Abstract
The inducible T cell costimulator (ICOS) is a potent promoter of organ inflammation in murine lupus. ICOS stimulates T follicular helper cell differentiation in lymphoid tissue, suggesting that it might drive autoimmunity by enhancing autoantibody production. Yet, the pathogenic relevance of this mechanism remains unclear. It is also unknown whether other ICOS-induced processes might contribute to lupus pathology. Here we show that selective ablation of ICOS ligand (ICOSL) in CD11c+ cells but not in B cells dramatically ameliorates kidney and lung inflammation in lupus-prone MRL. Faslpr mice. Autoantibody formation was largely unaffected by ICOSL deficiency in CD11c+ cells. However, ICOSL display by CD11c+ cells in inflamed organs had a nonredundant role in protecting invading T cells from apoptosis by elevating activity of the PI3K-Akt signaling pathway, thereby facilitating T cell accrual. These findings reveal a mechanism that locally sustains organ inflammation in lupus.
Introduction
Systemic lupus erythematosus (SLE) is a relapsing-remitting autoimmune syndrome in which aberrant T cell function leads to multi-organ inflammation (Bagavant and Fu, 2009). This pathological T cell response is orchestrated by various antigen-presenting cell (APC) subsets. Previous studies have demonstrated that B cells are critical for the induction of T cell autoimmunity in lupus mouse models (Chan and Shlomchik, 1998; Jacob et al., 2011). This function of B cells is partly dependent on their prior activation via the MyD88 pathway (Teichmann et al., 2013). In contrast, we recently demonstrated that CD11c+ cells, such as dendritic cells (DCs) and certain macrophages, contribute to T cell pathogenicity after disease is initiated, leading to tissue damage (Teichmann et al., 2010). Defining the signals by which specific types of APCs drive T cell autoimmunity and elucidating where and when these signals play a role will advance our capacity to develop new therapeutics that rely on disrupting T cell-APC interactions.
Accumulating evidence suggests that the T cell-expressed inducible costimulator (ICOS) is instrumental in T cell-driven multi-organ inflammation in lupus. In MRL. Faslpr mice, a mouse model of spontaneous systemic autoimmunity that is based on polygenic factors in the MRL genetic background and accelerated by Fas-deficiency, deletion of Icos confers protection from proteinuria and interstitial nephritis (Odegard et al., 2009). In sanroque mice a single amino acid substitution in Roquin-1 precipitates a lupus-like disease that is thought to arise from deregulated expression of multiple genes in the immune system, including Icos (Leppek et al., 2013). ICOSL (B7h, B7RP-1), the only known ligand for ICOS, is expressed by B cells, conventional DCs (cDCs), macrophages and non-hematopoietic cells. Notably, ICOSL is expressed by renal tubuloepithelial cells (de Haij et al., 2005), which might play a role in nephritis.
Upon ligation ICOS signals through PI3K. It contains a unique YMFM SH2 binding motif that has the ability to recruit a PI3K variant composed of the canonical p110 catalytic subunit and the p50α regulatory subunit (Fos et al., 2008). This form of PI3K has a particularly high lipid kinase activity. Its activation thus leads to robust production of phosphatidylinositol (3,4,5)-trisphosphate (PIP3) and concomitant stimulation of Akt kinase and mammalian target of rapamycin (mTOR). The PI3K-Akt pathway promotes cell proliferation and survival. Commensurately, in adoptive transfer studies, expansion and survival of effector OT-II CD4+ T cells deficient for ICOS was impaired, although it was not demonstrated whether this was due to reduced Akt activity (Burmeister et al., 2008).
ICOS signals are required for T follicular helper cell (Tfh) development. Tfh cells are a specialized subset of CD4+ T cells that localize to germinal centers and stimulate survival, proliferation, selection and differentiation of germinal center B cells. The transcriptional repressor Bcl6 is the defining transcription factor of Tfh cells. A sequential model was proposed in which Bcl6 expression is induced by ICOS-mediated signals during CD4+ T cell priming by DCs (Choi et al., 2011). After migration of T cells to the T:B border its sustained expression relies on ICOS ligation by cognate B cells, although this requirement can be overcome by high doses of antigen (Ag) (Weinstein et al., 2014). Recruitment of T cells from the T:B border into follicles appears to be facilitated by ICOSL on non-cognate B cells in a Bcl6-independent manner (Xu et al., 2013). c-Maf, a transcription factor that drives secretion of the Tfh cell signature cytokine interleukin-21 (IL-21), is also induced by ICOS (Bauquet et al., 2009).
Although these studies partially delineated the functions of ICOS in T cell responses to immunizations and infections, there remains a paucity of information regarding the mechanistic underpinning of ICOS-driven systemic autoimmunity. It is unknown which cell types are primarily responsible for promoting lupus by engaging ICOS. Whether ICOS has non-redundant functions in inhibiting apoptosis and cell cycle arrest in lupus has not been shown. Conceivably, the ubiquity of (self-) Ag and the inflammatory milieu obviates the need for ICOS induced activation of the PI3K-Akt pathway. In several mouse models of SLE, including MRL. Faslpr mice, differentiation of B cells into autoantibody (auto-Ab)-secreting cells takes place extrafollicularly. In these mice, auto-Ab generation is thought to be supported by CD4+ T cells in the red pulp that resemble Tfh cells in gene expression profile and B cell helper functionality (Odegard et al., 2008). Those cells are called T extrafollicular helper cells (Tefh). Like Tfh cells, they express Bcl6 and secrete IL-21. Deletion of Icos in MRL. Faslpr mice reduces the frequency of Tefh cells (Odegard et al., 2008) but it is unclear whether this is due to a lack of Bcl6 expression. If so, it is questionable whether Bcl6 is initially induced by DCs given that in this strain T cell activation is largely independent of DCs (Teichmann et al., 2010) but highly dependent on B cells (Chan and Shlomchik, 1998). Finally, a recent study described Bcl6+IL-21+ T helper (Th) cells in the pancreas and salivary glands of NOD mice, a murine model of type I diabetes, that facilitated CD8+ T cell expansion and β-islet destruction (McGuire et al., 2011). Thus, the critical function of Bcl6+ Th cells in lupus might not only be to help B cells and promote auto-Ab production but rather to assist cytotoxic T cells or to directly promote tissue damage.
To begin to address these issues, we used a tissue-specific gene deletion approach in a fully backcrossed lupus prone mouse strain. Specifically, we undertook a comprehensive analysis of MRL. Faslpr mice deficient for Icosl either in B cells or CD11c+ cells, which include DCs and CD11c+ macrophages. Our results uncover that mechanistic insights into ICOS-dependent T cell immunity gained from immunization or infection studies are of limited applicability to chronic systemic autoimmunity and provide a framework for understanding the effects of ICOS-targeted therapies that are currently being investigated.
Results
ICOS triggering by CD11c+ cells promotes interstitial nephritis and pneumonia
To investigate the contribution of ICOS ligation by CD11c+ cells and B cells to lupus development, we crossed Icosllfl/fl MRL. Faslpr mice to Itgax-Cre and CD19-Cre MRL. Faslpr lines, respectively. In Itgax-Cre Icoslfl/fl MRL. Faslpr mice (referred to as Itgax-IcoslΔ hereafter) 95.2% of Icoslfl alleles in splenic cDCs were deleted (Table S1) as determined by qRT-PCR on genomic DNA. Icoslfl ablation in red pulp macrophages was minimal (Table S1) but Itgax-Cre is active in peripheral tissue macrophages that express CD11c. Accordingly, 91.2% of Icoslfl alleles in the CD11c+MHCII+ cell subset in kidneys, which contains cDCs and macrophages (Satpathy et al., 2012), from Itgax-IcoslΔ mice were excised. In Cd19-Cre Icosllfl/fl MRL. Faslpr mice (Cd19-IcoslΔ) ablation of Icoslfl in B cells was very effective (95.8%, Table S1). The extent of Icoslfl deletion in Ab-forming cells (90.3%, Table S1) was comparable to that in B cells, suggesting that there was no selection for B cells that retained Icosl alleles during activation or differentiation. These data demonstrate that the Cre-loxP system is well suited to decipher the consequences of ICOS engagement by CD11c+ cells and B cells in lupus.
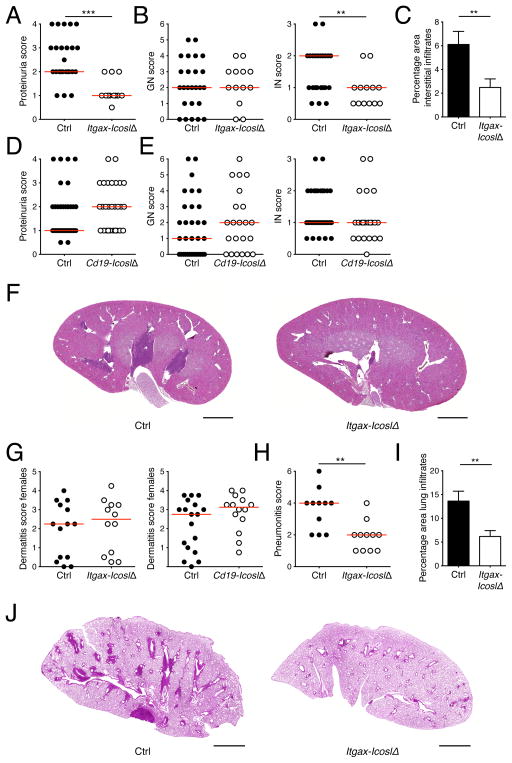
Multiorgan inflammation in MRL. Faslpr mice is dependent on both CD11c+ and B cells (Chan et al., 1999; Teichmann et al., 2010). To test whether ICOS triggering by these cell types promotes immune infiltration of tissues we performed a thorough analysis of several lupus target organs in Itgax-IcoslΔ and Cd19-IcoslΔ mice. Proteinuria, a measure of kidney function, was strikingly reduced in Itgax-IcoslΔ mice compared to controls (Fig. 1A). Histopathological evaluation of kidneys using a semiquantitative scoring system revealed that the decrease in proteinuria was associated with markedly less interstitial nephritis (IN, Fig. 1B) whereas glomerulonephritis (GN, Fig. 1B) was unaltered. There was a 59.0% reduction in relative infiltrate size in mice lacking ICOSL on CD11c+ cells (Fig. 1, C and F), quantitatively confirming the IN scoring. Unlike in Itgax-IcoslΔ mice, renal disease in Cd19-IcoslΔ mice was unaffected (Fig. 1, D and E). A similar examination of lungs further revealed a substantial reduction in lung inflammation in the absence of ICOSL on CD11c+ cells (Fig. 1, H–J). Finally, we assessed whether Icosl deletion in CD11c+ or B cells would affect the skin. Because skin inflammation is more common in MRL. Faslpr females than in males, we limited our analysis to females. Surprisingly, Icosl ablation in CD11c+ cells did not protect from dermatitis (Fig. 1G). Similarly, dermatitis severity in Cd19-IcoslΔ mice and controls was indistinguishable (Fig. 1G). Taken together, ICOS ligation by CD11c+ cells drives IN and interstitial pneumonia but does not contribute to dermatitis development indicating separate pathogenetic mechanisms.
Figure 1. ICOS triggering by CD11c+ cells promotes interstitial nephritis and pneumonia.
(A) Proteinuria scores of control and Itgax-IcoslΔ mice.
(B) Glomerular nephritis was scored from 0 to 6 and and interstitial nephritis from 0 to 3. Shown are scores of control and Itgax-IcoslΔ mice.
(C) The area of renal interstitial infiltrates expressed as a percentage of the total kidney section area of control (n = 25) and Itgax-IcoslΔ (n = 13) mice.
(D and E) As in (A) and (B) but for control and Cd19-IcoslΔ mice.
(F) Representative low magnification images of H&E stained kidneys sections (scale bar = 2 mm) illustrating perivascular infiltrates in kidneys from control and Itgax-IcoslΔ mice.
(G) Dermatitis scores of control and Itgax-IcoslΔ mice (left graph), or control and Cd19-IcoslΔ mice (right graph).
(H) Interstitial pneumonia was scored from 0 to 6 for control and Itgax-IcoslΔ mice.
(I) The pulmonary infiltrate area expressed as a percentage of the total lung section area of control (n = 11) and Itgax-IcoslΔ (n = 11) mice.
(J) Representative low magnification images of H&E stained lung sections (scale bar = 2 mm) illustrating pulmonary infiltrates in control and Itgax-IcoslΔ mice.
In scatter plots each dot represents an individual mouse and horizontal lines represent the median. Data in bar graphs are represented as mean ± SEM. See also Table S1.
ICOSL-deficiency in CD11c+ cells causes a loss of splenic Tefh and effector CD8+ T cells in lupus
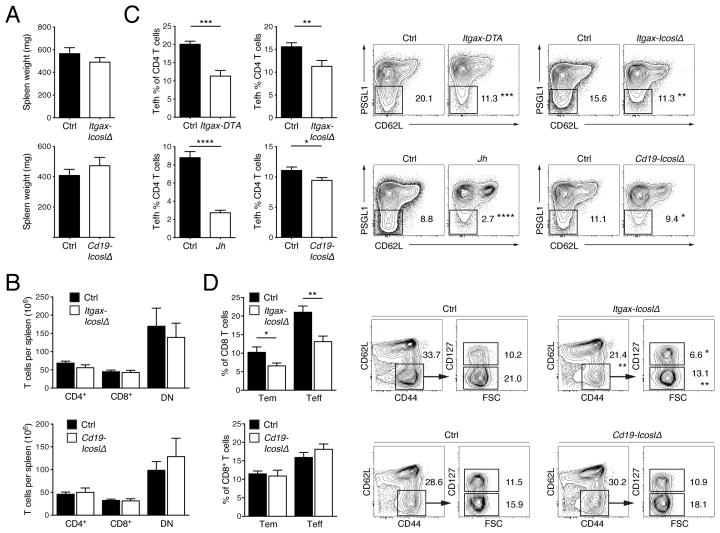
In previous work we have shown that T cell activation relies on B cells but not CD11c+ cells, whereas T cell expansion and differentiation into IFN-γ producing effectors is mediated by both cell types in the MRL. Faslpr strain (Chan and Shlomchik, 1998; Teichmann et al., 2010; Teichmann et al., 2013). We sought to clarify how ICOS triggering by distinct APCs impacts the spontaneous T cell response. Aged MRL. Faslpr mice develop splenomegaly and lymphadenopathy resulting from the accumulation of T cells, especially a subset characteristic of Fas-deficient mice that does not express the coreceptors CD4 and CD8. Spleen weight in Itgax-IcoslΔ and Cd19-IcoslΔ mice was similar to that in their respective controls (Fig. 2A). Accordingly, the loss of ICOSL on CD11c+ or B cells did not affect splenic CD4+, CD8+ or CD4− CD8− T cell numbers (Fig. 2B). Thus, T cell accumulation was not substantially impaired in the absence of ICOSL on CD11c+ or B cells.
Figure 2. ICOSL-deficiency in CD11c+ cells causes a selective loss of Tefh cells, CD8+ Teff and Tem cells in spleens of lupus-prone mice.
(A) Spleen weight of control (n = 34) and Itgax-IcoslΔ (n = 23) mice, or control (n = 32) and Cd19-IcoslΔ (n = 21) mice.
(B) Numbers of T cells per spleen of control (n = 25) and Itgax-IcoslΔ (n = 13) mice, or control (n = 25) and Cd19-IcoslΔ (n = 11) mice.
(C) Frequency of Tefh cells among CD4+ T cells in spleens from Itgax-IcoslΔ, Cd19-IcoslΔ, Itgax-DTA and Jh MRL. Faslpr mice with their respective controls. Contour plots illustrate the last gating step for Tefh cells (TCRβ+CD45R−CD4+CD44+CD62L−PSGL-1lo) (values indicate percentage of CD4+ T cells, mean). n = 13 for Itgax-IcoslΔ mice and n = 25 for controls; n = 20 for Cd19-IcoslΔ mice and n = 29 for controls; n = 9 Itgax-DTA and n = 12 for controls; n = 16 for Jh MRL. Faslpr mice and n = 12 for controls.
(D) Frequency of Teff and Tem cells among CD8+ T cells in spleens from Itgax-IcoslΔ and Cd19-IcoslΔ mice with their respective controls. Contour plots illustrate the last two gating steps for Tem (TCRβ+CD45R−CD8+CD44+CD62L−CD127+) and Teff (as Tem but CD127−) cells (values indicate percentage of CD8+ T cells, mean). n = 15 for Itgax-IcoslΔ mice and n = 13 for controls; n = 10 for Cd19-IcoslΔ mice and n = 12 for controls.
Data in bar graphs are represented as mean ± SEM. See also Figure S1.
Tfh cells have been the focus of intense research but little is known about their Tefh counterparts. To test whether Tefh cells require CD11c+ or B cells we determined the frequency of Tefh cells among CD4+ T cells in MRL. Faslpr mice that constitutively lack B cells (Jh MRL. Faslpr) or most CD11c+ cells (Itgax-Cre Rosa26-eGFP-DTA MRL. Faslpr, referred to as Itgax-DTA; (Teichmann et al., 2010)). Tefh cells were identified based on the surface phenotype PSGL-1loCD44+CD62L− TCRβ+CD45R−. Corresponding to their extrafollicular position, Tefh cells do not express CXCR5. Both B and CD11c+ cells contribute to Tefh development, as the CD4+ T cell pool contained considerably fewer Tefh cells in both B cell-deficient (Jh MRL. Faslpr, −69%) and CD11c+ cell-deficient (Itgax-DTA, −44%) mice (Fig. 2C). Tfh cells need ICOSL first on DCs for phenotype induction and then on B cells for maintenance. We therefore investigated Tefh cell frequencies in Itgax-IcoslΔ and Cd19-IcoslΔ mice. The loss of ICOSL on CD11c+ cells resulted in a 28% decrease in Tefh cells among CD4+ T cells (Fig. 2C) while the proportion of CD4+ T cells that were PSGL-1hi (PSGL-1hiCD44+CD62L− TCRβ+CD45R−) remained unchanged (Fig. S1A). B cell-specific ICOSL-deficiency caused only a slight decrement in Tefh cells (Fig. 2C) and did not affect PSGL-1hi CD4+ T cells (Fig. S1A). Because the Tfh cell differentiation pathway is co-opted by natural T regulatory (Treg) cells we studied FoxP3 expression by Tefh cells. Interestingly, a subset of Tefh cells in MRL. Faslpr mice expressed FoxP3 (Fig. S1B); however, the relative abundance of FoxP3+ Tefh cells was unchanged in Itgax-DTA, Itgax-IcoslΔ and Cd19-IcoslΔ mice compared to their littermate controls. Only Jh MRL. Faslpr mice showed a small reduction in FoxP3+ Tefh cells. Together, FoxP3− Tefh cells rely on ICOS engagement by CD11c+ cells and, to a minor extent, by B cells.
It has been suggested that ICOS signals augment CD8+ T cell immunity (Wallin et al., 2001). To explore ICOS-mediated effects on CD8+ T cells in lupus we measured the frequencies of CD8+ T cells with a terminal-effector (Teff, CD44+CD62L−CD127−) and effector-memory (Tem, CD44+CD62L−CD127+) phenotype in spleens from Itgax-IcoslΔ and Cd19-IcoslΔ mice. ICOSL-deficiency in CD11c+ cells led to a marked reduction of both subsets, whereas a lack of ICOSL on B cells had no discernible effect (Fig. 2D).
We conclude that in the context of chronic systemic autoimmunity the ablation of Icosl in CD11c+ cells leads to a partial loss of FoxP3− Tefh cells, and CD8+ Teff and Tem cells in the spleen without affecting overall T cell numbers.
Deletion of Icosl in CD11c+ or B cells does not block the Tefh cell program
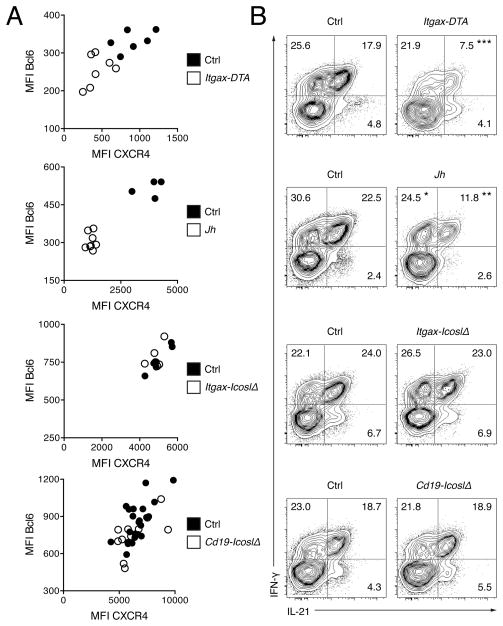
Although cells with a Tefh phenotype were to varying degree reduced in Itgax-DTA, Jh MRL. Faslpr, Itgax-IcoslΔ and Cd19-IcoslΔ mice it remained unclear whether this was due to a failure in Bcl6 expression. Residual Tefh cells in MRL. Faslpr mice lacking CD11c+ or B cells displayed lower protein expression of Bcl6 than those in littermate controls, indicating that either the induction or sustained expression of Bcl6 was impaired (Fig. 3A and S2). Accordingly, surface expression of the chemokine receptor CXCR4, which is directly regulated by Bcl6, was diminished on Tefh cells from Itgax-DTA and Jh MRL. Faslpr mice (Fig. 3A and S2). In contrast, the loss of ICOSL on CD11c+ or B cells had no effect on Bcl6 or CXCR4 expression by Tefh cells in Itgax-IcoslΔ and Cd19-IcoslΔ mice (Fig. 3A and S2). Next, we tested Tefh cell function by intracellular staining for IL-21 and interferon-γ (IFN-γ) after restimulation with phorbol myristate acetate (PMA) and ionomycin. IL-21 secretion, a key feature of Tefh cells, is induced by the transcription factor c-Maf (Bauquet et al., 2009). The substantial majority of Tefh cells that produced IL-21 also stained positive for IFN-γ in control mice (Fig. 3B). Notably, the frequency IL-21+IFN-γ+ cells among Tefh cells in Itgax-DTA and Jh MRL. Faslpr mice was greatly reduced (Fig. 3B). Deficiency for ICOSL in CD11c+ or B cells, however, did not impair IL-21 and IFN-γ production. In summary, Bcl-6 and IL-21 expression depend on both CD11c+ and B cells in lupus, but not ICOS triggering by either cell type.
Figure 3. Expression of the Tefh Cell Gene Program does not require ICOSL on CD11c+ or B cells.
(A) Mean fluorescence intensity (MFI) for Bcl6 (y-axis) and CXCR4 (expression of which is directly regulated by Bcl6, x-axis) of Tefh cells in spleens from Itgax-DTA, Jh MRL. Faslpr, Itgax-IcoslΔ and Cd19-IcoslΔ mice with their respective controls. n = 6 for Itgax-IcoslΔ mice and n = 6 for controls; n = 10 for Cd19-IcoslΔ mice and n = 23 for controls; n = 7 Itgax-DTA and n = 6 for controls; n = 4 for Jh MRL. Faslpr mice and n = 8 for controls.
(B) Flow cytometry plots show intracellular IL-21 and IFN-γ staining of gated Tefh cells of PMA-ionomycin stimulated splenocytes from Itgax-DTA, Jh MRL. Faslpr, Itgax-IcoslΔ and Cd19-IcoslΔ mice with their respective controls. Values indicate percentage of Tefh cells (mean). n = 17 for Itgax-IcoslΔ mice and n = 17 for controls; n = 8 for Cd19-IcoslΔ mice and n = 12 for controls; n = 6 Itgax-DTA and n = 10 for controls; n = 11 for Jh MRL. Faslpr mice and n = 8 for controls.
See also Figure S2.
Auto-Ab formation is largely unimpaired in mice deficient for ICOSL on B cells or CD11c+ cells
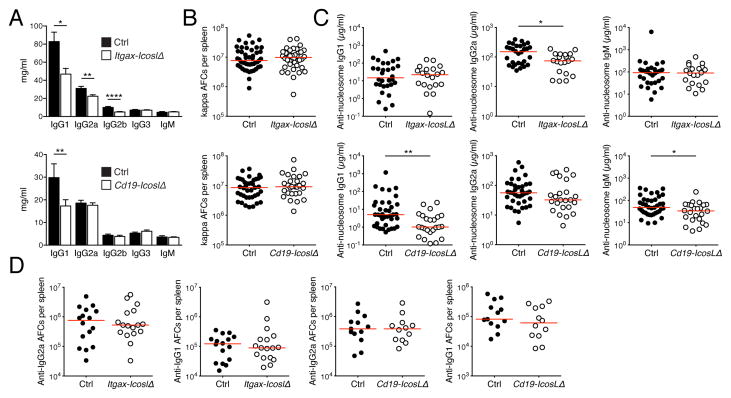
It has been proposed that Tfh cells enhance the production of auto-Abs, the deposition of which in tissues triggers immune infiltration (Linterman et al., 2009). Conceivably, organ inflammation in Itgax-IcoslΔ mice was therefore diminished because the decrease in Tefhs cells restricted auto-Ab formation. The drop in Tefh cells in Cd19-IcoslΔ mice was possibly too small to be pathogenically relevant, but this needed to be further investigated. To assess the roles of Ab, we first measured serum immunoglobulin isotype concentrations. IgG1 was reduced in both Itgax-IcoslΔ and Cd19-IcoslΔ mice (Fig. 4A). In addition, we observed decreases for IgG2a and IgG2b in Itgax-IcoslΔ mice. This demonstrates that ICOSL on CD11c+ and B cells has non-redundant functions in the production of class-switched Abs. Because auto-Abs in the MRL. Faslpr strain are predominantly secreted by short-lived plasmablasts in the spleen (Shlomchik, 2008), we enumerated splenic κ-light chain Ab-forming cells by ELISpot assay. Surprisingly, deletion of Icosl in CD11c+ or B cells did not diminish κ-light chain Ab-forming cell numbers (Fig. 4B). We then measured anti-nucleosome IgG1, IgG2a and IgM serum concentrations. Anti-nucleosome IgG2a was decreased in Itgax-IcoslΔ mice, as were anti-nucleosome IgG1 and IgM in Cd19-IcoslΔ mice (Fig 4C), but these effects were modest. The number of anti-IgG1 and anti-IgG2a rheumatoid factor producing cells was unaffected by the loss of CD11c+ and B cells (Fig. 4D). Overall, the contributions of ICOSL on CD11c+ and B cells to auto-Ab production were small. Importantly, if a loss of auto-Abs would have been responsible for the dramatically reduced IN and interstitial pneumonia in Itgax-IcoslΔ mice we would have expected that deletion of Icosl in CD11c+ cells would interfere more strongly with auto-Ab production than Icosl ablation in B cells, which had no detectable effect on tissue inflammation. This was, however, not the case. These data, arguing against the notion that ICOS-driven organ disease is mechanistically linked to auto-Abs, are commensurate with the presence of nephritis in MRL. Faslpr mice harboring B cells genetically rendered unable to secrete Ab (Chan et al., 1999).
Figure 4. Auto-Ab formation is largely unimpaired in mice deficient for ICOSL on B cells or CD11c+ cells.
(A) Serum Ig isotype concentrations of control (n = 20) and Itgax-IcoslΔ (n = 24) mice, or control (n = 21) and Cd19-IcoslΔ (n = 23) mice.
(B) Number of κ light chain Ab-forming cells per spleen determined by ELISpot assay.
(C) Serum concentrations of anti-nucleosome IgG1, IgG2a and IgGM.
(D) Number of anti-IgG1 and anti-IgG2a rheumatoid factor secreting cells per spleen determined by ELISpot assay.
In scatter plots each dot represents an individual mouse and horizontal lines represent the median. Data in bar graphs are represented as mean ± SEM.
Kidneys in lupus harbor Tefh-like cells that strongly depend on ICOS stimulation by CD11c+ cells
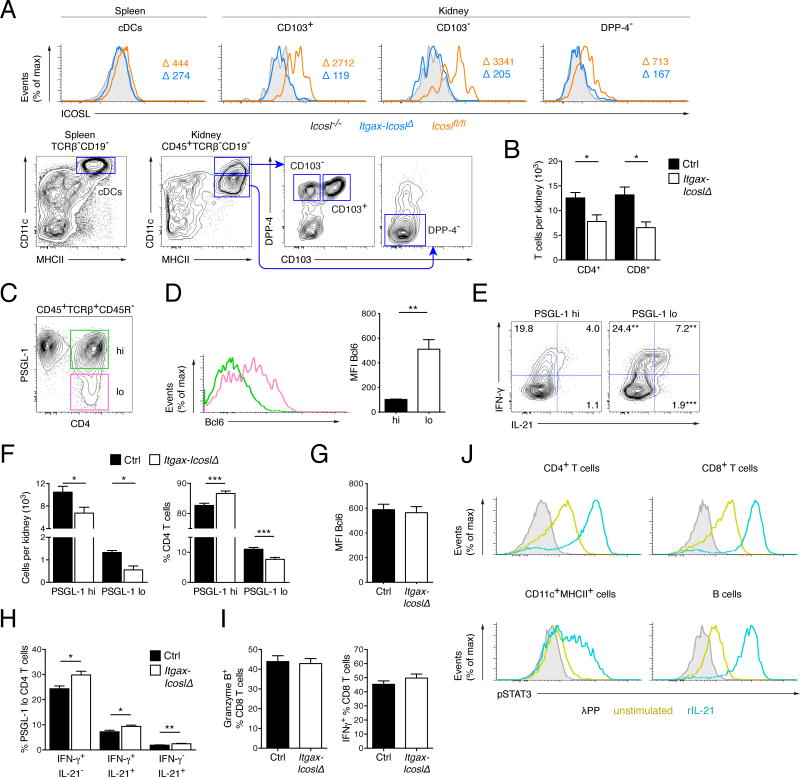
So far we had only examined the consequences of ICOS signals on immune cells in lymphoid tissue. We hypothesized that CD11c+ cells might sustain inflammation of target organs locally via ICOS stimulation. Notably, CD11c+ cells are abundantly present in inflamed kidneys (Teichmann et al., 2010) where B cells in contrast are scarce. To investigate this we compared ICOSL expression on CD11c+ cells in kidneys and spleen. Dipeptidyl peptidase-4 (DPP-4) was recently found to be higher expressed by cDCs than by macrophages, thus facilitating the distinction of these closely related cell types (Miller et al., 2012). Based on DPP-4 expression and traditional myeloid cell markers we distinguished three CD11c+MHCII+ populations in the kidneys: CD11chiMHCII+DPP-4+CD103+ cells that are considered cDCs, CD11chiMHCII+DPP-4+CD103− cells that likely consist of CD103− cDCs and monocyte-derived DCs, and a CD11cintMHCII+DPP-4− subset that probably includes macrophages (Fig. 5A). Remarkably, all three renal CD11c+ subsets expressed much higher amounts of ICOSL than splenic cDCs in MRL. Faslpr mice (Fig. 5A). ICOSL was also strongly expressed by CD11c+ cells in the lungs and skin (Fig. S3A). This expression pattern suggests that ICOS-ICOSL interactions might be of particular importance for immune reactions in peripheral organs. Deletion of Icosl was effective in all examined CD11c+ cell types in Itgax-IcoslΔ mice (Fig 5A and S3A).
Figure 5. Inflamed kidneys in lupus harbor Tefh-like cells that strongly depend on ICOS stimulation by CD11c+ cells.
(A) Histograms (upper row) for ICOSL expression of cDCs in spleens and several CD11c+MHCII+ subsets in kidneys from control MRL. Faslpr (Icoslfl/fl, n = 4) Itgax-IcoslΔ (n = 4) and Icosl−/− (n = 2) mice. The gating strategy for each subset is indicated in the contour plots (lower row). Values indicate MFI minus background staining (MFI of Icosl−/−).
(B) Numbers of CD4+ and CD8+ T cells per kidney of control (n = 9) and Itgax-IcoslΔ (n = 4) mice.
(C) Representative flow cytometry plot of PSGL-1lo and PSGL-1hi CD4+ T cells in kidneys from a 16 wk old wild type MRL. Faslpr mouse.
(D) Representative histogram (left) for Bcl6 expression of renal PSGL-1lo and PSGL-1hi CD4+ T cells as gated in (C). The bar graph (right) presents data from 5 animals.
(E) Flow cytometry plots show intracellular IL-21 and IFN-γ staining of gated PSGL-1lo and PSGL-1hi CD4+ T cells in PMA-ionomycin stimulated kidney cell suspensions from wild type MRL. Faslpr mice. Values indicate percentage of the respective cell type (mean).
(F) Numbers of PSGL-1lo and PSGL-1hi CD4+ T cells per kidney (left) and percentage of PSGL-1lo and PSGL-1hi cells among CD4+ T cells in kidneys (right) of control (n ≥ 9) and Itgax-IcoslΔ (n ≥ 4) mice.
(G) Mean fluorescence intensity (MFI) for Bcl6 of PSGL-1lo CD4+ T cells in kidneys from control (n = 6) and Itgax-IcoslΔ (n = 6) mice.
(H) IL-21 and IFN-γ staining characteristics of gated PSGL-1lo CD4+ T cells in PMA-ionomycin stimulated kidney cell suspensions in control (n = 17) and Itgax-IcoslΔ (n = 17) mice.
(I) Percentage of gated CD8+ T cells in kidney cell suspensions staining for granzyme B (left) or IFN-γ (right, after restimulation with PMA-ionomycin) of control and (n ≥ 6) and Itgax-IcoslΔ (n ≥ 6) mice.
(J) Histograms show p-STAT3 staining of gated CD4+ T cells, CD8+ T cells, CD11c+MHCII+ cells and B cells in kidney single-cell suspensions from WT MRL.Faslpr mice (n = 4). Kidney cells were treated with rIL-21 or left untreated for 30 min and then stained for p-STAT3. To determine background staining a set of untreated samples was dephosphorylated with λ protein phosphatase.
Data in bar graphs are represented as mean ± SEM. See also Figure S3.
ICOSL-deficiency in CD11c+ cells reduced the number of kidney infiltrating CD4+ and CD8+ T cells by 38.0% and 50.2%, respectively (Fig. 5B), consistent with the robust decrease in relative infiltrate size (Fig. 1, C and F). We detected a PSGL-1lo subset of CD4+ T cells in kidneys from aged MRL. Faslpr mice (Fig. 5C). This subset expressed Bcl6 and was enriched in IL-21 and IFN-γ producers, thus sharing key features with splenic Tefh cells (Fig. 5, D and E). These cells (called Tefh-like hereafter) resemble a Bcl6+IL-21+ CD4+ T cell population that infiltrates the pancreas and salivary glands in NOD mice, a model of type I diabetes (McGuire et al., 2011). Both Tefh-like and PSGL-1hi CD4+ T cells were diminished in kidneys from Itgax-IcoslΔ compared to control mice (−58,4% and −35,6%, respectively) (Fig. 5F, left). This contrasted with the selective decrease in Tefh cells in spleens. The greater relative reduction in Tefh-like cells, however, suggested that they are more dependent on ICOS stimulation by CD11c+ cells than PSGL-1hi cells and shifted the overall composition of the renal CD4+ T cell pool towards less Tefh-like cells in Itgax-IcoslΔ mice (Fig. 5F, right). Icosl deletion in CD11c+ cells neither impaired Bcl6 expression (Fig. 5G) nor the ability to produce IL-21 and IFN-γ (Fig. 5H) by renal Tefh-like cells. This supports the notion that ICOS triggering by CD11c+ cells is not required for the genetic programming of renal Tefh-like cells. As expected CD8+ T cells in kidneys from MRL. Faslpr mice largely displayed a Teff or Tem phenotype (Fig. S3B). The function of renal CD8+ T cells was unimpaired by the deletion of Icosl in CD11c+ cells based on granzyme B content and IFN-γ production after restimulation (Fig. 5I).
We asked on which cell types in inflamed kidneys the secreted IL-21 might act. To identify IL-21-responsive cell types we stimulated kidney single cell suspension with rIL-21 and quantified p-STAT3 by flow cytometry. Almost all CD4+ T cells, CD8+ T cells and B cells were sensitive to IL-21 (Fig 5J). In addition, p-STAT3 was increased in a subset of CD11c+MHCII+ cells. Thus, IL-21 is broadly active on nephritic cell infiltrates.
Taken together, CD11c+ cells in inflamed kidneys expressed high amounts of ICOSL. Deficiency for ICOSL in CD11c+ cells reduced the number of all kidney infiltrating T cell subsets in lupus with a disproportionately high decline in a novel population of Tefh-like cells among CD4+ T cells.
ICOS engagement by CD11c+ cells rescues Tfh cell types and effector CD8+ T cells from apoptosis
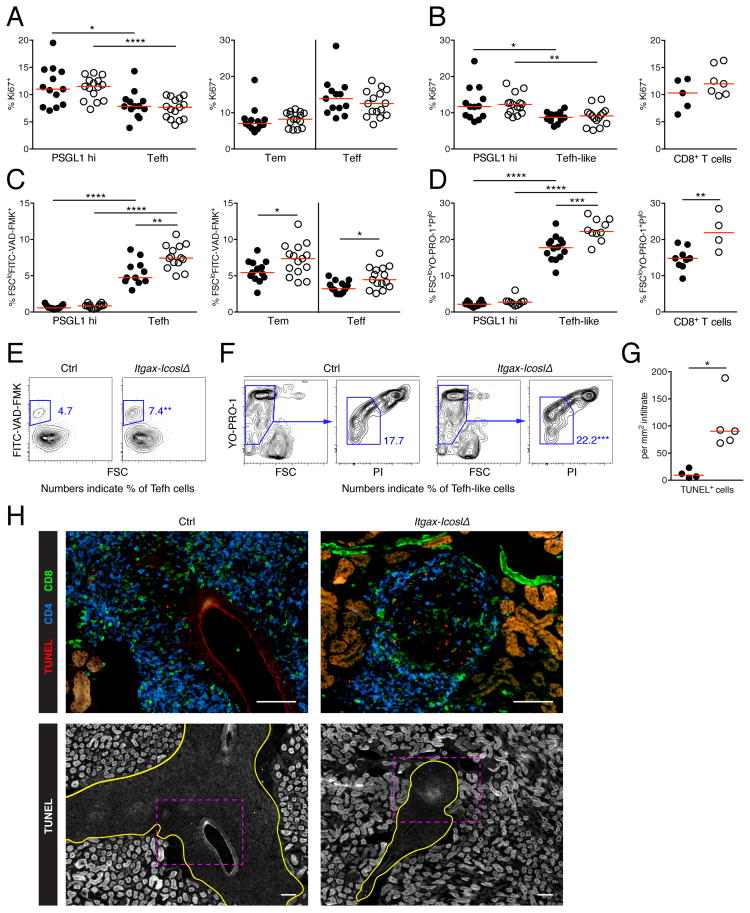
We sought to elucidate how the lack of ICOSL on CD11c+ cells reduces the abundance of discrete T cell subsets in spleens and kidneys. ICOS signals activate the PI3K-Akt pathway, which promotes proliferation and survival. First, we evaluated proliferative activity by staining for Ki-67 Ag, which is preferentially expressed during late G1, S, G2 and M phase of the cell cycle, by flow cytometry. Splenic Tefh cells and renal Tefh-like cells were dividing less than their PSGL-1hi counterparts (Fig. 6, A and B). ICOSL-deficiency in CD11c+ cells, however, did not inhibit proliferation of either cell type. Of note, in Itgax-DTA mice, which lack cDCs and CD11chi macrophages, fewer Tefh cells were cycling than in controls (Fig. S4A). Deletion of Icosl in CD11c+ cells did not impede proliferation of splenic CD8+ Teff and Tem cells (Fig. 6A), or renal CD8+ T cells (Fig. 6B).
Figure 6. ICOS engagement by CD11c+ cells rescues Tfh cell family members and effector CD8+ T cells from apoptosis in lymphoid and peripheral tissues.
(A) Percentage of PSGL1hi CD4+ T cells and Tefh cells (left), or Teff and Tem CD8+ T cells (right) expressing the proliferaton marker Ki67 in spleens from control (black) and Itgax-IcoslΔ (white) mice.
(B) Percentage of PSGL1hi CD4+ T cells and Tefh-like cells (left), or CD8+ T cells (right) expressing the proliferaton marker Ki67 in kidneys from control (black) and Itgax-IcoslΔ (white) mice.
(C) Percentage apoptotic cells (FSCloFITC-VAD-FMK+) among PSGL1hi CD4+ T cells and Tefh cells (left), or Tem and Teff CD8+ T cells (right) in spleens from control (black) and Itgax-IcoslΔ (white) mice.
(D) Percentage apoptotic cells (FSCloYO-PRO1+PIlo) among PSGL1hi CD4+ T cells and Tefh-like cells (left), or CD8+ T cells (right) in kidneys from control (black) and Itgax-IcoslΔ (white) mice.
(E) Flow cytometry plots show gating of apoptotic Tefh cells in spleens of control and Itgax-IcoslΔ mice. Numbers indicate percentage of Tefh cells. Results are summarized in (C, left).
(F) Flow cytometry plots show gating of apoptotic Tefh-like cells in kidneys of control and Itgax-IcoslΔ mice. Numbers indicate percentage of Tefh-like cells. Results are summarized in (D, left).
(G) Number of TUNEL+ cells per mm2 interstitial infiltrate in kidney sections of control (black) and Itgax-IcoslΔ (white) mice. One mid-sagittal longitudinal kidney section was analyzed per mouse. All TUNEL+ cells on the entire section were counted and the total infiltrate area size was measured with ImageJ.
(H) Representative images of kidney sections stained by TUNEL assay, and with anti-CD4 and anti-CD8 of a control and an Itgax-IcoslΔ mouse (scale bar = 100 μm). The upper images show the area marked with pink dashed rectangles in the lower images at higher magnification. Infiltrates are encircled with a yellow line in the lower images. Note that tubuloepithelial cells are strongly autofluorescent.
In scatter plots each dot represents an individual mouse and horizontal lines represent the median. See also Figure S4.
Next, we tested whether Tefh, CD8+ Teff and CD8+ Tem cells in spleen undergo apoptosis at an increased rate in the absence of ICOSL on CD11c+ cells. We used the FITC-labeled pan-caspase inhibitor VAD-FMK and scatter characteristics to identify apoptotic cells in spleens. Tefh cells were more often apoptotic than PSGL-1hi CD4+ T cells (Fig. 6C). ICOSL-deficiency in CD11c+ cells led to a considerable increase in apoptosis among Tefh cells, whereas there was no effect on PSGL-1hi CD4+ T cells (Fig. 6, C and E). Further, survival of splenic CD8+ Teff and Tem cells was impaired in Itgax-IcoslΔ mice compared to controls (Fig. 6C).
We also investigated apoptosis in kidney. Because caspase activation is an early event during apoptosis, FITC-VAD-FMK might label cells that became apoptotic either in vivo or during kidney processing. To circumvent this issue we used increased cell membrane permeability, which is only observed at late stages of apoptosis, as a measure for apoptosis in kidney single cell suspensions. Similar to the findings in spleens, Tefh-like cells in kidneys were much more frequently apoptotic than PSGL-1hi CD4+ T cells (Fig. 6D). Critically, in Itgax-IcoslΔ mice, the percentage of Tefh-like cells that underwent apoptosis was higher than in controls (Fig. 6, D and F). Surprisingly, ICOSL-deficiency in CD11c+ cells did not detectably affect survival of renal PSGL-1hi CD4+ T cells despite reducing their number. Similar to the situation for Tefh-like cells, more CD8+ T cells in kidneys from Itgax-IcoslΔ mice were apoptotic than in controls (Fig. 6D). To confirm our results from kidney single cell suspensions we detected apoptotic cells in-situ on tissue sections by TUNEL assay. The loss of ICOS stimulation by CD11c+ cells led to an 8.8-fold increase in TUNEL+ cells per mm2 renal infiltrate (Fig 6, G and H).
It remained unclear whether improved T cell survival represented a primary effect of ICOS triggering by CD11c+ cells or a secondary effect for example due to the release of cytokines induced by ICOS signaling. In the latter case stimulation of a cell via ICOS would also affect its neighboring cells. To distinguish these two possibilities we generated chimeric mice in which 50% of T cells originate from Icos−/− and 50% from Icos+/+ bone marrow. In these chimeras Icos−/− Tefh-like cells, PSGL-1hi CD4+ T cells and CD8+ T cells less efficiently accumulated in inflamed kidneys than their Icos+/+ counterparts (Fig. S4B). Icos−/− Tefh-like cells were underrepresented to the greatest extent. These data were consistent with the notion of a cell-intrinsic effect of ICOS ligation on T cell survival.
Taken together, the loss of cells belonging to the Tfh cell family and effector CD8+ T cells in both spleens and kidneys of Itgax-IcoslΔ mice resulted from impaired survival rather than proliferation.
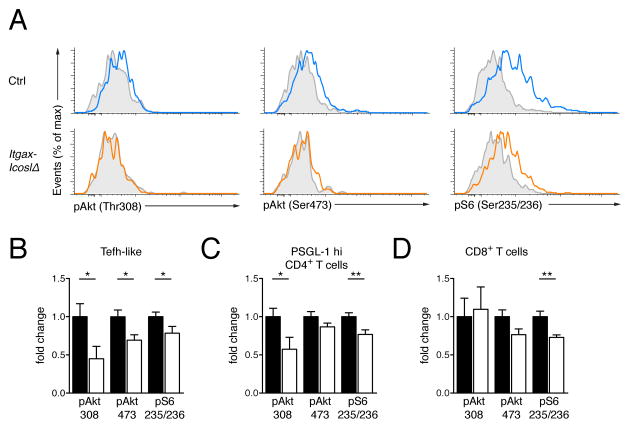
Lack of ICOS stimulation by CD11c+ cells dampens endogenous Akt activity in renal T cells
We recently described a phospho-flow technique that permits direct ex vivo determination of the spontaneous endogenous phosphorylation state of proteins (Khalil et al., 2012). We used this method to find out whether deletion of Icosl in CD11c+ cells might curtail Akt signaling, thus reducing survival of Tefh-like cells and CD8+ T cells in inflamed kidneys. Activation of Akt requires phosphorylation of threonine 308 (Thr308) and serine 473 (Ser473). Both phosphorylation events are dependent on PI3K. Once activated, Akt stimulates the rapamycin-sensitive mTOR complex-1 (TORC1) and subsequently S6K, which phosphorylates the ribosomal protein S6. Because p-S6 (Ser235/236) is well-detected by phospho-flow it is a sensitive downstream readout for Akt activity. Tefh-like cells in kidneys from aged Itgax-IcoslΔ mice demonstrated reduced amounts of basal p-Akt (Thr308), p-Akt (Ser473) and p-S6 (Ser235/236) compared to those from controls (Fig. 7, A and B). We also observed decreased amounts of p-Akt (Thr308) and p-S6 (Ser235/236) in renal PSGL-1hi CD4+ T cells (Fig. 7C). In addition there was less S6 phosphorylation in CD8+ T cells (Figure 7D). Our data indicate that in the absence of ICOSL on CD11c+ cells Akt activity is reduced in all renal T cell subsets. This is consistent with the finding that all T cell types were diminished in kidneys from Itgax-IcoslΔ mice. It is important to acknowledge that other PI3K-independent pathways downstream of ICOS exist but their role in apoptosis is unclear. We conclude that in lupus nephritis ICOSL-deficiency in CD11c+ cells is not compensated for by ICOSL on other cell types or Akt activation by receptors other than ICOS.
Figure 7. Lack of ICOS stimulation by CD11c+ cells dampens endogenous Akt activity in renal T cells.
(A) Spontaneous signaling in renal Tefh-like cells. Histograms display representative results for p-Akt (Thr308), p-Akt (Ser473) and p-S6 (Ser235/236) staining of gated Tefh-like cells in instantly fixed kidneys from a control and an Itgax-IcoslΔ mouse. Each histogram shows an overlay of λ protein phosphatase-treated (grey shaded) and untreated (colored) cells.
(B) Fold change of p-Akt (Thr308), p-Akt (Ser473) and p-S6 (Ser235/236) mean fluorescence intensity (MFI) of Tefh-like cells in instantly fixed kidneys of Itgax-IcoslΔ mice (n = 15, white bars) relative to those of controls (n = 11, black bars). Individual fold-change values were calculated as ΔMFIi (= MFIi, untreated – MFIi, λPP) divided by the average of ΔMFIi values obtained from controls. Data are represented as mean ± SEM.
(C) As in (B) but gated on PSGL1hi CD4+ T cells.
(D) As in (B) but gated on CD8+ T cells.
Discussion
ICOS engagement drives the development of systemic autoimmunity, which has been attributed to its key function in Tfh differentiation and thus auto-Ab formation (Vinuesa et al., 2005). Here we demonstrate that multi-organ inflammation in lupus critically relies on ICOS stimulation by CD11c+ cells, which comprise DCs and CD11c+ macrophages. In contrast, tissue infiltration was independent of ICOSL on B cells. Surprisingly, promotion of lupus via ICOSL on CD11c+ cells was largely unrelated to the production of auto-Abs. Rather, reduced T cell accrual in kidneys, a major target organ in lupus, of Itgax-IcoslΔ mice was caused by impaired survival of various tissue-infiltrating T cell subsets. Notably, these included a population of renal IL-21-secreting Bcl6+ Th cells that likely interacts with T, B, and myeloid cells. Increased apoptosis correlated with reduced endogenous activity of the PI3K-Akt cascade in renal T cells. CD11c+ Ag-presenting cell types in kidneys expressed high amounts of ICOSL, suggesting that these cells locally induced essential PI3K-mediated pro-survival signals in organ-infiltrating T cells via ICOS triggering, thus actively maintaining peripheral inflammation.
Conflicting results have been published regarding the impact of global ICOS deficiency on organ pathology in lupus (Odegard et al., 2009; Zeller et al., 2006). Our study agrees with Odegard et al. in demonstrating that ICOS is a critical determinant of tissue inflammation and identifies CD11c+ cells as the main drivers of ICOS-dependent organ disease. Indeed, the degree to which proteinuria and IN was reduced in Itgax-IcoslΔ mice was comparable to that previously described in globally ICOS-deficient MRL. Faslpr mice (Odegard et al., 2009). Considering the wide hematopoietic and parenchymal expression of ICOSL, the strikingly selective importance of ICOSL on CD11c+ cells was unexpected.
A plausible explanation for diminished organ disease in Itgax-IcoslΔ mice was that the lack of ICOS stimulation by CD11c+ cells might have constrained auto-Ab production. However, several lines of evidence indicate that this was not the case. First, the impact of CD11c+ cell-specific Icosl deficiency on the anti-self humoral response was minor. Second, tissue inflammation in Cd19-IcoslΔ mice was unabated despite effects on auto-Ab production comparable to those seen Itgax-IcoslΔ mice. Third, we have previously shown that the development of IN in MRL. Faslpr mice does not require soluble immunoglobulin (Chan et al., 1999). Thus, ICOS signals likely drive autoimmune pathology mainly through mechanisms that do not depend on auto-Abs.
Consistent with a limited role for auto-Abs in ICOS-driven inflammation in lupus, the Tefh cell differentiation program in Itgax-IcoslΔ and Cd19-IcoslΔ mice was not disrupted. Deletion of Icosl in CD11c+ or B cells of lupus-prone mice neither interfered with expression of the Tfh master transcription factor Bcl6 nor with production of the Tfh signature cytokine IL-21 by Th cells. This contrasts with the established model for Tfh differentiation in viral infection, in which sequential ICOS engagement by DCs and B cells is required to institute the Tfh phenotype (Choi et al., 2011). Notably, in Itgax-DTA and Jh MRL. Faslpr mice that completely lack CD11c+ and B cells, respectively, Bcl6 and IL-21 expression were reduced. Thus, the Tefh cell differentiation program in lupus depends on CD11c+ and B cells, but not ICOS triggering by either cell type. A recent report showed in an ovalbumin immunization model that ICOSL display by B cells is dispensable for Tfh polarization when Ag is abundant (the loss of ICOSL on DCs was not investigated) (Weinstein et al., 2014). Ubiquity of self-Ag is a characteristic feature of lupus. We thus hypothesize that the lack of effect on Tefh differentiation in lupus-prone mice deficient for Icosl in CD11c+ or B cells results from broad Ag availability rather than from fundamental differences between Tefh in autoimmunity and Tfh cells in infection.
Inflamed kidneys in lupus-prone mice harbored Th cells that downregulated PSGL-1, expressed Bcl6 and secreted IL-21, thereby resembling Tefh cells. A recent report demonstrated that kidney samples from SLE patients contain ICOS+ Th cells that partly interact with B cells (Liarski et al., 2014). Whether these interactions lead to local plasmablast differentiation was not addressed. Of note, β-islet destruction in the NOD mouse model of type I diabetes depends on a subset of pancreas infiltrating CCR9+Bcl6+IL-21+ Th cells. Despite their relatedness to Tfh cells this subset primarily provides IL-21 to CD8+ T cells, which facilitates CD8+ T cell expansion and survival (McGuire et al., 2011). Further, IL-21 in NOD-NP mice is required for pancreatic cDCs to acquire chemokine receptor 7, which enables migration to the draining lymph node and priming of self-reactive T cells (Van Belle et al., 2012). Because of these observations we sought to identify the cell types residing in kidney infiltrates of lupus mice on which IL-21 secreted by local Tefh-like cells might impact. Virtually all CD4+ T cells, CD8+ T cells, B cells and a subset of CD11c+ Ag-presenting cells were responsive to IL-21. Although it was not feasible to more definitively establish the function of IL-21 released by renal Tefh-like cells, given that no tools exist to manipulate discrete cell types only in specific locations, our experiments indicate that Tefh-like cell-derived IL-21 has a wide range of targets in inflamed kidneys and thus may play a pivotal role.
Our study directly demonstrates that ICOSL display by CD11c+ cells has non-redundant functions in preventing apoptosis of Tfh cell family members and effector CD8+ T cells in lymphoid tissue and, most importantly, in inflammatory organ infiltrates. Icosl deletion in CD11c+ cells was linked to constrained spontaneous activity of the pro-survival PI3K-Akt cascade in kidney infiltrating T cells. Consequently, other possible stimuli of Akt in T cells, such as Ag-MHC complexes, CD80, CD86, CD40L, cytokines and chemokines, most of which are probably abundantly available to T cells in systemic autoimmunity, cannot compensate for the loss of ICOSL expressed by CD11c+ cells. Given that PI3K activity is thought to collapse within minutes when the triggering stimulus is withdrawn (Huppa et al., 2003) it is likely that sustained PI3K-Akt signaling in renal T cells reflects local ICOS stimulation. Importantly, this notion is supported by our experiments revealing that ICOSL expression on MHCII+CD11c+ cell subsets in inflamed kidneys is considerably higher than on cDCs in lymphoid tissue. It is also noteworthy that MHCII+CD11c+ cells are present at high density in kidneys from aged MRL. Faslpr mice (~10% of CD45+ cells). B cells are rare in this location and Cd19-IcoslΔ mice showed no reduction in nephritis. Interestingly, selective deficiency for MyD88 in CD11c+ cells does not mitigate nephritis in lupus prone mice (Teichmann et al., 2013). This suggests that the ability of CD11c+ cells to maintain kidney infiltrating T cells is not imparted by Toll-like receptor or IL-1R-IL-18R signals but likely depends on signals induced by the T cells they interact with.
In conclusion, we define CD11c+ Ag-presenting cells to be the main drivers of ICOS-dependent lupus pathology and establish that ICOS triggering by this population perpetuates target organ inflammation by protecting organ infiltrating T cells from apoptosis via induction of PI3K-Akt signaling rather than by biasing T cell differentiation and propelling auto-Ab production. This mechanism potentially applies to many other autoimmune and alloimmune conditions that rely on ICOS, for example type I diabetes (Hawiger et al., 2008), rheumatoid arthritis (Iwai et al., 2002), polymyositis (Katsumata et al., 2007) and chronic allograft rejection (Ozkaynak et al., 2001). Interestingly, in each of these diseases B cells are implicated as the upstream APCs for global T cell activation (Takemura et al., 2001; Wong et al., 2004; Zeng et al., 2014). ICOS blockade may serve as an effective approach to turn off chronic, destructive organ inflammation regardless of the nature of the disease initiating processes: by truncating the late effector stage of the pathological T cell response.
Materials and Methods
Mice
Icoslfl/+ C57BL/6 mice were as described (Nurieva et al., 2003) and Itgax-Cre BAC transgenic C57BL/6 mice (Caton et al., 2007) were kindly provided by Boris Reizis. Cd19-Cre C57BL/6, Rosa26-eGFP-DTA C57BL/6 and Jh (Igh-Jtm1Dhu) Balb/c mice were purchased from the Jackson Laboratory or Taconic. All animals were backcrossed to MRL-MpJ-Faslpr/J mice (Jackson Laboratory) for at least 10 generations. Itgax-IcoslΔ and Cd19-IcoslΔ mice were generated by interbreeding Icoslfl/fl with Itgax-Cre Icoslfl/fl or CD19-Cre Icoslfl/fl MRL. Faslpr mice, respectively. Littermates without a Cre allele were used as controls. Mice were analyzed at 16–18 wks of age unless stated otherwise. All animals were maintained under specific pathogen-free (SPF) conditions and handled according to protocols approved by the Yale Institutional Animal Care and Use Committee.
Preparation of kidney single cell suspensions
After perfusion of mice with DPBS kidneys were removed, minced and digested in HBSS containing 100 Mandl U/ml collagenase D (Roche) and 50 Kunitz U/ml DNase I (Sigma-Aldrich). Tissue pieces were then pressed through a 70 μm strainer to obtain single cell suspensions. Cells were enriched in leukocytes using an 80/20 percoll gradient.
Standard flow cytometry
Surface staining was performed in ice-cold PBS with 3% calf serum in the presence of FcR blocking Ab 2.4G2. Ab clones used for FACS can be found in the Supplemental Information. Intracellular staining was performed using the BD Cytofix/Cytoperm and Perm/Wash buffers or, for intracellular Bcl6, Ki67 and FoxP3 staining, the eBioscience FoxP3 staining buffer set. For intracellular IL-21 and IFN-γ staining, 5 × 106 splenocytes were cultured for 5 hr at 37°C in 24-well plates in 2 ml culture medium containing ionomycin (750 ng/ml) and PMA (20 ng/ml). For the last 4 hrs brefeldin A (10 μg/ml) was added to the cultures. IL-21 was detected with a recombinant mouse IL-21R subunit/human IgG1 Fc chimera (R&D systems) with goat anti-human Fcγ conjugated to PE (Jackson ImmunoResearch). Cells were analyzed on a LSRII instrument (BD). For purification of populations, cells were sorted on a FACSAria II (BD).
Phospho-flow
For identification of IL-21 responsive cell types in kidneys, freshly prepared kidney single cell suspensions were incubated with rIL-21 (20 ng/ml, R&D systems) for 30 min or left untreated. Cells were fixed with 1% paraformaldehyde, permeabilized with 90% methanol and washed. Aliquots of untreated cells were dephosphorylated with λ protein phosphatase (NEB BioLabs). All samples were then stained with p-STAT3 (Tyr705) antibody (Cell Signaling). To determine the endogenous phosphorylation state of Akt and S6 in renal T cell populations, kidneys were directly disrupted in RPMI containing 1% paraformaldehyde. Fixed cells were permeabilized with 90% methanol, washed and aliquots of all samples were incubated with λ protein phosphatase. Cells were stained with p-Akt (Ser308), p-Akt (Thr473) or p-S6 (Ser235/236) antibody (Cell Signaling).
Apoptosis
For flow cytometric detection of apoptotic cells in spleens, freshly prepared cell suspensions were stained for active caspases with FITC-VAD-FMK (SM Biochemicals). To identify apoptotic cells by FACS in kidneys, we monitored cell membrane permeability by staining with YO-PRO1 in combination with propidium iodide. Apoptotic cells in kidney tissue sections were visualized by TUNEL method using the ApoAlert DNA Fragmentation Assay Kit (Clontech) as per the manufacturer’s instructions.
ELISpots, Luminex and ELISA
ELISpot assays to enumerate Ab-forming cells that produce κ light chain Abs, anti-IgG1 or anti-IgG2a rheumatoid factor in spleens, Luminex assays to measure serum Ig isotype concentrations, and ELISAs to determine serum anti-nucleosome IgG, IgG1 and IgG2a concentrations were all performed as reported (Teichmann et al., 2013).
Evaluation of clinical disease
Nephritis, proteinuria and dermatitis were scored as previously described (Teichmann et al., 2010). For pulmonary disease, formalin-fixed and paraffin-embedded sections of the left lung, stained with H&E, were scored for cellular interstitial pneumonia from 0 to 6 (0 = none, 6 = severe) in a blinded fashion. The percentage area of renal and pulmonary interstitial infiltrates was determined with ImageJ software.
Statistical analysis
Statistics were calculated by two-tailed Mann-Whitney U test with p values indicated throughout as *p < 0.05, **p < 0.01, ***p < 0.001.
Supplementary Material
Acknowledgments
We thank Jason Weinstein, Kevin Nickerson and Kevin Cieslak for helpful comments and technical expertise. L.L.T. is supported by Deutsche Forschungsgemeinschaft (TE 785/3-1) and BONFOR (2013-1-25). M.J.S. is supported by NIH (R01-AR044077). J.C. is supported by NIH (R01-AR40072) and Alliance for Lupus Research. C.D. is supported by NIH (R01-AI106654).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
L.L.T. and M.J.S. conceived of the study and wrote the manuscript. L.L.T. designed and performed experiments. J.L.C. assisted with experiments. M.K. scored kidney disease. J.C. and C.D. provided mice and commented on the paper.
References
- Bagavant H, Fu SM. Pathogenesis of kidney disease in systemic lupus erythematosus. Curr Opin Rheumatol. 2009;21:489–494. doi: 10.1097/BOR.0b013e32832efff1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauquet AT, Jin H, Paterson AM, Mitsdoerffer M, Ho IC, Sharpe AH, Kuchroo VK. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nat Immunol. 2009;10:167–175. doi: 10.1038/ni.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmeister Y, Lischke T, Dahler AC, Mages HW, Lam KP, Coyle AJ, Kroczek RA, Hutloff A. ICOS controls the pool size of effector-memory and regulatory T cells. J Immunol. 2008;180:774–782. doi: 10.4049/jimmunol.180.2.774. [DOI] [PubMed] [Google Scholar]
- Caton ML, Smith-Raska MR, Reizis B. Notch-RBP-J signaling controls the homeostasis of CD8- dendritic cells in the spleen. J Exp Med. 2007;204:1653–1664. doi: 10.1084/jem.20062648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan O, Shlomchik MJ. A new role for B cells in systemic autoimmunity: B cells promote spontaneous T cell activation in MRL-lpr/lpr mice. J Immunol. 1998;160:51–59. [PubMed] [Google Scholar]
- Chan OT, Hannum LG, Haberman AM, Madaio MP, Shlomchik MJ. A novel mouse with B cells but lacking serum antibody reveals an antibody-independent role for B cells in murine lupus. J Exp Med. 1999;189:1639–1648. doi: 10.1084/jem.189.10.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YS, Kageyama R, Eto D, Escobar TC, Johnston RJ, Monticelli L, Lao C, Crotty S. ICOS Receptor Instructs T Follicular Helper Cell versus Effector Cell Differentiation via Induction of the Transcriptional Repressor Bcl6. Immunity. 2011;34:932–946. doi: 10.1016/j.immuni.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fos C, Salles A, Lang V, Carrette F, Audebert S, Pastor S, Ghiotto M, Olive D, Bismuth G, Nunès JA. ICOS ligation recruits the p50alpha PI3K regulatory subunit to the immunological synapse. J Immunol. 2008;181:1969–1977. doi: 10.4049/jimmunol.181.3.1969. [DOI] [PubMed] [Google Scholar]
- de Haij S, Woltman AM, Trouw LA, Bakker AC, Kamerling SW, van der Kooij SW, Chen L, Kroczek RA, Daha MR, van Kooten C. Renal tubular epithelial cells modulate T-cell responses via ICOS-L and B7-H1. Kidney Int. 2005;68:2091–2102. doi: 10.1111/j.1523-1755.2005.00665.x. [DOI] [PubMed] [Google Scholar]
- Hawiger D, Tran E, Du W, Booth CJ, Wen L, Dong C, Flavell RA. ICOS mediates the development of insulin-dependent diabetes mellitus in nonobese diabetic mice. J Immunol. 2008;180:3140–3147. doi: 10.4049/jimmunol.180.5.3140. [DOI] [PubMed] [Google Scholar]
- Huppa JB, Gleimer M, Sumen C, Davis MM. Continuous T cell receptor signaling required for synapse maintenance and full effector potential. Nat Immunol. 2003;4:749–755. doi: 10.1038/ni951. [DOI] [PubMed] [Google Scholar]
- Iwai H, Kozono Y, Hirose S, Akiba H, Yagita H, Okumura K, Kohsaka H, Miyasaka N, Azuma M. Amelioration of collagen-induced arthritis by blockade of inducible costimulator-B7 homologous protein costimulation. J Immunol. 2002;169:4332–4339. doi: 10.4049/jimmunol.169.8.4332. [DOI] [PubMed] [Google Scholar]
- Jacob N, Guo S, Mathian A, Koss MN, Gindea S, Putterman C, Jacob CO, Stohl W. B Cell and BAFF dependence of IFN-α-exaggerated disease in systemic lupus erythematosus-prone NZM 2328 mice. J Immunol. 2011;186:4984–4993. doi: 10.4049/jimmunol.1000466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsumata Y, Harigai M, Sugiura T, Kawamoto M, Kawaguchi Y, Matsumoto Y, Kohyama K, Soejima M, Kamatani N, Hara M. Attenuation of experimental autoimmune myositis by blocking ICOS-ICOS ligand interaction. J Immunol. 2007;179:3772–3779. doi: 10.4049/jimmunol.179.6.3772. [DOI] [PubMed] [Google Scholar]
- Khalil AM, Cambier JC, Shlomchik MJ. B Cell Receptor Signal Transduction in the GC Is Short-Circuited by High Phosphatase Activity. Science. 2012;336:1178–1181. doi: 10.1126/science.1213368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppek K, Schott J, Reitter S, Poetz F, Hammond MC, Stoecklin G. Roquin Promotes Constitutive mRNA Decay via a Conserved Class of Stem-Loop Recognition Motifs. Cell. 2013;153:869–881. doi: 10.1016/j.cell.2013.04.016. [DOI] [PubMed] [Google Scholar]
- Liarski VM, Kaverina N, Chang A, Brandt D, Yanez D, Talasnik L, Carlesso G, Herbst R, Utset TO, et al. Cell distance mapping identifies functional T follicular helper cells in inflamed human renal tissue. Sci Transl Med. 2014;6:230ra46. doi: 10.1126/scitranslmed.3008146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linterman MA, Rigby RJ, Wong RK, Yu D, Brink R, Cannons JL, Schwartzberg PL, Cook MC, Walters GD, Vinuesa CG. Follicular helper T cells are required for systemic autoimmunity. J Exp Med. 2009;206:561–576. doi: 10.1084/jem.20081886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire HM, Vogelzang A, Ma CS, Hughes WE, Silveira PA, Tangye SG, Christ D, Fulcher D, Falcone M, King C. A subset of interleukin-21+ chemokine receptor CCR9+ T helper cells target accessory organs of the digestive system in autoimmunity. Immunity. 2011;34:602–615. doi: 10.1016/j.immuni.2011.01.021. [DOI] [PubMed] [Google Scholar]
- Miller JC, Brown BD, Shay T, Gautier EL, Jojic V, Cohain A, Pandey G, Leboeuf M, Elpek KG, et al. Deciphering the transcriptional network of the dendritic cell lineage. Nat Immunol. 2012;13:888–899. doi: 10.1038/ni.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurieva RI, Mai XM, Forbush K, Bevan MJ, Dong C. B7h is required for T cell activation, differentiation, and effector function. Proc Natl Acad Sci U S A. 2003;100:14163–14168. doi: 10.1073/pnas.2335041100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegard JM, DiPlacido LD, Greenwald L, Kashgarian M, Kono DH, Dong C, Flavell RA, Craft J. ICOS controls effector function but not trafficking receptor expression of kidney-infiltrating effector T cells in murine lupus. J Immunol. 2009;182:4076–4084. doi: 10.4049/jimmunol.0800758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegard JM, Marks BR, DiPlacido LD, Poholek AC, Kono DH, Dong C, Flavell RA, Craft J. ICOS-dependent extrafollicular helper T cells elicit IgG production via IL-21 in systemic autoimmunity. J Exp Med. 2008;205:2873–2886. doi: 10.1084/jem.20080840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozkaynak E, Gao W, Shemmeri N, Wang C, Gutierrez-Ramos JC, Amaral J, Qin S, Rottman JB, Coyle AJ, Hancock WW. Importance of ICOS-B7RP-1 costimulation in acute and chronic allograft rejection. Nat Immunol. 2001;2:591–596. doi: 10.1038/89731. [DOI] [PubMed] [Google Scholar]
- Satpathy AT, Kc W, Albring JC, Edelson BT, Kretzer NM, Bhattacharya D, Murphy TL, Murphy KM. Zbtb46 expression distinguishes classical dendritic cells and their committed progenitors from other immune lineages. J Exp Med. 2012;209:1135–1152. doi: 10.1084/jem.20120030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlomchik MJ. Sites and stages of autoreactive B cell activation and regulation. Immunity. 2008;28:18–28. doi: 10.1016/j.immuni.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Takemura S, Klimiuk A, Braun A, Goronzy J, Weyand M. T Cell Activation in Rheumatoid Synovium Is B Cell Dependent. J Immunol. 2001;167:4710–4718. doi: 10.4049/jimmunol.167.8.4710. [DOI] [PubMed] [Google Scholar]
- Teichmann LL, Ols ML, Kashgarian M, Reizis B, Kaplan DH, Shlomchik MJ. Dendritic cells in lupus are not required for activation of T and B cells but promote their expansion, resulting in tissue damage. Immunity. 2010;33:967–978. doi: 10.1016/j.immuni.2010.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichmann LL, Schenten D, Medzhitov R, Kashgarian M, Shlomchik MJ. Signals via the Adaptor MyD88 in B Cells and DCs Make Distinct and Synergistic Contributions to Immune Activation and Tissue Damage in Lupus. Immunity. 2013;38:528–540. doi: 10.1016/j.immuni.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Belle TL, Nierkens S, Arens R, von Herrath MG. Interleukin-21 Receptor-Mediated Signals Control Autoreactive T Cell Infiltration in Pancreatic Islets. Immunity. 2012 doi: 10.1016/j.immuni.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinuesa CG, Cook MC, Angelucci C, Athanasopoulos V, Rui L, Hill KM, Yu D, Domaschenz H, Whittle B, et al. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature. 2005;435:452–458. doi: 10.1038/nature03555. [DOI] [PubMed] [Google Scholar]
- Wallin JJ, Liang L, Bakardjiev A, Sha WC. Enhancement of CD8+ T cell responses by ICOS/B7h costimulation. J Immunol. 2001;167:132–139. doi: 10.4049/jimmunol.167.1.132. [DOI] [PubMed] [Google Scholar]
- Weinstein JS, Bertino SA, Hernandez SG, Poholek AC, Teplitzky TB, Nowyhed HN, Craft J. B cells in T follicular helper cell development and function: separable roles in delivery of ICOS ligand and antigen. J Immunol. 2014;192:3166–3179. doi: 10.4049/jimmunol.1302617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong FS, Wen L, Tang M, Ramanathan M, Visintin I, Daugherty J, Hannum LG, Janeway CA, Shlomchik MJ. Investigation of the role of B-cells in type 1 diabetes in the NOD mouse. Diabetes. 2004;53:2581–2587. doi: 10.2337/diabetes.53.10.2581. [DOI] [PubMed] [Google Scholar]
- Xu H, Li X, Liu D, Li J, Zhang X, Chen X, Hou S, Peng L, Xu C, et al. Follicular T-helper cell recruitment governed by bystander B cells and ICOS-driven motility. Nature. 2013;496:523–527. doi: 10.1038/nature12058. [DOI] [PubMed] [Google Scholar]
- Zeller GC, Hirahashi J, Schwarting A, Sharpe AH, Kelley VR. Inducible co-stimulator null MRL-Faslpr mice: uncoupling of autoantibodies and T cell responses in lupus. J Am Soc Nephrol. 2006;17:122–130. doi: 10.1681/ASN.2005080802. [DOI] [PubMed] [Google Scholar]
- Zeng Q, Ng YH, Singh T, Jiang K, Sheriff KA, Ippolito R, Zahalka S, Li Q, Randhawa P, et al. B cells mediate chronic allograft rejection independently of antibody production. J Clin Invest. 2014;124:1052–1056. doi: 10.1172/JCI70084. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.