Changes in the Purkinje cells (PCs) and climbing fibers (CFs) have been postulated to be involved in essential tremor (ET) disease pathogenesis.1 PCs receive 2 excitatory inputs: CFs and parallel fibers (PFs). CFs form synapses predominantly on the thick, proximal PC dendrites, whereas PFs form synapses on the thin, distal PC dendritic branchlets. CF-PC and PF-PC innervation territories on PC dendritic arbors are tightly regulated for proper PC function. We recently reported more CF-PC synapses on the thin, distal PC dendritic branchlets in ET cases than controls,2 and this pathologic feature was associated with tremor severity in a small sample of 8 ET cases. We now expand the ET case sample nearly fivefold (37 cases) and assess the association between abnormal CF-PC connections and a wider range of clinical features. The overarching goal was to begin to mark out clinical characteristics that track with pathologic features in ET.
Methods.
Thirty-seven ET brains were obtained from the New York Brain Bank. During life, severity of action tremor in the arms and hands was rated (total tremor scores [TTS] [range 0–36]) and the presence of rest tremor and head and voice tremors was noted.3 All cases consented to brain donation and pathology studies. We performed dual immunofluorescence of vesicular glutamate transporter type 2 (VGlut2) and calbindin to visualize CF-PC synapses in 7-μm-thick paraffin cerebellar cortical sections. We used random digits to choose PC dendritic trees for image acquisition. Different from our previous study using random field selections of a given PC dendritic arbor,2 we acquired serial images (Leica TSC SP2 microscope) to reconstruct the dendritic arbors from the PC layer to the pial surface (figure, A). In Image J (NIH, imagej.nih.gov), we calculated the percentage of CF-PC synapses on PC dendrites <1 μm thickness (%CFPC1). Therefore, we quantified the average %CFPC1 in 5 PCs in each ET case. A priori, the primary clinical variable of interest was the TTS. We further assessed additional clinical variables of interest: sex, age at tremor onset, age at death, presence of voice tremor, head tremor, and rest tremor. For these additional comparisons, we set the significance level at 0.0083 (i.e., 0.05/6) after Bonferroni adjustment.
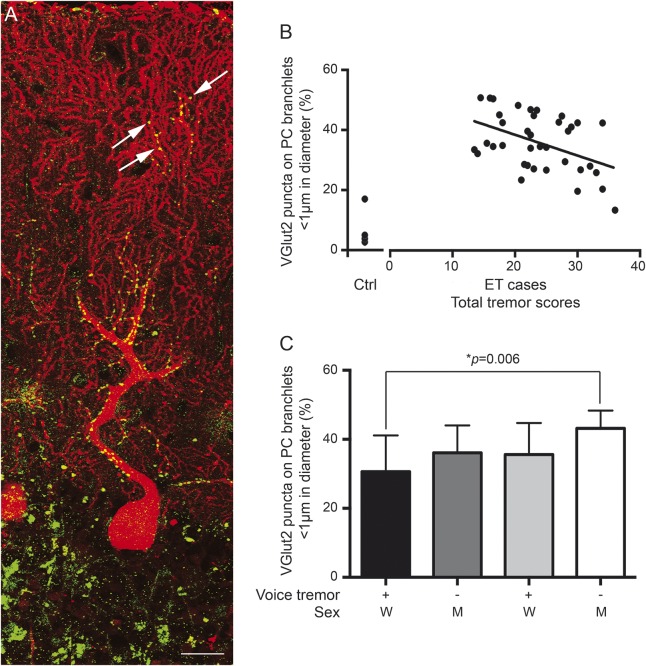
Figure. Clinicopathologic correlations of climbing fiber–Purkinje cell synaptic pathology and tremor.
(A) Dual immunofluorescence with anti-VGlut2 (Alexa 488, green) and anti-calbindin D28k antibody (Alexa 594, red) of a cerebellar section in an essential tremor (ET) case. Each Purkinje cell (PC) dendritic arbor was imaged, from the PC layers to pial surface, and reconstructed in Image J. VGlut2 puncta followed the climbing fibers and were distributed over proximal, thick PC dendrites and occasionally VGlut2 puncta localized over the distal, thin PC branchlets (arrows). Scale bar: 25 μm. (B) The percentage of VGlut2 puncta on PC branchlets <1 μm inversely correlated with the total tremor scores in ET cases. We also included data on 4 controls, collected previously.2 (C) Men without voice tremor had the highest percentage of VGlut2 puncta on PC branchlets <1 μm whereas women with voice tremor had the lowest percentage of VGlut2 puncta on PC branchlets <1 μm. M = men; W = women.
Results.
There were 37 ET cases (25 women) with mean ± SD age at onset 42.1 ± 22.6 years, age at death 86.8 ± 7.1 years, and TTS 23.7 ± 6.3. Of these, 58.3% had head tremor and 38.9% had voice tremor. There was a robust, inverse correlation between TTS and %CFPC1 (r = −0.45, p = 0.005) (figure, B). The %CFPC1 was not related to the age at tremor onset (r = −0.04, p = 0.79) or age at death (r = 0.02, p = 0.90). The %CFPC1 was lower in ET cases with vs without voice tremor (31.8 ± 10.0% vs 38.3 ± 8.7%, p = 0.045) and was lower in women than men (33.4 ± 9.9%, vs 41.4 ± 6.2%, p = 0.02), although the differences were not statistically significant after Bonferroni adjustment. The greatest difference was observed between women with voice tremor vs men without voice tremor (30.6 ± 10.5% vs 43.2 ± 5.1%, p = 0.006) (figure, C). %CFPC1 was not associated with presence of head tremor or rest tremor (p = 0.34 and 0.13, respectively).
Discussion.
With an expanded sample size, we showed a robust association between CF-PC synaptic pathology and tremor severity in ET cases, and uncovered additional sources of clinical-pathologic heterogeneity.
In mild ET, we observed increased CF-PC synaptic connections on the thin PC dendrites, which should have been receiving inputs from PFs. The abnormal CF-PC connections could lead to disturbed PC physiology, contributing to abnormal oscillation networks in ET. Alternatively, the abnormal CF-PC synaptic connections could represent a compensatory change, resulting from longstanding rhythmic firing of CFs onto PCs. In ET cases, a loss of PC dendritic arborization and spines has been observed.4 With disease progression, it is possible that distal pruning of PC dendritic spines could lead to decreased distal distribution of CF-PC synapses, thereby increasing cerebellar dysfunction in ET. These possibilities deserve further exploration.
Both sex and voice tremor seemed to track with the extent of abnormal CF-PC connections in ET. Prior work in ET has shown that both sex and cranial tremors seem to be associated with certain clinical differences.5,6 The present study highlights the notion that ET may represent not one but several clinical-pathologic entities, and future studies to link specific clinical features with identifiable pathologic characteristics are critically important.
Acknowledgments
Acknowledgment: The authors thank Dr. Elan Louis for editing the manuscript.
Footnotes
Author contributions: Ravi Louis: acquisition of data, analysis and interpretation, critical revision of the manuscript for important intellectual content. Chi-Ying Lin: study concept and design, analysis and interpretation, critical revision of the manuscript for important intellectual content. Phyllis Faust: analysis and interpretation, critical revision of the manuscript for important intellectual content. Arnulf Koeppen: critical revision of the manuscript for important intellectual content. Sheng-Han Kuo: study concept and design, analysis and interpretation, critical revision of the manuscript for important intellectual content.
Study funding: Dr. Kuo has received funding from the National Institute of Neurological Disorders and Stroke #K08 NS08738 (principal investigator), Louis V. Gerstner Jr. Scholar Award, Parkinson's Disease Foundation, American Brain Foundation Research Fellowship, Parkinson's Disease Foundation, American Parkinson's Disease Association, International Essential Tremor Foundation, NIEHS pilot award #ES009089.
Disclosure: The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
References
- 1.Hallett M. Tremor: pathophysiology. Parkinsonism Relat Disord 2014;20:S118–S122. [DOI] [PubMed] [Google Scholar]
- 2.Lin CY, Louis ED, Faust PL, Koeppen AH, Vonsattel JP, Kuo SH. Abnormal climbing fibre-Purkinje cell synaptic connections in the essential tremor cerebellum. Brain 2014;137:3149–3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Louis ED, Ottman R, Clark LN. Clinical classification of borderline cases in the family study of essential tremor: an analysis of phenotypic features. Tremor Other Hyperkinet Mov 2014;4:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Louis ED, Lee M, Babij R, et al. Reduced Purkinje cell dendritic arborization and loss of dendritic spines in essential tremor. Brain 2014;137:3142–3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Louis ED, Rios E, Rao AK. Tandem gait performance in essential tremor: clinical correlates and association with midline tremors. Mov Disord 2010;25:1633–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Louis ED. When do essential tremor patients develop head tremor? Influences of age and duration and evidence of a biological clock. Neuroepidemiology 2013;41:110–115. [DOI] [PMC free article] [PubMed] [Google Scholar]