One of the greatest challenges in the study and treatment of cancer has been that the disease is too heterogeneous: too many tissue types, too many etiologic factors, too much genetic diversity. After the discovery of oncogenes, it was thought that a limited number of genes, the proto-oncogenes, might turn a normal cell into a cancerous one. But soon it was discovered that tumor-suppressor genes antagonize the action of oncogenes, thus increasing genetic diversity in the context of cancer pathogenesis. To date, about 140 genes have been identified that can drive cancer growth when genetically altered.1 The discovery that driver genes can be classified into 1 or more of 12 pathways led to one of the greatest achievements in drug development and the establishment of targeted therapy in cancer.2–4
Recently, whole-genome sequencing revealed a surprising fact: every tumor contains hundreds to thousands of somatic mutations, which are obtained throughout life, and their number is directly correlated with age. Certain types of tumors display many more or many fewer mutations. Melanomas and lung cancers are the outliers and contain approximately 200 nonsynchronous mutations per tumor. It has been hypothesized that this large number of mutations reflects the effect of potent mutagens (e.g., ultraviolet light and cigarette smoking).5 The unique genetic fingerprint of almost every tumor raises the concern that treatments might be destined to fail owing to tumor heterogeneity and the continuous development of mutations.
Despite the long debate about the ability of T cells to destroy tumors,6 the unprecedented recent success of immunotherapy in malignant disorders has provided evidence that the patient’s endogenous immune system can be altered to attack established tumors. A major hurdle in tumor immunotherapy is the fact that mechanisms of self-tolerance that prevent autoimmunity also impair T-cell responses against tumors, which do not differ substantially from self. Blockade of the major checkpoint inhibitors cytotoxic T-lymphocyte antigen 4 (CTLA-4) and programmed cell death 1 (PD-1) has resulted in durable responses in many patients.7,8 However, why other patients have only transient responses or no responses at all remains unclear. It is also unclear how patients should be identified as appropriate candidates for immunotherapy.
In a study now reported in the Journal, Snyder and colleagues9 asked whether the genetic landscape of melanoma might affect clinical benefit from immunotherapy with CTLA-4 blockade. The investigators analyzed tumor DNA using whole-exome sequencing and, as expected, detected a large number of somatic mutations. Mutational burden was higher in patients with a sustained clinical benefit than in those without a sustained benefit. Specific tumor neoepitopes encoded by these mutations were identified, and, after translation of missense mutations into mutant and nonmutant peptides, their ability to initiate major histocompatibility complex (MHC) class I– mediated responses in T cells was assessed by means of a bioinformatics algorithm incorporating prediction for MHC class I binding, T-cell receptor binding, and patient-specific HLA type. Mutant peptides were predicted to bind MHC class I molecules with higher affinity than the corresponding nonmutant peptides.
Moreover, it was determined that a number of tetrapeptide sequences corresponding to mutation-derived neoepitopes were shared by patients with a long-term clinical benefit but were completely absent from patients with a minimal benefit or no benefit. These neoepitopes defined a signature that could predict long-term clinical benefit from CTLA-4 blockade. The quality — not the number — of mutations had the strongest predictive value. Strikingly, many neoepitopes that were common to patients who had a sustained clinical benefit were homologous to viral and bacterial antigens.
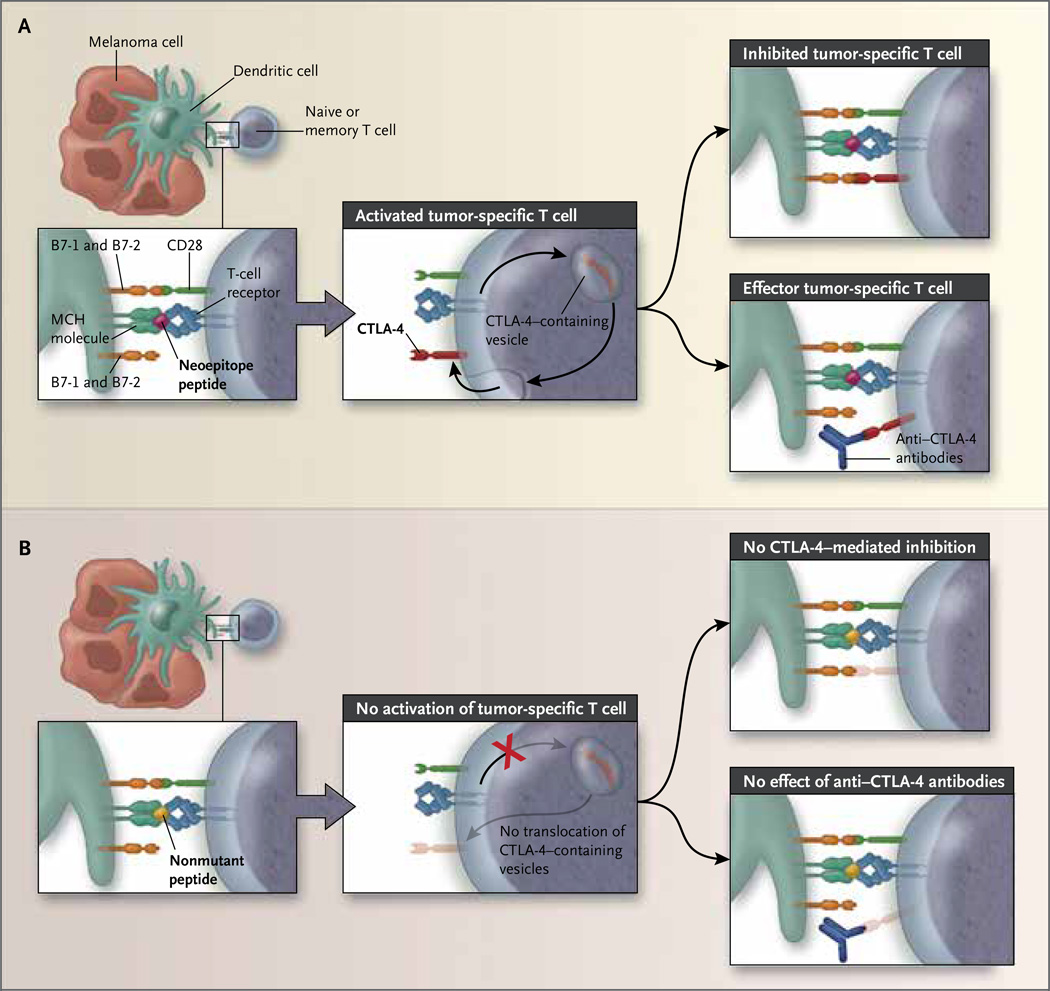
These findings are exciting for two main reasons. First, these data provide convincing evidence that in order for a CTLA-4 checkpoint blockade to mediate clinical benefit, T cells must be activated in the context of tumor-associated antigens. In unstimulated T cells, a small amount of CTLA-4 resides in intracellular vesicles of the endosomal and trans-Golgi network and recycles continuously to the cell surface, followed by rapid endocytosis and degradation. T-cell activation leads to an increase in CTLA-4, translocation of CTLA-4–containing vesicles to the immunologic synapse, and the release and expression of CTLA-4 on the cell surface. The stronger the T-cell–receptor signal, the more CTLA-4 accumulates at the immunologic synapse. 10 It is conceivable that in patients with melanomas expressing immunogenic neoepitopes, mutant peptides capable of binding MHC class I molecules with high affinity induce T-cell activation, leading to relocalization of CTLA-4–containing intracellular vesicles to the immunologic synapse, release of CTLA-4, and up-regulation of CTLA-4 on the cell surface. Under these conditions, anti–CTLA-4 antibodies reverse the inhibitory effect of CTLA-4, which is mediated by B7-1 and B7-2 ligation, and induce long-term antitumor responses (Fig. 1A). In contrast, in patients lacking such mutations, nonmutant peptides corresponding to the nonmutated counterparts of immunogenic neoepitopes have lower affinity for MHC class I molecules and do not induce T-cell activation. Under these conditions, CTLA-4 remains in the intracellular vesicles and is not up-regulated on the cell surface. Consequently, anti–CTLA-4 antibodies yield no clinical benefit (Fig. 1B).
Figure 1. Somatic Neoepitopes of Melanomas and Benefit from CTLA-4 Blockade.
Recognition of epitopes by T-cell receptors can be mediated by consensus tetrapeptides in immunogenic peptides. As shown in Panel A, mutant tetrapeptides that bind major histocompatibility complex (MHC) class I molecules with high affinity induce activation of antigen-specific naive or memory T cells that recognize this tumor-specific antigen, leading to translocation of cytotoxic T-lymphocyte antigen 4 (CTLA-4)–containing vesicles to the immunologic synapse and up-regulation of CTLA-4 expression on the cell surface. Ligation of CTLA-4 by B7-1 and B7-2 mediates an inhibitory signal in these tumor-specific T cells. Under these conditions, anti–CTLA-4 antibodies prevent CTLA-4 ligation and CTLA-4–mediated inhibition, resulting in the generation of functional tumor-specific effector T cells, which induce anti-tumor responses. As shown in Panel B, in the absence of such mutations, nonmutant tetrapeptides corresponding to the nonmutated counterparts of immunogenic somatic neoepitopes have lower affinity for MHC class I molecules and do not induce activation of antigen-specific naive or memory T cells that recognize this tumor-specific antigen. Under these conditions, CTLA-4–containing vesicles do not translocate to the immunologic synapse, CTLA-4 expression is not up-regulated, and anti–CTLA-4 antibodies confer no clinical benefit.
Second, these findings indicate that clinical benefit from CTLA-4 blockade depends on responses against epitopes that T cells are likely to recognize, such as those present on viral and bacterial pathogens. It is tempting to speculate that development of these antitumor responses might also be mediated by memory T cells generated during prior exposure to such antigens that were foreign to the host and happened to be homologous to neoepitopes induced by somatic mutations in melanomas. Importantly, a high mutational burden increased the likelihood of the development of specific neoepitopes that would confer clinical benefit from CTLA-4 blockade. In the era of immunotherapy, genetic diversity of cancer may, in fact, be a good thing.
Footnotes
Disclosure forms provided by the author are available with the full text of this article at NEJM.org.
References
- 1.Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA, Jr, Kinzler KW. Cancer genome landscapes. Science. 2013;339:1546–1558. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non�small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 3.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non�small-cell lung cancer. N Engl J Med. 2010;363:1693–1703. doi: 10.1056/NEJMoa1006448. [Erratum, N Engl J Med 2011;364:588.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alexandrov LB, Nik-Zainal S, Wedge DC, et al. Signatures of mutational processes in human cancer. Nature. 2013;500:415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenberg SA, Spiess P, Lafreniere R. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science. 1986;233:1318–1321. doi: 10.1126/science.3489291. [DOI] [PubMed] [Google Scholar]
- 7.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [Erratum, N Engl J Med 2010;363:1290.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti�PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Snyder A, Makarov V, Merghoub T, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Egen JG, Allison JP. Cytotoxic T lymphocyte antigen-4 accumulation in the immunological synapse is regulated by TCR signal strength. Immunity. 2002;16:23–35. doi: 10.1016/s1074-7613(01)00259-x. [DOI] [PubMed] [Google Scholar]