Abstract
Objective:
We sought to determine the association of dietary factors and risk of cognitive decline in a population at high risk of cardiovascular disease.
Methods:
Baseline dietary intake and measures of the Mini-Mental State Examination were recorded in 27,860 men and women who were enrolled in 2 international parallel trials of the ONTARGET (Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial) and TRANSCEND (Telmisartan Randomised Assessment Study in ACE Intolerant Subjects with Cardiovascular Disease) studies. We measured diet quality using the modified Alternative Healthy Eating Index. Cox proportional hazards regression was used to determine the association between diet quality and risk of ≥3-point decline in Mini-Mental State Examination score, and reported as hazard ratio with 95% confidence intervals with adjustment for covariates.
Results:
During 56 months of follow-up, 4,699 cases of cognitive decline occurred. We observed lower risk of cognitive decline among those in the healthiest dietary quintile of modified Alternative Healthy Eating Index compared with lowest quintile (hazard ratio 0.76, 95% confidence interval 0.66–0.86, Q5 vs Q1). Lower risk of cognitive decline was consistent regardless of baseline cognitive level.
Conclusion:
We found that higher diet quality was associated with a reduced risk of cognitive decline. Improved diet quality represents an important potential target for reducing the global burden of cognitive decline.
Diet is reported to be a potential risk factor for noncommunicable diseases including cardiovascular (CV) disease, Alzheimer disease, and vascular cognitive impairment. Dietary intake may modify the risk of cognitive decline through multiple mechanisms including increased risk of stroke (both overt and covert) and through deficiency of nutrients required for neuronal regeneration (e.g., group B vitamins, and vitamin C).1 However, the association between overall diet quality and cognitive impairment is uncertain. Although some cohort studies did not report any association between a Mediterranean-style diet and cognitive decline,2–4 others reported that adherence to a Mediterranean diet is associated with slower cognitive decline.5,6 Three recent systematic reviews reported that moderate adherence to a Mediterranean diet is associated with reduced risk of cognitive impairment.7–9 In addition, one study reported that a Western diet (vs Oriental diet) was associated with a reduced risk of Alzheimer disease.10 More precise associations between diet (assessed using standardized methodology) and cognitive outcomes may be observed in a large multinational prospective cohort study. The ONTARGET11 (Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial) and TRANSCEND12 (Telmisartan Randomised Assessment Study in ACE Intolerant Subjects with Cardiovascular Disease) studies provide a unique opportunity to investigate the association between diet quality and cognitive decline in a population at high risk of CV disease.
METHODS
Study population.
We included participants from the ONTARGET and TRANSCEND studies, which were 2 parallel, multinational, double-blind, randomized trials with similar protocols, conducted in 733 centers in 40 middle- and high-income countries. These trials included 31,456 men and women aged 55 years and older with a history of one or more of coronary, cerebral, or peripheral artery disease, or high-risk diabetes mellitus. Neither ONTARGET nor TRANSCEND included patients with acute coronary syndrome, acute stroke, congestive heart failure (CHF), or important renal insufficiency. The primary outcome in both trials was the first occurrence of the composite of CV disease death, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for CHF. For ONTARGET, 99.8% of participants, and for TRANSCEND, 99.7% of participants were followed until the first primary outcome or the end of the study. The designs and main findings of both studies have been previously reported.11,12
For these analyses, we included only participants who completed a Mini-Mental State Examination (MMSE) at baseline and at least once more during study follow-up. Of 31,456 participants enrolled in the ONTARGET and TRANSCEND trials, 30,959 (98.1%) completed the MMSE at baseline and 90% of these completed the MMSE during follow-up, making 27,860 participants eligible for inclusion in these analyses. The median follow-up for participants included in these analyses was 4.9 (4.4–5.0) years. We compared the included and excluded participants and found included participants to be slightly younger, more likely to be male, to have normal creatinine, and less likely to have proteinuria (table e-1 on the Neurology® Web site at Neurology.org).
Data collection, measures, and cognitive assessment.
We obtained information at baseline on age, education, fasting lipids, glucose, and lifestyle, including diet, smoking, and alcohol intake. Medications, physical activity, blood pressure, and body mass index (BMI) were recorded at baseline, 2 years, and study end.
Trained members of the study team administered the MMSE at baseline, at 2 years of follow-up, and at the penultimate trial visit (5 years in ONTARGET and 5.5 years in TRANSCEND). The MMSE includes 10 domain items that measure orientation to time (5 points) and place (5 points), registration (3 points), attention and calculation (5 points), recall (3 points), naming and repetition (3 points), comprehension (3 points), reading ability (1 point), writing ability (1 point), and design copy (1 point), the latter being a measure of visual construction. For each successfully completed item on the MMSE, a score of 1 point was awarded for a total score from 0 to 30, with a higher score indicating better cognitive performance.
Outcome measures.
Cognitive decline was defined as a decrease of 3 or more points in MMSE score at any time during follow-up,13,14 computed by subtracting the score at the last follow-up visit from the baseline score. We defined the outcome of decline in MMSE subdomains based on the maximum possible score for each subdomain. For components with a maximum score of 5, the outcome was defined as a decline of 2 or more points; for those with a maximum score of 3, the outcome was defined as a decline of 1 or more points.
Assessment of diet quality.
At baseline, we recorded participants' food intake using a qualitative Food Frequency Questionnaire (FFQ) containing 20 items (table e-2). Despite regional differences, this FFQ is applicable in different countries.15 The FFQ was administered after patients were checked for compliance with run-in drugs and confirmed eligibility (week 0). Participants were asked, “In the last 12 months, how often did you eat foods from each of the following categories?” for a standard list of food items. For these analyses, frequencies of consumption were converted to “times per day,” and the association between diet quality and cognitive decline was determined using the modified Alternative Healthy Eating Index (mAHEI), which was developed to measure overall diet quality.16 The mAHEI has 7 components comprising the consumption of vegetables, fruits, nuts and soy proteins, whole grain, deep-fried foods, ratio of fish to meat and egg, and alcohol. Cutoff points for scoring were based on dietary recommendations, with a maximum of 10 points assigned when the dietary recommendation was met. A higher score indicates more frequent intake of healthy food choices (e.g., fruits, nuts and soy protein).16
Covariates.
Geographical region was defined as West (including Canada, Mexico, United States, Belgium, Czech Republic, France, Austria, Germany, Greece, Hungary, Italy, the Netherlands, Poland, Portugal, Russia, Denmark, Finland, Norway, Sweden, Slovakia, Spain, Switzerland, United Kingdom, Ireland, Ukraine, Australia, and New Zealand), East (including South Africa, Turkey, United Arab Emirates, China, Hong Kong, Philippines, Singapore, Malaysia, South Korea, Taiwan, and Thailand), and South American (including Argentina and Brazil). Education was categorized as ≤12 or >12 years and smoking categorized as never, former, or current smoker. Physical activity was self-reported and categorized as sedentary (active less than once per week), moderate (active 2 to 4 times per week), or high (active more than 4 times per week). We defined microalbuminuria as urine albumin to creatinine ratio of 30 to 300 mg/g and macroalbuminuria was defined as albumin to creatinine ratio of >300 mg/g. Antithrombotic use included antiplatelet or anticoagulant agents. Depression was self-reported and defined as either previous treatment for depression or a yes response to the question, “Have you ever had a time when you felt sad, low in your spirits, or depressed for 2 weeks or more in a row?”
Statistical analysis.
Mean (SD) and medians (range) were calculated to summarize continuous variables and participant characteristics compared using χ2 and analysis of variance tests as appropriate. Cox proportional hazards regression was used to determine the association between diet quality (mAHEI divided into quintiles) and cognitive decline. Multivariable adjustment included continuous (age, BMI, blood pressure, baseline MMSE score) and categorical variables (sex, trial enrollment, treatment allocation, geographical region, education, smoking, physical activity, medical history [stroke/TIA, hypertension, diabetes mellitus, and myocardial infarction], and medication use [statin, β-blocker, antithrombotic]). To account for intraclass correlations within centers, the standard error of coefficients were estimated using the robust sandwich approach. We performed sensitivity analysis to address reverse causation of dietary modification by excluding (1) patients with a major CV event (stroke, myocardial infarction, or hospitalization for CHF) during follow-up, (2) patients with history of cancer before or during follow-up, (3) patients with baseline MMSE score <24, and (4) patients with cognitive decline during the first 2 years of follow-up. Furthermore, we explored whether the association between diet quality and the outcome varied between participants categorized by baseline MMSE score (<26 vs 26–28 vs >28)17 and levels of physical activity at baseline. We also explored whether the association varied using alternative definitions for cognitive decline (alternative definition 1 = decline ≥2 points; alternative definition 2 = decline ≥4 points). Subgroup analyses were conducted for each component of the MMSE. For all analyses, the criterion for statistical significance was <0.05. We used SAS version 8.2 for UNIX (SAS Institute, Cary, NC) for all analyses.
Standard protocol approvals, registrations, and patient consents.
The local ethics committee in each country approved the study according to local regulations. Both studies were coordinated by the Population Health Research Institute, McMaster University, and Hamilton Health Sciences (Hamilton, Canada); Oxford University, United Kingdom, and University of Auckland, New Zealand. Participants provided consent before enrollment in the study. The study was registered with www.clinicaltrials.gov (NCT00153101).
RESULTS
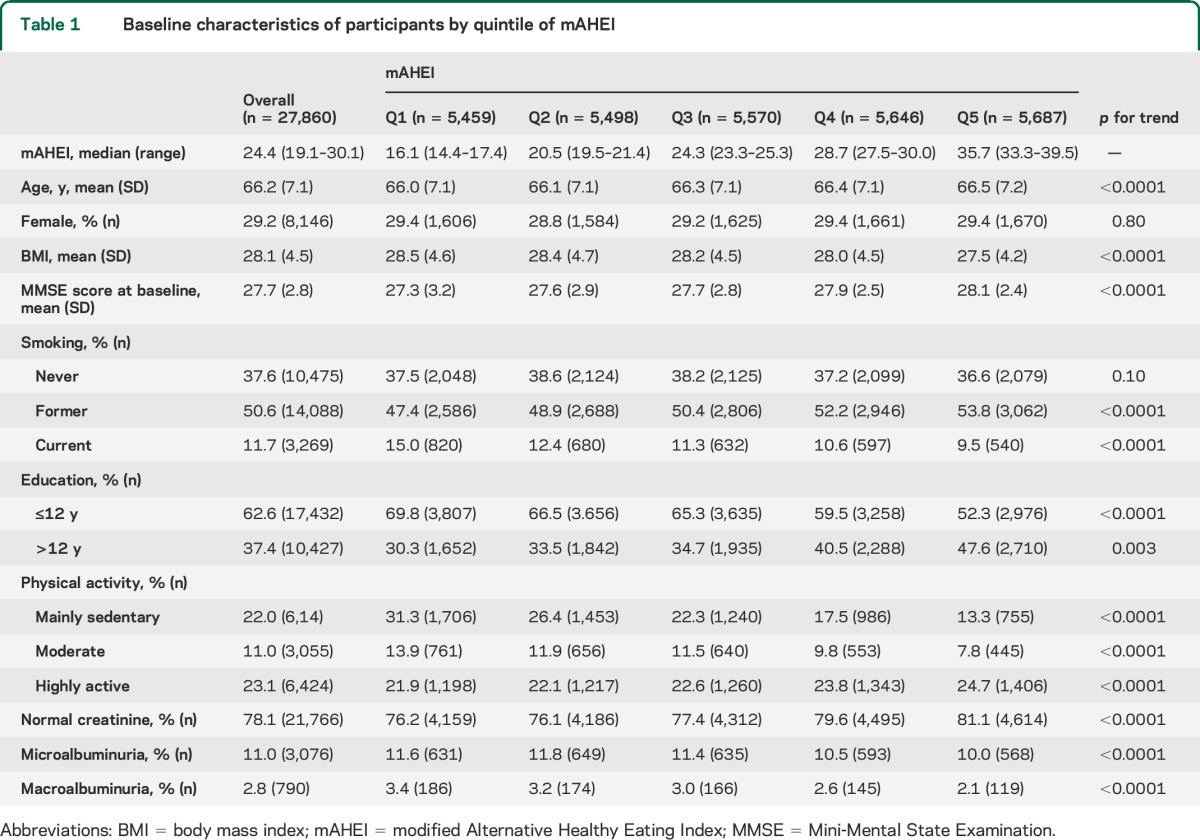
In 27,860 participants included in these analyses, the median mAHEI score was 24.4 (minimum 3.1, maximum 66.7) and baseline mean (SD) MMSE score was 27.7 (2.8). Participants with higher mAHEI scores (healthiest diet) were slightly older, more active, less likely to smoke, had a lower BMI, normal serum creatinine, and had higher MMSE score (p < 0.001) (table 1).
Table 1.
Baseline characteristics of participants by quintile of mAHEI
Diet quality and risk of cognitive decline.
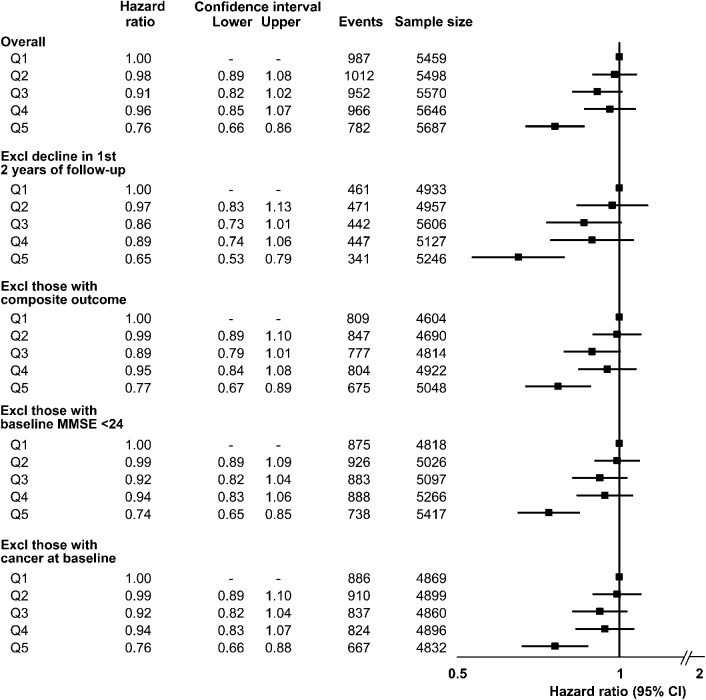
Cognitive decline occurred in 4,699 participants (16.8%) during follow-up. After multivariable adjustment, we observed an inverse association between diet quality and risk of cognitive decline. Comparing healthiest vs unhealthiest diet, the highest quintile of mAHEI was associated with a reduction in risk of cognitive decline (hazard ratio [HR] 0.76, 95% confidence interval [CI] 0.66–0.86) (figure 1) (p for trend <0.01).
Figure 1. Diet quality (mAHEI) and cognitive decline.
Adjusted HR and 95% CIs for the association between quintiles of diet quality (mAHEI) and cognitive decline, where Q1 represents the unhealthiest diet and Q5 the healthiest diet. The primary analyses are presented as the overall cohort; sensitivity analyses are presented excluding early events (first 2 years of follow-up), those with the composite (cardiovascular) outcome from the primary studies, those with baseline MMSE score <24, and those with cancer at baseline. All HRs adjusted for age, education, sex, trial enrollment (ONTARGET or TRANSCEND), treatment allocation, geographical region, baseline MMSE score, systolic blood pressure, history of stroke/TIA, diabetes mellitus, myocardial infarction, microalbuminuria, macroalbuminuria, serum creatinine, statin therapy, β-blocker therapy, antithrombotic use (antiplatelet or anticoagulant), smoking, body mass index, physical activity, and depression. CI = confidence interval; Excl = excluding; HR = hazard ratio; mAHEI = modified Alternative Healthy Eating Index; MMSE = Mini-Mental State Examination.
Sensitivity analyses.
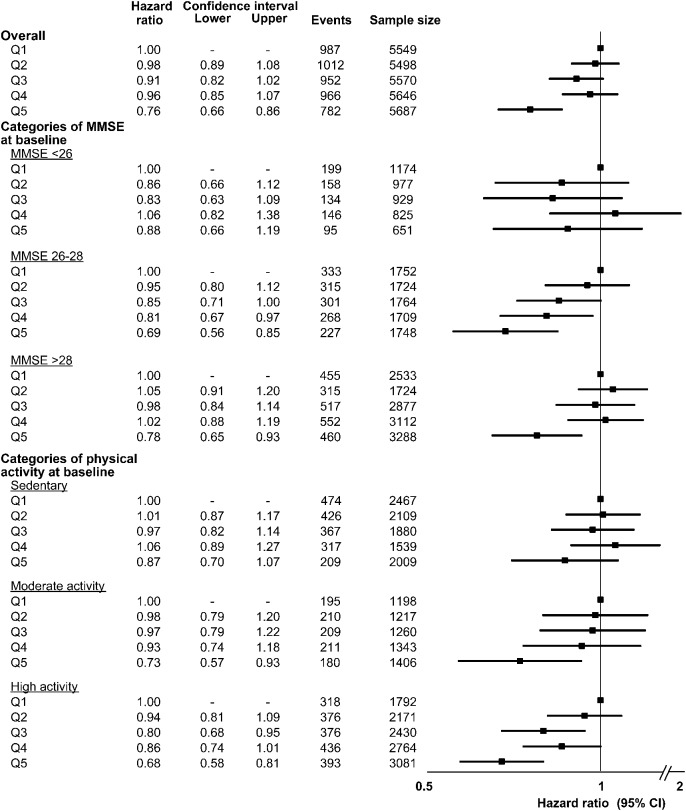
The healthiest diet (Q5) continued to be associated with a reduced risk of cognitive decline after excluding those with a major CV event (HR 0.77, 95% CI 0.67–0.89), those with cancer (HR 0.76, 95% CI 0.66–0.88), those with baseline MMSE score <24 (HR 0.74, 95% CI 0.65–0.85), and those with cognitive decline during the first 2 years (HR 0.65, 95% CI 0.53–0.79). We observed similar associations between categories of baseline MMSE (although a small number of participants had baseline MMSE score <26) and physical activity (figure 2). The overall pattern of association between mAHEI and cognitive decline was similar using multiple definitions of cognitive decline (figure 3).
Figure 2. Subgroup analyses of diet quality and cognitive decline.
Adjusted HR (95% CI) for the association between quintiles of diet quality and cognitive decline by subgroup of baseline MMSE score and physical activity. Sedentary physical activity defined as active less than once per week, moderate activity defined as active 2 to 4 times per week, high activity defined as active more than 4 times per week. All HRs adjusted for age, education, sex, trial enrollment (ONTARGET or TRANSCEND), treatment allocation, geographical region, baseline MMSE score, systolic blood pressure, history of stroke/TIA, diabetes mellitus, myocardial infarction, microalbuminuria, macroalbuminuria, serum creatinine, statin therapy, β-blocker therapy, antithrombotic use (antiplatelet or anticoagulant), smoking, body mass index, and depression. CI = confidence interval; HR = hazard ratio; MMSE = Mini-Mental State Examination.
Figure 3. Sensitivity analyses of different definitions of cognitive decline.
Adjusted HR (95% CI) for the association between quintiles of diet quality and cognitive decline using 3 definitions of cognitive decline. All HRs adjusted for age, education, sex, trial enrollment (ONTARGET or TRANSCEND), treatment allocation, geographical region, baseline MMSE score, systolic blood pressure, history of stroke/TIA, diabetes mellitus, myocardial infarction, microalbuminuria, macroalbuminuria, serum creatinine, statin therapy, β-blocker therapy, antithrombotic use (antiplatelet or anticoagulant), smoking, BMI, physical activity, and depression. CI = confidence interval; HR = hazard ratio.
MMSE domains.
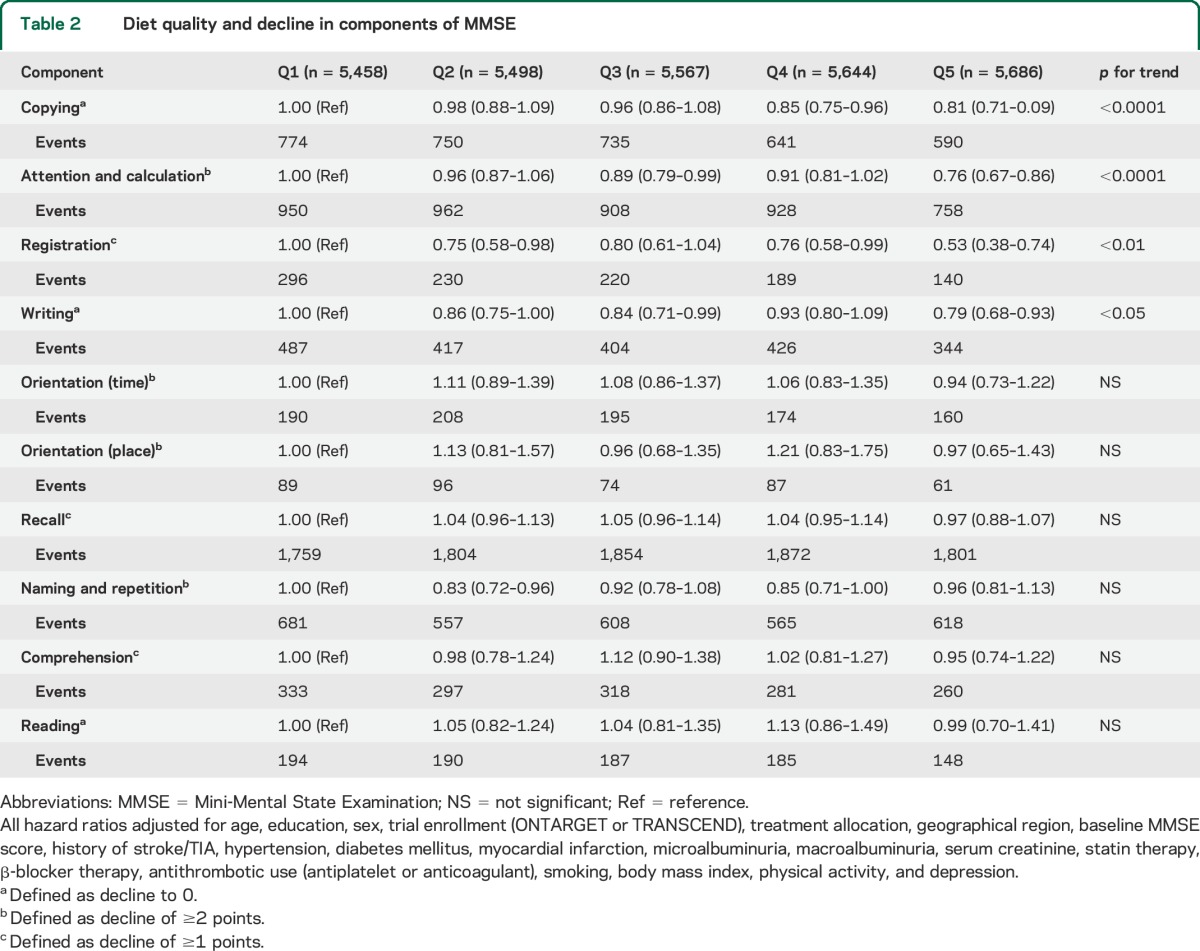
After multivariable adjustment, we observed a significant association between higher diet quality and reduced risk of decline in 4 components of the MMSE, including copying, attention and calculation, registration, and writing (p < 0.05) (table 2).
Table 2.
Diet quality and decline in components of MMSE
DISCUSSION
To our knowledge, this is the first study to investigate the association between diet quality and cognitive impairment in a large multinational cohort of middle-aged and elderly people at high CV risk. In this large multinational cohort, we report that high diet quality is associated with a lower risk of cognitive decline, after controlling for known confounding factors over 5 years of follow-up. The association persisted after excluding those with major CV events during follow-up, those with cancer, those with MMSE score <24 at baseline, and those with cognitive decline during the first 2 years of follow-up, and using multiple definitions of cognitive decline.
To date, observational studies evaluating the association between diet quality, or its constituents (high intake of fruit and vegetables, nuts, fish, moderate alcohol intake, and low consumption of red meat), and cognitive decline report mixed findings; some report a positive association18 while others no association.6,19,20 Three recent systematic reviews explored the association between a Mediterranean diet and cognitive impairment and pooled analyses of 8 studies reported that moderate adherence to a Mediterranean diet was associated with a reduced risk of cognitive impairment.7,8,21 Similar to these reports, in this large multinational cohort study of middle-aged and elderly people, we observed a graded independent association between diet quality and cognitive decline. We also report novel information on the association between healthy eating and several subdomains of the MMSE.
The mechanism by which a healthy diet may lead to a reduced risk of cognitive decline requires further study. In addition to the established association between healthy diet and a reduction in overt stroke, a healthy diet may also reduce covert stroke (with resultant ischemia from microbleeds), although unproven. Foods rich in omega-3 fatty acids, vitamins C and E, folate, and other carotenoids may alter inflammatory pathways via reductions in oxidative stress and lipid peroxidation.8 In our cohort of people at high CV risk, the risk of covert stoke was expected to be high, as well as other CV events, such as hospitalization for CHF, that may also increase the risk of cognitive decline. Of note, the association between diet quality and cognitive decline persisted when participants with major CV events during follow-up were excluded. Modifying risk factors for covert stroke such as hypertension, independent of diet quality, could also indirectly modify cognitive decline. For example, diets high in fruits and vegetables, which are rich in potassium, may lower blood pressure and reduce the risks of stroke and cognitive decline. We previously reported that a healthy diet was associated with a reduced risk of recurrent CV events, which lends support to the suggested mechanism in those with established CV disease.16 In addition, our analyses of MMSE components demonstrate a preferential association with domains that are prominent in vascular cognitive impairment (e.g., executive function).
Our study has several strengths including the large number of patients and outcome events (>4,000), the international cohort, detailed information on covariates, and the high completeness of data (99.8%). The method used to measure diet, the short FFQ, is reported to be a reliable measure of dietary intake by previous studies.15,16 Similarly, the method used to measure cognitive function, the MMSE, is a validated and widely used method of cognitive assessment, although it may have poor sensitivity for the detection of mild cognitive impairment, subtle changes in word recall, or impairment in executive function.22,23
Our study has a number of limitations. First, because this is an observational study, we can only establish association and not causation, and the effect of residual confounding cannot be ruled out, as dietary habits tend to be lifelong and may be a proxy for other poor health behaviors that were either unknown or unmeasured. Although we adjusted for BMI and physical activity, we chose not to also adjust for energy (caloric) intake because of the effect of multicollinearity and risk of overadjustment.24 Second, diet quality was measured only at baseline and we were unable to assess change in diet during follow-up. However, significant changes in dietary habits would be unlikely to occur over the length of follow-up of this study, including in those with incident CV events.25 In addition, those with incident CV disease would be more likely to change from unhealthy to healthy dietary patterns during follow-up, which would more likely bias toward the null. Third, our findings may partly be explained by reverse causation, as those at higher disease burden at baseline may be more likely to experience outcomes during follow-up. However, when we excluded participants with early cognitive decline (during the first 2 years of follow-up), the observed associations were materially unchanged. Fourth, dietary assessment by short FFQ in those with cognitive impairment at baseline may not accurately represent diet–disease association because the dietary assessment may not be as reliable and diet quality is unlikely to reverse established cognitive impairment. However, sensitivity analyses, excluding those with MMSE score <24 at baseline, did not materially alter the observed associations.
In conclusion, we report that higher diet quality is associated with a reduced risk of cognitive decline. Improved diet quality represents an important potential target for reducing the global burden of cognitive decline.
Supplementary Material
GLOSSARY
- BMI
body mass index
- CHF
congestive heart failure
- CI
confidence interval
- CV
cardiovascular
- FFQ
Food Frequency Questionnaire
- HR
hazard ratio
- mAHEI
modified Alternative Healthy Eating Index
- MMSE
Mini-Mental State Examination
- ONTARGET
Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial
- TRANSCEND
Telmisartan Randomised Assessment Study in ACE Intolerant Subjects with Cardiovascular Disease
Footnotes
Supplemental data at Neurology.org
Contributor Information
Collaborators: Salim Yusuf, Rafael Diaz, Leopoldo Piegas, Koon Teo, Gilles Dagenais, Ernesto Cardona, Jeffrey Probstfield, Michael Weber, James Young, Robert Fagard, Jean Mallion, Johannes Mann, Michael Bohm, Nicholas Karatzas, Matyas Keltai, Bruno Trimarco, Paolo Verdecchia, Leszek Ceremuzynski, Andrzej Budaj, Rafael Ferreira, Irina Chazova, Lars Ryden, George Fodor, Patrick Commerford, Josep Redon, Thomas Luescher, Ali Oto, Azan Binbrek, Peter Sleight, Alexander Parkhomenko, Garry Jennings, Lisheng Liu, John Sanderson, Chin Choy Lang, Jae-Hyung Kim, Jyh-Hong Chen, Suphachai Chaithiraphan, Petr Jansky, Ernesto Paolasso, Alvaro Avezum, Bernd Eber, Aldo Maggioni, Giuseppe Mancia, Nicholas Holwerda, Tage Lysbo Svendsen, Kaj Metsarinne, Kenneth Dickstein, and Bernark Kwok
AUTHOR CONTRIBUTIONS
All authors contributed to the discussions and interpretation of the data, and to the writing of the report. A.S. and M.D. planned and supervised data analysis and had primary responsibility for writing the report. Data were analyzed by P.G. All authors had full access to the data. No medical writer or other people were involved in the design, analysis, or writing of the manuscript.
STUDY FUNDING
The sponsors of the study (Boehringer Ingelheim) had no role in study design, data collection, data analysis, data interpretation, or writing of the report. All authors had full access to all study data and had final responsibility for the decision to submit for publication.
DISCLOSURE
A. Smyth, M. Dehghan, M. O'Donnell, and C. Anderson report no disclosures relevant to the manuscript. K. Teo received grants, honoraria, consulting, lecture fees, or research grants from Boehringer Ingelheim and from other companies manufacturing angiotensin receptor blockers. P. Gao reports no disclosures relevant to the manuscript. P. Sleight received grants, honoraria, consulting, lecture fees, or research grants from Boehringer Ingelheim and from other companies manufacturing angiotensin receptor blockers. G. Dagenais received grants, honoraria, consulting, lecture fees, or research grants from Boehringer Ingelheim and from other companies manufacturing angiotensin receptor blockers. J. Probstfield received grants, honoraria, consulting, lecture fees, or research grants from Boehringer Ingelheim and from other companies manufacturing angiotensin receptor blockers. A. Mente reports no disclosures relevant to the manuscript. S. Yusuf received grants, honoraria, consulting, lecture fees, or research grants from Boehringer Ingelheim and from other companies manufacturing angiotensin receptor blockers. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Gillette-Guyonnet S, Secher M, Vellas B. Nutrition and neurodegeneration: epidemiological evidence and challenges for future research. Br J Clin Pharmacol 2013;75:738–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Psaltopoulou T, Kyrozis A, Stathopoulos P, Trichopoulos D, Vassilopoulos D, Trichopoulou A. Diet, physical activity and cognitive impairment among elders: the EPIC-Greece cohort (European Prospective Investigation into Cancer and Nutrition). Public Health Nutr 2008;11:1054–1062. [DOI] [PubMed] [Google Scholar]
- 3.Samieri C, Okereke OI, E Devore D, Grodstein F. Long-term adherence to the Mediterranean diet is associated with overall cognitive status, but not cognitive decline, in women. J Nutr 2013;143:493–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vercambre MN, Grodstein F, Berr C, Kang JH. Mediterranean diet and cognitive decline in women with cardiovascular disease or risk factors. J Acad Nutr Diet 2012;112:816–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feart C, Samieri C, Rondeau V, et al. Adherence to a Mediterranean diet, cognitive decline, and risk of dementia. JAMA 2009;302:638–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tangney CC, Kwasny MJ, Li H, Wilson RS, Evans DA, Morris MC. Adherence to a Mediterranean-type dietary pattern and cognitive decline in a community population. Am J Clin Nutr 2011;93:601–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Psaltopoulou T, Sergentanis TN, Panagiotakos DB, Sergentanis IN, Kosti R, Scarmeas N. Mediterranean diet, stroke, cognitive impairment, and depression: a meta-analysis. Ann Neurol 2013;74:580–591. [DOI] [PubMed] [Google Scholar]
- 8.Feart C, Samieri C, Barberger-Gateau P. Mediterranean diet and cognitive health: an update of available knowledge. Curr Opin Clin Nutr Metab Care 2015;18:51–62. [DOI] [PubMed] [Google Scholar]
- 9.Lourida I, Soni M, Thompson-Coon J, et al. Mediterranean diet, cognitive function, and dementia: a systematic review. Epidemiology 2013;24:479–489. [DOI] [PubMed] [Google Scholar]
- 10.Ross GW, Petrovitch H, White LR, et al. Characterization of risk factors for vascular dementia: the Honolulu-Asia Aging Study. Neurology 1999;53:337–343. [DOI] [PubMed] [Google Scholar]
- 11.Yusuf S, Teo KK, Pogue J, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008;358:1547–1559. [DOI] [PubMed] [Google Scholar]
- 12.Yusuf S, Teo K, Anderson C, et al. Effects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intolerant to angiotensin-converting enzyme inhibitors: a randomised controlled trial. Lancet 2008;372:1174–1183. [DOI] [PubMed] [Google Scholar]
- 13.Hensel A, Luck T, Luppa M, Glaesmer H, Angermeyer MC, Riedel-Heller SG. Does a reliable decline in Mini Mental State Examination total score predict dementia? Diagnostic accuracy of two reliable change indices. Dement Geriatr Cogn Disord 2009;27:50–58. [DOI] [PubMed] [Google Scholar]
- 14.Tzourio C, Anderson C, Chapman N, et al. Effects of blood pressure lowering with perindopril and indapamide therapy on dementia and cognitive decline in patients with cerebrovascular disease. Arch Intern Med 2003;163:1069–1075. [DOI] [PubMed] [Google Scholar]
- 15.Iqbal R, Anand S, Ounpuu S, et al. Dietary patterns and the risk of acute myocardial infarction in 52 countries: results of the INTERHEART study. Circulation 2008;118:1929–1937. [DOI] [PubMed] [Google Scholar]
- 16.Dehghan M, Mente A, Teo KK, et al. Relationship between healthy diet and risk of cardiovascular disease among patients on drug therapies for secondary prevention: a prospective cohort study of 31 546 high-risk individuals from 40 countries. Circulation 2012;126:2705–2712. [DOI] [PubMed] [Google Scholar]
- 17.O'Donnell M, Teo K, Gao P, et al. Cognitive impairment and risk of cardiovascular events and mortality. Eur Heart J 2012;33:1777–1786. [DOI] [PubMed] [Google Scholar]
- 18.Scarmeas N, Stern Y, Mayeux R, Manly JJ, Schupf N, Luchsinger JA. Mediterranean diet and mild cognitive impairment. Arch Neurol 2009;66:216–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cherbuin N, Anstey KJ. The Mediterranean diet is not related to cognitive change in a large prospective investigation: the PATH Through Life Study. Am J Geriatr Psychiatry 2012;20:635–639. [DOI] [PubMed] [Google Scholar]
- 20.Shatenstein B, Ferland G, Belleville S, et al. Diet quality and cognition among older adults from the NuAge study. Exp Gerontol 2012;47:353–360. [DOI] [PubMed] [Google Scholar]
- 21.Singh B, Parsaik AK, Mielke MM, et al. Association of Mediterranean diet with mild cognitive impairment and Alzheimer's disease: a systematic review and meta-analysis. J Alzheimers Dis 2014;39:271–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kasten M, Bruggemann N, Schmidt A, Klein C. Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology 2010;75:478; author reply 478–479. [DOI] [PubMed] [Google Scholar]
- 23.Spencer RJ, Wendell CR, Giggey PP, et al. Psychometric limitations of the Mini-Mental State Examination among nondemented older adults: an evaluation of neurocognitive and magnetic resonance imaging correlates. Exp Aging Res 2013;39:382–397. [DOI] [PubMed] [Google Scholar]
- 24.Leal C, Bean K, Thomas F, Chaix B. Multicollinearity in associations between multiple environmental features and body weight and abdominal fat: using matching techniques to assess whether the associations are separable. Am J Epidemiol 2012;175:1152–1162. [DOI] [PubMed] [Google Scholar]
- 25.Teo K, Lear S, Islam S, et al. Prevalence of a healthy lifestyle among individuals with cardiovascular disease in high-, middle- and low-income countries: the Prospective Urban Rural Epidemiology (PURE) study. JAMA 2013;309:1613–1621. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.