Abstract
FCRL4 is an immunoregulatory receptor expressed by a subpopulation of memory B cells. These tissue-based cells express increased levels of the src-family kinases HCK and FGR. In this study, we investigate the roles of these src-family kinases in FCRL4-mediated immunoregulation of B cells in the context of previously unrecognized palmitoylation of the receptor. We observed enhanced phosphorylation of FCRL4 on tyrosine residues in the presence of the HCK p59 or FGR. This phosphorylation was markedly reduced in assays using a palmitoylation-defective mutant of FCRL4. In reporter gene studies, we observe that FCRL4 expression enhances CpG-mediated activation of NF-κB signaling. Surprisingly, using a reporter gene linked to activation of the MAPK substrate Elk-1 in response to Ag receptor ligation, we find that FCRL4 has inhibitory activity in cells coexpressing FGR but an activating function in cells coexpressing HCK p59. We provide evidence that in primary memory B cells, expression of FCRL4 leads to increased expression of IL-10 in the presence of FGR or HCK p59 in response to CpG, but increased levels of IFN-γ only in the context of coexpression of FGR. Our study supports the specific requirement of HCK p59 and FGR src-family kinases for FCRL4-mediated immunomodulatory activity and indicates that palmitoylation serves as an additional level of regulatory control of FCRL4.
Introduction
Memory B (Bmem) cells are a key component in providing immunity to previously encountered Ags during the course of either natural infections or vaccinations (1–3). Originally identified by expression of the CD27 Ag (4), it has become clear that the pool of Bmem consists of functionally distinct subpopulations in humans and mice (5, 6). Despite the prominent position of Bmem cells in humoral immune responses, our knowledge of regulatory control mechanisms governing the activation of Bmem subpopulations is incomplete.
A novel family of ITIM- and/or ITAM-bearing receptors implicated in regulating Bmem responses are the FcR-like (FCRL) molecules. This family of immunoreceptors has been identified by their sequence similarities to FcRs, neighboring genomic localization, and conserved gene organization (7–11). The FCRL family in humans contains six cell-surface receptors, five of which (FCRL1–5) are preferentially expressed by B lineage cells. The FCRL members are often most highly expressed by Bmem cells, implying that Bmem cells are subject to important immunoregulatory mechanisms that are poorly understood. FCRL4 is unique in that it is expressed exclusively by a limited population of Bmem cells that are normally based in mucosal lymphoid tissues (5). Interestingly, an exhausted population of FCRL4+ Bmem cells has been detected in blood samples from viremic HIV-1+ individuals, as well as in individuals from malaria-endemic regions (12–14) and in the joint fluid of rheumatoid arthritis patients (15).
The presence of ITAM and/or ITIM sequences in the intracellular domains of FCRL molecules suggests an immunoregulatory function of these receptors. This is supported by biochemical evidence for several of the FCRL receptors. The intracellular region of FCRL4 contains three ITIM sequences, the two membrane-proximal of which could also form a noncanonical ITAM, and a potent inhibitory function on BCR signaling has been demonstrated (16). This inhibitory activity was mediated via recruitment of the SHP-1 and SHP-2 tyrosine phosphatases to the intracellular domain of FCRL4 after coligation with the Ag receptor. Similarly, the FCRL2 and FCRL5 molecules display inhibitory activity upon coligation with the BCR by a mechanism dependent on tyrosine phosphorylation and recruitment of the SHP-1 phosphatase (17, 18). Interestingly, a recent study demonstrated that ligation of FCRL5 in viable motheaten mice lacking a functional SHP-1 phosphatase enhanced BCR signaling, whereas coligation of FCRL5 with the BCR on B lineage cells derived from Lyn−/− mice inhibited BCR signals (19). In contrast with the inhibitory activity of FCRL2, FCRL4, and FCRL5, FCRL1 functions as coactivator on human B lineage cells via a mechanism that remains to be elucidated (20). FCRL3 on human B cells has been found to enhance CpG signaling but interfere with CpG-mediated plasma cell differentiation (21).
In previous studies we observed that FCRL4− Bmem responded equally well to T-dependent and T-independent stimulation, whereas FCRL4+ Bmem selectively responded to simulated T-dependent stimulation (5). To further define this distinction, we performed a comparative transcriptome analysis and identified differentially regulated transcripts in the FCRL4+ and FCRL4− Bmem cells, including cell-surface receptors, transcription factors, cell-cycle regulators, and signal transduction molecules (22). The src-family members hemopoietic cell kinase (HCK) and feline Gardner-Rasheed sarcoma viral oncogene homolog (FGR) were among the most strongly upregulated transcripts in FCRL4+ cells. Given the key role of src-family kinases in phosphorylating tyrosine residues of ITIM- and ITAM-bearing receptors (23), we investigated in this study the role of the HCK and FGR kinases in FCRL4-mediated modulation of BCR signaling. Recent evidence shows FCRL4-mediated enhancement of CD23 expression induced by TLR9 stimulation (24), suggesting a function for FCRL4 at the interface of adaptive and innate immunity. Therefore, we extended our study to the potential regulation of signaling by innate receptors. Our findings indicate a potential role for HCK and FGR in the immunoregulatory activity of FCRL4. Furthermore, we provide evidence for FCRL4 palmitoylation and lipid raft localization of the FCRL4 receptor.
Materials and Methods
Cells and reagents
HEK293T and BOSC23 cells were cultured in DMEM supplemented with glutamine, 100 U/ml penicillin/streptomycin, and 10% FBS. The IgM+ BJAB human Burkitt’s lymphoma cell line was cultured in RPMI 1640 supplemented with glutamine, 100 U/ml penicillin/streptomycin, 50 μM 2-ME, and 10% FBS. Cells were maintained in a humidified atmosphere at 37°C with 5% CO2. cDNAs for src-family kinases were obtained from American Type Culture Collection, and FCRL4 sequences have been described previously (10, 16). Anti-phosphotyrosine Abs were obtained from Millipore (Billerica, MA), and anti-myc epitope tag Abs were purchased from Cell Signaling Technology (Danvers, MA). Abs to the cell-surface Ags mCD4 (PE-Cy7, clone RM4-5), CD20 (PerCP-Cy5.5, clone H1(FB1)), CD27 (FITC, clone M-T271), as well as anti–IL-6 (PE, clone MQ2-6A3) and anti–IFN-γ (PE, clone B27), were purchased from BD Biosciences (San Jose, CA), and anti–IL-10 (PE, clone JES3-19F2), anti–TNF-α (PE, clone MAb11), and anti-CD3 (allophycocyanin-Cy7, clone HIT3a) Abs were purchased from Biolegend (San Diego, CA). Unlabeled anti-IgM (clone G20-127) was also purchased from BD Biosciences. F(ab′)2 preparations of goat anti-human Ig Abs for stimulations were obtained from Jackson Immunoresearch and CpG (ODN2006) from Invivogen (San Diego, CA). IgA preparations from colostrum were obtained from Sigma (St. Louis, MO) and heat-aggregated by incubating at 60°C for 30 min. Recombinant cytokines were purchased from Peprotech (Rocky Hill, NJ).
Generation of expression constructs and transfections
Full-length FCRL4 and the various FCRL4 mutants were modified with an N-terminal HA epitope tag and cloned into the pMX-PIE vector, which allows the expression of the gene of interest upstream of an internal ribosome entry site (IRES) sequence and the enhanced GFP marker (25). The LYN, HCK, and FGR src-family kinases were modified to express a C-terminal c-myc epitope tag. To generate the long isoform HCK p61, we replaced the naturally occurring atypical CTG translational initiation sequence (26) with a conventional ATG sequence for increased expression. For transient transfections in HEK293T cells, the wild-type (wt) and constitutively active mutants of the src-family kinases were cloned into the pIRESpuro2 vector backbone (Invitrogen, Carlsbad, CA). Site-directed mutagenesis to generate constitutively active mutants of the various src-family kinases was performed using standard molecular biology techniques with the following antisense oligonucleotides: LYN Y508F (5′-taagatctaggctgctgctggaattgcccttccg-3′), FGR Y523F (5′-taagatcttgtctgatccccgggctggaactgtggttc-3′), HCK p59 Y500F, and HCK p61 Y522F (5′-taagatcttggctgctgttggaactggctctctgt-3′). The PCR fragments were subsequently cloned into the pmyc-IRES vector, a construct based on the pIRESpuro2 vector designed to add a C-terminal c-myc epitope tag to the src-family kinases, and sequence fidelity was verified by DNA sequencing. For stable transfections, the wt src-family kinases were cloned into a modified version of the pLPCX vector in which the extracellular and transmembrane regions of murine CD4 were inserted downstream of an IRES element, thereby generating a cell-surface marker that allows for purification of cells stably expressing intracellular proteins. All expression constructs were verified by DNA sequencing. Transient transfections and retroviral transductions were performed as described previously (16). In brief, 750,000/well HEK293T cells were plated in a volume of 2 ml 24 h before transfection. The cells were transfected using a poly(ethyleneimine) (PEI) approach at a ratio of 3 μg PEI/1 μg plasmid DNA (27). At these low concentrations of PEI we did not observe toxic effects on HEK293T cells or BOSC23 cells (viability >98%). To use retroviral supernatants from the ecotropic BOSC23 packaging line to transduce human cell lines, we transiently transfected BJAB cells by electroporation (275 V, 975 μF) 36–48 h before viral transduction with a plasmid expressing the ecotropic receptor (mCAT1), thereby creating a brief window of opportunity for viral transduction with ecotropic viral particles. Expression of the various FCRL4 or src-family kinase constructs in BJAB cells did not alter the expression levels of the cell-surface Ag receptor (Supplemental Fig. 2).
Immunoprecipitations and Western blot analysis
Stimulations of BJAB cells with anti-Ig Abs or CpG were performed after starvation of the cells for 2–3 h in medium without FBS, supplemented with 20 mM HEPES, pH 7.4. Ag receptor cross-linkage was performed either with anti-IgM, thereby not engaging IgA-coated FCRL4, or with Ab preparations recognizing IgA, IgM, and IgG (IgA/M/G), leading to coligation of IgM on the surface of BJAB cells and IgA-coated FCRL4. For each experiment, 3 × 106 cells were subjected to stimulation. Western blot analysis was performed as described earlier (16, 25, 28). In brief, the cells were pelleted by centrifugation and lysed in Nonidet P-40 lysis buffer (1% Nonidet P-40, 150 mM NaCl, 5 mM EDTA, 50 mM Tris, pH 7.5) in the presence of protease and phosphatase inhibitors (leupeptin 5 μg/ml, pepstatin 1 μg/ml, aprotinin 5 μg/ml, soybean trypsin inhibitor 10 μg/ml, PMSF 40 μg/ml, Na3VO4 0.2 mM, Na2MoO4 1 mM, β-glycerophosphate 10 μM). The cells were lysed for 10 min on ice, centrifuged at 20,000 × g for 10 min at 4°C, and the supernatants were subjected to immunoprecipitation using 2 μg anti-HA Abs and 30 μl of a 50% slurry of protein G Sepharose (GE Healthcare, Pittsburgh, PA). Alternatively, the supernatants were prepared for analyses of whole-cell lysates by addition of 5× sample loading buffer (final concentration 2% SDS, 10% glycerol, 40 mM Tris pH 6.8). Subsequently, the cell lysates and immunoprecipitates were resolved by SDS-PAGE and transferred onto nitrocellulose membranes (Pall, Port Washington, NY). The membranes were blocked in 5% BSA in TBS/1%Tween 20 (TBS-T) for 3 h and incubated with primary Abs overnight at 4°C in 1% BSA in TBS-T, followed by incubation with secondary Abs for 45 min at room temperature. Signals were visualized using ECL reagent (Millipore, Billerica, MA) and a BioRad imaging system (BioRad, Hercules, CA).
Metabolic labeling of cells with [3H]palmitic acid
BJAB cells expressing FCRL4 wt or FCRL4 C410A were incubated with 0.5 mCi [9,10(n)-3H] palmitic acid (Amersham Pharmacia Biotech, Little Chalfont, U.K.) for 4 h at 37°C in the presence of 10% dialyzed FBS before lysis. The cells were lysed as described earlier, and the cell lysates were subjected to immunoprecipitation using anti-HA tag Abs. Eighty percent of the immunoprecipitates were separated by SDS-PAGE on a 7.5% acrylamide gel, followed by treatment with EN3HANCE solution (Perkin Elmer, Waltham, MA) and subsequent autoradiography. The remaining 20% of the samples was separated by SDS-PAGE, and immunoprecipitated FCRL4 proteins were detected by anti-HA epitope tag Western blotting as described earlier. Palmitoylation of FCRL4 was assessed in two independently performed metabolic labeling experiments.
Density gradient centrifugation assays
Density gradient centrifugation was performed as described previously (29). In brief, 1.5 × 107 cells were stimulated as indicated followed by lysis for 30 min on ice in 600 μl TNE buffer (1% Triton X-100, 150 mM NaCl, 5 mM EDTA, 10 mM Tris, pH 7.5) supplemented with leupeptin (5 μg/ml), aprotinin (5 μg/ml), soybean trypsin inhibitor (10 μg/ml), and Na3VO4 (0.2 mM). The cell lysates were treated with 10 strokes of a Dounce homogenizer with a B-type pestle, followed by centrifugation for 10 min at 900 × g at 4°C. The supernatants were mixed with an equal volume of 85% sucrose in TNE, transferred to an ultracentrifugation tube, and overlaid with 2 ml of 35% sucrose in TNE and 1 ml of 5% sucrose in TNE. Subsequently, the samples were subjected to ultracentrifugation for 18 h at 230,000 × g. After centrifugation, the samples were removed in 11 aliquots of 400 μl from the top of the ultracentrifugation tubes, mixed with 5× SDS sample loading buffer, and analyzed by SDS-PAGE and Western blotting with anti-HA tag Abs, anti-LYN Abs, and anti-CD45 Abs. Lipid raft localized proteins were typically detected in fractions 3 and 4 from the top of the gradient.
Immunofluorescence microscopy and data quantification/analysis
Lipid rafts were visualized using 30 μg/ml Alexa 555–conjugated cholera toxin subunit B (CTB; Life Technologies) in RPMI 1640 for 30 min on ice and then washed two times with PBS. Cells were then incubated with 20 μg/ml heat-aggregated IgA for 20 min, washed with PBS, and subsequently stimulated with 20 μg/ml F(ab′)2 goat anti-human IgA/M/G at 37°C for 6.5 min. To stop the stimulation, we fixed cells with 4% PFA for 15 min at 37°C, washed, and dispensed into a Lab-Tek II chamber (Nalge Nunc International). Images were captured using a WaveFX-X1 spinning disc confocal microscope (Quorum Technologies) equipped with a 63× oil-immersion objective and an EM-CCD camera (Hamamatsu Photonics). Approximately 0.2- to 0.3-μm z-stacks comprising sequential x-y sections ± 1.5 μm from the center plane of the cells (3 μm total) were acquired for at least 50 cells for each condition. Images were processed and analyzed using Volocity software (Improvision) and ImageJ (National Institutes of Health). Surface colocalization between lipid rafts and GFP-FCRL4 was assessed in extended focus z-stack images from a 3-μm-thick central section of the cell. Cell surface was manually selected by drawing around the cell membrane using Volocity, and Pearson’s correlation coefficient was calculated for each cell. Background intensity was set as a minimum threshold for each input channel for calculation of correlation coefficient. In total, ∼400 cells for each condition were pooled for colocalization coefficient analysis from three independent experiments. Two-tailed t tests were performed on data using Prism 6 (GraphPad Software).
Reporter gene expression assays
Elk-1 activity was assessed using the PathDetect system (Stratagene, La Jolla, CA) at a transfection ratio of 1:2 of the reporter gene expression plasmids Gal-4/Elk and 5xGal4/luciferase. NF-κB activity was evaluated using a luciferase reporter gene under control of five NF-κB binding sites. BJAB cells (5 × 106) were transiently transfected with 5 μg of the indicated plasmids by electroporation using a BioRad Gene Pulser Xcell instrument (275 V, 975 μF). Twenty-four hours after transfection, the cells were incubated with 20 μg/ml heat-aggregated IgA, washed with PBS, and stimulated as indicated for an additional 24 h before harvesting. Reporter gene activity was determined using the Dual-Luciferase reporter assay system (Promega, Madison, WI) according to the manufacturer’s instructions and normalized to reporter activities from 0.5 μg cotransfected plasmids encoding Renilla luciferase under control of the TK-promoter.
Flow-cytometry and intracellular cytokine staining
Peripheral blood was obtained from healthy donors and heparinized. The study was approved by the Ethics Review Board of the University of Toronto in accordance with the Declaration of Helsinki, and all participants gave written, informed consent. PBMCs were isolated by density gradient centrifugation over lymphocyte separation medium for 20 min at 750 × g. PBMCs (5 × 106 cells) were transfected with 5 μg of the indicated FCRL4 and src-family kinase expression plasmids at a ratio of 2:1 by electroporation using the B cell nucleofection system (LONZA, Allendale, NJ). Transfection efficiencies of PBMCs were typically between 20 and 40%. After transfection, the cells were cultured in the presence of 200 μg/ml CD40L and 50 ng/ml IL-2 for 20 h before addition of CpG (5 μg/ml) or an equal volume of PBS as negative control for an additional 48 h. Five hours before harvesting, the cells were treated with 50 ng/ml PMA, 1 μg/ml ionomycin, and 1:1000 dilution of brefeldin A solution followed by fixation/permeabilization with Cytofix/Cytoperm solution according to the manufacturer’s manual (BD Biosciences, San Jose, CA). Transfected cells were identified by expression of mCD4. Frequencies of mCD4+ Bmem cells ranged from 10 to 15% of total Bmem cells. Cell data were acquired using a BD Canto flow cytometer, and the data were analyzed using the FlowJo software package (Ashland, OR).
Results
Src-family kinases mediate phosphorylation of FCRL4
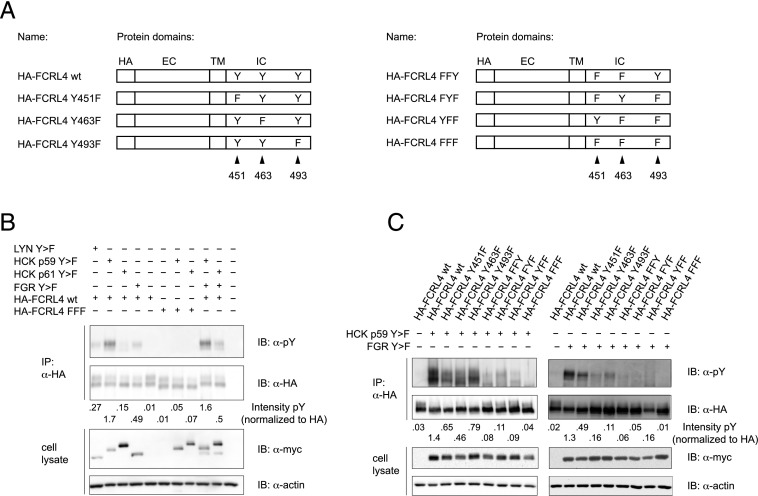
Because increased levels of expression of the HCK and FGR src-family kinases were found in FCRL4+ Bmem cells, we investigated the potential involvement of the HCK (p59 and p61 isoforms), FGR, and LYN kinases in the phosphorylation of the intracellular domain of FCRL4. Cotransfection of constitutively active HCK p59 Y > F with FCRL4 in HEK293T cells resulted in a strong tyrosine phosphorylation signal in FCRL4 immunoprecipitates. Moderate tyrosine phosphorylation was also detected after cotransfection of constitutively active FGR Y > F or LYN Y > F with FCRL4, whereas HCK p61 Y > F cotransfection had only marginal effect on tyrosine phosphorylation of FCRL4 (Fig. 1B). As expected, tyrosine phosphorylation could not be detected upon cotransfection of src-kinases with the phosphorylation-deficient FCRL4-FFF mutant, in which the tyrosine residues in the ITIM sequences are mutated to phenylalanines. Phosphorylation of FCRL4 in response to cotransfection of constitutively active FGR and HCK p59 did not differ from that seen with single transfection of HCK p59, nor did cotransfection of constitutively active FGR with HCK p61 differ from the effects of FGR alone. To further investigate the phosphorylation of FCRL4 in response to constitutively active HCK p59 or FGR, we generated single or double tyrosine to phenylalanine mutants (Fig. 1A) and evaluated their phosphorylation status after cotransfection with the src-family kinases. As expected, we observed the strongest tyrosine phosphorylation signals in experiments in which HA-FCRL4 wt was cotransfected with the active src-kinases and reduced signals in experiments involving the single and double tyrosine-to-phenylalanine mutants, and the FCRL4-FFF mutant was devoid of any phosphotyrosine signal (Fig. 1C). Interestingly, the HA-FCRL4 Y493F mutant consistently showed stronger phospho-tyrosine signals in the context of cotransfection with HCK p59 Y > F, whereas the HA-FCRL4 Y451F mutant yielded stronger phosphotyrosine signals in cotransfection experiments with FGR Y > F. These results demonstrate that FCRL4 can be differentially phosphorylated by distinct src-kinase family members.
FIGURE 1.
HCK- and FGR-induced phosphorylation of FCRL4. (A) Graphic representation of HA-epitope tagged FCRL4 with introduced point mutations. Positions of the tyrosine residues of ITIM sequences are indicated. (B) Phosphorylation of FCRL4 after cotransfection with constitutively active myc-epitope tagged src-family kinases. FCRL4 wt or FCRL4 FFF was cotransfected with the indicated src-family kinases in HEK293T cells. The cell lysates were subjected to immunoprecipitation with anti-HA Abs, and the cell lysates and immunoprecipitates were analyzed by Western blotting using anti-phosphotyrosine and anti-myc Abs. Equal loading was verified by reprobing the membranes with anti-HA and anti-actin Abs, respectively. A representative of five independently performed experiments is shown. The degree of tyrosine phosphorylation of FCRL4 was assessed by densitometry and is shown normalized to the signal obtained after reprobing of the membranes with anti-HA Abs. (C) Phosphorylation of FCRL4 wt and the indicated FCRL4 mutants in response to cotransfection with constitutively active HCK p59 Y > F or FGR Y > F. The transfected cells were lysed and FCRL4 proteins were immunoprecipitated and analyzed for tyrosine phosphorylation as in (B). A representative of five independently performed experiments is shown.
Binding of aggregated IgA to cells expressing FCRL4
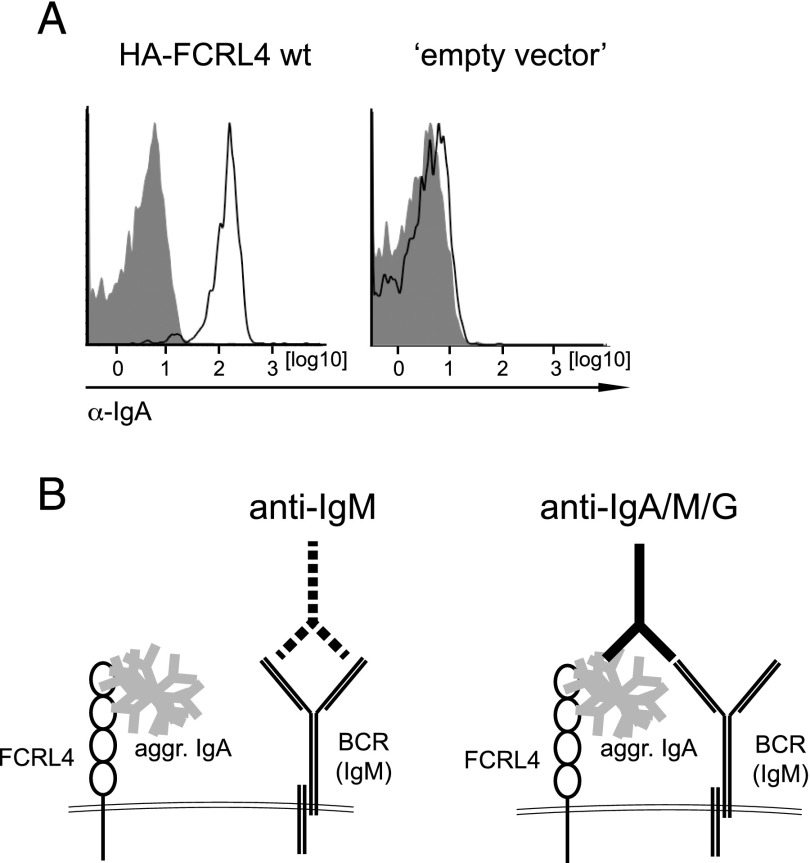
To further investigate the roles of these src-kinase family members in FCRL4-mediated immune regulation, we took advantage of the recently reported IgA-binding activity of FCRL4 (30). We confirmed binding of heat-aggregated human IgA to the Burkitt’s lymphoma BJAB cell line expressing FCRL4 (Fig. 2A). This allowed us to establish an experimental system in which BJAB cells expressing the various FCRL4 mutants were preincubated with heat-aggregated IgA followed by stimulation with IgM-specific Igs (anti-IgM) or Igs reactive with IgA, IgM, and IgG isotypes (anti-IgA/M/G), thereby triggering an Ag receptor signal with or without coengaged FCRL4 (Fig. 2B).
FIGURE 2.
IgA binding by BJAB cells expressing FCRL4. (A) BJAB cells expressing HA-FCRL4 wt or empty vector control cells were incubated with heat-aggregated IgA (open histograms) or left untreated (filled histograms) followed by incubation with anti-human IgA Abs and analysis by flow cytometry. A representative of four independent experiments is shown. (B) Experimental system to analyze the effects of full-length FCRL4 on Ag receptor engagement. Treatment of BJAB cells with aggregated IgA (gray) followed by stimulation with anti-IgM (dotted lines) Abs leads to engagement of the Ag receptor on BJAB cells without coengagement of FCRL4 (left). Stimulation of these cells with anti–Ig Abs (solid lines) reactive with IgA, IgM, and IgG (anti-IgA/M/G) leads to coengagement of FCRL4 (right).
Posttranslational modification of the intracellular domain of FCRL4
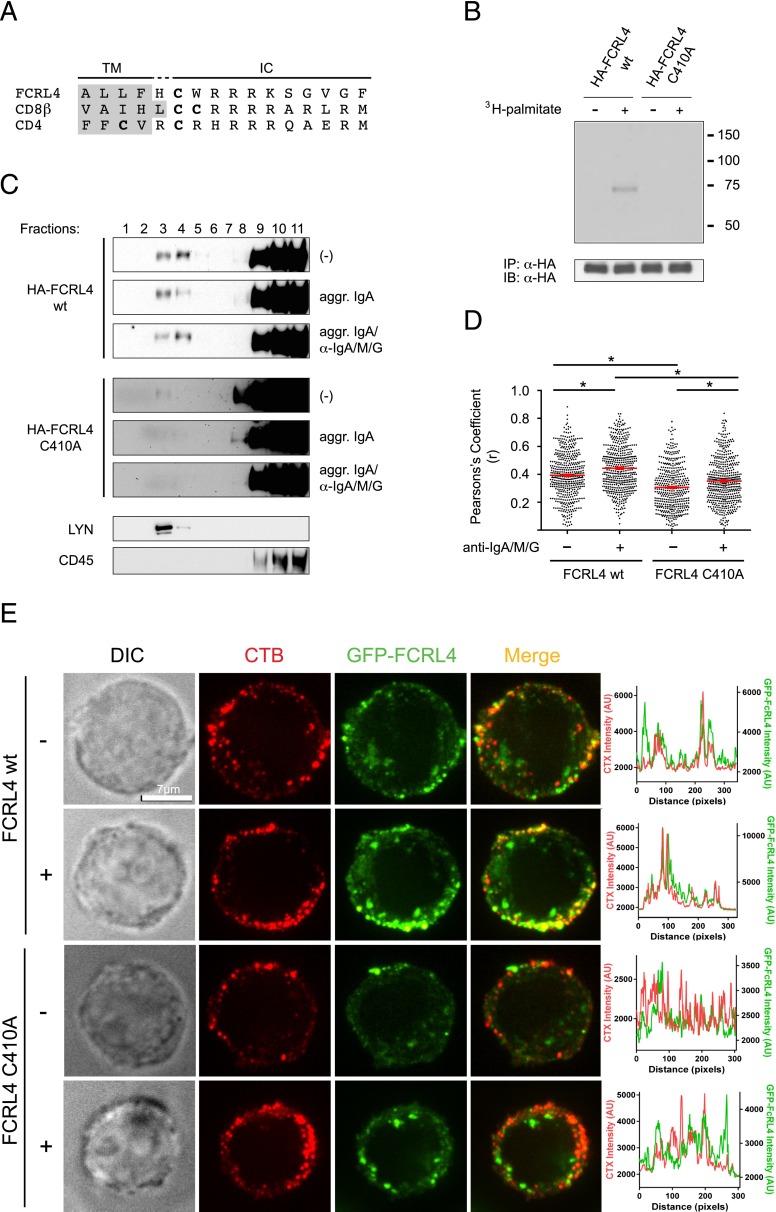
The intracellular domain of FCRL4 contains a cysteine residue at position 410 in a sequence environment similar to cysteine residues in the CD4 and CD8β coreceptors, which are targets for posttranslational palmitoylation (31–34) (Fig. 3A). We therefore generated a predicted palmitoylation-deficient mutant of FCRL4 (HA-FCRL4 C410A) by mutating cysteine 410 to alanine to determine whether cysteine 410 of the FCRL4 molecule could serve as acceptor site for palmitic acid. Metabolic labeling experiments showed that FCRL4 wt, but not the FCRL4 C410A mutant, incorporated [3H]-labeled palmitic acid (Fig. 3B), thereby implicating palmitoylation as a potential immunoregulatory factor affecting FCRL4 activity.
FIGURE 3.
Palmitoylation and lipid raft localization of FCRL4 wt. (A) Sequence analysis of the membrane-proximal regions of the intracellular domains of FCRL4, CD8β, and CD4. Cysteine residues that are targets for palmitoylation in CD4 and CD8β or are a predicted target for palmitoylation in FCRL4 are shown in bold. Residues that form part of the transmembrane domains are shaded in gray. (B) BJAB cells expressing FCRL4 wt or FCRL4 C410A were incubated with or without [3H]palmitic acid before cell lysis and immunoprecipitation with anti-HA epitope tag Abs. The immunoprecipitates were separated on SDS-PAGE followed by detection of incorporated [3H]palmitic acid by autoradiography (top) using 80% of the samples. The remaining 20% of the immunoprecipitates was probed for FCRL4 protein using anti-HA epitope tag Abs (bottom). (C) Lipid raft localization of FCRL4. HA-FCRL4 wt and HA-FCRL4 C410A–expressing cells were treated as indicated before cell lysis and sucrose density centrifugation, and the location of the FCRL4 proteins was determined by Western blotting with anti-HA Abs (top and middle panels). Separation of detergent soluble and insoluble fractions was demonstrated by probing of the membranes with anti-CD45 and anti-LYN Abs, respectively (bottom panel). A representative of six independently performed experiments is shown. (D and E) Preferential colocalization of FCRL4 wt with CTB. BJAB cells expressing FCRL4 wt-GFP or FCRL4 C410A-GFP were stained with CTB and analyzed for colocalization (D) or cocapping (E) of the fluorescent signals. Cells were treated with aggregated IgA and stimulated with anti-IgA/M/G where indicated. Statistical significance was determined using a two-tailed Student t test. Statistical significance is indicated by an asterisk (*p < 0.05).
Translocation of cell-surface receptors after receptor engagement into ordered membrane microdomains resistant to anionic detergents (lipid rafts) has been reported for multiple receptor systems, including the B cell Ag receptor (35). Furthermore, palmitoylation of cell-surface receptors is also recognized as a mechanism supporting lipid raft localization (36, 37). We therefore performed density gradient centrifugation experiments to investigate the potential of FCRL4 to localize to lipid rafts and found a fraction of HA-FCRL4 wt in detergent insoluble fractions (Fig. 3C, top panels). Using this experimental approach, we did not detect quantitative differences of FCRL4 localized in detergent insoluble fractions when comparing cells without IgA preincubation, with IgA preincubation or with IgA preincubation followed by Ag receptor engagement. In contrast, we detected only minute quantities of HA-FCRL4 C410A in detergent insoluble fractions after extended exposure of the membranes (Fig. 3C, middle panels). We used Abs to endogenous CD45 and LYN to determine the fractions containing detergent soluble and detergent insoluble proteins (Fig. 3C, bottom panels). To independently validate these results, we used BJAB cells expressing fusion proteins consisting of the GFP fused to either FCRL4 wt or FCRL4 C410A and used immunofluorescence microscopy to evaluate colocalization of FCRL4 with the fluorescently labeled lipid raft marker CTB. This approach also allowed us to quantitate potential lipid raft translocation of FCRL4 after coligation with the Ag receptor. As expected, we found that FCRL4 wt colocalized with CTB at a higher frequency than the FCRL4 C410A palmitoylation-deficient mutant (Fig. 3D, 3E). We further determined that the frequencies of FCRL4 wt and FCRL4 C410A colocalization with CTB increased after coengagement of FCRL4 with the Ag receptor. These experiments indicate that palmitoylation and lipid raft localization may represent a regulatory factor in the immunomodulation mediated by FCRL4.
Preferential phosphorylation of FCRL4 wt but not FCRL4 C410A after coengagement with the B cell Ag receptor
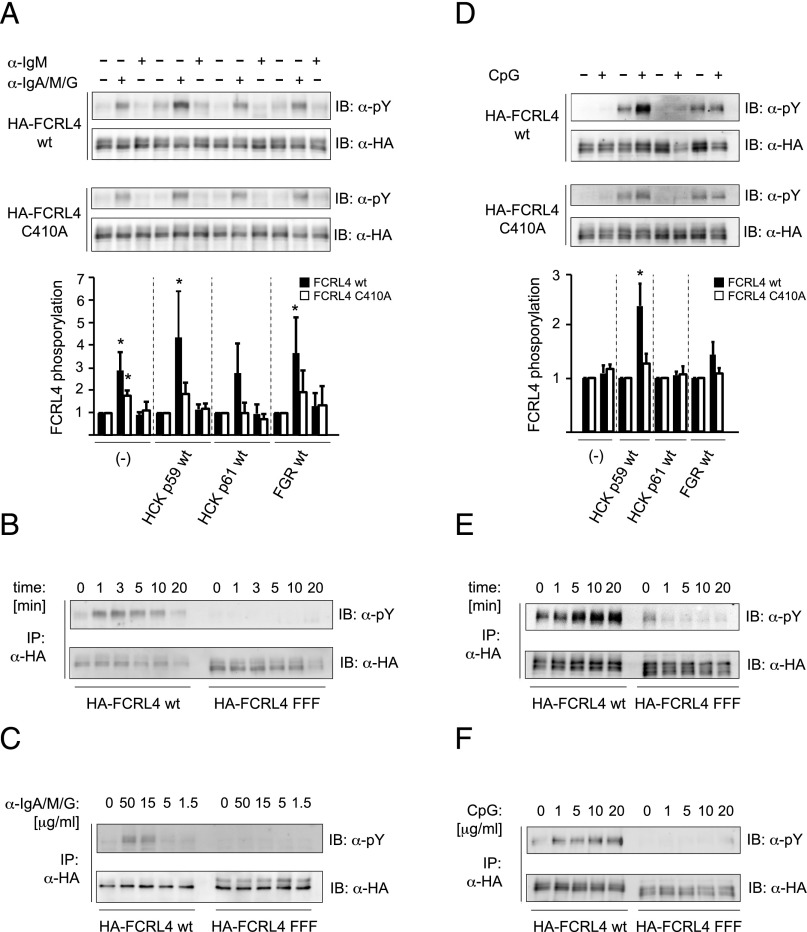
To address the effects of the FGR and HCK kinases on FCRL4-mediated B cell regulation, we expressed epitope-tagged FGR wt, HCK p59 wt, or HCK p61 wt together with HA-FCRL4 wt, the palmitoylation-deficient HA-FCRL4 C410A mutant, or the HA-FCRL4 FFF mutant in the BJAB Burkitt’s lymphoma cell line (Supplemental Fig. 1). Quantitative RT-PCR determined that FCRL4 expression had no influence on the endogenous levels of these src-family kinases and LYN; similarly, stable expression of the src-family kinases did not induce expression of endogenous FCRL4 (data not shown). We observed slightly increased background phosphorylation in cells expressing HCK p59 or FGR (Fig. 4A, top panel). Treatment of cells with aggregated IgA, which is expected to cross-link the FCRL4 molecule, did not result in increased tyrosine phosphorylation in the absence of anti-IgA/M/G treatment (data not shown). When the various cell types were preincubated with heat-aggregated IgA before BCR ligation with anti-IgM Abs or anti-IgA/M/G Abs, densitometric analyses of phosphorylation of FCRL4 and normalization to the signals obtained from unstimulated controls revealed statistical significance only after anti-IgA/M/G treatment (Fig. 4A). In experiments with cells expressing HCK p61, the level of tyrosine phosphorylation, although detectable, did not reach statistical significance (Fig. 4A). The reduced FCRL4 phosphorylation is in agreement with the low phosphorylation levels observed in transient transfection assays using constitutively active HCK p61 Y > F; however, lower levels of expression of HCK p61 wt in BJAB cells (Supplemental Fig. 1C) could contribute to this result. Although we detected tyrosine phosphorylation of FCRL4 C410A, the degree of phosphorylation of the palmitoylation-deficient mutant of FCRL4 did not reach statistical significance (Fig. 4A, bottom panel). Phosphorylation of FCRL4 in HCK p59 cells occurred rapidly after coligation (Fig. 4B) and required Ab concentration >5 μg/ml (Fig. 4C). As expected, no tyrosine phosphorylation signal could be detected with the FCRL4 FFF mutant, indicating specificity of the assays.
FIGURE 4.
Phosphorylation of FCRL4 wt and FCRL4 C410A in response to Ag receptor ligation and TLR9 stimulation. FCRL4 wt or FCRL4 C410 BJAB cells coexpressing the indicated wt src-family kinases were pretreated with heat-aggregated IgA (A–C) or left untreated (D–F). Time courses (B and E) and titrations (C and F) were performed with HCK p59–expressing cells. After pretreatment with IgA, the cells were then stimulated with 15 μg/ml anti-IgM or anti-IgA/M/G for 3 min (A), with 15 μg/ml anti-IgA/M/G for the indicated time points (B), and for 3 min with the indicated concentrations of anti-IgA/M/G (C). The cell lysates were subjected to immunoprecipitation with anti-HA Abs and analyzed with anti-phosphotyrosine Abs. Membranes were reprobed with anti-HA tag Abs to ascertain equal loading. The degree of FCRL4 phosphorylation was assessed by densitometry and was normalized to the signal obtained by reprobing of the membranes with anti-HA Abs, and is displayed as “fold induction” over the corresponding unstimulated control. Bar diagrams show mean ± SD (n = 4 for anti-IgA/M/G stimulation, n = 3 for CpG stimulation), and statistical significance (one-sample Student t test) is indicated by an asterisk (*p < 0.05). Experiments with cells expressing the FCRL4-FFF mutant were included as specificity controls for intracellular tyrosine phosphorylation of FCRL4. TLR9 stimulations of BJAB cells expressing FCRL4 wt or FCRL4 C410A and the indicated src-family kinases were performed by stimulating cells with 10 μg/ml CpG for 10 min (D), with 10 μg/ml CpG for the indicated time points (E), and for 10 min with the indicated CpG concentrations (F). Negative controls received an equal volume of PBS. The cell lysates were subjected to immunoprecipitation with anti-HA Abs, analyzed with anti-phosphotyrosine Abs, and the membranes were reprobed with anti-HA tag Abs to ascertain equal loading as described for Ag receptor stimulations.
A recent study by Sohn et al. (24) implicated FCRL4 as an immunoregulatory factor positioned at the interface of innate and adaptive immune signaling. Similar to our observation of FCRL4 phosphorylation after Ag receptor ligation, we observed increased phosphorylation of the intracellular domain of FCRL4 in response to CpG stimulation of HCK p59-expressing cells (Fig. 4D, top panel); the observed phosphorylation was greatly decreased in cells with the FCRL4 C410A palmitoylation-deficient mutant (Fig. 4D, bottom panel). Unlike the observed FCRL4 phosphorylation at early time points in the response to Ag receptor ligation, FCRL4 phosphorylation was most prominent at later time points after TLR9 stimulation (Fig. 4E) and could be detected with CpG concentrations of 5 μg/ml (Fig. 4F). These results are in accordance with FCRL4 phosphorylation observed in response to transfection of constitutively active src-family kinases. They support a role of HCK in FCRL4-mediated immune regulation and indicate palmitoylation as a factor impacting FCRL4 function.
Regulatory effects of FCRL4 on Elk-1– and NF-κB–mediated reporter gene activation in HCK p59– and FGR-expressing cells
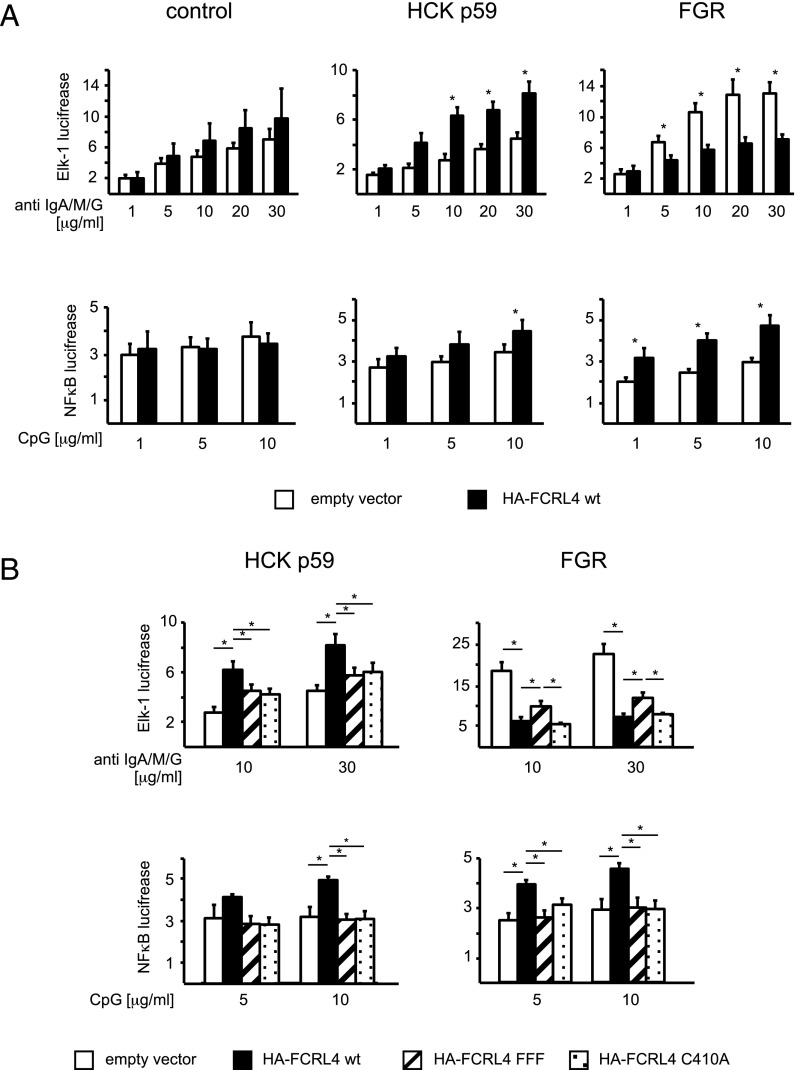
To investigate the potential effects of FCRL4 on MAPK activation by BCR ligation, we used the luciferase reporter gene driven by the ETS-family Elk-1 transcription factor, a target for phosphorylation by activated MAPKs (38). These experiments were performed in cells expressing FGR or HCK p59 in the context of anti-IgA/M/G treatment because we observed significant phosphorylation of the intracellular domain of FCRL4 under these conditions. Unexpectedly, we found that anti-IgA/M/G stimulation of BJAB transfectants expressing HCK p59 resulted in a significant increase of Elk-1 reporter gene activity (Fig. 5A, top row). In contrast, BCR ligation in cells expressing FCRL4 and FGR resulted in decreased Elk-1 reporter gene activity (Fig. 5A, top row). These effects were not seen in FCRL4- and HCK p59–expressing cells when the FCRL4 wt was replaced by the FCRL4-FFF mutant or the palmitoylation-deficient FCRL4 C410A mutant (Fig. 5B, top row). The reduced reporter gene activity observed in FCRL4 and FGR coexpressing cells could be partially re-established in cells expressing the FCRL4-FFF mutant, but not in cells expressing the FCRL4-C410A mutant (Fig. 5B, top row), indicating that additional factors may contribute to the reduced luciferase activity in this experimental system. We did not observe significant induction of Elk-1–mediated reporter gene activity in response to CpG stimulation in BJAB cells. Similarly, in subsequent experiments, we did not observe significant NF-κB reporter gene activity after Ag receptor ligation (data not shown).
FIGURE 5.
Modulation of reporter gene activation in BJAB cells expressing FCRL4 and the HCK p59 or FGR src-family kinases. (A) BJAB cells expressing FCRL4 wt (filled bars) or “empty vector” control cells (open bars) in the absence of exogenous wt src-family kinase (left panel), or in the presence of HCK p59 (middle panel) or FGR (right panel) were transfected with the firefly luciferase reporter gene under control of the Elk-1 promoter (top row) or under control of an NF-κB–inducible promoter (bottom row) and the Renilla luciferase reporter gene under control of the TK promoter for normalization. For Ag receptor ligations, the cells were pretreated with heat-aggregated IgA. The cells were stimulated for 24 h with the indicated concentrations of anti-IgA/M/G Abs (top row) or CpG (bottom row). (B) HCK p59 and FGR cells expressing “empty vector” (open bars), FCRL4 wt (closed bars), FCRL4 C410A (hatched bars), or FCRL4 FFF (dotted bars) were transfected with the firefly luciferase reporter gene under control of the Elk-1 promoter (top row) or under control of an NF-κB–inducible promoter (bottom row) and the Renilla luciferase reporter gene under control of the TK promoter and stimulated as in (A). Reporter gene activity was normalized to the cotransfected Renilla luciferase reporter gene activity. Values are displayed as mean ± SD from at least 11 independently performed experiments. Statistical significance was assessed using the two-tailed Student t test. *p < 0.05.
To investigate potential effects of FCRL4 on TLR signaling, we transfected cells with plasmids encoding the luciferase reporter gene under the control of the NF-κB transcription factor. FCRL4 expression in HCK p59 transfectants and in FGR transfectants resulted in enhanced NF-κB reporter gene activity (Fig. 5A, bottom row). In both cases, the enhancement was abolished when FCRL4 wt was replaced with the FCRL4-FFF mutant or the palmitoylation-deficient FCRL4 C410A mutant (Fig. 5B, bottom row). The enhancement of NF-κB reporter gene activity by FCRL4 is independent of IgA binding because we observed the same effect on NF-κB activation in cells subjected to pretreatment with aggregated IgA before CpG stimulation (data not shown). These findings indicate a potential regulatory role for FCRL4 in TLR9-mediated signaling.
FCRL4 and HCK p59 or FGR promote expression of IL-10 and IFN-γ in human Bmem cells
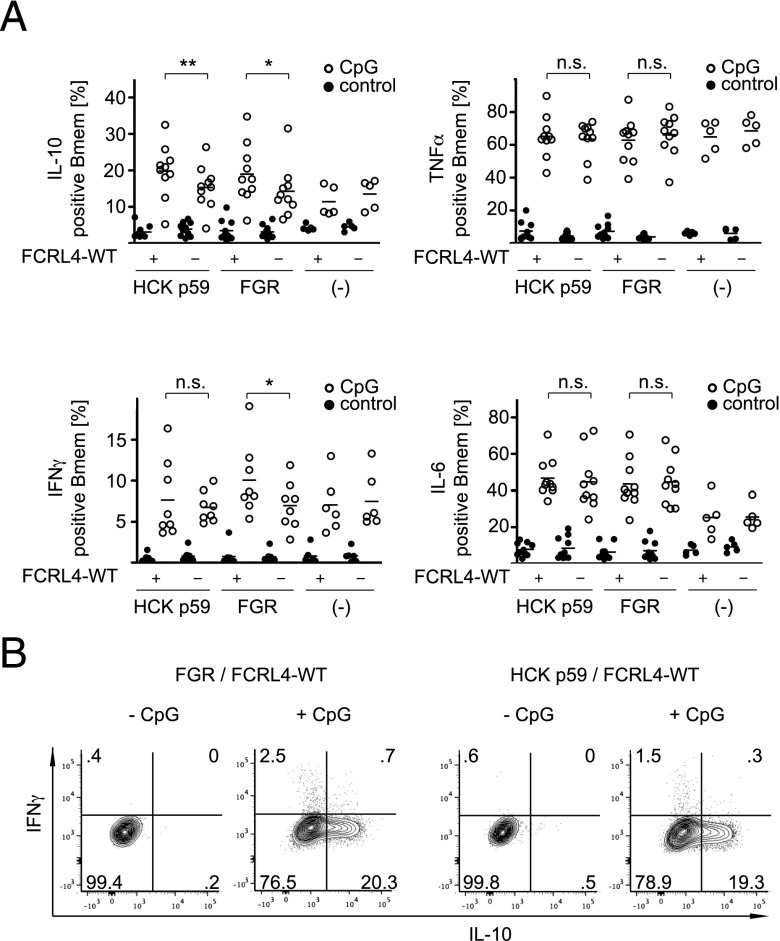
The positive regulatory effect of FCRL4 on the NF-κB reporter gene activity prompted us to investigate the role of FCRL4 on TLR9-mediated responses in primary human B cells. To this end, we transiently transfected PBMCs with FCRL4 and the HCK p59 and FGR kinases, and evaluated the induction of the cytokines IL-10, IL-6, and TNF-α. Expression of these cytokines by human B cells in response to CpG is well documented (39–41). In cotransfection experiments of FCRL4 wt with wt HCK p59 or wt FGR, we observed a significant increase of IL-10 production in the Bmem cell compartment (Fig. 6A), but not in naive B cells (data not shown). Ex vivo culture of PBMCs in the presence of IL-2 and CD40L did not lead to induction of endogenous FCRL4 (data not shown). The IL-10 signal obtained for HCK p59 transfectants was frequently stronger than the IL-10 signal in FGR transfectants, although this difference did not reach statistical significance. In contrast, FCRL4 transfection did not affect CpG-mediated induction of IL-6 or TNF-α in memory or naive B cell compartments. The increased levels of IL-10 in cells expressing FCRL4 wt together with HCK p59 or FGR led us to investigate whether the regulation of additional B cell effector molecules was affected. Using cDNA from primary human B cells cotransfected with FCRL4 wt and HCK p59 or FGR to survey a panel of transcripts modulated by IL-10 revealed the induction of IFN-γ by CpG in cells expressing FGR and FCRL4 wt (data not shown). Flow-cytometric analysis of transfected CpG-stimulated B cells confirmed increased IFN-γ production by FCRL4 wt and FGR-expressing cells (Fig. 6A). In contrast with the induction of IL-10, modulation of IFN-γ production by FCRL4 in HCK p59–expressing cells did not reach statistically significant levels. Furthermore, we noticed that Bmem cells transfected with FGR and FCRL4 wt induced IFN-γ and IL-10 expression in a mutually exclusive manner (Fig. 6B). These results are consistent with our earlier observation of enhanced NF-κB reporter gene activity in BJAB transfectants and indicate that FCRL4 expression may have distinct influences on immune responses dependent on expression or activation levels of the FGR and HCK src-family kinases.
FIGURE 6.
Modulation of cytokine secretion by Bmem cells. (A) Transiently transfected PBMCs with HCK p59 or FGR in the presence of FCRL4 wt or “empty vector” control were stimulated with CpG at 5 μg/ml or PBS as negative control for 48 h, followed by intracellular staining of Bmem cells for IL-10, TNF-α, IFN-γ, and IL-6, and assessment of changes in the percentage of cytokine-expressing cells. Bmem gating was set on CD3−/CD20+/CD27+ lymphocytes. Between 6 and 10 independent samples were cotransfected with plasmids encoding FCRL4 wt and the indicated src-family members (identified as mCD4+, as shown in Supplemental Fig. 1D). Statistically significant differences for CpG-induced cytokine expression in the presence or absence of FCRL4 wt are indicated by asterisks (*p < 0.05, **p < 0.01) and were determined using the paired Student t test. (B) Mutually exclusive pattern of expression of IL-10 and IFN-γ in transfected Bmem cells.
Discussion
FCRL4 expression defines a distinctive population of Bmem cells in MALTs. Gene expression profiling indicated that these cells express increased levels of the src-family kinases HCK and FGR, thus raising the possibility that these kinases are involved in FCRL4-mediated immunoregulation. Although FGR and HCK are typically regarded as src-family members involved in the regulation of myeloid lineage cells, expression of HCK and FGR in human B lineage cells has been reported (42–44). The results presented in this article indicate that FCRL4, after binding to aggregated IgA, exerts highly flexible modulation of Ag receptor signaling in the context of distinct src-family kinases, specifically HCK p59 or FGR. In addition, we showed that FCRL4 augments CpG-induced NF-κB activation and IL-10 production in the presence of HCK p59 or FGR. In contrast, increased levels of IFN-γ could be observed only in the presence of FGR and FCRL4 wt.
The finding that FCRL4 expression in HCK p59-expressing cells results in increased Elk-1 reporter gene activity in response to Ag receptor ligation was unexpected. In earlier experiments, we had shown that coligation of the BCR with a fusion protein consisting of the extracellular and transmembrane domains of CD32 and the intracellular domain of FCRL4 with the BCR resulted in strong inhibition of Ag receptor signaling in the A20-IIA1.6 mouse Bmem cell line (16). However, our initial analysis using fusion proteins did not allow us to monitor any effects of the transmembrane region of FCRL4 or of the membrane proximal cysteine 410. Similarly, Sohn et al. (24) reported inhibitory activity of FCRL4 in response to Ag receptor ligation in the R2G6 cell line, a subline of Ramos B cells. Expression of FCRL4 in this model system also resulted in significant constitutive tyrosine phosphorylation of FCRL4 and its constitutive association with SHP-1 and SHP-2 phosphatases, and these characteristics might contribute to the observed discrepancy. It will be interesting to investigate the expression levels of FGR and HCK in this cell line and to evaluate BCR signal modulation in cells engineered to express increased levels of HCK p59.
The intracellular domain of FCRL4 contains three consensus ITIMs; however, the two membrane-proximal ITIMs could also form a potential ITAM upon dual phosphorylation. In support of this, a synthetic peptide spanning the two membrane-proximal ITIM sequences with phosphorylated tyrosine residues was able to bind to PLCγ (16), indicating a potential activating function. In our initial studies we noticed that tyrosine residue 451, located in the membrane-proximal ITIM of the intracellular domain of FCRL4, was not phosphorylated in response to Ag receptor ligation in A20-IIA1.6 cells (16). In contrast, we observed increased phosphorylation of the HA-FCRL4 Y493F mutant in the context of cotransfected HCK p59 when compared with the Y451F or Y463F mutants, suggestive of increased phosphorylation of the two membrane-proximal tyrosine residues. This raises the possibility that HCK p59 may be required for dual phosphorylation of the two membrane-proximal tyrosine residues of the intracellular domain of FCRL4 and that, dependent on specific src-family kinase activation, FCRL4 engagement may provide activating or inhibitory immunomodulatory signals. Our observations come from an experimental system based on IgA binding to FCRL4, allowing us to monitor FCRL4 phosphorylation and modulation of Ag receptor signaling. However, this system does not exclude the possible engagement of additional receptors by aggregated IgA distinct from FCRL4.
Regulation of TLR signaling by ITAM/ITIM-bearing receptors has been reported, and an incompletely understood mechanism involving src-family kinases has been suggested (45–47). Our observations of enhanced cytokine expression levels in FCRL4 wt transfected Bmem cells after CpG treatment are in agreement with observations of increased CD23 expression in R2G6 cells and primary human B cells in response to TLR9 engagement (24). Investigations into cytokine secretion by B cells have increased over the last decade and led to the recognition of regulatory B cell functions rooted in the modulation of proinflammatory and anti-inflammatory cytokine levels. The positioning of FCRL4+ Bmem cells at mucosal surfaces places them into an environment where they will readily come into contact with innate immune stimulators. In this regard, it is notable that we observed differential effects of FCRL4 on B cell cytokine expression that were dependent on the coexpressed FGR or HCK p59 src-family kinases. Induction of IFN-γ after CpG stimulation was stronger in FGR-transfected cells, whereas IL-10 induction was stronger in HCK p59–transfected cells; loss-of-function experiments targeting the individual src-family kinases in primary human Bmem cells will help to further delineate the function of HCK and FGR in FCRL4-mediated immunoregulation. Our previous work demonstrated that the unfractionated tonsillar FCRL4+ Bmem cells express high levels of HCK and FGR (22). This study also indicated that FCRL4+ Bmem cells are heterogenous with respect to other cell-surface markers such as CD27, CD11b, and CD23. It will be important to determine whether the HCK and FGR kinases are uniformly expressed in all FCRL4+ Bmem cells (and thus may require differential levels of activation of src-family kinases to lead to induction of IL-10 or IFN-γ) or whether distinct subpopulations of FCRL4-bearing Bmem cells express varying levels of FGR and HCK.
Palmitoylation of proteins is a reversible posttranslational modification that is implicated in regulation of intracellular trafficking, as well as in the partition of proteins in ordered microdomains of biomembranes (37, 48). Our finding that FCRL4 is subject to palmitoylation and that it localizes to lipid rafts reveals an additional level of regulation by which this receptor modulates B cell responses. This may affect the positive regulatory activity of FCRL4 on CpG stimulation in the context of FGR or HCK p59 expression, or on BCR signaling in the context of HCK p59 expression because the increase in the observed reporter gene activity was dependent on expression of a palmitoylation-proficient FCRL4. Palmitoylation of FCRL4 may therefore facilitate the colocalization of FCRL4 with FGR and HCK p59 src-family kinases, which are subject to the same posttranslational modification and are found in detergent insoluble fractions (49–51). In support of this possibility, it is noteworthy that we observed regulatory activity of FCRL4 in the context of FGR or HCK p59, but not HCK p61. The two HCK isoforms are generated by usage of an alternative translation start site and differ by 21 aa at the N terminus (26). Whereas the shorter HCK p59 isoform is palmitoylated and predominantly located at the plasma membrane, HCK p61 is nonpalmitoylated and found in endocytic compartments; generation of a palmitoylation-deficient mutant of HCK p59 led to colocalization of HCK p59 with HCK p61 on lysosomes (52–54).
In conclusion, we provide evidence for involvement of HCK and FGR in FCRL4-mediated immunoregulation and for the functional importance of posttranslational modifications of the FCRL4 molecule. The system we used for our studies, namely, pretreating cells with aggregated IgA as a surrogate for IgA immune complexes, is likely to reflect the condition of FCRL4 on Bmem cells in the mucosal microenvironment. Our results indicate that FCRL4+ Bmem cells are a functionally distinct subpopulation of Bmem cells and that FCRL4 is positioned to exert positive or negative regulatory roles for these cells in a context-dependent manner. We do not have sufficient information on the factors governing FCRL4 expression on only a subpopulation of Bmem cells. However, our findings underscore the complexity of human Bmem cells and suggest a key role for FCRL4 in the regulation of adaptive recall responses in the mucosa.
Supplementary Material
Acknowledgments
We thank Dr. Max D. Cooper (Emory University) for help determining palmitoylation of FCRL4 and for critical reading of the manuscript.
This work was supported by Canadian Institutes of Health Research Grants MOP-12614 and THA-11900 (to G.R.A.E.).
The online version of this article contains supplemental material.
- Bmem
- memory B
- CTB
- cholera toxin subunit B
- FCRL
- FcR-like
- FGR
- feline Gardner-Rasheed sarcoma viral oncogene homolog
- HCK
- hemopoietic cell kinase
- IRES
- internal ribosome entry site
- PEI
- poly(ethyleneimine)
- wt
- wild-type.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Ahmed R., Gray D. 1996. Immunological memory and protective immunity: understanding their relation. Science 272: 54–60. [DOI] [PubMed] [Google Scholar]
- 2.Sallusto F., Lanzavecchia A., Araki K., Ahmed R. 2010. From vaccines to memory and back. Immunity 33: 451–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bevan M. J. 2011. Understand memory, design better vaccines. Nat. Immunol. 12: 463–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klein U., Rajewsky K., Küppers R. 1998. Human immunoglobulin (Ig)M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J. Exp. Med. 188: 1679–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ehrhardt G. R., Hsu J. T., Gartland L., Leu C. M., Zhang S., Davis R. S., Cooper M. D. 2005. Expression of the immunoregulatory molecule FcRH4 defines a distinctive tissue-based population of memory B cells. J. Exp. Med. 202: 783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zuccarino-Catania G. V., Sadanand S., Weisel F. J., Tomayko M. M., Meng H., Kleinstein S. H., Good-Jacobson K. L., Shlomchik M. J. 2014. CD80 and PD-L2 define functionally distinct memory B cell subsets that are independent of antibody isotype. Nat. Immunol. 15: 631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ehrhardt G. R., Cooper M. D. 2011. Immunoregulatory roles for fc receptor-like molecules. Curr. Top. Microbiol. Immunol. 350: 89–104. [DOI] [PubMed] [Google Scholar]
- 8.Davis R. S. 2007. Fc receptor-like molecules. Annu. Rev. Immunol. 25: 525–560. [DOI] [PubMed] [Google Scholar]
- 9.Davis R. S., Ehrhardt G. R., Leu C. M., Hirano M., Cooper M. D. 2005. An extended family of Fc receptor relatives. Eur. J. Immunol. 35: 674–680. [DOI] [PubMed] [Google Scholar]
- 10.Davis R. S., Wang Y. H., Kubagawa H., Cooper M. D. 2001. Identification of a family of Fc receptor homologs with preferential B cell expression. Proc. Natl. Acad. Sci. USA 98: 9772–9777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hatzivassiliou G., Miller I., Takizawa J., Palanisamy N., Rao P. H., Iida S., Tagawa S., Taniwaki M., Russo J., Neri A., et al. 2001. IRTA1 and IRTA2, novel immunoglobulin superfamily receptors expressed in B cells and involved in chromosome 1q21 abnormalities in B cell malignancy. Immunity 14: 277–289. [DOI] [PubMed] [Google Scholar]
- 12.Moir S., Ho J., Malaspina A., Wang W., DiPoto A. C., O’Shea M. A., Roby G., Kottilil S., Arthos J., Proschan M. A., et al. 2008. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J. Exp. Med. 205: 1797–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weiss G. E., Crompton P. D., Li S., Walsh L. A., Moir S., Traore B., Kayentao K., Ongoiba A., Doumbo O. K., Pierce S. K. 2009. Atypical memory B cells are greatly expanded in individuals living in a malaria-endemic area. J. Immunol. 183: 2176–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kardava L., Moir S., Wang W., Ho J., Buckner C. M., Posada J. G., O’Shea M. A., Roby G., Chen J., Sohn H. W., et al. 2011. Attenuation of HIV-associated human B cell exhaustion by siRNA downregulation of inhibitory receptors. J. Clin. Invest. 121: 2614–2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yeo L., Lom H., Juarez M., Snow M., Buckley C. D., Filer A., Raza K., Scheel-Toellner D. 2015. Expression of FcRL4 defines a pro-inflammatory, RANKL-producing B cell subset in rheumatoid arthritis. Ann. Rheum. Dis. 74: 928–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ehrhardt G. R., Davis R. S., Hsu J. T., Leu C. M., Ehrhardt A., Cooper M. D. 2003. The inhibitory potential of Fc receptor homolog 4 on memory B cells. Proc. Natl. Acad. Sci. USA 100: 13489–13494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jackson T. A., Haga C. L., Ehrhardt G. R., Davis R. S., Cooper M. D. 2010. FcR-like 2 Inhibition of B cell receptor-mediated activation of B cells. J. Immunol. 185: 7405–7412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haga C. L., Ehrhardt G. R., Boohaker R. J., Davis R. S., Cooper M. D. 2007. Fc receptor-like 5 inhibits B cell activation via SHP-1 tyrosine phosphatase recruitment. Proc. Natl. Acad. Sci. USA 104: 9770–9775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu Z., Li R., Li H., Zhou T., Davis R. S. 2013. FCRL5 exerts binary and compartment-specific influence on innate-like B-cell receptor signaling. Proc. Natl. Acad. Sci. USA 110: E1282–E1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leu C. M., Davis R. S., Gartland L. A., Fine W. D., Cooper M. D. 2005. FcRH1: an activation coreceptor on human B cells. Blood 105: 1121–1126. [DOI] [PubMed] [Google Scholar]
- 21.Li F. J., Schreeder D. M., Li R., Wu J., Davis R. S. 2013. FCRL3 promotes TLR9-induced B-cell activation and suppresses plasma cell differentiation. Eur. J. Immunol. 43: 2980–2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ehrhardt G. R., Hijikata A., Kitamura H., Ohara O., Wang J. Y., Cooper M. D. 2008. Discriminating gene expression profiles of memory B cell subpopulations. J. Exp. Med. 205: 1807–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barrow A. D., Trowsdale J. 2006. You say ITAM and I say ITIM, let’s call the whole thing off: the ambiguity of immunoreceptor signalling. Eur. J. Immunol. 36: 1646–1653. [DOI] [PubMed] [Google Scholar]
- 24.Sohn H. W., Krueger P. D., Davis R. S., Pierce S. K. 2011. FcRL4 acts as an adaptive to innate molecular switch dampening BCR signaling and enhancing TLR signaling. Blood 118: 6332–6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ehrhardt G. R., Leslie K. B., Lee F., Wieler J. S., Schrader J. W. 1999. M-Ras, a widely expressed 29-kD homologue of p21 Ras: expression of a constitutively active mutant results in factor-independent growth of an interleukin-3-dependent cell line. Blood 94: 2433–2444. [PubMed] [Google Scholar]
- 26.Lock P., Ralph S., Stanley E., Boulet I., Ramsay R., Dunn A. R. 1991. Two isoforms of murine hck, generated by utilization of alternative translational initiation codons, exhibit different patterns of subcellular localization. Mol. Cell. Biol. 11: 4363–4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Godbey W. T., Wu K. K., Mikos A. G. 1999. Poly(ethylenimine) and its role in gene delivery. J. Control. Release 60: 149–160. [DOI] [PubMed] [Google Scholar]
- 28.Ehrhardt G. R., Korherr C., Wieler J. S., Knaus M., Schrader J. W. 2001. A novel potential effector of M-Ras and p21 Ras negatively regulates p21 Ras-mediated gene induction and cell growth. Oncogene 20: 188–197. [DOI] [PubMed] [Google Scholar]
- 29.Cheng P. C., Dykstra M. L., Mitchell R. N., Pierce S. K. 1999. A role for lipid rafts in B cell antigen receptor signaling and antigen targeting. J. Exp. Med. 190: 1549–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilson T. J., Fuchs A., Colonna M. 2012. Cutting edge: human FcRL4 and FcRL5 are receptors for IgA and IgG. J. Immunol. 188: 4741–4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crise B., Rose J. K. 1992. Identification of palmitoylation sites on CD4, the human immunodeficiency virus receptor. J. Biol. Chem. 267: 13593–13597. [PubMed] [Google Scholar]
- 32.Fragoso R., Ren D., Zhang X., Su M. W., Burakoff S. J., Jin Y. J. 2003. Lipid raft distribution of CD4 depends on its palmitoylation and association with Lck, and evidence for CD4-induced lipid raft aggregation as an additional mechanism to enhance CD3 signaling. J. Immunol. 170: 913–921. [DOI] [PubMed] [Google Scholar]
- 33.Arcaro A., Grégoire C., Boucheron N., Stotz S., Palmer E., Malissen B., Luescher I. F. 2000. Essential role of CD8 palmitoylation in CD8 coreceptor function. J. Immunol. 165: 2068–2076. [DOI] [PubMed] [Google Scholar]
- 34.Pang D. J., Hayday A. C., Bijlmakers M. J. 2007. CD8 Raft localization is induced by its assembly into CD8alpha beta heterodimers, Not CD8alpha alpha homodimers. J. Biol. Chem. 282: 13884–13894. [DOI] [PubMed] [Google Scholar]
- 35.Dykstra M., Cherukuri A., Sohn H. W., Tzeng S. J., Pierce S. K. 2003. Location is everything: lipid rafts and immune cell signaling. Annu. Rev. Immunol. 21: 457–481. [DOI] [PubMed] [Google Scholar]
- 36.Levental I., Grzybek M., Simons K. 2010. Greasing their way: lipid modifications determine protein association with membrane rafts. Biochemistry 49: 6305–6316. [DOI] [PubMed] [Google Scholar]
- 37.Levental I., Lingwood D., Grzybek M., Coskun U., Simons K. 2010. Palmitoylation regulates raft affinity for the majority of integral raft proteins. Proc. Natl. Acad. Sci. USA 107: 22050–22054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharrocks A. D. 2001. The ETS-domain transcription factor family. Nat. Rev. Mol. Cell Biol. 2: 827–837. [DOI] [PubMed] [Google Scholar]
- 39.Bouaziz J. D., Calbo S., Maho-Vaillant M., Saussine A., Bagot M., Bensussan A., Musette P. 2010. IL-10 produced by activated human B cells regulates CD4(+) T-cell activation in vitro. Eur. J. Immunol. 40: 2686–2691. [DOI] [PubMed] [Google Scholar]
- 40.Liang X., Moseman E. A., Farrar M. A., Bachanova V., Weisdorf D. J., Blazar B. R., Chen W. 2010. Toll-like receptor 9 signaling by CpG-B oligodeoxynucleotides induces an apoptotic pathway in human chronic lymphocytic leukemia B cells. Blood 115: 5041–5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lund F. E., Randall T. D. 2010. Effector and regulatory B cells: modulators of CD4+ T cell immunity. Nat. Rev. Immunol. 10: 236–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taguchi T., Kiyokawa N., Sato N., Saito M., Fujimoto J. 2000. Characteristic expression of Hck in human B-cell precursors. Exp. Hematol. 28: 55–64. [DOI] [PubMed] [Google Scholar]
- 43.Podar K., Mostoslavsky G., Sattler M., Tai Y. T., Hayashi T., Catley L. P., Hideshima T., Mulligan R. C., Chauhan D., Anderson K. C. 2004. Critical role for hematopoietic cell kinase (Hck)-mediated phosphorylation of Gab1 and Gab2 docking proteins in interleukin 6-induced proliferation and survival of multiple myeloma cells. J. Biol. Chem. 279: 21658–21665. [DOI] [PubMed] [Google Scholar]
- 44.Hu Y., Liu Y., Pelletier S., Buchdunger E., Warmuth M., Fabbro D., Hallek M., Van Etten R. A., Li S. 2004. Requirement of Src kinases Lyn, Hck and Fgr for BCR-ABL1-induced B-lymphoblastic leukemia but not chronic myeloid leukemia. Nat. Genet. 36: 453–461. [DOI] [PubMed] [Google Scholar]
- 45.Wang L., Gordon R. A., Huynh L., Su X., Park Min K. H., Han J., Arthur J. S., Kalliolias G. D., Ivashkiv L. B. 2010. Indirect inhibition of Toll-like receptor and type I interferon responses by ITAM-coupled receptors and integrins. Immunity 32: 518–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ivashkiv L. B. 2008. A signal-switch hypothesis for cross-regulation of cytokine and TLR signalling pathways. Nat. Rev. Immunol. 8: 816–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ivashkiv L. B. 2009. Cross-regulation of signaling by ITAM-associated receptors. Nat. Immunol. 10: 340–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lingwood D., Simons K. 2010. Lipid rafts as a membrane-organizing principle. Science 327: 46–50. [DOI] [PubMed] [Google Scholar]
- 49.Zaman S. N., Resek M. E., Robbins S. M. 2008. Dual acylation and lipid raft association of Src-family protein tyrosine kinases are required for SDF-1/CXCL12-mediated chemotaxis in the Jurkat human T cell lymphoma cell line. J. Leukoc. Biol. 84: 1082–1091. [DOI] [PubMed] [Google Scholar]
- 50.Séverin S., Nash C. A., Mori J., Zhao Y., Abram C., Lowell C. A., Senis Y. A., Watson S. P. 2012. Distinct and overlapping functional roles of Src family kinases in mouse platelets. J. Thromb. Haemost. 10: 1631–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saeki K., Miura Y., Aki D., Kurosaki T., Yoshimura A. 2003. The B cell-specific major raft protein, Raftlin, is necessary for the integrity of lipid raft and BCR signal transduction. EMBO J. 22: 3015–3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carréno S., Caron E., Cougoule C., Emorine L. J., Maridonneau-Parini I. 2002. p59Hck isoform induces F-actin reorganization to form protrusions of the plasma membrane in a Cdc42- and Rac-dependent manner. J. Biol. Chem. 277: 21007–21016. [DOI] [PubMed] [Google Scholar]
- 53.Möhn H., Le Cabec V., Fischer S., Maridonneau-Parini I. 1995. The src-family protein-tyrosine kinase p59hck is located on the secretory granules in human neutrophils and translocates towards the phagosome during cell activation. Biochem. J. 309: 657–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carréno S., Gouze M. E., Schaak S., Emorine L. J., Maridonneau-Parini I. 2000. Lack of palmitoylation redirects p59Hck from the plasma membrane to p61Hck-positive lysosomes. J. Biol. Chem. 275: 36223–36229. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.