the formation of the alternative pathway C5 convertase (C3bBbC3b). The C5 convertase then cleaves C5 into C5a and C5b. C5a is the most powerful of the anaphylatoxins and C5b is the first component of the terminal pathway of complement.
Regardless of how complement is activated (CP, LP or AP), it has been estimated that the AP accounts for about 80% of generated complement activation products, and as such it is a major driver of inflammation25.
The Terminal Pathway of Complement
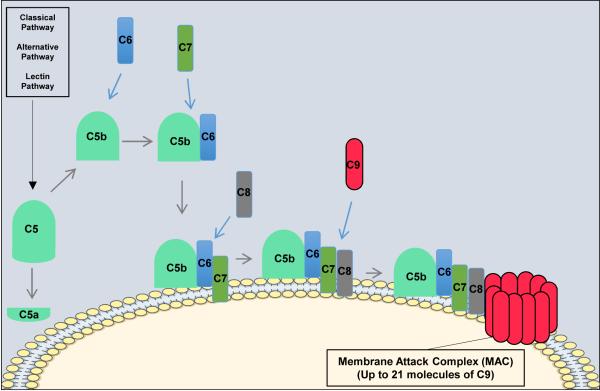
All three pathways of complement activation converge at the formation of the C3 and C5 convertases, the latter of which cleaves C5. Cleavage of C5 produces the anaphylatoxin C5a, and a larger C5b fragment that initiates the non-enzymatic terminal pathway of complement (Figure 4).
Figure 4. The Terminal Pathway of Complement.
All three pathways of complement activation converge in the terminal pathway. The terminal pathway begins when C5b binds to C6. C7 then binds to the C5b-C6 complex and the newly formed C5b-C7 complex inserts into the target membrane. C8 subsequently binds to the C5b-C7 complex and creates a small pore in the target membrane. The final step in the terminal pathway is the binding of C9 molecules (up to 21) to the C5b-C8 complex forming the membrane attack complex.
The cleavage of C5 exposes a binding site on C5b for C6. The resulting C5b-6 complex then binds C7 to form C5b-7, and upon formation of this trimeric complex, amphipathic sites are exposed that allow insertion into lipid bilayers26-29. In the event that C7 fails to bind to C5b-6, the complex is released and in the presence of C7 in able to bind to other nearby membranes. However, membrane insertion is an inefficient process, and most of the complexes are inactivated in the fluid phase. The C5b-7 complex than binds C8, exposing hydrophobic sites that form a stronger association with lipid bilayers. Finally, C5b-8 binds C9, which then unfolds in the membrane and which exposes further C9 binding sites. Thus, C5b-8 binds and polymerizes several C9 molecules, the result of which is a cytolytic transmembrane pore known as the membrane attack complex (MAC, C5b-9n).
Additional Pathways of Complement Activation
In addition to the classical, alternative and lectin pathways, it is now known that the complement system can be activated via other means. The best described of such interactions is the crosstalk between the complement, coagulation and fibrinolysis pathways. As described above, FXIIa is able to activate the classical pathway of complement activation. Additionally, thrombin has been shown to both cleave C330,31 and also act as a C5 convertase, hence, bypassing the early pathways of complement activation13. It is also well described that plasmin and kallikrein directly cleave C3 and its activation fragments18,31-33.
Relevant to the current discussion is recent data investigating complement activation in the pathophysiology of the thrombotic microangiopathies. Evidence suggests that thrombotic thrombocytopenic purpura (TTP), atypical hemolytic uremic syndrome (aHUS) and typical shiga toxin-induced hemolytic uremic syndrome (STEC-HUS) are all diseases of aberrant complement activation34. It is well known that aHUS results from uncontrolled complement activation secondary to mutation in complement regulatory proteins35. However, until recently STEC-HUS and TTP were not considered complement-mediated diseases. In TTP, current reports suggest widespread activation of complement as an important pathogenic mechanism36-38. In STEC-HUS, it is thought that complement is activated via p-selectin, which is upregulated as a result of endothelial damage caused by shiga toxin39,40.
REGULATION OF THE COMPLEMENT SYSTEM
Complement activation leads to the induction of a number of potent immunological processes. This powerful effector system has the potential to do significant damage to the host if not kept in check, and it is therefore tightly regulated. A “built-in” mechanism of regulation for all complement pathways is that many of the intermediate activation products are very unstable and are spontaneously inactivated if they do not rapidly interact with other components of the cascade. In addition, there are several complement regulatory proteins expressed by the host that include both membrane bound and fluid phase inhibitors. These inhibitors can be general, or can be activation pathway or terminal pathway specific. Before engaging in a discussion of the functions of individual complement inhibitors, it is pertinent to discuss the mechanisms that regulate the expression of complement inhibitors. While the exact mechanisms leading to the modulation of complement inhibitor expression in vivo are not well understood, in vitro studies indicate that cytokines play a significant role. Several cytokines including IL-4, TNF-α, IFN-γ, IL-1β and TGFβ have been shown to induce an increase in the expression of complement inhibitors on endothelial cells in vitro41-44.
Complement inhibitors protect the host against collateral complement damage during episodes of complement activation (e.g. inflammatory responses). Hence, there is interconnection between important players in an inflammatory response (i.e. cytokines) and mechanisms leading to complement inhibitor expression. The following is a review of some important soluble and membrane-bound complement inhibitors:
Soluble Inhibitors of Complement
C1 Inhibitor (C1-inh)
The first step in the classical pathway is mediated by the C1 complex (C1q, C1r and C1s), and the first step in the lectin pathway is mediated by MBL or a ficolin complex (MBL/ficolin, MASP-1, MASP-2, MASP-3). C1-inhibitor (C1-inh) inhibits C1r and C1s proteases of the CP, and MASP-1 and MASP-2 proteases of the LP, with the result of preventing cleavage of C4 and C245.
C4-binding Protein (C4bp) and Factor I (fI)
C4-binding protein (C4bp) binds to C4b and accelerates the decay of the CP C3 convertase, and also serves as cofactor for the serine protease factor I (fI). Factor I cleaves C4b into the inactive fragments C4c and C4d46,47. Factor I can also cleave and inactivate C3b. C4bp regulates the classical and lectin pathways, while fI is involved in regulating the classical, alternative and lectin pathways.
Factor H (fH)
Factor H is a plasma protein that regulates the alternative pathway both in the fluid phase and on cell surfaces. After binding to C3b, fH functions by preventing the formation of, or causing the dissociation the AP C3 convertase. Factor H also serves as a cofactor for fI, leading to the cleavage and inactivation of C3b and preventing further progress of the complement cascade48,49. Since the alternative pathway spontaneously activates on any surface and amplifies the other complement pathways, fH provides an important regulatory mechanism to prevent host cell injury.
MAp44
The initial step in activation of the lectin pathway is the binding of MBL or ficolins to carbohydrate patterns and the subsequent cleavage of C4 and C2 by associated MASPs. An additional protein known as Map44 or MAP1 is also associated with the MBL/ficolin-MASP complex, and this non-proteolytic protein inhibits the LP by displacing the MASPs from MBL/ficolins and thus preventing C4 and C2 cleavage50-52.
Membrane-bound Inhibitors of Complement
Complement Receptor Type 1 (CR1, CD35)
Complement receptor 1 (CR1 or CD35) is a glycoprotein expressed predominantly on blood cell types including erythrocytes and leukocytes53,54. It is a receptor for C3b and C4b, and one important function of CR1 is the removal of complement opsonized microbes or immune complexes via their transport to the liver or spleen (see below). CR1 expressed on certain leukocytes can also facilitate phagocytosis of complement-opsonized cells. CR1 functions as a complement inhibitor by acting as a cofactor for fI-mediated cleavage and inactivation of C3b and C4b, a mechanism analogous to the function of C4bp and fH. In addition, CR1 can accelerate the decay of the C3 and C5 convertases55.
Membrane Cofactor Protein (MCP, CD46)
MCP is a transmembrane protein that is mostly associated with control of the alternative pathway convertases, although it is reported to modulate the classical and lectin pathways as well56. It is expressed on most cell types except erythrocytes. The complement regulatory functions of MCP are very similar to those of CR1. Like CR1, MCP acts a cofactor for fI in the cleavage and inactivation of C4b and C3b. However, unlike CR1 it does not promote the decay of the C3 or C5 convertases. MCP can also bind to C4b and C3b and block the formation of the C3 and C5 convertases. This protein has also been shown to down-regulate T helper type 1 (Th1) immune responses57.
Decay Accelerating Factor (DAF, CD55)
DAF is attached to the cell membrane via a glycosyl-phosphatidylinositol (GPI) anchor and it is expressed in most tissues58. It inhibits the formation of both the CP and AP C3 convertases, as well as accelerates their decay. Additionally, an alternatively spliced DAF cDNA has been reported and it is thought to encode for a secreted form of the protein59.
CD59
Like DAF, CD59 is a GPI-anchored protein. It is abundantly and widely expressed53. CD59 is the only well-characterized inhibitor of the terminal pathway, and it functions by binding to C8 and C9 in the assembling MAC, thus interfering with C9 binding, polymerization and pore formation60,61.
Additional Pathways of Complement Regulation
As described previously, there is significant crosstalk between the complement, coagulation and fibrinolysis cascades. This crosstalk not only leads to complement activation, but it can also lead to complement regulation. For example, thrombomodulin, an important component of the fibrinolytic pathway, can accelerate the factor I-mediated inactivation of C3b62. In addition, thrombomodulin also enhances the thrombin-mediated activation of procarboxypeptidase B, an inhibitor of the fibrinolytic pathway, resulting in inactivation of C3a and C5a62.
FUNCTIONS OF THE COMPLEMENT SYSTEM
The complement system has diverse physiological functions, and is additionally intertwined with several other biological networks thus expanding the universe of its activity. Here, we discuss only some of the more well-known and better-documented functions of complement.
Protection of the Host Against Infection
As an essential component of the innate immune system, complement is a first line of defense against many invading pathogens. Initial infection by microorganisms such as bacteria, viruses and parasites, can differently activate any complement pathway depending on the composition of the microbe surface. This activation is triggered by components such as lipopolysaccharides, peptidoglycans and certain carbohydrates (e.g. mannose). The CP and LP can also be triggered by natural IgM antibodies that recognize non-self microbial antigens63-65. In addition, the classical pathway can be activated if pathogen-specific antibodies are also present in the host. Complement activation can lead to formation of the MAC on the surface of the invading microorganism, but MAC-mediated cytolysis is not effective against most microorganisms. For example, with regard to bacterial infection, deficiency in one of the terminal complement proteins leads to increased susceptibility to Neisserial infection only.
A more important complement-dependent mechanism of host defense involves complement receptor-dependent phagocytosis of complement-opsonized pathogens. As described above, factor I cleaves C3 into iC3b and C3f. Factor I further cleaves iC3b into C3c and C3dg. Subsequently, serum proteases cleave C3dg into C3g and C3d. These C3 activation products are covalently bound to activating surfaces and thus can opsonize microbes. While these C3 fragments are unable to participate further in complement activation, they can bind to complement receptors on immune effectors cells and mediate phagocytosis of microorganisms. Complement-dependent phagocytosis is mediated primarily by complement receptors 3 and 4 (CR3 and CR4) (Table 2). CR3 is a member of the 02 integrin family and it is a promiscuous receptor as it binds to iC3b, ICAM-1, LPS, fibrinogen, factor X and some carbohydrates66. CR3 is expressed on monocytes, macrophages, neutrophils, NK cells, follicular dendritic cells (FDC) and subsets of T-cells (6). CR4 belongs to the same family as CR3, and its ligands and tissue distribution are similar to that of CR3. CR3 is recognized as being the more important mediator of phagocytosis as determined by studies of patients with a deficiency in β2 integrins67,68.
Table 2.
Distribution of Complement Receptors
| Cell Type | Receptor | CR1 | CR2 | CR3 | CR4 | C3aR | C5aR | C1qR |
|---|---|---|---|---|---|---|---|---|
| Ligand | C3b C4b |
C3d C3dg iC3b |
iC3b | iC3b | C3a | C5a | C1q | |
| Erythrocytes | X | |||||||
| Platelets | X | X | ||||||
| Neutrophils | X | X | X | X | X | |||
| Monocytes | X | X | X | X | ||||
| Macrophages | X | X | X | X | X | |||
| Eosinophils | X | |||||||
| Basophils | X | |||||||
| Mast Cells | X | |||||||
| Dendritic Cells | X | X | ||||||
| B Cells | X | X | X | |||||
| T Cells | X | X | X | X | X | X | X | |
| NK Cells | X | X |
Another complement-mechanism that contributes to host defense is the induction of an inflammatory response. The complement fragments C3a and C5a are anaphylatoxins and have varied and powerful effects in mediating inflammation. By interacting with their respective receptors, C3a and C5a mediate inflammation by variously increasing vascular permeability, inducing smooth muscle contraction, and the recruitment and activation of immune cells. They can also modulate the adaptive immune response to infectious agents (see below). With regard to infection, C5a is generally considered the more potent of the anaphylatoxins at mediating inflammatory processes. The receptor for C5a is expressed on mast cells, basophils, neutrophils, monocytes, macrophages, endothelial cells, smooth muscle cells and lymphocytes. In addition to causing degranulation of basophils and mast cells, C5a is a strong chemotactic agent for neutrophils, eosinophils, basophils and monocytes69,70. C5a also plays an important role in the function of neutrophils and macrophages as it primes these phagocytic cells for enhanced functional responses71.
Immune homeostasis and disposal of waste
The clearance of immune complexes and the clearance of dead and dying cells are important functions of complement. Binding of antibody to soluble antigen leads to the formation of immune complexes. These complexes become coated with C3b and subsequently bind to CR1 receptors on erythrocytes. Erythrocytes then carry the immune complexes to the liver and spleen where they are destroyed72. The failure to clear immune complexes results in their tissue deposition and complement activation, leading to inflammation and tissue injury. Complement also plays an important role in the clearance of apoptotic and injured cells. Apoptotic cells express neoepitopes on their surface that are recognized by natural circulating IgM antibodies. These antibodies activate both the CP and LP, leading to complement opsonization of the apoptotic cell and complement-receptor-mediated phagocytosis. C1q can also bind directly to apoptotic cells, thus activating the CP. The importance of this clearance process and of the role of complement is demonstrated by the fact that deficiency in certain complement proteins is strongly linked to the development of autoimmune disease21,73.
Tissue repair, regeneration and development
Few organs are able to regenerate in adult mammals, with the liver being a notable exception. Preclinical studies into the mechanism of hepatic regeneration have demonstrated an essential role for C3a and C5a in priming the hepatocyte proliferative response after injury or resection. This occurs via the effect of C3a/5a receptor signaling on cytokine and transcription factor expression74,75. Complement, (principally C3a and C5a) has also been implicated in the regeneration of bone, cardiac muscle and skeletal muscle, as well as stem cell engraftment (reviewed in 76-78 ). Additionally, several groups have reported that complement also plays an important role in several key processes in the central nervous system (CNS)77-79. While the early consensus was that complement had mainly neurodegenerative and deleterious effects in the CNS, it is now apparent that complement is also involved in neuron regeneration, synapse formation and neuroprotection. Particularly interesting is the finding that C1q and C3 opsonins play an essential role in synaptic pruning and neuron remodeling during development, which appears to occur via a mechanism analogous to that for immune homeostasis and apoptotic cell clearance80 (see above).
Bridging of the Innate and Adaptive Immune Responses
Role of Complement in Humoral Immune Responses
The complement system is not only an essential component of innate immunity, but also an important player in the induction of adaptive immune responses4,21,7,81,82. The role of complement in humoral immunity is well characterized and it is mediated via complement receptor 2 (CR2). CR2 is expressed primarily on B-cells, follicular dendritic cells (FDC) and certain T-cell subsets. Binding of complement (C3d)-opsonized antigen to CR2 on B-cells reduces the threshold required for B-cell activation83,84. In addition, complement aids in the localization of antigen to FDCs within lymphoid follicles, promotes the development of B-cell memory and it can also enhance avidity maturation and class switching7,82,85-88.
Role of Complement in Cell-mediated Immune Responses
Complement can modulate all three phases of T-cell immune responses (i.e. inductor phase, effector phase and contraction phase)82,89-91, and plays an important role during cognate interactions between antigen presenting cells (APCs) and T-cells. Signaling via co-stimulatory molecules (CD28, CD80, CD86, CD154 and CD40) results in 1) secretion of C3, factor B, factor D and C5, 2) upregulation of the surface expression of C3a and C5a receptors (C3aR and C5aR) and 3) downregulation of surface expression of the complement inhibitor CD55 (DAF)92-95. These changes in complement protein expression occur in both APCs and T cells, and via the local production of C3a and C5a results in autocrine and paracrine stimulation of the engaged APCs and T cells via C3aR and C5aR92-94. The role of complement in the effector phase of T-cell immunity has been shown in preclinical models showing that lack of C3 or C5aR leads to impaired CD4+ and CD8+ T cell responses against viruses96-98. A role for complement in the contraction phase has also been demonstrated; concurrent crosslinking of the TCR and CD46 (MCP) on T-cells leads to the development of regulatory T-cells (Tregs) in vitro89,99. Recently, it has been shown that human T cells can process C3 to C3a and C3b intracellularly by cathepsin L cleavage, resulting in complement-dependent autocrine stimulation of T cells. Intracelluarly generated C3a was found to be required for T cell survival, and following T cell stimulation induced the autocrine production of pro-inflammatory cytokines100. Finally, C3a and C5a receptor signaling can inhibit both natural and induced T regulatory cell (T reg) function, thereby further modulating the overall strength of a T cell immune response101,102. Depending on the context of complement activation and regulation, these effects of complement on the shaping of an adaptive immune response not only impacts immunity to infection, but also autoimmunity, alloimmunity and tumor immunity.
Synopsis.
The complement system is an essential component of the immune system. It is a highly integrative system and has a number of functions that include host defense, removal of injured cells and debris, modulation of metabolic and regenerative processes, and the regulation of adaptive immunity. Complement is activated via different pathways and it is tightly regulated by several mechanisms to prevent host injury. Imbalance between complement activation and regulation can manifest in disease and injury to self. This article provides an outline of complement activation pathways, regulatory mechanisms, and normal physiological functions of the system.
Key Points.
The complement system is composed of over 50 interacting serum and membrane-bound proteins that provides an effective immune surveillance.
Complement can be activated via three different pathways: the classical, lectin and alternative pathways. These pathways converge at the cleavage and activation of C3 with the subsequent generation of various biological effector molecules.
Strict regulation of the complement system is mediated by a number of soluble and membrane-bound proteins to prevent damage to self.
Complement plays a key role in a number of biological processes that include host defense, removal of injured cells and debris, modulation of metabolic and regenerative processes, and the regulation of adaptive immunity.
Inappropriate complement activation and impaired regulation can lead to self-directed attack and contributes to various diseases and disease-related conditions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have nothing to disclose
REFERENCES
- 1.Carroll MC, Fischer MB. Complement and the immune response. Curr. Opin. Immunol. 1997;9:64–69. doi: 10.1016/s0952-7915(97)80160-4. [DOI] [PubMed] [Google Scholar]
- 2.Bordet J, Gengou O. Sur l’existence de substances sensibilisatrices dans la plupart des serum antimicrobiens. Ann Inst Pasteur. 1901;15:289–302. [Google Scholar]
- 3.Metschnikoff E. Sur la lutte des cellule de l’organisme contre l'invasion des microbes. Ann Inst Pasteur. 1887;1:321. [Google Scholar]
- 4.Walport MJ. Complement. First of two parts. N. Engl. J. Med. 2001;344:1058–66. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- 5.Goldsby R, Kindt T, Osborne, Kuby J. Immunoloy. Freeman and Company; 2003. [Google Scholar]
- 6.Paul W. Fundamental Immunology. Lippincott; Williams and Wilkins: 2003. [Google Scholar]
- 7.Carroll MC. The complement system in regulation of adaptive immunity. Nat. Immunol. 2004;5:981–6. doi: 10.1038/ni1113. [DOI] [PubMed] [Google Scholar]
- 8.Rus H, Cudrici C, Niculescu F. The role of the complement system in innate immunity. Immunol. Res. 2005;33:103–12. doi: 10.1385/IR:33:2:103. [DOI] [PubMed] [Google Scholar]
- 9.Kemper C, Pangburn MK, Fishelson Z. Complement nomenclature 2014. Mol. Immunol. 2014;61:56–8. doi: 10.1016/j.molimm.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat. Immunol. 2010;11:785–97. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stover C. Dual role of complement in tumour growth and metastasis (Review) Int. J. Mol. Med. 2010;25:307–13. doi: 10.3892/ijmm_00000346. [DOI] [PubMed] [Google Scholar]
- 12.Markiewski MM, et al. Modulation of the antitumor immune response by complement. Nat. Immunol. 2008;9:1225–35. doi: 10.1038/ni.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huber-Lang M, et al. Generation of C5a in the absence of C3: a new complement activation pathway. Nat. Med. 2006;12:682–7. doi: 10.1038/nm1419. [DOI] [PubMed] [Google Scholar]
- 14.Mold C, Gewurz H, Du Clos TW. Regulation of complement activation by C-reactive protein. Immunopharmacology. 1999;42:23–30. doi: 10.1016/s0162-3109(99)00007-7. [DOI] [PubMed] [Google Scholar]
- 15.Mold C, Du Clos TW, Nakayama S, Edwards KM, Gewurz H. C-reactive protein reactivity with complement and effects on phagocytosis. Ann. N. Y. Acad. Sci. 1982;389:251–62. doi: 10.1111/j.1749-6632.1982.tb22141.x. [DOI] [PubMed] [Google Scholar]
- 16.Gewurz H, Mold C, Siegel J, Fiedel B. C-reactive protein and the acute phase response. Adv. Intern. Med. 1982;27:345–72. [PubMed] [Google Scholar]
- 17.Ghebrehiwet B, Silverberg M, Kaplan AP. Activation of the classical pathway of complement by Hageman factor fragment. J. Exp. Med. 1981;153:665–76. doi: 10.1084/jem.153.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rittirsch D, Flierl MA, Ward PA. Harmful molecular mechanisms in sepsis. Nat. Rev. Immunol. 2008;8:776–87. doi: 10.1038/nri2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iobst ST, Wormald MR, Weis WI, Dwek RA, Drickamer K. Binding of sugar ligands to Ca(2+)-dependent animal lectins. I. Analysis of mannose binding by site-directed mutagenesis and NMR. J. Biol. Chem. 1994;269:15505–11. [PubMed] [Google Scholar]
- 20.Iobst ST, Drickamer K. Binding of sugar ligands to Ca(2+)-dependent animal lectins. II. Generation of high-affinity galactose binding by site-directed mutagenesis. J. Biol. Chem. 1994;269:15512–9. [PubMed] [Google Scholar]
- 21.Walport MJ. Complement. Second of two parts. N. Engl. J. Med. 2001;344:1140–4. doi: 10.1056/NEJM200104123441506. [DOI] [PubMed] [Google Scholar]
- 22.Volanakis JE, Narayana SV. Complement factor D, a novel serine protease. Protein Sci. 1996;5:553–64. doi: 10.1002/pro.5560050401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chapitis J, Lepow IH. Multiple sedimenting species of properdin in human serum and interaction of purified properdin with the third component of complement. J. Exp. Med. 1976;143:241–57. doi: 10.1084/jem.143.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cortes C, Ohtola JA, Saggu G, Ferreira VP. Local release of properdin in the cellular microenvironment: role in pattern recognition and amplification of the alternative pathway of complement. Front. Immunol. 2012;3:412. doi: 10.3389/fimmu.2012.00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harboe M, Mollnes TE. The alternative complement pathway revisited. J. Cell. Mol. Med. 2008;12:1074–84. doi: 10.1111/j.1582-4934.2008.00350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Podack ER, Kolb WP, Muller-Eberhard HJ. The C5b-9 complex: subunit composition of the classical and alternative pathway-generated complex. J. Immunol. 1976;116:1431–4. [PubMed] [Google Scholar]
- 27.Podack ER, Biesecker G, Müller-Eberhard HJ. Membrane attack complex of complement: generation of high-affinity phospholipid binding sites by fusion of five hydrophilic plasma proteins. Proc. Natl. Acad. Sci. U. S. A. 1979;76:897–901. doi: 10.1073/pnas.76.2.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Preissner KT, Podack ER, Müller-Eberhard HJ. The membrane attack complex of complement: relation of C7 to the metastable membrane binding site of the intermediate complex C5b-7. J. Immunol. 1985;135:445–51. [PubMed] [Google Scholar]
- 29.Podack ER, Müller-Eberhard HJ. Binding of desoxycholate, phosphatidylcholine vesicles, lipoprotein and of the S-protein to complexes of terminal complement components. J. Immunol. 1978;121:1025–30. [PubMed] [Google Scholar]
- 30.Clark A, et al. Evidence for non-traditional activation of complement factor C3 during murine liver regeneration. Mol. Immunol. 2008;45:3125–32. doi: 10.1016/j.molimm.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amara U, et al. Molecular intercommunication between the complement and coagulation systems. J. Immunol. 2010;185:5628–36. doi: 10.4049/jimmunol.0903678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thoman ML, Meuth JL, Morgan EL, Weigle WO, Hugli TE. C3d-K, a kallikrein cleavage fragment of iC3b is a potent inhibitor of cellular proliferation. J. Immunol. 1984;133:2629–33. [PubMed] [Google Scholar]
- 33.Goldberger G, et al. NH2-terminal structure and cleavage of guinea pig pro-C3, the precursor of the third complement component. J. Biol. Chem. 1981;256:12617–9. [PubMed] [Google Scholar]
- 34.Noris M, Mescia F, Remuzzi G. STEC-HUS, atypical HUS and TTP are all diseases of complement activation. Nat. Rev. Nephrol. 2012;8:622–33. doi: 10.1038/nrneph.2012.195. [DOI] [PubMed] [Google Scholar]
- 35.Noris M, Remuzzi G. Atypical hemolytic-uremic syndrome. N. Engl. J. Med. 2009;361:1676–87. doi: 10.1056/NEJMra0902814. [DOI] [PubMed] [Google Scholar]
- 36.Ruiz-Torres MP, et al. Complement activation: the missing link between ADAMTS-13 deficiency and microvascular thrombosis of thrombotic microangiopathies. Thromb. Haemost. 2005;93:443–52. doi: 10.1160/TH04-07-0450. [DOI] [PubMed] [Google Scholar]
- 37.Réti M, et al. Complement activation in thrombotic thrombocytopenic purpura. J. Thromb. Haemost. 2012;10:791–8. doi: 10.1111/j.1538-7836.2012.04674.x. [DOI] [PubMed] [Google Scholar]
- 38.Chapin J, Weksler B, Magro C, Laurence J. Eculizumab in the treatment of refractory idiopathic thrombotic thrombocytopenic purpura. Br. J. Haematol. 2012;157:772–4. doi: 10.1111/j.1365-2141.2012.09084.x. [DOI] [PubMed] [Google Scholar]
- 39.Morigi M, et al. Alternative pathway activation of complement by Shiga toxin promotes exuberant C3a formation that triggers microvascular thrombosis. J. Immunol. 2011;187:172–80. doi: 10.4049/jimmunol.1100491. [DOI] [PubMed] [Google Scholar]
- 40.Del Conde I, Crúz MA, Zhang H, López JA, Afshar-Kharghan V. Platelet activation leads to activation and propagation of the complement system. J. Exp. Med. 2005;201:871–9. doi: 10.1084/jem.20041497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mason JC, Lidington EA, Ahmad SR, Haskard DO. bFGF and VEGF synergistically enhance endothelial cytoprotection via decay-accelerating factor induction. Am. J. Physiol. Cell Physiol. 2002;282:C578–87. doi: 10.1152/ajpcell.00339.2001. [DOI] [PubMed] [Google Scholar]
- 42.Mason JC, et al. Induction of decay-accelerating factor by cytokines or the membrane-attack complex protects vascular endothelial cells against complement deposition. Blood. 1999;94:1673–82. [PubMed] [Google Scholar]
- 43.Kawano M. Complement regulatory proteins and autoimmunity. Arch. Immunol. Ther. Exp. (Warsz) 2000;48:367–72. [PubMed] [Google Scholar]
- 44.Moutabarrik A, et al. Cytokine-mediated regulation of the surface expression of complement regulatory proteins, CD46(MCP), CD55(DAF), and CD59 on human vascular endothelial cells. Lymphokine Cytokine Res. 1993;12:167–72. [PubMed] [Google Scholar]
- 45.Kirschfink M, Nürnberger W. C1 inhibitor in anti-inflammatory therapy: from animal experiment to clinical application. Mol. Immunol. 36:225–32. doi: 10.1016/s0161-5890(99)00048-6. [DOI] [PubMed] [Google Scholar]
- 46.Blom AM, Kask L, Dahlbäck B. CCP1-4 of the C4b-binding protein alpha-chain are required for factor I mediated cleavage of complement factor C3b. Mol. Immunol. 2003;39:547–56. doi: 10.1016/s0161-5890(02)00213-4. [DOI] [PubMed] [Google Scholar]
- 47.Sim RB, Laich A. Serine proteases of the complement system. Biochem. Soc. Trans. 2000;28:545–50. doi: 10.1042/bst0280545. [DOI] [PubMed] [Google Scholar]
- 48.Liszewski MK, Farries TC, Lublin DM, Rooney IA, Atkinson JP. Control of the complement system. Adv. Immunol. 1996;61:201–83. doi: 10.1016/s0065-2776(08)60868-8. [DOI] [PubMed] [Google Scholar]
- 49.Pangburn MK, Schreiber RD, Müller-Eberhard HJ. Human complement C3b inactivator: isolation, characterization, and demonstration of an absolute requirement for the serum protein beta1H for cleavage of C3b and C4b in solution. J. Exp. Med. 1977;146:257–70. doi: 10.1084/jem.146.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Banda NK, et al. Essential role for the lectin pathway in collagen antibody-induced arthritis revealed through use of adenovirus programming complement inhibitor MAp44 expression. J. Immunol. 2014;193:2455–68. doi: 10.4049/jimmunol.1400752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pavlov VI, et al. Endogenous and natural complement inhibitor attenuates myocardial injury and arterial thrombogenesis. Circulation. 2012;126:2227–35. doi: 10.1161/CIRCULATIONAHA.112.123968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Degn SE, et al. MAp44, a human protein associated with pattern recognition molecules of the complement system and regulating the lectin pathway of complement activation. J. Immunol. 2009;183:7371–8. doi: 10.4049/jimmunol.0902388. [DOI] [PubMed] [Google Scholar]
- 53.Miwa T, Song WC. Membrane complement regulatory proteins: insight from animal studies and relevance to human diseases. Int. Immunopharmacol. 2001;1:445–59. doi: 10.1016/s1567-5769(00)00043-6. [DOI] [PubMed] [Google Scholar]
- 54.Ahearn JM, Fearon DT. Structure and function of the complement receptors, CR1 (CD35) and CR2 (CD21) Adv. Immunol. 1989;46:183–219. doi: 10.1016/s0065-2776(08)60654-9. [DOI] [PubMed] [Google Scholar]
- 55.Iida K, Nussenzweig V. Functional properties of membrane-associated complement receptor CR1. J. Immunol. 1983;130:1876–80. [PubMed] [Google Scholar]
- 56.Kojima A, et al. Membrane cofactor protein (CD46) protects cells predominantly from alternative complement pathway-mediated C3-fragment deposition and cytolysis. J. Immunol. 1993;151:1519–27. [PubMed] [Google Scholar]
- 57.Yamamoto H, Fara AF, Dasgupta P, Kemper C. CD46: the “multitasker” of complement proteins. Int. J. Biochem. Cell Biol. 2013;45:2808–20. doi: 10.1016/j.biocel.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 58.Lublin DM, Atkinson JP. Decay-accelerating factor: biochemistry, molecular biology, and function. Annu. Rev. Immunol. 1989;7:35–58. doi: 10.1146/annurev.iy.07.040189.000343. [DOI] [PubMed] [Google Scholar]
- 59.Caras IW, et al. Cloning of decay-accelerating factor suggests novel use of splicing to generate two proteins. Nature. 325:545–9. doi: 10.1038/325545a0. [DOI] [PubMed] [Google Scholar]
- 60.Meri S, et al. Human protectin (CD59), an 18,000-20,000 MW complement lysis restricting factor, inhibits C5b-8 catalysed insertion of C9 into lipid bilayers. Immunology. 1990;71:1–9. [PMC free article] [PubMed] [Google Scholar]
- 61.Rollins SA, Sims PJ. The complement-inhibitory activity of CD59 resides in its capacity to block incorporation of C9 into membrane C5b-9. J. Immunol. 1990;144:3478–83. [PubMed] [Google Scholar]
- 62.Delvaeye M, et al. Thrombomodulin mutations in atypical hemolytic-uremic syndrome. N. Engl. J. Med. 2009;361:345–57. doi: 10.1056/NEJMoa0810739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baumgarth N, Herman OC, Jager GC, Brown L, Herzenberg LA. Innate and acquired humoral immunities to influenza virus are mediated by distinct arms of the immune system. Proc. Natl. Acad. Sci. U. S. A. 1999;96:2250–5. doi: 10.1073/pnas.96.5.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boes M, Prodeus AP, Schmidt T, Carroll MC, Chen J. A critical role of natural immunoglobulin M in immediate defense against systemic bacterial infection. J. Exp. Med. 1998;188:2381–6. doi: 10.1084/jem.188.12.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang M, et al. Activation of the lectin pathway by natural IgM in a model of ischemia/reperfusion injury. J. Immunol. 2006;177:4727–34. doi: 10.4049/jimmunol.177.7.4727. [DOI] [PubMed] [Google Scholar]
- 66.Ehlers MR. CR3: a general purpose adhesion-recognition receptor essential for innate immunity. Microbes Infect. 2000;2:289–94. doi: 10.1016/s1286-4579(00)00299-9. [DOI] [PubMed] [Google Scholar]
- 67.Coxon A, et al. A novel role for the beta 2 integrin CD11b/CD18 in neutrophil apoptosis: a homeostatic mechanism in inflammation. Immunity. 1996;5:653–66. doi: 10.1016/s1074-7613(00)80278-2. [DOI] [PubMed] [Google Scholar]
- 68.Hogg N, et al. A novel leukocyte adhesion deficiency caused by expressed but nonfunctional beta2 integrins Mac-1 and LFA-1. J. Clin. Invest. 1999;103:97–106. doi: 10.1172/JCI3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fernandez HN, Henson PM, Otani A, Hugli TE. Chemotactic response to human C3a and C5a anaphylatoxins. I. Evaluation of C3a and C5a leukotaxis in vitro and under stimulated in vivo conditions. J. Immunol. 1978;120:109–15. [PubMed] [Google Scholar]
- 70.Morita E, Schröder JM, Christophers E. Differential sensitivities of purified human eosinophils and neutrophils to defined chemotaxins. Scand. J. Immunol. 1989;29:709–16. doi: 10.1111/j.1365-3083.1989.tb01175.x. [DOI] [PubMed] [Google Scholar]
- 71.Ward PA. The dark side of C5a in sepsis. Nat. Rev. Immunol. 2004;4:133–42. doi: 10.1038/nri1269. [DOI] [PubMed] [Google Scholar]
- 72.Schifferli JA, Ng YC, Peters DK. The role of complement and its receptor in the elimination of immune complexes. N. Engl. J. Med. 1986;315:488–95. doi: 10.1056/NEJM198608213150805. [DOI] [PubMed] [Google Scholar]
- 73.Korb LC, Ahearn JM. C1q binds directly and specifically to surface blebs of apoptotic human keratinocytes: complement deficiency and systemic lupus erythematosus revisited. J. Immunol. 1997;158:4525–8. [PubMed] [Google Scholar]
- 74.Strey CW, et al. The proinflammatory mediators C3a and C5a are essential for liver regeneration. J. Exp. Med. 2003;198:913–23. doi: 10.1084/jem.20030374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.He S, et al. A complement-dependent balance between hepatic ischemia/reperfusion injury and liver regeneration in mice. J. Clin. Invest. 2009;119:2304–16. doi: 10.1172/JCI38289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Phieler J, Garcia-Martin R, Lambris JD, Chavakis T. The role of the complement system in metabolic organs and metabolic diseases. Semin. Immunol. 2013;25:47–53. doi: 10.1016/j.smim.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rutkowski MJ, et al. Complement and the central nervous system: emerging roles in development, protection and regeneration. Immunol. Cell Biol. 88:781–6. doi: 10.1038/icb.2010.48. [DOI] [PubMed] [Google Scholar]
- 78.Mastellos DC, Deangelis RA, Lambris JD. Complement-triggered pathways orchestrate regenerative responses throughout phylogenesis. Semin. Immunol. 2013;25:29–38. doi: 10.1016/j.smim.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stephan AH, Barres BA, Stevens B. The complement system: an unexpected role in synaptic pruning during development and disease. Annu. Rev. Neurosci. 2012;35:369–89. doi: 10.1146/annurev-neuro-061010-113810. [DOI] [PubMed] [Google Scholar]
- 80.Perry VH, O’Connor V. C1q: the perfect complement for a synaptic feast? Nat. Rev. Neurosci. 2008;9:807–11. doi: 10.1038/nrn2394. [DOI] [PubMed] [Google Scholar]
- 81.Toapanta FR, Ross TM. Complement-mediated activation of the adaptive immune responses: role of C3d in linking the innate and adaptive immunity. Immunol. Res. 2006;36:197–210. doi: 10.1385/IR:36:1:197. [DOI] [PubMed] [Google Scholar]
- 82.Kemper C, Atkinson JP. T-cell regulation: with complements from innate immunity. Nat. Rev. Immunol. 2007;7:9–18. doi: 10.1038/nri1994. [DOI] [PubMed] [Google Scholar]
- 83.Carter RH, Fearon DT. CD19: lowering the threshold for antigen receptor stimulation of B lymphocytes. Science. 1992;256:105–7. doi: 10.1126/science.1373518. [DOI] [PubMed] [Google Scholar]
- 84.Dempsey PW, Allison ME, Akkaraju S, Goodnow CC, Fearon DT. C3d of complement as a molecular adjuvant: bridging innate and acquired immunity. Science. 1996;271:348–50. doi: 10.1126/science.271.5247.348. [DOI] [PubMed] [Google Scholar]
- 85.Fang Y, Xu C, Fu YX, Holers VM, Molina H. Expression of complement receptors 1 and 2 on follicular dendritic cells is necessary for the generation of a strong antigen-specific IgG response. J. Immunol. 1998;160:5273–9. [PubMed] [Google Scholar]
- 86.Carroll MC. The complement system in B cell regulation. Mol. Immunol. 2004;41:141–6. doi: 10.1016/j.molimm.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 87.Molina H, et al. Markedly impaired humoral immune response in mice deficient in complement receptors 1 and 2. Proc. Natl. Acad. Sci. U. S. A. 1996;93:3357–61. doi: 10.1073/pnas.93.8.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Test ST, Mitsuyoshi J, Connolly CC, Lucas AH. Increased immunogenicity and induction of class switching by conjugation of complement C3d to pneumococcal serotype 14 capsular polysaccharide. Infect. Immun. 2001;69:3031–40. doi: 10.1128/IAI.69.5.3031-3040.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kemper C, et al. Activation of human CD4+ cells with CD3 and CD46 induces a T-regulatory cell 1 phenotype. Nature. 2003;421:388–92. doi: 10.1038/nature01315. [DOI] [PubMed] [Google Scholar]
- 90.Morgan BP, Marchbank KJ, Longhi MP, Harris CL, Gallimore AM. Complement: central to innate immunity and bridging to adaptive responses. Immunol. Lett. 2005;97:171–9. doi: 10.1016/j.imlet.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 91.Longhi MP, Harris CL, Morgan BP, Gallimore A. Holding T cells in check--a new role for complement regulators? Trends Immunol. 2006;27:102–8. doi: 10.1016/j.it.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 92.Lalli PN, et al. Locally produced C5a binds to T cell-expressed C5aR to enhance effector T-cell expansion by limiting antigen-induced apoptosis. Blood. 2008;112:1759–66. doi: 10.1182/blood-2008-04-151068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Strainic MG, et al. Locally produced complement fragments C5a and C3a provide both costimulatory and survival signals to naive CD4+ T cells. Immunity. 2008;28:425–35. doi: 10.1016/j.immuni.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Heeger PS, et al. Decay-accelerating factor modulates induction of T cell immunity. J. Exp. Med. 2005;201:1523–30. doi: 10.1084/jem.20041967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu J, et al. The complement inhibitory protein DAF (CD55) suppresses T cell immunity in vivo. J. Exp. Med. 2005;201:567–77. doi: 10.1084/jem.20040863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kopf M, Abel B, Gallimore A, Carroll M, Bachmann MF. Complement component C3 promotes T-cell priming and lung migration to control acute influenza virus infection. Nat. Med. 2002;8:373–8. doi: 10.1038/nm0402-373. [DOI] [PubMed] [Google Scholar]
- 97.Suresh M, et al. Complement component 3 is required for optimal expansion of CD8 T cells during a systemic viral infection. J. Immunol. 2003;170:788–94. doi: 10.4049/jimmunol.170.2.788. [DOI] [PubMed] [Google Scholar]
- 98.Kim AHJ, et al. Complement C5a receptor is essential for the optimal generation of antiviral CD8+ T cell responses. J. Immunol. 2004;173:2524–9. doi: 10.4049/jimmunol.173.4.2524. [DOI] [PubMed] [Google Scholar]
- 99.Grossman WJ, et al. Human T regulatory cells can use the perforin pathway to cause autologous target cell death. Immunity. 2004;21:589–601. doi: 10.1016/j.immuni.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 100.Liszewski MK, et al. Intracellular complement activation sustains T cell homeostasis and mediates effector differentiation. Immunity. 2013;39:1143–57. doi: 10.1016/j.immuni.2013.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Strainic MG, Shevach EM, An F, Lin F, Medof ME. Absence of signaling into CD4+ cells via C3aR and C5aR enables autoinductive TGF-β1 signaling and induction of Foxp3+ regulatory T cells. Nat. Immunol. 2013;14:162–71. doi: 10.1038/ni.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kwan W, van der Touw W, Paz-Artal E, Li MO, Heeger PS. Signaling through C5a receptor and C3a receptor diminishes function of murine natural regulatory T cells. J. Exp. Med. 2013;210:257–68. doi: 10.1084/jem.20121525. [DOI] [PMC free article] [PubMed] [Google Scholar]