Abstract
General arousal has been operationally defined as enhanced motor activity and enhanced intensity of response to sensory stimuli. Even though the effects of gonadal hormones on mating behavior have been much studied, their potential effect on generalized arousal, as defined above, has never been evaluated. In the present study we employed a thoroughly validated assay of general arousal to determine the effects of estradiol (E) and testosterone (T) in gonadectomized female and male mice, respectively. The steroids were administered in three different ways: A fast-acting, water soluble preparation given intraperitoneally, an oil solution given subcutaneously, and an oil solution in a subcutaneous Silastic capsule. Motor activity and responses to sensory stimuli were recorded for 24 h, 91 h, and seven days following hormone administration, respectively. All measures of arousal varied according to the day/night cycle. The water soluble steroid preparation had no reliable effect. When the same doses of estradiol and testosterone were administered subcutaneously in an oil vehicle no effect of either treatment on arousal was observed. The subcutaneously implanted capsule containing estradiol or testosterone had a delayed effect on motor activity in females (four to seven days) but no effect in males. The long time required by the gonadal hormones for affecting arousal would be consistent with, but does not prove, a genomic action. The limited effects of E and T in our arousal assay suggest to us that the strongest actions of these hormones on arousal occur in the context of sequences of responses to sexually relevant stimuli.
Keywords: Arousal, Estrogen, Testosterone, Activity, Stimuli reaction, Mice
1. Introduction
One of the most elusive issues in neuroscience is how an organism’s reactivity to environmental events, arousal, is controlled. Part of the lack of progress in this area is most likely due to a lack of clarity in the definition of arousal. Sometimes the term is used to refer to the internal factors that energize and potentiate behaviors or that accounts for the readiness to initiate behavior [1]. This use is most unfortunate because it confounds arousal with motivation, a term with an entirely different meaning in the behavioral literature (see [2] for a discussion). Instead, arousal may be “operationally defined” as follows: An organism in high arousal will (1) be more responsive to sensory stimuli in all sensory modalities, (2) emit more voluntary motor activity and (3) be more reactive emotionally than an organism in low arousal [3–5]. According to this definition, general arousal can be quantified by measuring the level of motor activity and/or the magnitude of response to any environmental stimulus or to an emotionally relevant stimulus. This, in turn, means that the abstract notion of arousal can be subjected to experimental study.
Several ascending transmitter systems, as the noradrenergic, dopaminergic, histaminergic, cholinergic and serotonergic systems, are known to modulate arousal [3]. Likewise, gonadal steroids may affect arousal, the most notorious effect probably being the large increase in running wheel activity around ovulation in rodents [6], known to be caused by estrogens (e.g. [7, 8]). In addition to their effects on motor activity, estrogens have been reported to modulate fear and anxiety. In the elevated plus maze, dark/light choice test or the open field, estrogen reduced locomotion on the open arms, the number of transitions and activity in the center, respectively [7, 8]. These responses were interpreted as a result of enhanced anxiety. Interestingly, estrogen treatment increased running wheel and home cage activity in these studies. It was suggested that estrogen-induced general arousal manifests itself as increased activity in safe environments. To the contrary, when estrogen-induced arousal is added to the already heightened arousal caused by the threatening procedures (plus maze, strong light, unknown open field) the ensuing overarousal inhibits behavior according to the Yerkes-Dodson principle [9]. Similar opposing actions of estrogen in safe and threatening contexts have been reported in studies in rats [10–12].
The varied behavior responses to estrogens may be interpreted as suggesting that (i) estrogens modify arousal in non-reproductive activities; (ii) the effects of estrogens depend on the procedure employed. In a familiar environment, such as a running wheel in the home cage, estrogen-enhanced arousal manifests itself in increased locomotion. On the other hand, when animals are exposed to novel or otherwise threatening contexts, estrogen-enhanced arousal leads to behavioral manifestations interpreted as fear or anxiety [13]. The preceding proposals would be considerably strengthened if it could be shown that estrogens indeed modified general arousal in a procedure specifically designed for the evaluation of arousal levels.
Androgens are also known to modify locomotor activity. Castration leads to reduced home cage activity in mice, and testosterone replacement prevents this reduction [14]. In intact male mice, treatment with large doses of testosterone does not increase or decrease open field activity [15] and there is no correlation between running wheel activity and serum testosterone concentration [16]. In intact rats, however, supplemental testosterone has been reported to enhance activity in the running wheel [17]. Whether these contradictory observations are due to a species difference with regard to testosterone action or to different experimental procedures is unknown at present. It may also be mentioned that the actions of testosterone on running wheel activity in male rats seem to depend on aromatization, since dihydrotestosterone lacks effect and estradiol is even more effective than testosterone [18]. Provided that locomotor activity is one of the expressions of arousal, these observations could suggest that gonadal hormones affect the arousal level in male rodents. It is possible that estrogen rather than androgen receptors are involved. Again, these proposals need to be substantiated by experimental data showing that gonadal steroids affect general arousal in males.
Many gonadal steroid actions are mediated by slow, transcriptional processes (e.g. [19]). The time lag between hormone administration and effect varies from hours to several days [20, 21]. Among the slow actions are modifications of the morphology of dendrites and patterns of synaptic connectivity as well as the neuronal soma size and even the overall regional volume [22, 23]. In addition to a genomic action some steroid effects depend on a fast-acting membrane receptor. This receptor may alter neuronal membrane excitability within seconds [24–26], modify intracellular Ca2+ availability and activate G-protein-dependent processes, among other things [27, 28]. The membrane receptor also regulates gene expression, which can lead to long term effects [21]. Consequently, estrogens as well as androgens may have actions starting within seconds of receptor activation as well as slow onset effects requiring days before becoming evident. However the time needed for gonadal hormone-induced modifications of the behavioral manifestations of general arousal has not been studied.
For the first time, we have measured potential effects of sex hormones on generalized arousal using a novel assay. In the present study, ovariectomized female and castrated male mice were treated with estradiol and testosterone, respectively. The hormones were administered in a fast acting preparation as well as in the classical oil vehicle and Silastic capsule. The subjects’ arousal level following hormone treatment was determined in a previously validated assay of general arousal [29–31]. It is based on continuous recording of several behavioral parameters, including locomotion and rearing as well as the reaction to sensory stimuli, i.e. emotional reactivity. Data from this experiment would allow us to determine the potential effects of gonadal hormones, administered in different ways, on general arousal in males and females. We also analyzed sex differences and differences between phases of the dark/light cycle.
2. Methods and materials
2.1 Subjects
Eight female and eight male C57BL/6 mice were purchased from Taconic farms, Inc. They were five weeks old at arrival. Animals were maintained on a 12:12LD cycle with light off at 0600h with food and water available ad libitum. Female ovariectomy and male castration were conducted under isoflurane anesthesia shortly after arrival. The animals were left to recover for one week following gonadectomy.
All animal procedures were approved by The Rockefeller University’s Animal Care and Use Committee in accordance with the Animal Welfare Act and the Department of Health and Human Services Guide for the Care and Use of Laboratory Animals.
2.2 Apparatus
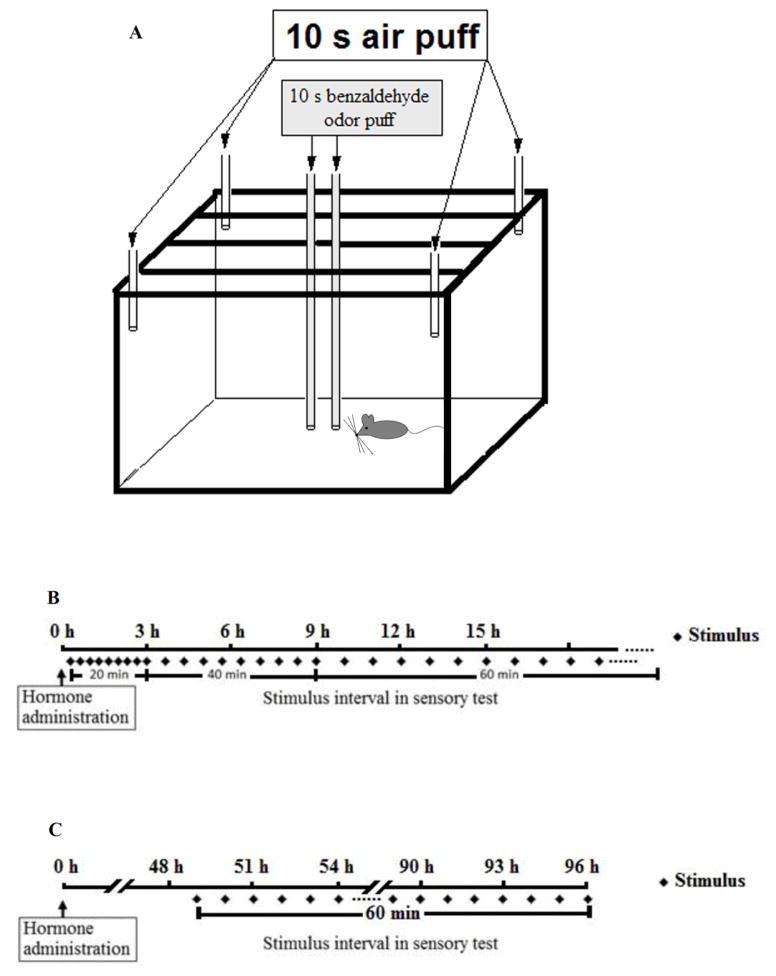
Mice were housed individually inside a VersaMax monitor (Accuscan Instruments) consisting of an acrylic cage (18 cm × 29 cm × 13 cm) equipped with horizontal infrared beams and sensors spaced 2.54 cm (1 inch) apart in the horizontal plane. Each VersaMax monitor was placed inside a larger wooden chamber with its dedicated light and ventilation systems used to minimize the potential for transmission of sounds and other signals between animals. The experimental setup is illustrated in Figure 1A.
Figure 1.
(A) Schematic diagram of experimental setup; (B) Timeline for the administration of the sensory stimuli in Experiment 1 and 2; (C) Timeline for the administration of the sensory stimuli in Experiment 3.
Activity was sampled every second. Three different modes of activity were recorded: (1) horizontal activity, fidgeting movements collected by the home cage Accuscan system and representing the number of infrared beams broken in the horizontal plane; (2) total distance, ambulation, collected by the home cage Accuscan system and representing non-repeating infrared beam breaks in the horizontal plane; and (3) vertical activity, rearing movement collected by the home cage Accuscan system and representing beam breaks in the vertical plane. The data were recorded onto a personal computer (Dell) by using VersaMax analyzer software version 3.41.
Tactile stimulation in the form of a 10 psi air puff could be provided through four metal tubes connected to a compressed air tank. The tubes ended in the cage roof corners. Olfactory stimulation (10 psi air through 100% benzaldehyde) was administered through two deeply inserted plastic tubes ending at floor level in the middle of the cage (Figure 1A). Both types of stimulus (tactile and olfactory) lasted for 10 seconds. They could be scheduled at varying intervals by using a computer program (LabLinc V, Coulbourn Instruments, Allentown, PA). A similar procedure has been used earlier [31–33].
2.3 Hormones
For Experiment 1, estradiol (E2) and testosterone (T) were dissolved in 25 % 2-hydroxypropyl-β-cyclodextrin in saline to a concentration of 0.1 mg/ml and 1 mg/ml, respectively (all compounds were from Sigma). Subjects were injected intraperitoneally (i.p.) with 100 μl of hormone solution or vehicle at 0900 on the test day. In Experiment 2, E2 and T were dissolved in sesame oil to a concentration of 0.2 mg/ml for E2 and 2 mg/ml for T. Subjects were injected 50 μl hormone solution or vehicle subcutaneously (s.c.) at 0900 on test day. For Experiment 3, E2 and T were dissolved in sesame oil to a concentration of 36 μg/ml for E2 and 360 μg/ml for T. Implantable capsules (I.D. x O.D. x length = 1.98 × 3.18 × 20 mm) made from silicone tubing (Silastic, Dow Corning) were filled with hormone solution and then sealed by medical grade silicone (Type A, Dow Corning) in both ends. They were implanted subcutaneously under isoflurane anesthesia.
In all experiments, the hormones and vehicle were administered according to a counterbalanced design, with half the subjects given vehicle and the other half hormone. The vehicle was the 25 % 2-Hydroxypropyl-β-cyclodextrin solution without any added hormone in Experiment 1, sesame oil in Experiment 2 and a Silastic capsule filled with sesame oil in Experiment 3. The interval between treatments was 48 h in Experiment 1, one week in Experiment 2 and one month in Experiment 3.
2.4 Design and procedure
There were eight females and eight males in these experiments. Four animals of each sex were given vehicle first, while the four others were given hormone first. A seven days baseline observation was started one week after gonadectomy. Immediately thereafter, Experiment 1 was initiated. First, spontaneous activity was recorded for 24 hours after hormone injection. Then, the experiment was repeated but now a tactile stimulus in the form of an air puff was introduced. The first air puff was presented 20 minutes after treatment and then repeated every 20 minutes for the first three hours, every 40 minutes from four to nine hours posttreatment, and every 60 minutes from the 10th hour to the end of the test (Figure 1B). Activity was recorded during the 10 sec of stimulus presentation and for 100 sec after stimulus offset. In a second repetition of the experiment, an olfactory stimulus was used instead of the air puff.
Experiment 2 started when the subjects used in Experiment 1 were 14 weeks old. After a seven days baseline, data were collected for 91 hours, beginning immediately after the hormone injection. The interval between the replications of this experiment was three days. Otherwise the procedure was the same as in Experiment 1.
Experiment 3 was initiated when the subjects were 21 weeks old. Again, a seven days baseline period preceded the experiment. Data were collected for one week following capsule implantation. Thereafter, the capsules were removed. The interval between replications of this experiment was three weeks. In the first and second replications, the sensory stimuli were presented once an hour from 49 hours after capsule implantation until 96 hours after (Figure 1C).
2.5 Data preparation and statistics
Activity data from the three baselines were compared with one-factor ANOVA for repeated measures. The sum of the entire seven days period was used for this analysis. A more detailed analysis of the data from the baseline preceding Experiment 1 was also made. Three-factor (Sex, Day, Light/Dark) ANOVAs were used.
In Experiment 1, activity data during the 24 h observation period was divided in 12 intervals of two h each. The first interval began immediately after treatment. In sensory tests of Experiment 1, activity data during the 10 sec of exposure to the stimulus, from the 10 sec period following stimulus offset and from the 100 sec poststimulus period were analyzed. The mean of all 10 sec or 100 sec periods occurring in each two h interval was calculated, and those means were used for analysis. There were a total of 11 intervals. Due to a technical failure, no stimulus was applied during the last hour of the 24 h observation. Consequently, the last interval was not used. The three activity parameters (total distance, horizontal activity and vertical activity) were analyzed with a three-factor ANOVA with two within-subjects factors (Treatment and Interval) and one between-subjects factor (Sex).
In Experiment 2, the SC hormone injection was given during the dark phase on Day 1. The 91 h postinjection observation was divided in eight periods. Four of these periods (postinjection hour 0–8, hour 20–31, hour 44–55 and hour 68–79) corresponded to the dark phase on Day one – four respectively. The other four periods (postinjection hour 8–19, hour 32–43, hour 56–67 and hour 80–91) corresponded to the light phase on Day one – four. In sensory tests of Experiment 2, activity data recorded during the 10 sec stimulus and during the 10 sec and 100 sec periods following the stimulus were analyzed as in Experiment 1. Mixed ANOVA with three within-groups factors (Treatment, Phase and Day) and one between-groups factor (Sex) was used for analysis [34].
In Experiment 3, the seven days observation was divided in dark or light periods in the same way as in Experiment 2. Data were also evaluated as in that experiment, except that results from the first day were not included because of the disturbance caused by the anesthesia and surgery associated with capsule implantation.
3. Results
3.1 Baseline tests
Analysis of the data from the seven days baseline period preceding Experiment 1 showed that the subjects were more active during the dark than during the light phase (ps < 0.02). Females were more active than males in total distance and vertical activity (ps < 0.04) and they showed a faster decline over days than males in these two parameters (Day by Sex, ps < 0.001). Data are shown in Figure 2.
Figure 2.
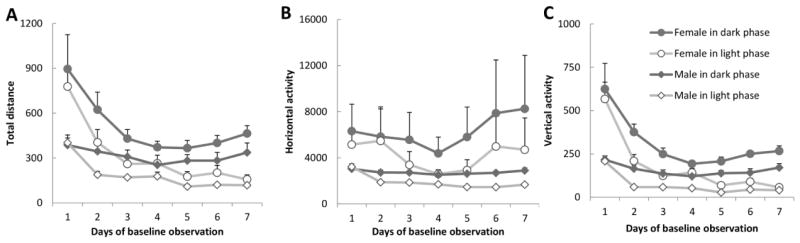
Motor activity in baseline tests. (A) Total distance; (B) Horizontal activity and (C) Vertical activity. There were eight males and eight females in the experiment. Data are represented as mean ± SEM.
Comparison of the three baseline recordings prior to three experiments revealed that neither the total distance (F2,30 = 1.085, p = 0.351) nor the horizontal activity (F2,30 = 1.128, p = 0.337) differed between baselines. However there was a significant difference with regard to vertical activity (F2,30 = 3.477, p = 0.044), but Tukey’s HSD test failed to confirm that difference (data not shown). These data show that the potential hormone effects were evaluated from essentially the same baseline activity in all three experiments reported here.
3.2 Experiment 1 - effect of gonadal hormones over 24 h
3.2.1 Motor activities
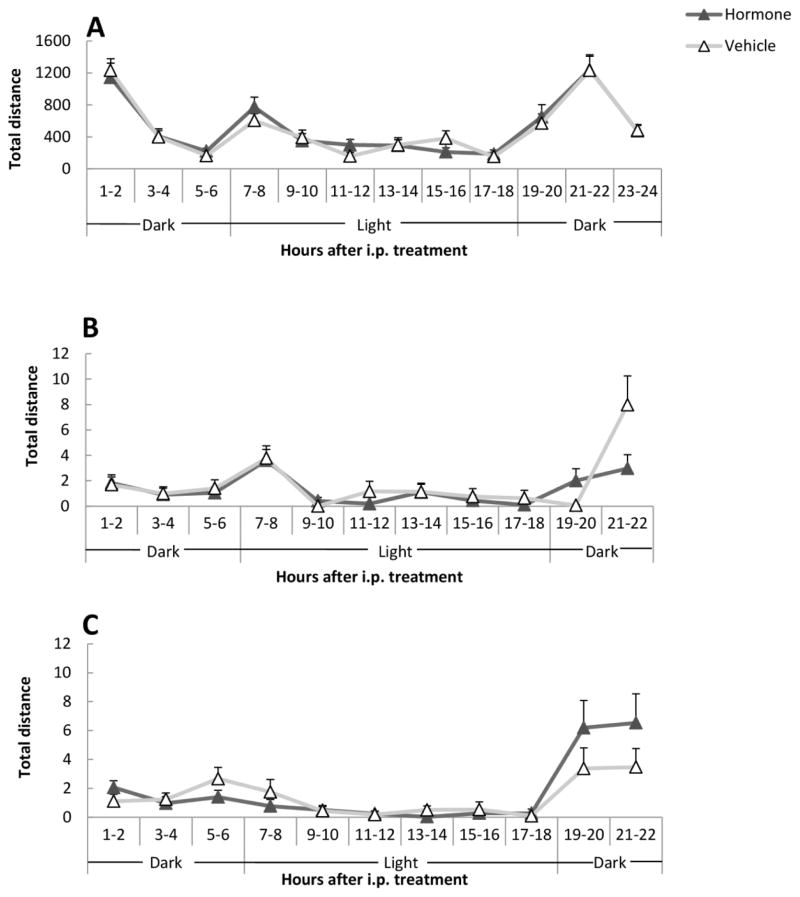
None of the two-factor interactions between Treatment, Sex and Interval reached significance (ps > 0.44). The results show that neither estradiol nor testosterone affects locomotor activity within 24 h of administration, suggesting that general arousal was unaltered by the hormones. Data are illustrated in Figure 3A.
Figure 3.
Motor activity following i.p. steroid administration. (A) total distance, (B) total distance during the tactile stimulus and (C) total distance during the 10 s following the end of the olfactory stimulus. There were eight males and eight females in the experiment. Since there was no sex difference, results from males and females were collapsed. Data represent mean ± SEM counts/period. For further details, see text.
3.2.2 Response to a tactile stimulus
Activity during the 10 sec of exposure to the stimulus was first analyzed. The interaction Treatment by Interval was significant for total distance (F10,140 = 2.55, p = 0.007; see Figure 3B) but not for horizontal and vertical activity (ps > 0.18). The interactions between Treatment and Sex as well as between Interval and Sex were also non-significant (ps > 0.08). It is most likely that the isolated effect on total distance does not represent a real hormone effect. The activity during the 10 sec period following stimulus offset was also analyzed. The interactions of Treatment by Interval and Treatment by Sex as well as Interval by Sex were not significant in any activity measure (ps > 0.16).
The absence of a treatment effect on the response to the tactile stimulus could be due to the short poststimulus interval used for analysis. Therefore, we also analyzed the data for the 100 sec periods following stimulation. No statistically significant effect was obtained (ps > 0.26).
3.2.3 Response to an olfactory stimulus
There was no effect of treatment on activity measures during the 10 sec of stimulus exposure (ps > 0.11). The data from the 10 sec poststimulus period from the 11 intervals (Figure 3C) showed that the interaction between Treatment and Interval was significant for total distance (F10,140 = 2.61, p = 0.006). The subjects presented a higher total activity in the last two intervals when hormone-treated than when given vehicle. However, the interaction between Treatment and Interval was non-significant for horizontal and vertical activity (ps > 0.16). There was no other significant interaction in any parameter (ps > 0.17). When analyzing the 100 sec following stimulus offset, no effect was found (ps > 0.44).
3.3 Experiment 2 - effect of gonadal hormones over 91 h
3.3.1 Motor activity
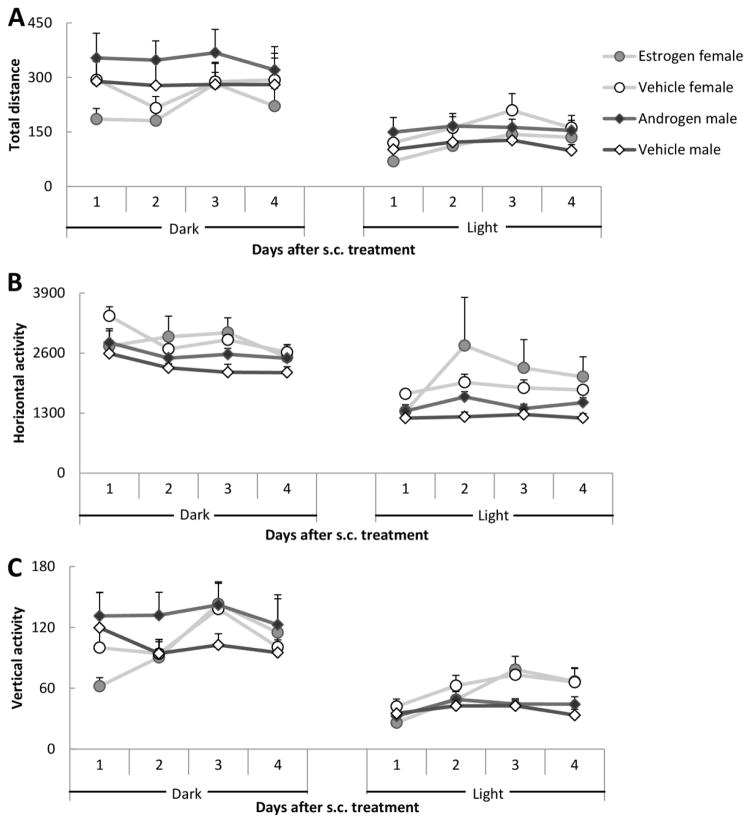
The interaction between Treatment and Sex as well as the interactions of Treatment by Day and Treatment by Phase were non-significant (ps > 0.08). To the contrary, the interaction Sex by Day was significant both with regard to total distance (F3,42 = 6.10, p = 0.002) and vertical activity (F3,42 = 8.90, p < 0.001). According to Tukey’s HSD test, there was an increase in activity in the females but not in the in males. Data are illustrated in Figure 4.
Figure 4.
Motor activity after s.c. administration. (A) Total distance; (B) Horizontal activity and (C) Vertical activity. There were eight males and eight females in the experiment. Data are presented as mean ± SEM.
The increase in activity over days observed in the females could be caused by random factors, such as the unusually low activity shown by the estrogen-treated females during period 1. The important observation here is that treatment with gonadal hormones failed to modify any of the indices of arousal employed here.
3.3.2 Response to a tactile stimulus
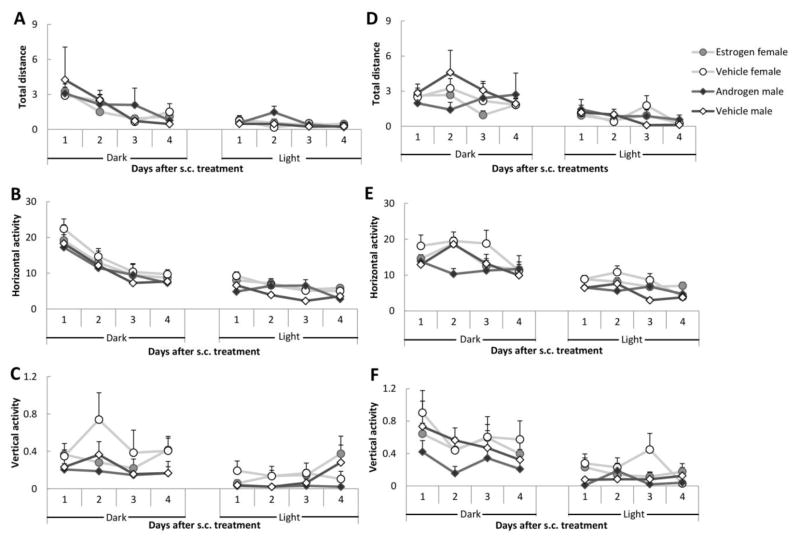
As illustrated in Figure 5, the subjects’ response to the air puff was not affected by hormone treatment. All interactions between two factors were non-significant (ps > 0.26). In addition, the results of the analysis of the activity during the 10 sec following the end of the tactile stimulus failed to reveal any Treatment effect on any parameter (ps > 0.18). None of the two-factor interactions reached significance (ps > 0.12).
Figure 5.
Response to the tactile stimulus after s.c. administration. (A) Total distance during the tactile stimulus; (B) Horizontal activity during the tactile stimulus; (C) Vertical activity during the tactile stimulus; (D) Total distance during the 10 sec following the end of the tactile stimulus; (E) Horizontal activity during the 10 sec following the end of the tactile stimulus and (F) Vertical activity during the 10 sec following the end of the tactile stimulus. There were eight males and eight females in the experiment. Data are represent as mean ± SEM.
3.3.3 Response to an olfactory stimulus
The activity during exposure to the olfactory stimulus was not affected by Treatment, and all interactions were non-significant (ps > 0.10). The analysis of the 10 sec following odor presentation indicated that Treatment did not have any effect on any parameter. None of the other two-factor interactions was significant (ps > 0.08).
3.4 Experiment 3 - effects of gonadal hormones over seven days
3.4.1 Motor activity
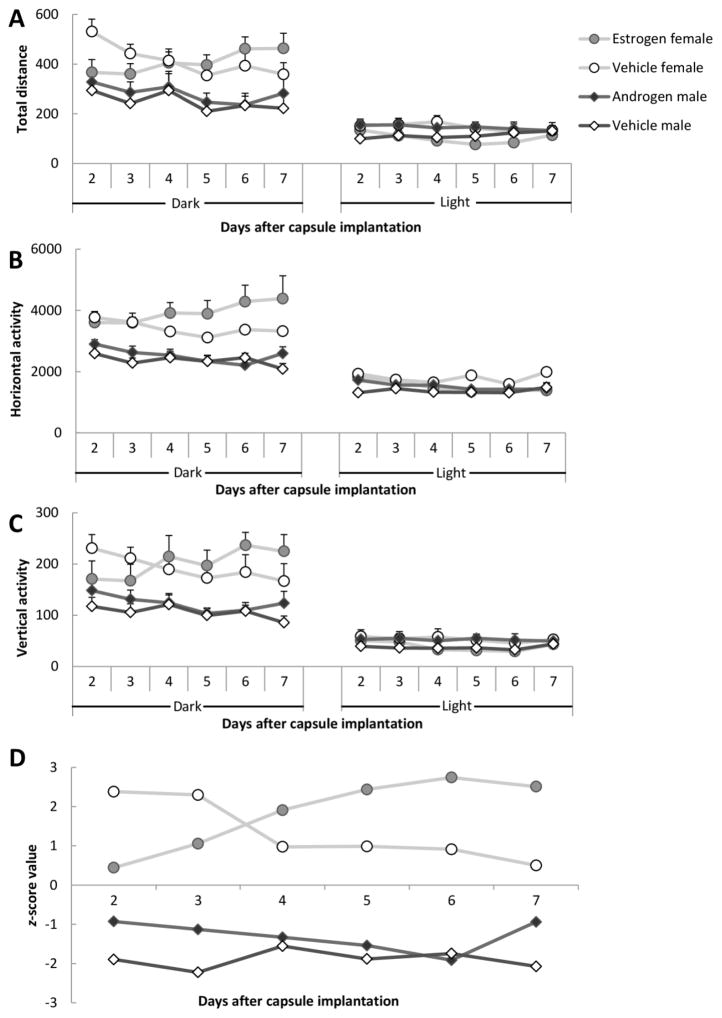
Although none of the two-factor interactions related to Treatment had any significant effect (ps > 0.057), there was a 3-factor interaction between Treatment, Sex and Day in all activity measures (total distance, F5,70 = 4.09, p = 0.003; horizontal activity, F5,70 = 4.91, p = 0.001; vertical activity, F5,70 = 3.66, p = 0.005). There was also a 3-factor interaction between Treatment, Day and Phase (total distance, F5,70 = 3.52, p = 0.007; horizontal activity, F5,70 = 4.19, p = 0.002; vertical activity, F5,70 = 2.77, p = 0.02). These interactions indicate that the gonadal hormones altered motor activity of males and females differently and that the hormone effect emerged some days after the capsule implantation. As we can see in Figure 6A, B and C, in the dark phase all three activities of estrogen-treated females increased from approximately three to four days after the implantation. Androgen treated males, on the other hand, did not show any hormone-induced modulation of their motor activities. The data relevant for the interaction Treatment by Day by Sex are illustrated as normalized values in Figure 6D.
Figure 6.
Motor activity after hormone capsule implantation. (A) Total distance; (B) Horizontal activity; (C) Vertical activity and (D) Normalized values of total activity during the dark phase after hormone capsule implantation. Since total distance moved, vertical activity and horizontal activity showed a similar pattern we here illustrated total activity, the z scores from the three parameters were added, and the resulting sum is shown in the figure. There were eight males and eight females in the experiment. Data are presented as mean ± SEM.
3.4.2 Response to a tactile and an olfactory stimulus
In order to reduce the subjects’ potential habituation to the repeated presentation of the stimuli, the data collection started two days after capsule implantation and lasted for another two days. No significant treatment effect or any meaningful interaction was obtained (data not shown).
4. Discussion
A novel assay of generalized arousal was used to look for potential steroid sex hormone effects. The results contained a mixture of predicted and unpredicted findings.
4.1 Baseline measurements
The data obtained before the beginning of the experimental treatments (baseline) and in the subjects given vehicle confirm that the behaviors recorded here show variations coinciding with known variations in level of general arousal. Not only was general motor activity higher during the night (e.g. [4, 31, 35–38]), but also the response to the tactile and olfactory stimuli was larger. It seems, then, that the procedure employed in these experiments is sensitive enough to detect the circadian variation in arousal level. Moreover, the behavioral changes coincide exactly with what would be expected according to the operational definition of general arousal (see Introduction) upon which the present experiments are based. Thus, the procedure seems most appropriate for detecting possible changes in general arousal caused by the administration of gonadal hormones.
Females were found to be more active at baseline and more reactive after vehicle than males. In rats, overall daily activity is 20 % – 50 % higher in females than in males [39]. A comparison of activity data from males and females in several mouse strains confirmed that females were more active in most, but not all, strains [40, 41]. However, open field activity was not different in male and female gonadectomized C57/BL6 mice [42]. This and other observations (e.g. [43]) suggest that estrogen effects on activity are mainly activational. Present data suggest otherwise, at least with regard to home cage activity and responses to sudden stimuli. The sex difference in the distribution of running wheel activity during the circadian cycle observed in intact C57BL/6 mice also persists in gonadectomized animals [44]. Even though males and females display the same total amount of activity, the females have longer duration of activity when housed in constant darkness. A more clear-cut sex difference in running wheel activity was found in intact mice of the ERaL3−/+ strain [45]. Both these studies suggest that organizational effects of gonadal steroids early in life as well as activational effects in the adult animal may influence the level of general arousal, at least when expressed as running wheel activity.
It may also be noted that the fact that we reproduced the well-established effects of sex and time of day on general arousal shows that the modest sample size employed here is sufficient for detecting even rather small effects. In contrast to the robust effect of time of day, the sex difference is quite small, yet it was replicated here.
4.2 Effect of gonadal hormones over 24 h
Data from Experiment 1 show that there was no rapid hormone effect on spontaneous activity. Likewise, the response to the tactile stimulus was unaltered by the hormones. To the contrary, there was a small effect on the response to the olfactory stimulus. The significant Treatment by Interval interaction for total distance during the 10 sec following stimulus offset was caused by an enhanced response in the hormone-treated groups during the last two intervals, incidentally coinciding with the beginning of the dark period. Since there was no interaction between Sex and Treatment, it must be concluded that the effect was similar in females and males. However, the hormone effect was rather small, and neither horizontal nor vertical activity was significantly affected. Likewise, the complete absence of effect on responses to the tactile stimulus suggests that the hormones only marginally affected arousal processes. Since the only effect observed became evident about 20 h after hormone treatment it seems unlikely that any of the fast actions of estrogens or androgens are related to arousal processes.
The half-life of estradiol has been reported to be 20 min after intravenous administration and 77 min after intraperitoneal injection [46]. The fact that the effects observed here occurred about 20 h after hormone administration, when serum concentrations should be negligible, shows that the gonadal steroids initiate actions that continue even in the absence of further hormone exposure and that they become evident after a substantial delay. Interestingly, this is also the case for another of the slow actions of estrogens, the stimulation of lordosis [47, 48]. Thus, the arousal enhancing effects of gonadal steroids do not seem to be entirely different from their actions on sexual behavior. In fact, the temporal coincidence between general arousal, expressed as locomotor activity, and sexual responsiveness, expressed as receptivity/proceptivity, in intact, cycling females shows that the time course of these estrogen-dependent behavioral events is similar. This is also the case for the effects of estradiol on wheel running (e.g. [12]).
4.3 Effect of gonadal hormones over 91 h
Subcutaneous administration of the hormones in an oil vehicle completely failed to affect any response during the observation period of 91 h. This might appear contradictory to the results obtained when the hormones were administered in a fast acting form. The contradiction, however, disappears when considering that the administration procedure was entirely different in these experiments (fast-releasing preparation in water vs. slow-releasing oil solution) while both the estradiol and testosterone doses were the same. It is most likely that maximum serum concentration was far higher in Experiment 1 than in Experiment 2. It is even possible that the rate of release from the oil vehicle was not much larger than the rate of catabolism, which might mean that hormone availability was below effective levels. Furthermore, even when administered subcutaneously in an oil vehicle, the half-life of estradiol is only about two h [49], suggesting that the mice were exposed to a low level estrogen for a rather short time. Thus, the lack of effect is not surprising. However, since no measurements of serum hormone concentrations were made, this explanation is purely speculative.
4.4 Effect of gonadal hormones over seven days
The time course of the response obtained in Experiment 3 was different from the modest effects on response to the tactile and olfactory stimuli observed in Experiment 1. The estrogen effect became apparent three to four days after capsule implantation in Experiment 3 compared to less than 24 h needed after IP administration in Experiment 1. A similar difference between routes of administration has been found with regard to the induction of receptivity. An intravenous injection of estradiol produces a response within 16 hours [47] as already mentioned whereas capsule implantation needs several days [50]. Likewise, estradiol-filled subcutaneous capsules start to increase running wheel activity after four to six days [8]. This confirms the notion that a short lived exposure to high concentrations of estradiol rapidly initiates intracellular processes whereas a sustained exposure to low concentrations needs far more time to activate these same processes. It also strongly suggests that rapid membrane actions of estradiol or testosterone are not important for arousal responses. Alternatively, such actions might interact with necessary genomic processes (e.g. [51]).
The fact that the estradiol response needed three to four days to develop indicates a genomic action, requiring synthesis of new mRNA and subsequently of new proteins. This mode of action was established long ago for the activation of lordosis [52–55] suggesting that gonadal hormones have similar mechanisms of action for stimulating lordosis and for enhancing general arousal.
4.5 Technical limitations of this work
The same subjects were used in all three experiments. This means that they were about 14 weeks older in Experiment 3 than in Experiment 1. It must be admitted that 14 weeks comprises more than 10 percent of the typical life span of mice in our laboratory, and that in the attempt to get as much data as possible from each subject there may have been unintended changes in their underlying physiology. Likewise, it must be admitted that they were given gonadal hormones on several occasions, and the doses were such that serum concentrations could be expected to be just above physiological levels. Nevertheless, our data, meant to be thought-provoking and to lead to further work, are valid as far as they go, for the following five reasons: (a.) Despite the age difference, all experiments were performed in young adults - - all mice were post-pubertal and within the age range when females are cycling normally. Further, there is no indication that the effects of gonadal hormones on mating behaviors change significantly during this time period. The effects of estrogens on learning and memory and on progesterone receptor induction also remain constant from young adulthood to at least middle age [56–58]. (b.) Also, the baseline activity did not change from the first to the last experiment. (c.) Between each hormone treatment, there was a wash out period eliminating or at least strongly reducing cumulative effects. (d.) Furthermore, a potential cumulative effect is of little concern, since the purpose of these experiments was to determine whether gonadal hormones can modify general arousal and not to determine whether they do so in physiological doses. (e.) This strategic requirement also explains the deliberate choice of the doses employed.
4.6 Perspectives
We were surprised at how limited the effects of gonadal hormones on measures of the abstract concept ‘generalized arousal’ were. It could, perhaps, be argued that a larger sample size would have allowed us to detect more effects. However, this is quite unlikely. First, the kind of within-subjects design used here is exquisitely sensitive to treatment effects. Second, there is no reason to believe that a larger sample size would have led to larger hormone effects. Thus, the modest effects observed here are probably not due to poor design or small sample. Nevertheless, these limited effects contrast with the large effect seen in estradiol-treated females observed in the running wheel. On the other hand they are not entirely different from the much smaller effects of testosterone reported in males (see Introduction for references). It is possible that running-wheel activity is functionally different from home cage activity. In a well-known, small environment such as the home cage increased activity is most unlikely to bring the mouse in contact with biologically relevant stimuli. To the contrary, increased running in a wheel might be equivalent to the increased forward locomotion typical of females in estrus. This kind of locomotion would enhance the female’s probability of encountering a mate, and would thus be a most adaptive response. Even though quantitative determinations of forward locomotion during the estrus cycle have not been performed in wild rodent females, it has been reported that estrous females appear to be more active than non-estrous females [59–61]. In a seminatural environment, females leave the burrow a lot more when sexually receptive than when not [62], and most sexual activities occur outside the burrow system [63]. Forward locomotion is an essential part of the female’s behavior as soon as she leaves the burrow, but not when remaining in it. Considering that the home cage could be equivalent to the burrow in a natural environment, small effects of gonadal hormones would actually be expected.
There were also limited effects of the gonadal hormones on the response to the tactile and olfactory stimulus. Assuming that these hormones indeed enhance general arousal, a larger effect could reasonably be expected. For example, a male’s response to female odors varies dramatically according to his gonadal status, being limited in a castrated male and substantial in a gonadally intact male (e.g. [64, 65]). Female responses to male odors are similarly dependent on ovarian steroids [66, 67]. The small effect on the reaction to sexually irrelevant tactile and olfactory stimuli observed in the present study might suggest that the gonadal steroids enhance responsivity to sexually relevant stimuli only, or that responsivity is enhanced only in a sexual context. Perhaps the elements of the natural sequence of reproductive behaviors are exquisitely sensitive to the arousal-enhancing actions of gonadal hormones whereas behavioral responses outside of that sequence are far less so. A context-dependent, arousal-enhancing effect of gonadal steroids needs to be further explored.
Finally, the results of the present experiments lead us to speculate that the greatest effects of sex hormones on behavioral measures of arousal may be limited to those environmental circumstances in which animals are following temporally ordered chains of behaviors - communicative and proceptive - that, under conditions outside the laboratory would lead to copulatory behaviors.
Highlights.
Generalized arousal manifests itself in motor activity and reactivity to stimuli
Sex hormone effects were tested in a novel generalized arousal assay
Gonadal steroids had modest and restricted effects in the generalized arousal assay
No rapid effect of gonadal steroids was found in the generalized arousal assay
Hormone effects on arousal may be most evident in a reproductive context
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jing J, Gillette R, Weiss KR. Evolving concepts of arousal: insights from simple model systems. Reviews in the Neurosciences. 2009;20:405–27. doi: 10.1515/revneuro.2009.20.5-6.405. [DOI] [PubMed] [Google Scholar]
- 2.Ågmo A. On the intricate relationship between sexual motivation and arousal. Hormones and Behavior. 2011;59:681–8. doi: 10.1016/j.yhbeh.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 3.Pfaff DW. Brain Arousal and Information Theory: Neural and Genetic Mechanisms. Cambridge, MA, USA: Harvard University Press; 2006. [Google Scholar]
- 4.Garey J, Goodwillie A, Frohlich J, Morgan M, Gustafsson JA, Smithies O, et al. Genetic contributions to generalized arousal of brain and behavior. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:11019–22. doi: 10.1073/pnas.1633773100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quinkert AW, Vimal V, Weil ZM, Reeke GN, Schiff ND, Banavar JR, et al. Quantitative descriptions of generalized arousal, an elementary function of the vertebrate brain. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:15617–23. doi: 10.1073/pnas.1101894108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Long A, Evans HM. The oestrus cycle of the rat and its associated phenomena. Memoirs of the University of California. 1922;6:1–148. [Google Scholar]
- 7.Morgan MA, Pfaff DW. Estrogen’s effects on activity, anxiety, and fear in two mouse strains. Behavioural Brain Research. 2002;132:85–93. doi: 10.1016/s0166-4328(01)00398-9. [DOI] [PubMed] [Google Scholar]
- 8.Morgan MA, Pfaff DW. Effects of estrogen on activity and fear-related behaviors in mice. Hormones and Behavior. 2001;40:472–82. doi: 10.1006/hbeh.2001.1716. [DOI] [PubMed] [Google Scholar]
- 9.Yerkes RM, Dodson JD. The relation of strength of stimulus to rapidity of habit-formation. Journal of Comparative Neurology and Psychology. 1908;18:459–82. [Google Scholar]
- 10.Mora S, Dussaubat N, Diaz-Veliz G. Effects of the estrous cycle and ovarian hormones on behavioral indices of anxiety in female rats. Psychoneuroendocrinology. 1996;21:609–20. doi: 10.1016/s0306-4530(96)00015-7. [DOI] [PubMed] [Google Scholar]
- 11.Spiteri T, Musatov S, Ogawa S, Ribeiro A, Pfaff DW, Ågmo A. The role of the estrogen receptor alpha in the medial amygdala and ventromedial nucleus of the hypothalamus in social recognition, anxiety and aggression. Behavioural Brain Research. 2010;210:211–20. doi: 10.1016/j.bbr.2010.02.033. [DOI] [PubMed] [Google Scholar]
- 12.Spiteri T, Ogawa S, Musatov S, Pfaff DW, Ågmo A. The role of the estrogen receptor a in the medial preoptic area in sexual incentive motivation, proceptivity and receptivity, anxiety, and wheel running in female rats. Behavioural Brain Research. 2012;230:11–20. doi: 10.1016/j.bbr.2012.01.048. [DOI] [PubMed] [Google Scholar]
- 13.Morgan MA, Schulkin J, Pfaff DW. Estrogens and non-reproductive behaviors related to activity and fear. Neuroscience & Biobehavioral Reviews. 2004;28:55–63. doi: 10.1016/j.neubiorev.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 14.Broida J, Svare B. Genotype modulates testosterone-dependent activity and reactivity in male mice. Hormones and Behavior. 1983;17:76–85. doi: 10.1016/0018-506x(83)90017-x. [DOI] [PubMed] [Google Scholar]
- 15.Salvador A, Moya-Albiol L, Martinez-Sanchis S, Simon VM. Lack of effects of anabolic-androgenic steroids on locomotor activity in intact male mice. Perceptual and Motor Skills. 1999;88:319–28. doi: 10.2466/pms.1999.88.1.319. [DOI] [PubMed] [Google Scholar]
- 16.Eleftheriou BE, Elias MF, Cherry C, Lucas LA. Relationship of wheel-running activity to post-wheel running plasma testosterone and corticosterone levels: A behavior-genetic analysis. Physiology & Behavior. 1976;16:431–8. doi: 10.1016/0031-9384(76)90321-8. [DOI] [PubMed] [Google Scholar]
- 17.McGinnis MY, Lumia AR, Tetel MJ, Molenda-Figueira HA, Possidente B. Effects of anabolic androgenic steroids on the development and expression of running wheel activity and circadian rhythms in male rats. Physiology & Behavior. 2007;92:1010–8. doi: 10.1016/j.physbeh.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roy EJ, Wade GN. Role of estrogens in androgen-induced spontaneous activity in male rats. Journal of Comparative and Physiological Psychology. 1975;89:573–9. doi: 10.1037/h0077436. [DOI] [PubMed] [Google Scholar]
- 19.Nilsson S, Makela S, Treuter E, Tujague M, Thomsen J, Andersson G, et al. Mechanisms of estrogen action. Physiological Reviews. 2001;81:1535–65. doi: 10.1152/physrev.2001.81.4.1535. [DOI] [PubMed] [Google Scholar]
- 20.Farach-Carson MC, Davis PJ. Steroid hormone interactions with target cells: Cross talk between membrane and nuclear pathways. Journal of Pharmacology and Experimental Therapeutics. 2003;307:839–45. doi: 10.1124/jpet.103.055038. [DOI] [PubMed] [Google Scholar]
- 21.Marino M, Galluzzo P, Ascenzi P. Estrogen signaling multiple pathways to impact gene transcription. Current Genomics. 2006;7:497–508. doi: 10.2174/138920206779315737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arnold AP, Breedlove SM. Organizational and activational effects of sex steroids on brain and behavior: A reanalysis. Hormones and Behavior. 1985;19:469–98. doi: 10.1016/0018-506x(85)90042-x. [DOI] [PubMed] [Google Scholar]
- 23.Cooke BM, Woolley CS. Gonadal hormone modulation of dendrites in the mammalian CNS. Journal of Neurobiology. 2005;64:34–46. doi: 10.1002/neu.20143. [DOI] [PubMed] [Google Scholar]
- 24.Allera A, Wildt L. Glucocorticoid-recognizing and -effector sites in rat liver plasma membrane. Kinetics of corticosterone uptake by isolated membrane vesicles--II. Comparative influx and efflux. The Journal of Steroid Biochemistry and Molecular Biology. 1992;42:757–71. doi: 10.1016/0960-0760(92)90116-z. [DOI] [PubMed] [Google Scholar]
- 25.Allera A, Wildt L. Glucocorticoid-recognizing and -effector sites in rat liver plasma membrane. Kinetics of corticosterone uptake by isolated membrane vesicles--I. Binding and transport. The Journal of Steroid Biochemistry and Molecular Biology. 1992;42:737–56. doi: 10.1016/0960-0760(92)90115-y. [DOI] [PubMed] [Google Scholar]
- 26.Daufeldt S, Lanz R, Alléra A. Membrane-initiated steroid signaling (MISS): Genomic steroid action starts at the plasma membrane. The Journal of Steroid Biochemistry and Molecular Biology. 2003;85:9–23. doi: 10.1016/s0960-0760(03)00141-9. [DOI] [PubMed] [Google Scholar]
- 27.Foradori CD, Weiser MJ, Handa RJ. Non-genomic actions of androgens. Frontiers in Neuroendocrinology. 2008;29:169–81. doi: 10.1016/j.yfrne.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Michels G, Hoppe UC. Rapid actions of androgens. Frontiers in Neuroendocrinology. 2008;29:182–98. doi: 10.1016/j.yfrne.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 29.Arrieta-Cruz I, Pfaff DW, Shelley DN. Mouse model of diffuse brain damage following anoxia, evaluated by a new assay of generalized arousal. Experimental Neurology. 2007;205:449–60. doi: 10.1016/j.expneurol.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shelley DN, Dwyer E, Johnson C, Wittkowski KM, Pfaff DW. Interactions between estrogen effects and hunger effects in ovariectomized female mice. I. Measures of arousal. Hormones and Behavior. 2007;52:546–53. doi: 10.1016/j.yhbeh.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weil ZM, Zhang Q, Hornung A, Blizard D, Pfaff DW. Impact of generalized brain arousal on sexual behavior. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:2265–70. doi: 10.1073/pnas.0914014107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Proekt A, Banavar JR, Maritan A, Pfaff DW. Scale invariance in the dynamics of spontaneous behavior. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:10564–9. doi: 10.1073/pnas.1206894109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quinkert AW, Pfaff DW. Temporal patterns of deep brain stimulation generated with a true random number generator and the logistic equation: Effects on CNS arousal in mice. Behavioural Brain Research. 2012;229:349–58. doi: 10.1016/j.bbr.2012.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Winer BJ, Brown DR, Michels KM. Statistical Principles in Experimental Design. 3. New York: McGraw-Hill; 1991. [Google Scholar]
- 35.Bacon Y, Ooi A, Kerr S, Shaw-Andrews L, Winchester L, Breeds S, et al. Screening for novel ENU-induced rhythm, entrainment and activity mutants. Genes, Brain, and Behavior. 2004;3:196–205. doi: 10.1111/j.1601-183X.2004.00070.x. [DOI] [PubMed] [Google Scholar]
- 36.de Visser L, van den Bos R, Spruijt BM. Automated home cage observations as a tool to measure the effects of wheel running on cage floor locomotion. Behavioural Brain Research. 2005;160:382–8. doi: 10.1016/j.bbr.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 37.Girard I, McAleer MW, Rhodes JS, Garland T., Jr Selection for high voluntary wheel-running increases speed and intermittency in house mice (Mus domesticus) The Journal of Experimental Biology. 2001;204:4311–20. doi: 10.1242/jeb.204.24.4311. [DOI] [PubMed] [Google Scholar]
- 38.Refinetti R. Daily activity patterns of a nocturnal and a diurnal rodent in a seminatural environment. Physiology & Behavior. 2004;82:285–94. doi: 10.1016/j.physbeh.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 39.Bronstein PM, Wolkoff FD, Levine MJ. Sex-related differences in rats open-field activity. Behavioral Biology. 1975;13:133–8. doi: 10.1016/s0091-6773(75)90913-x. [DOI] [PubMed] [Google Scholar]
- 40.Lightfoot JT, Turner MJ, Pomp D, Kleeberger SR, Leamy LJ. Quantitative trait loci for physical activity traits in mice. Physiological Genomics. 2008;32:401–8. doi: 10.1152/physiolgenomics.00241.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lightfoot JT, Leamy L, Pomp D, Turner MJ, Fodor AA, Knab A, et al. Strain screen and haplotype association mapping of wheel running in inbred mouse strains. Journal of Applied Physiology. 2010;109:623–34. doi: 10.1152/japplphysiol.00525.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ogawa S, Chan J, Gustafsson JA, Korach KS, Pfaff DW. Estrogen increases locomotor activity in mice through estrogen receptor alpha: specificity for the type of activity. Endocrinology. 2003;144:230–9. doi: 10.1210/en.2002-220519. [DOI] [PubMed] [Google Scholar]
- 43.Gentry RT, Wade GN. Sex differences in sensitivity of food intake, body weight, and running-wheel activity to ovarian steroids in rats. Journal of Comparative and Physiological Psychology. 1976;90:747–54. doi: 10.1037/h0077246. [DOI] [PubMed] [Google Scholar]
- 44.Kuljis DA, Loh DH, Truong D, Vosko AM, Ong ML, McClusky R, et al. Gonadal- and sex-chromosome-dependent sex differences in the circadian system. Endocrinology. 2013;154:1501–12. doi: 10.1210/en.2012-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blattner MS, Mahoney MM. Circadian parameters are altered in two strains of mice with transgenic modifications of estrogen receptor subtype 1. Genes Brain and Behavior. 2012;11:828–36. doi: 10.1111/j.1601-183X.2012.00831.x. [DOI] [PubMed] [Google Scholar]
- 46.De Hertogh R, Vanderheyden I, Delait AM, Ekka E. Enhanced metabolism of [2,4,6,7-3H] estradiol-17β in the diabetic rat. Journal of Steroid Biochemistry. 1984;21:433–8. doi: 10.1016/0022-4731(84)90307-8. [DOI] [PubMed] [Google Scholar]
- 47.Green R, Luttge WG, Whalen RE. Induction of receptivity in ovariectomized female rats by a single intravenous injection of estradiol-17β. Physiology & Behavior. 1970;5:137–41. doi: 10.1016/0031-9384(70)90056-9. [DOI] [PubMed] [Google Scholar]
- 48.Södersten P, Eneroth P, Hansen S. Induction of sexual receptivity in ovariectomized rats by pulse administration of oestradiol-17 β. Journal of Endocrinology. 1981;89:55–62. doi: 10.1677/joe.0.0890055. [DOI] [PubMed] [Google Scholar]
- 49.Bauss F, Esswein A, Reiff K, Sponer G, Muller-Beckmann B. Effect of 17β-estradiol-bisphosphonate conjugates, potential bone-seeking estrogen pro-drugs, on 17β-estradiol serum kinetics and bone mass in rats. Calcified Tissue International. 1996;59:168–73. doi: 10.1007/s002239900104. [DOI] [PubMed] [Google Scholar]
- 50.Ydstebo R, Södersten P. Induction of sexual receptivity in ovariectomized female rats by subcutaneous implants of estradiol-17β. Hormones and Behavior. 1977;9:130–40. doi: 10.1016/0018-506x(77)90080-0. [DOI] [PubMed] [Google Scholar]
- 51.Kow LM, Pfaff DW. The membrane actions of estrogens can potentiate their lordosis behavior-facilitating genomic actions. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:12354–7. doi: 10.1073/pnas.0404889101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Quadagno DM, Ho GKW. The reversible inhibition of steroid-induced sexual behavior by intracranial cycloheximide. Hormones and Behavior. 1975;6:19–26. doi: 10.1016/0018-506x(75)90019-7. [DOI] [PubMed] [Google Scholar]
- 53.Ogawa S, Taylor JA, Lubahn DB, Korach KS, Pfaff DW. Reversal of sex roles in genetic female mice by disruption of estrogen receptor gene. Neuroendocrinology. 1996;64:467–70. doi: 10.1159/000127154. [DOI] [PubMed] [Google Scholar]
- 54.Gordan JD, Attardi BJ, Pfaff DW. Mathematical exploration of pulsatility in cultured gonadotropin-releasing hormone neurons. Neuroendocrinology. 1998;67:2–17. doi: 10.1159/000054293. [DOI] [PubMed] [Google Scholar]
- 55.Lee AW, Kow LM, Devidze N, Ribeiro A, Martin-Alguacil N, Schober J, et al. Genetic mechanisms in neural and hormonal controls over female reproductive behaviors. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. 2. 1–5. San Diego: Elsevier Academic Press; 2009. [Google Scholar]
- 56.Fan L, Zhao Z, Orr PT, Chambers CH, Lewis MC, Frick KM. Estradiol-induced object memory consolidation in middle-aged female mice requires dorsal hippocampal extracellular signal-regulated kinase and phosphatidylinositol 3-kinase activation. The Journal of Neuroscience. 2010;30:4390–4400. doi: 10.1523/JNEUROSCI.4333-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fortress AM, Kim J, Poole RL, Gould TJ, Frick KM. 17b-Estradiol regulates histone alterations associated with memory consolidation and increases Bdnf promoter acetylation in middle-aged female mice. Learning Memory. 2014;21:457–67. doi: 10.1101/lm.034033.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Funabashi T, Kleopoulos SP, Brooks PJ, Kimura F, Pfaff DW, Shinohara K, Mobbs CV. Changes in estrogenic regulation of estrogen receptor a mRNA and progesterone receptor mRNA in the female rat hypothalamus during aging: an in situ hybridization study. Neuroscience Research. 2000;38:85–92. doi: 10.1016/s0168-0102(00)00150-4. [DOI] [PubMed] [Google Scholar]
- 59.Calhoun JB. The Ecology and Sociology of the Norway Rat. Washington, D.C: US Governnment Printing Office; 1962. [Google Scholar]
- 60.Robitaille JA, Bovet J. Field observations on the social behavior of the Norway rat, Rattus norvegicus (Berkenhout) Biology of Behaviour. 1976;1:289–308. [Google Scholar]
- 61.Telle HJ. Beitrag zur kenntnis der verhaltensweise von ratten, vergleichend dargestellt bei Rattus norvegicus und Rattus rattus. Zeitschrift für Angewandte Zoologie. 1965;53:129–96. [Google Scholar]
- 62.Garey J, Kow LM, Huynh W, Ogawa S, Pfaff DW. Temporal and spatial quantitation of nesting and mating behaviors among mice housed in a semi-natural environment. Hormones and Behavior. 2002;42:294–306. doi: 10.1006/hbeh.2002.1823. [DOI] [PubMed] [Google Scholar]
- 63.Chu X, Ågmo AA. Sociosexual behaviours in cycling, intact female rats (Rattus norvegicus) housed in a seminatural environment. Behaviour. 2014;151:1143–84. [Google Scholar]
- 64.Carr WJ, Caul WF. The effect of castration in rat upon the discrimination of sex odours. Animal Behaviour. 1962;10:20–7. [Google Scholar]
- 65.Carr WJ, Loeb LS, Wylie NR. Responses to feminine odors in normal and castrated male rats. Journal of Comparative and Physiological Psychology. 1966;62:336–8. doi: 10.1037/h0023693. [DOI] [PubMed] [Google Scholar]
- 66.Baum MJ, Keverne EB. Sex difference in attraction thresholds for volatile odors from male and estrous female mouse urine. Hormones and Behavior. 2002;41:213–9. doi: 10.1006/hbeh.2001.1749. [DOI] [PubMed] [Google Scholar]
- 67.Sorwell KG, Wesson DW, Baum MJ. Sexually dimorphic enhancement by estradiol of male urinary odor detection thresholds in mice. Behavioral Neuroscience. 2008;122:788–93. doi: 10.1037/0735-7044.122.4.788. [DOI] [PMC free article] [PubMed] [Google Scholar]