Abstract
The factors contributing to the development of spatial imagery skills are not well understood. Here we ask whether visual experience shapes these skills. Although differences between sighted and the blind on spatial imagery have been reported, it is unclear whether they are truly due to visual deprivation or extraneous factors such as reduced opportunities for the blind to interact with their environment. A direct way of assessing vision’s contribution to spatial imagery development lies in determining whether these skills change soon after the onset of sight in a congenitally blind individual. We describe our results with ten children who gained sight after several years of congenital blindness. We find significant improvements in their spatial imagery skills following sight-restoring surgeries. These results provide evidence of vision’s contribution to spatial imagery and also have implications for the nature of internal spatial representations.
Introduction
Being able to imagine and reason about the spatial structure of our environment is a crucially important skill. We rely upon it for various tasks such as handling and manipulating objects, and planning routes through complex layouts. However, the factors that contribute to the development of spatial imagery and reasoning skills are still unclear.
Sensory input across multiple modalities (vision, audition, touch and proprioception) is rich with information about the spatial structure of our environment and the objects therein1,2. The redundancy of these multiple sources, and their strong interactions3,4 while providing robustness, also makes it difficult to titrate their individual contributions to spatial imagery. One way of gaining insight into this issue is to determine whether spatial skills change after the introduction of a sensory stream that an individual had been deprived of since birth. Obvious ethical considerations rule out forced sensory deprivation as an experimental manipulation with human subjects. This question has, therefore, remained largely unaddressed thus far.
One promising way forward is to study those rare cases wherein people have not received treatment for disorders that cause profound sensory loss in a particular modality, even though their conditions are curable. Previous studies have demonstrated the effectiveness of sight-restoring surgeries approach for studying various aspects of visual development5. However the influence of vision on spatial imagery task is not well researched. Do the spatial skills of such individuals change from before to after treatment? Specifically, we ask whether the spatial imagery skills of congenitally blind children change after they receive sight-restoring surgeries.
Our work builds upon earlier studies by other investigators who have compared spatial imagery skills of normally sighted and blind individuals. Although these experiments cannot reveal the influence of sight initiation on spatial skills after a lifetime of blindness, they do provide interesting cross-sectional data showing whether and how long-term visual deprivation affects spatial abilities.
The basic finding from these studies is that people born without sight are able to mentally experience spatial representations6-10, showing abilities similar to those of sighted individuals in generating pictures by means of haptic stimuli11,12, performing classic mental rotation13, mental scanning14, and motor imagery tasks15. Moreover, it appears that blind and sighted participants rely on similar processes while carrying out imagery tasks, since they exhibit similar disruptions in performance by a spatial interference task when analyzing the shape of a series of mentally generated objects or when following a pattern on a mentally generated matrix16.
Several studies have found that congenitally blind individuals perform less accurately than age-matched sighted participants in spatial imagery tasks17-26. However, the robustness of these group differences is debatable. Some studies have argued that visual experience is neither necessary nor sufficient for the development of spatial representations27-32. Furthermore, the differences may be tied to specific task scenarios. For instance, Vecchi and his colleagues33 suggested that the difficulty the blind experience may be tied more to the maintenance of multiple spatial structures in memory simultaneously rather than in manipulating any single one. It is also unclear whether any observed differences in the spatial skills of sighted and blind individuals are due to the lack of visual experience per se or due to the long-term (typically several years in duration) limitations on environmental exploration imposed by blindness. Furthermore, even if we accept that visual experience contributes to spatial skills, is its influence subject to a critical window of time during development, or can it be effective much later in life as well?
To summarize, past results on the contribution of vision to spatial imagery skills provide a mixed picture. Blind individuals are able to perform various imagery tasks, and the differences they exhibit relative to sighted participants cannot be definitively attributed to the lack of their visual experience. We believe that a more reliable way forward would be to adopt a longitudinal approach which would allow us to determine whether the onset of sight in a blind individual leads to changes in his/her spatial skills.
Methods
Subjects
We worked with three groups of participants.
Group 1
30 early blind children (15 males; 12.5-15 years, mean age 13 years). Individual subject information (age, cause of blindness) is appended to this manuscript as supplementary information. All children were enrolled in a school for the blind in New Delhi and knew Braille.
Group 2
30 normally sighted children (15 males; 9-11 years, mean age 10.5 years).
Group 3
10 congenitally blind children with treatable blindness (10 males; 12-22 years, mean age 15 years). All children in this group were blind due to dense bilateral congenital cataracts. Assessment of the congenitality of visual deprivation was based on parental reports and also the presence of nystagmus which is known to be induced by profound visual impairment very early in life34. Individual subject information is appended to this manuscript. The children were identified via outreach activities undertaken as part of Project Prakash35,36. All children were provided surgeries which involved cataract extraction and intra-ocular lens implantation. Post-operative visual acuity ranged from 1.51 logMAR to 1.17 logMAR, with a mean of 1.38 logMAR.
There was no history of neurological or psychiatric illness in any of the subjects. Informed consent was obtained from all subjects prior to our study.
Groups 1 and 2 were matched in size and gender composition to allow a cross-sectional comparison of their results. Group 3 was intended to derive longitudinal data (before and after sight-restoring surgery) allowing for a within-group analysis.
Stimuli and Procedure
The experimental stimuli comprised a series of square matrices with different numbers of elements (2×2, 3×3 and 4×4). Using raised pegs on a flat plastic board, the configurations of these matrices were designed to be conveyed easily by touch (figure 1). Subjects were seated comfortably and asked to tactually explore the peg-matrix configurations for 30 seconds to become familiar with their arrangement. They were free to use one or both hands to explore the stimuli. The lower-left peg was designated to be the ‘origin’. The normally sighted (group 2) and congenitally blind subjects (group 3) after sight onset were blindfolded during this and remaining phases of the experiment. They had no visual experience with the peg board.
Figure 1.
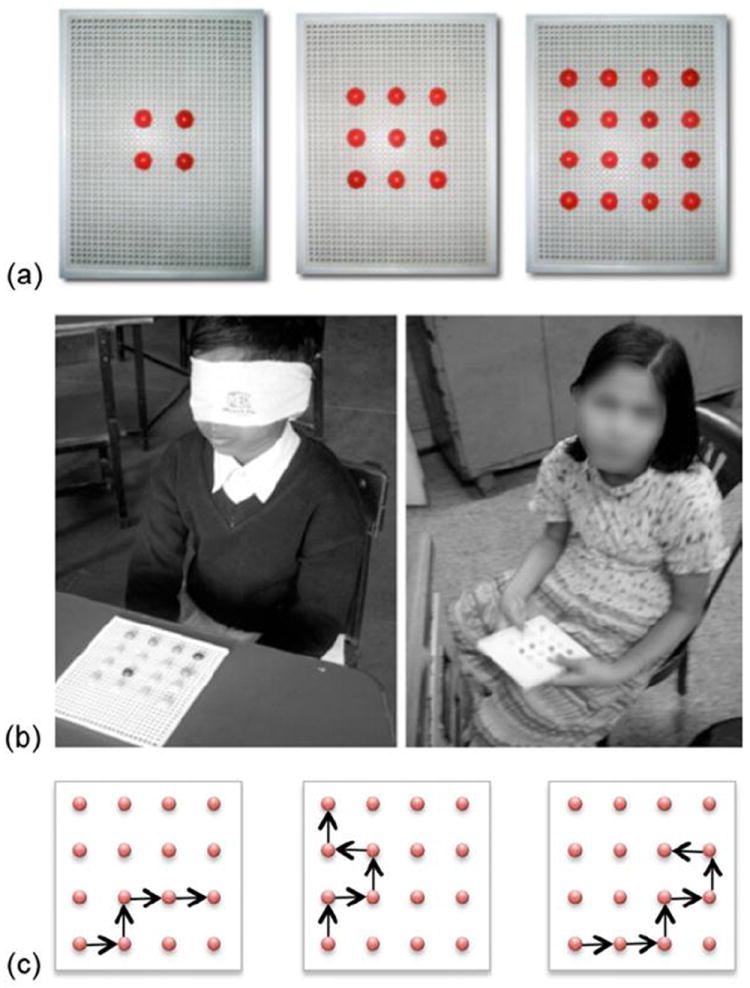
(a) The different peg matrices used in our studies. (b) Two participants performing spatial reasoning tasks. Left panel: Sighted child with a blindfold (group 1); Right panel: Congenitally blind child (group 2). (c) Sample command chains of length 4, 5 and 6 on a 4×4 grid.
During the test, the training grid was removed from the reach of the subjects. They were directed to follow chains of directional commands given verbally by the experimenter. Each command involved a one step movement in the horizontal or vertical direction, starting at the origin. Subjects were asked to keep their hands still during the testing to prevent them from using any exogenous tactile reference frames.
There were three levels of command chains: levels 4 through 6 (corresponding to the number of sequential steps on the pegs of the matrix), as shown in figure 1c. Each subject underwent three trials at each command-chain level for each matrix size. For group 3 (newly sighted), we additionally included a level 3 command chain.
After the delivery of a command-chain, the matrix grid was again placed in a subject’s hands and he/she was asked to point to the final position of the peg on the grid. No feedback was provided to the subjects.
Members of group 3 participated in two experimental sessions. The first was conducted prior to their surgery and the second was conducted post-surgery. The mean time to follow-up was 4.5 months.
Results
We report two results. The first is a cross-group comparison of the performance of sighted and congenitally blind participants. The second is a within-group comparison of the performance of group 3 participants pre- and post-surgery. The first result serves as a precursor to the second, and primary, result. It establishes differences between blind and sighted participants and sets the stage for an investigation of whether these differences can be bridged longitudinally by the introduction of sight.
Result 1
We conducted a 3-way ANOVA to investigate the main effects of children’s visual status (sighted versus blind), sizes of matrices (2×2, 3×3 and 4×4) and length of command chains (4,5 and 6 level commands) and their possible interactions. The dependent variable was performance defined as the proportion of trials in which a subject correctly indicated the terminal position of a command chain.
The main effect of visual status was significant (F1, 58) = 175.34, p < 0.001,η2=0.751); sighted participants overall outperformed visually impaired ones. Accuracy averaged across all conditions was 90.3% for sighted participants and 58.4% for the visually impaired. The main effect of matrix size was significant (F2,116 = 46.781, p<0.001, η2=0.446) with larger matrix sizes eliciting poorer performance. The interaction between matrix size and command chains was significant (F2,116 = 8.966, p < 0.001, η2=0.133); overall accuracy decreased with increased level of command complexity. The interactions between visual status and matrix size as well as between visual status and command chain length were significant ((F2,116 = 45.154, p<0.001, η2=0.437) and (F2,116 = 16.693, p<0.00, η2=0.223) respectively). Blind subjects performed well with small matrix sizes and low levels of command-chain length. With increasing task complexity, their performance decreased significantly relative to that of the sighted subjects.
The good performance of blind participants on low complexity grids/tasks indicates that they understand the basic task requirements. Differences in performance across sighted and blind groups replicates previous results22-24, 33, and more importantly, sets the stage for examining whether the observed differences between the sighted and blind can be mitigated if sight is initiated in the latter group.
Result 2
Figure 3 shows the pre- and post-surgical performance of group 3. Recapitulating results from group 1, pre-operative performance is high for small matrices and short command-chains, but declines as either of these parameters take on higher values. Post-treatment, the children were re-tested during a follow-up visit, on average 4.5 months after their surgeries. The children had spent the intervening period in their homes. No training or other visual rehabilitation was provided to them during this period.
Figure 3.
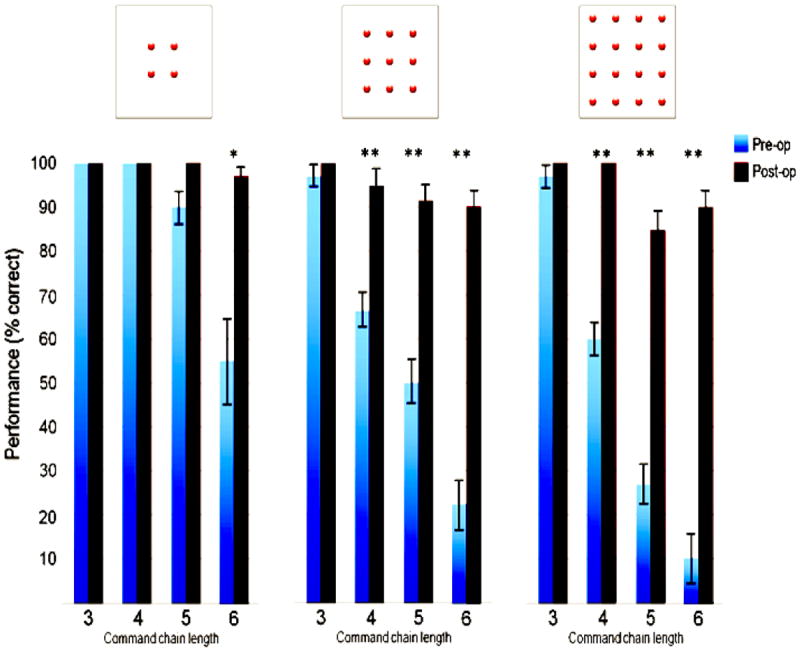
Performance of congenitally blind children on spatial reasoning tasks of different complexities before and after gaining sight (blue and black bars, respectively). Levels of statistical significance: *: 0.05, **: 0.01.
The children exhibited marked improvements in their performance post-operatively. They were proficient, often near ceiling levels, on the matrix sizes and command-chain levels that pre-operatively they had performed poorly on. From the results of 3-way repeated-measures ANOVA with surgical status (pre vs. post), matrix size and command chain length as within-subject factors, we found a main effect of sight acquisition; post-operative performance (95.8%) significantly exceeded pre-operative performance (64.8%) (F1,9 = 691.38, p<0.001, η2=0.987), aggregating data across all matrix sizes and command chain levels. The main effect of matrix size was significant at p<0.001 (F2,18 = 42.859, η2=0.988) as was the main effect of command chain length (F3,27=220.14, η2=0.96). The interactions between surgical status and matrix size as well as command chain length were both significant at p<0.001 (F2,18 = 34.724, η2= 0.794 and F3,27 = 69.893, η2=0.885 respectively).
Discussion
Our goal was to examine whether visual experience contributes to spatial imagery skills. We find that a basic level of spatial imagery can be developed even with very limited visual experience, as demonstrated by the ability of group 1 and group 3 pre-operatively to perform well on simple matrices and short command-chains. However, spatial imagery skills of congenitally blind children improve significantly and rapidly after the onset of sight. Taken together, these results suggest that visual experience can significantly enhance spatial imagery capabilities. This appears to be consistent with the distinctions between the different senses: audition, touch and proproception convey spatial information37-39, but do not match the richness of spatial detail provided by the visual modality40-42. This is echoed in our results showing that in the presence of profound visual impairment, the spatial abilities that develop are less able to handle complex imagery tasks than those following the onset of sight.
Besides demonstrating that internal spatial representations are enriched by visual information, the results reported here also bear upon the question of when such enrichment can happen. Analogous to the notion of sensory ‘critical periods’43, there could also be a critical period for the development of spatial imagery skills. In other words, perhaps a sensory modality can influence spatial reasoning abilities only during a critical window early in the developmental timeline. If that period elapses without a sensory modality being available, then later restoration of that input will not have an impact on spatial abilities. Our results argue against such a notion in the context of vision. To the extent that congenitally blind children as old as 18 years of age show significant improvements in their spatial abilities after the onset of sight, we are led to conclude that the ability of vision to contribute to spatial skills is either not subject to a strict critical period, or the critical period, if it exists, extends beyond the late teenage years.
These findings bring up several interesting questions that await further study. We highlight three. First, does the nature of spatial imagery change qualitatively in progressing from blindness to sight44,45 ? If so, do the newly sighted use a fundamentally different imagery system post-operatively from the one they used pre-operatively? Or, is the post-operative system a more elaborated version of the same one that they used pre-operatively? Perhaps one way of addressing this issue will be by characterizing differences in tactile exploration strategies of the children from before to after sight onset. Functional brain imaging studies to examine neural correlates of mental imagery46-49 pre- and post-operatively will also help address this issue.
Second, what kinds of learning and representational change mechanisms can account for the rapidity with which spatial imagery abilities are seen to change after the onset of sight? In this context, some of our other results from Project Prakash deserve mention. While investigating the Molyneux question50 with the newly sighted, we found that even though these children did not appear to possess a mapping between the spatial information provided by touch and that provided by vision immediately after sight restoring surgery, the mapping developed rapidly, in some cases over the course of a week. It will be interesting to ask whether the learning processes that yield cross-modal spatial mapping are related to the ones that enhance spatial imagery abilities, and precisely what their neural substrates might be.
Third, from an applied perspective, these results point to the capacity for improvement in spatial skills well into childhood. It is worth asking whether this improvement can be achieved in any way other than sight surgeries. This is of relevance to the many blind individuals whose blindness is currently not treatable.
Supplementary Material
Figure 2.
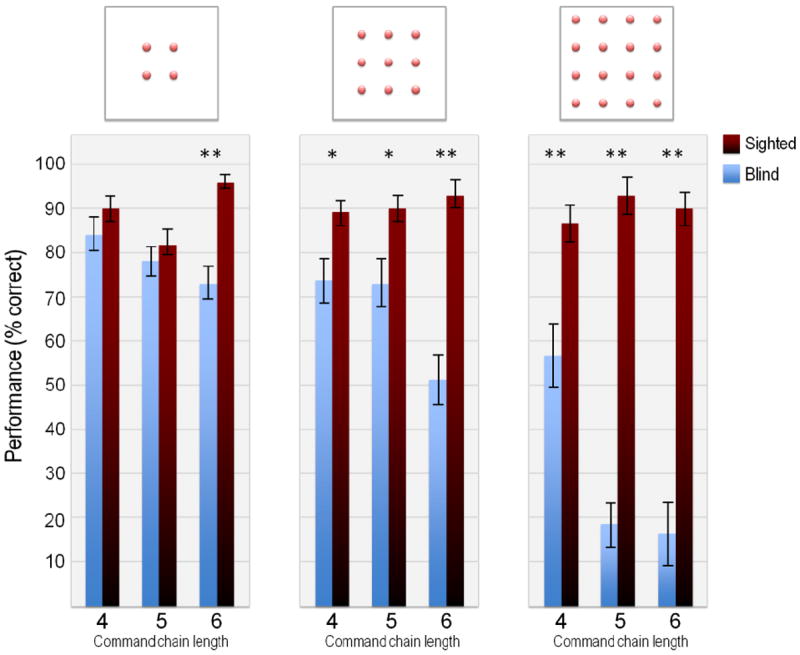
Mean performance accuracy for blind and sighted (blindfolded) groups. The three panels correspond to different matrix sizes (2×2, 3×3 and 4×4) and the three bar pairs within each panel show performance on command chains of different lengths (4, 5 and 6). Level of statistical significance: *: 0.05, **: 0.01
Acknowledgments
The authors wish to thank all children who participated in these studies, members of the Project Prakash team who were instrumental in identifying treatably blind children, Dr. Amy Kalia for her help with data analysis and Prof. Daphne Maurer for her helpful comments on an earlier draft of this manuscript. The research reported here was supported by the James McDonnell Foundation and the National Eye Institute of NIH.
References
- 1.Woods AT, Newell FN. Visual, haptic and cross-modal recognition of objects and scenes. J Physiol Paris. 2004;98(1-3):147–159. doi: 10.1016/j.jphysparis.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 2.Lacey S, Sathian K. Multisensory object representation: insights from studies of vision and touch. Prog Brain Res. 2011;191:165–176. doi: 10.1016/B978-0-444-53752-2.00006-0. [DOI] [PubMed] [Google Scholar]
- 3.Stein BE, Meredith MA. The merging of the senses. MIT Press; 1993. [Google Scholar]
- 4.Deneve S, Pouget A. Bayesian multisensory integration and cross-modal spatial links. Journal of Physiology-Paris. 2004;98:1–3. 249–258. doi: 10.1016/j.jphysparis.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 5.Maurer D, Lewis TL, Mondloch CJ. Missing sights: consequences for visual cognitive development. Trends Cogn Sci. 2005;9:144–151. doi: 10.1016/j.tics.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Arditi A, Holtzman JD, Kosslyn SM. Mental imagery and sensory experience in congenital blindness. Neuropsychologia. 1988;26(1):1–12. doi: 10.1016/0028-3932(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 7.Forrest EB. The innate vs. the learned: visual imagery and the role of experience. J Am Optom Assoc. 1984;55(1):43–46. [PubMed] [Google Scholar]
- 8.Haber RN, Haber LR, Levin CA, Hollyfield R. Properties of spatial representations: data from sighted and blind subjects. Perception & Psychophysics. 1993;54(1):1–13. doi: 10.3758/bf03206932. [DOI] [PubMed] [Google Scholar]
- 9.Vecchi T. Visuo-spatial limitations in congenitally totally blind people. Memory. 1998;6:91–102. doi: 10.1080/741941601. [DOI] [PubMed] [Google Scholar]
- 10.Zimler J, Keenan JM. Imagery in the congenitally blind: how visual are visual images? J Exp Psychol Learn Mem Cogn. 1983;9(2):269–282. doi: 10.1037//0278-7393.9.2.269. [DOI] [PubMed] [Google Scholar]
- 11.Carreiras M, Codina M. Spatial cognition of the blind and sighted: visual and amodal hypotheses. Curr Psychol Cogn. 1992;12:51–78. [Google Scholar]
- 12.Klatzky RL, Golledge RG, Loomis JM, Cicinelli JG, Pellegrino JW. 1995 [Google Scholar]
- 13.Marmor GS, Zaback LA. Mental rotation by the blind: does mental rotation depends on visual imagery? J Exp Psychol Hum Percept Perform. 1976;2:515–521. doi: 10.1037//0096-1523.2.4.515. [DOI] [PubMed] [Google Scholar]
- 14.Kerr NH. The role of vision in “visual imagery” experiments: evidence from the congenitally blind. J Exp Psychol Gen. 1983;112:265–277. doi: 10.1037//0096-3445.112.2.265. [DOI] [PubMed] [Google Scholar]
- 15.Imbiriba LA, Rodrigues EC, Magalhaes J, Vargas CD. Motor imagery in blind subjects: The influence of the previous visual experience. Neuroscience Letters. 2006;400:181–185. doi: 10.1016/j.neulet.2006.02.042. [DOI] [PubMed] [Google Scholar]
- 16.Aleman A, van Lee L, Mantione MH, Verkoijen IG, de Haan EH. Visual imagery without visual experience: evidence from congenitally totally blind people. NeuroReport. 2001;12:2601–2604. doi: 10.1097/00001756-200108080-00061. [DOI] [PubMed] [Google Scholar]
- 17.Warren DH. Blindness and early childhood development. New York: AmericanFoundation for the Blind Press; 1977. [Google Scholar]
- 18.Hatwell Y. From perception and related issues in blind humans. In: Held R, Leibowitz HW, Teuber HL, editors. Handbook of sensory physiology. Berlin: Springer Verlag; 1978. [Google Scholar]
- 19.Byrne RW, Salter E. Distance and directions in the cognitive maps of the blind. Canadian Journal of Psychology. 1983;37:293–299. doi: 10.1037/h0080726. [DOI] [PubMed] [Google Scholar]
- 20.Eimer M. Multisensory integration: How visual experience shapes spatial perception. Current Biology. 2004;14:115–117. [PubMed] [Google Scholar]
- 21.Knauff M, May E. Mental imagery, reasoning and blindness. Q J Exp Psychol. 2006;59(1):161–177. doi: 10.1080/17470210500149992. [DOI] [PubMed] [Google Scholar]
- 22.Cattaneo Z, Vecchi T, Monegato M, Pece A, Cornoldi C. Effects of visual impairment on mental representations activated by visual and tactile stimuli. Brain Research. 2007;1148:170–176. doi: 10.1016/j.brainres.2007.02.033. [DOI] [PubMed] [Google Scholar]
- 23.Cornoldi C, Cortesi A, Preti D. Individual differences in the capacity limitation of visuo-spatial short-term memory: research on sighted and totally congenitally blind people. Mem Cogn. 1991;19:459–468. doi: 10.3758/bf03199569. [DOI] [PubMed] [Google Scholar]
- 24.Cornoldi C, Bertuccelli B, Rocchi P, Sbrana B. Processing capacity limitations in pictorial and spatial representations in the totally congenitally blind. Cortex. 1993;29:675–689. doi: 10.1016/s0010-9452(13)80290-0. [DOI] [PubMed] [Google Scholar]
- 25.De Beni R, Cornoldi C. Imagery limitations in totally congenitally blind subjects. Journal Experimental Psychology: Learning, Memory and Cognition. 1988;14(4):650–655. doi: 10.1037/0278-7393.14.4.650. [DOI] [PubMed] [Google Scholar]
- 26.Gandhi TK, Khurana A, Santhosh J, Anand S. Configurational imagery experience in sighted and visually impaired children. Indian Academy of Applied Psychology. 2011;37:128–133. [Google Scholar]
- 27.Millar S. Understanding and representing space: Theory andevidence from studieswith blind and sighted children. Oxford: Oxford University Press; 1994. [Google Scholar]
- 28.Thinus-Blanc C, Gaunet F. Representation of space in blind persons: vision as a spatial sense? Psychol Bull. 1997;121(1):20–42. doi: 10.1037/0033-2909.121.1.20. [DOI] [PubMed] [Google Scholar]
- 29.Cornoldi C, Vecchi T. Mental imagery in blind people: The role of passiveand active visuo-spatial processes. In: Heller M, editor. Touch, representation and blindness. Oxford: Oxford University Press; 2000. [Google Scholar]
- 30.Cattaneo Z, Vecchi T, Cornoldi C, Mammarella I, Bonino D, Ricciardi E, et al. Imagery and spatial processes in blindness and visual impairment. Neuroscience and Biobehavioral Reviews. 2008;32:1346–1360. doi: 10.1016/j.neubiorev.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 31.Iachini T, Ruggiero G. The role of visual experience in mental scanning of actual pathways: Evidence from blind and sighted people. Perception. 2010;39:953–969. doi: 10.1068/p6457. [DOI] [PubMed] [Google Scholar]
- 32.Afonso A, Blum A, Katz BFG, Tarroux P, Borst G, Denis M. Structural properties of spatial representations in blind people: Scanning images constructedfrom haptic exploration or from locomotion in a 3-D audio virtual environment. Memory & Cognition. 2010;38:591–604. doi: 10.3758/MC.38.5.591. [DOI] [PubMed] [Google Scholar]
- 33.Vecchi T, Tinti C, Cornoldi C. Spatial memory and integration processes in congenital blindness. NeuroReport. 2004;15:2787–2790. [PubMed] [Google Scholar]
- 34.Tusa RJ, Repka MX, Smith CB, Herdma SJ. Early visual deprivation results in persistent strabismus and nystagmus in monkeys. Investigative Ophthalmology & Visual Science. 1991;32(1):134–141. [PubMed] [Google Scholar]
- 35.Sinha P. Once blind and now they see. Scientific American. 2013 Jul;2013:48–55. doi: 10.1038/scientificamerican0713-48. [DOI] [PubMed] [Google Scholar]
- 36.Sinha P, Held R. Sight restoration. F1000 Medicine Reports. 2012;4:17. doi: 10.3410/M4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caclin A, Soto-Faraco S, Kingstone A, Spence C. Tactile “capture” of audition. Percept Psychophys. 2002;64(4):616–630. doi: 10.3758/bf03194730. [DOI] [PubMed] [Google Scholar]
- 38.Perrott D, Saberi K. Minimum audible angle thresholds for sources varying in both elevation and azimuth. J Accoust Soc Am. 1990;87:1728–1731. doi: 10.1121/1.399421. [DOI] [PubMed] [Google Scholar]
- 39.Pick HL, Warren DH, Hay JC. Sensory conflict in judgments of spatial direction. Perception & Psychophysics. 1969;6(4):203–205. [Google Scholar]
- 40.Witten IB, Knudsen EI. Why seeing is believing: merging auditory and visual worlds. Neuron. 2005;48:489–496. doi: 10.1016/j.neuron.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 41.Kassuba T, Klinge C, Hölig C, Röder B, Siebner HR. Vision holds a greater share in visuo-haptic object recognition than touch. Neuroimage. 2013;65:59–68. doi: 10.1016/j.neuroimage.2012.09.054. [DOI] [PubMed] [Google Scholar]
- 42.Warren DH, Welch RB, McCarthy TJ. The role of visual-auditory “compellingness” in the ventriloquism effect: implications for transitivity among the spatial senses. Percept Psychophys. 1981;30(6):557–564. doi: 10.3758/bf03202010. [DOI] [PubMed] [Google Scholar]
- 43.Daw N. Visual Development. Plenum Press; New York: 2006. [Google Scholar]
- 44.Kaski D. Revision: Is visual perception a requisite for visual imagery? Perception. 2002;31(6):717–731. doi: 10.1068/p3360. [DOI] [PubMed] [Google Scholar]
- 45.Röder B, Rösler F, Hennighausen E. Different cortical activation patterns in blind and sighted humans during encoding and transformation of haptic images. Psychophysiology. 1997;34(3):292–307. doi: 10.1111/j.1469-8986.1997.tb02400.x. [DOI] [PubMed] [Google Scholar]
- 46.D’Esposito M, Detre JA, Aguirre GK, Stallcup M, Alsop DC, Tippet LJ, Farah MJ. A functional MRI study of mental imagery generation. Neuropsychologia. 1997;35(5):725–730. doi: 10.1016/s0028-3932(96)00121-2. [DOI] [PubMed] [Google Scholar]
- 47.Knauff M, Kassubek J, Mulack T, Greenlee MW. Cortical activation evoked by visual mental imagery as measured by fMRI. Neuroreport. 2000;11(18):3957–62. doi: 10.1097/00001756-200012180-00011. [DOI] [PubMed] [Google Scholar]
- 48.Kosslyn SM, Thompson WL, Kim IJ, Alpert NM. Topographical representations of mental images in primary visual cortex. Nature. 1995;378:496–498. doi: 10.1038/378496a0. [DOI] [PubMed] [Google Scholar]
- 49.Kosslyn SM, Pascual-Leone A, Felician O, Camposano S, Keenan JP, Thompson WL, Ganis G, Sukel KE, Alpert NM. The role of area 17 in visual imagery: convergent evidence from PET and rTMS. Science. 1999;284(5411):167–170. doi: 10.1126/science.284.5411.167. [DOI] [PubMed] [Google Scholar]
- 50.Held R, Ostrovsky Y, de Gelder B, Gandhi T, Ganesh S, Mathur U, Sinha P. The newly sighted fail to match seen with felt. Nature Neuroscience. 2011;14:551–554. doi: 10.1038/nn.2795. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


