Synoposis
The skeletal dysplasias are a group of more than 450 heritable disorders of bone. They frequently present in the newborn period with disproportion, radiographic abnormalities, and occasionally other organ system abnormalities. For improved clinical care it is important to determine a precise diagnosis to aid in management, familial recurrence and identify those disorders highly associated with mortality. Long-term management of these disorders is predicated on an understanding of the associated skeletal system abnormalities and these children are best served by a team approach to health care surveillance.
Keywords: osteochondrodysplasias, skeletal dysplasias, nonassortive mating, Achondroplasia, type II collagenopathies, osteogenesis imperfecta
The skeletal dysplasias or osteochondrodysplasias are a heritable group of more than 450 well delineated disorders that affect primarily bone and cartilage, but can also have significant effects on muscle, tendons and ligaments.1 By definition, skeletal dysplasias are heritable diseases that have generalized abnormalities in cartilage and bone, while dysostoses are genetic disorders characterized by abnormalities in a single or group of bones.2 Over time, the distinction between these disorders has become blurred, as the field has recognized that there is radiographic, clinical and molecular overlap. Recent advances in genetic technologies have identified the molecular basis in more than 350 of these disorders, providing us with the opportunity to translate research findings into clinical service. Understanding the genes that produce these disorders allows us to delineate the extent of spectrum of disease associated with a particular disorder, provides diagnostic service for families at risk for recurrence based on mode of inheritance, as well as furthering our understanding of pathways involved in the development and maintenance of the skeleton.
Embryology
The skeletal forms under two distinct processes; endochondral and membraneous ossification. Endochondral ossification is responsible for the formation of most of the mammalian appendicular skeleton and it involves a sequence of carefully orchestrated developmental processes. These include embryonic limb bud initiation and its outgrowth from lateral plate mesoderm, specification of mesenchymal cells for the future limb elements, mesenchymal condensations triggering cartilage differentiation, ossification of developing bones, and finally, their proper growth and maturation in the postnatal period.3,4 Membraneous ossification is the developmental event in which condensing mesenchymal cells progress almost directly to bone cells. The bones of the skull, lateral clavicle and pubis form via mesenchymal ossification. Postnatally, growth continues through the cartilage growth plate in which resting chondrocytes proliferate, undergo hypertrophy, then apoptosis, becoming the growing scaffold of bone.5 Multiple molecular mechanisms (genes) underlie skeleton formation and perturbations to these highly orchestrated processes can lead to skeletal dysplasias. 6
Genetics
The skeletal dysplasias are inherited in an autosomal recessive, autosomal dominant, X-linked recessive, X-linked dominant, and Y-linked manner.1 Appreciation of the mode of inheritance is important because it imparts information to families regarding future recurrences. Family history, including parental and familial heights and growth patterns, should be obtained from the parents of any affected child to determine if there are similarly affected siblings, or other family members, which can lead to a diagnosis or establish the mode of inheritance. There are several X-linked skeletal disorders that recur in male family members and carrier females are either unaffected or only mildly affected (e.g. X-linked Spondyloepiphyseal Tarda).7 Table 1 lists some of these more commonly seen disorders seen in the neonatal period with their respective inheritance pattern. The Nosology and Classification of Genetic Skeletal Disorders1 and On-line Inheritance of Man (OMIM:www.omim.org/) are sources that include information on these disorders and include data on their patterns of inheritance. There are some uncommonly seen patterns of inheritances in the skeletal dysplasias. These include somatic mosaicism, in which one of the parents is mildly affected and their offspring is more severely affected.8 Evaluation of the parents should be considered if there is any question that one of the parents could have a mild skeletal disorder. Gonadal mosaicism is characterized by familial recurrence of a known dominant disorder resultant from one parent carrying heterozygosity for a mutation in one of the cell lineages that comprise the pool of progenitor germ cells and the parent is clinically unaffected.9 This is a rare occurrence, but influences counseling of all dominant disorders because if a newborn is diagnosed with an autosomal dominant disorder, counseling should include a less than 1% recurrence risk based on gonadal mosaicism.
| Group or name of the disorder FGFR3 disorders | Mode of Inheritance Gene Symbol | |
| Thanatophoric dysplasia | AD | FGFR3 |
| Achonodroplasia | AD | FGFR3 |
| Hypochondroplasia | AD | FGFR3 |
| SADDNA | AD | FGFR3 |
| Type II collagen disorders | ||
| Achondrogenesis II | AD | COL2A1 |
| Hypochondrogenesis | AD | COL2A1 |
| Spondyloepiphyseal dysplasia congenita (SEDC) | AD | COL2A1 |
| Kniest dysplasia | AD | COL2A1 |
| Type X1 collagen disorders | ||
| Fibrochondrogenesis | AR | COL11A1 |
| Fibrochondrogenesis | AD | COL11A1, COL11A2 |
| Otospondylomegaepiphyseal dysplasia (OSMED) | AR | COL11A2 |
| Sulfation disorders | ||
| Achondrogenesis IB | AR | SLC26A2 |
| Atelosteogenesis II | AR | SLC26A2 |
| Diastrophic dysplasia | AR | SLC26A2 |
| Chondrodysplasia with congenital joint dslocations | AR | CHST3 |
| Perlecan disorders | ||
| Dyssegmental dysplasia | AR | PLC |
| Dyssegmental dysplasia, Silverman-Handmaker type | AR | PLC |
| Dyssegmental dysplasia, Rolland Desbuquois type | AR | PLC |
| Filamin Disorders and similar disorders | ||
| Otopalatodigital syndrome I and II | XLD | FLNA |
| Osteodysplasty, Melnick-Needles | XLD | FLNA |
| Atelosteogenesis types I and III | AD | FLNB |
| Larsen syndrome | AD | FLNB |
| Spondylo-carpal-tarsal dysplasia | AR | FLNB |
| Serpentine fibula-polycystic kidney syndrome | AD | NOTCH2 |
| TRPV4 disorders | ||
| Metatopic dysplasia | AD | TRPV4 |
| Short-rib dysplasias (with and without polydactyly) | ||
| Chondroectodermal dysplasia (Ellis-van Creveld (EVC) | AR | EVC1, EVC2 |
| Short-rib polydactyly syndrome I, II, III and IV including Asphxiating Thoracic Dystrophy | AR | DYNC2H1 |
| IFT80 | ||
| NEK | ||
| WDR35 | ||
| WDR19 | ||
| WDR34 | ||
| Thoracolaryngeal dysplasia | AD | unknown |
| Metaphyseal dysplasias | ||
| Cartilage-hair hypoplasia | AR | RMRP |
| Metaphyseal dysplasia, Jansen type | AD | PTHR1 |
| Spondylo-epi-(meta)-physeal dysplasia | ||
| SEMD, short limb abnormal calcification type | AR | DDR2 |
| Severe spondylodysplastic dysplasias | ||
| Achondrogenesis 1A | AR | GMAP210 |
| Schneckenbecken dysplasia | AR | SLC35D1 |
| Opsismodysplasia | AR | INPPL1 |
| Acromesomelic disorders | ||
| Acromesomelic dysplasia, type Maroteaux | AR | NPR2 |
| Mesomelic and rhizo-mesomelic dysplasias | ||
| Langer type (homozygoud dyschondrosteosis | pseudo-AR/XLD | SHOX |
| Omodysplasia | AR | GPC6 |
| Robinow syndrome, recessive | AR | ROR2 |
| Robinow syndrome, dominant | AD | WNT5 |
| Bent bone dysplasias | ||
| Campomelic dysplasia | AD | SOX9 |
| Stuve-Wiedemann dysplasia | AR | LIFR |
| Bent bone dysplasia FGFR2 type | AD | FGFR2 |
| Slender bone dysplasias | ||
| Microcephalic osteodysplastic primordial dwarfism (MOPD1) | AR | RNU4ATAC |
| Microcephalic osteodysplastic primordial dwarfism (MOPD2) | AR | PCNT |
| Osteocraniostenosis | FAM111A | |
| Dysplasias with multiple joint dislocations | ||
| Desbuquois dysplasia | AR | CANT1, XYLT1 |
| Pseudodiatrophic dysplasia | AR | unknown |
| Chondrodysplasia punctata group (CDP) | ||
| CDP, X-linked dominant | XLD | EBP |
| Conradi-Hunermann type (CDPX2) | XLR | ARSE |
| brachytelephalangic type (CDPX1) | XLD | NSDHL |
| CHILD syndrome | XLD | EBP |
| Greenberg dysplasia | AR | LBR |
| Rhizomelic CDP type 1 | AR | PEX7 |
| Rhizomelic CDP type 2 | AR | DHPAT |
| Rhizomelic CDP type 3 | AR | AGPS |
| Neonatal osteosclerotic dysplasias | ||
| Bloomstrand dysplasia | AR | PTHR1 |
| Desmosterolosis | AR | DHCR24 |
| Caffey disease (infantile) | AD | COL1A1 |
| Raine dysplasia | AR | FAM20C |
| Increased bone density group | ||
| Osteopetrosis (severe neonatal or infantile forms) | AR | TCIRG1 |
| Osteopetrosis (severe neonatal or infantile forms) | AR | CLCN7 |
| Dysosteosclerosis | AR | SLC29A3 |
| Lenz-Majewski hyperostostic dysplasia | SP | PTDSS1 |
| Osteogenesis imperfecta and decreased bone density group | ||
| Osteogenesis imperfecta, moderate, severe and perinatal lethal | AD | COL1A1, COL1A2 |
| IFITM5 | ||
| Osteogenesis imperfecta, moderate, severe and perinatal lethal | AR | CRTAP |
| P3H1 | ||
| PPBI | ||
| FKBP10 | ||
| HSP47 | ||
| SP7 | ||
| WNT1 | ||
| TMEM33B | ||
| Bruck syndrome | PLOD2 | |
| FKBP10 | ||
| Osteoporosis-pseudoglioma syndrome | AR | LRP5 |
| Cole-Carpenter dysplasia | SP | unknown |
| Abnormal mineralization group | ||
| Hypophosphatasia, perinatal and infantile forms | AR | ALPL |
In the short stature community, it is common to see nonassortive mating, meaning that both parents have skeletal dysplasias. Under these circumstances it is common to evaluate pregnancies at risk for differing outcomes. In cases where one parent has a recessively inherited skeletal disorder and the other parent has a dominantly inherited skeletal disorder, the fetus is at 50% risk for only the dominantly inherited disorder. Whether carrying the recessive allele for another skeletal disorder influences adult height or skeletal complications is unknown, though the concept of genetic load and disease could be applicable in this situation. More commonly, couples are seen that both have the same autosomal dominant disorder, or have two different autosomal dominant disorders. In either scenario, the fetuses or newborns are at 25% risk for inheriting both dominant conditions. Compound heterozygosity has been seen for achondroplasiaachondroplasia, 10 achonodroplasia-hypochondroplasia (non-FGFR3)11, achondroplasiaspondyloepiphyseal congenita12,13,14 pseudoachondroplasia-achondroplasia15,16, pseudoachondroplasia-spondyloepiphyseal dysplasia17, achondroplasia-osteogenesis imperfecta, mild type,11 Leri-Weil dyschondrosteosis-achondroplasia,18 pseudoachondroplasia-osteogenesis imperfecta, severe type, achondroplasia-osteogeneis imperfecta,19 severe type (personal communication) and achondroplasia-acromicric dysplasia (personal communication). The outcomes for these infants are based on the severity of each individual skeletal disorder. Many of these children have guarded prognoses based on respiratory insufficiency due to restrictive lung disease and die within the first year of life. For longer term survivors, issues of severe cervical canal stenosis, foramen magnum stenosis with spinal cord compression, cervical spine instabilities, abnormalities of the brainstem, hydrocephalous, brain dysgenesis (FGFR3 compound heterozygosity), seizures, poor feeding with gastrointestinal reflux, apnea, joint hypermobility, truncal hypermobility, scoliosis, fixed angle kyphophysis, fractures, and orthopaedic complications have been reported, but the body of literature remains small.
Information regarding the severity and natural history of an individual disorder is critical for the family and providers. There is no large single source summarizing the findings, and in some cases dependent on the private mutations that each parent carries. If possible, it is important to establish that the child carries two deleterious mutations if the genes for the disorders are known, assuring the care takers and family that the child indeed has two autosomal dominant disorders. If the child survives the neonatal period, then care should be individualized based on the each organ system abnormality and its severity.
The explosion in molecular genetics has allowed for gene identification in more than two thirds of the skeletal dysplasias. This technical advancement has allowed for more precise diagnosis and physicians and health care providers can direct their care based on the established natural history of each disorder. When a disorder is diagnosed based on family history, clinical or radiograph data, clinical gene testing is performed by many laboratories (GeneTests: https://www.genetests.org/), as well as through skeletal dysplasia gene panels offered by many diagnostic laboratories. The historical approach of clinical diagnosis through physical evaluation and radiographic review then directed molecular testing has now in many centers been supplanted by use of skeletal dysplasia panels that are somewhat unbiased to clinical findings. The limitations to this approach include delay in diagnosis, cost and potential nondiagnosis, because panels may not be comprehensive for all skeletal disorders. However, the benefits include molecular diagnosis in a group of disorders that are rare and often difficult to diagnose, and for some of these disorders there are multiple potential responsible genes (locus heterogeneity), allowing for diagnosis not based on a serial testing approach. As advancing technology evolves, the cost, and the precision of newer gene sequencing approaches improves, it should become available to a larger portion of the population.
Molecular diagnosis can be important particularly for disorders associated with both allelic and locus heterogeneity. For some disorders, the type and location of the mutation within the disease-producing gene (protein) can impart long term natural history information, e.g. nonsense or loss of protein mutations can lead to a different severity of disease relative to missense mutations. This is well illustrated in osteogenesis inperfecta (OI); nonsense or nonstop mutations in the genes that encode type I collagen, COL1A1 and COL1A2, cause the mildest form of the disease,20 while missense mutations in the same genes produce more severe progressive deforming forms of OI.21 Further complicating counseling is that approximately 90% of case of OI result from mutations type I collagen, however most of the remaining 10% result from mutations in recessively inherited genes that are primarily involved in the processing and trafficking of type I collagen.22 These recessively inherited forms of OI illustrate the explosion in our molecular understanding of a single osteochondrodysplasia.23 While immediate care of a neonate with OI is similar regardless of the molecular basis, the familial recurrence risk and long term natural history differs based on the underlying genetic basis of disease. For example, severe progressing OI is typically associated with normal intelligence, while the same radiographic form of OI due to homozygosity for Wintless 1 (WNT 1) mutations are associated with subnormal intelligence. 24,25
Prenatal Diagnosis of Osteochondrodyslasias
Rapid advances of in both imaging modalities and the aforementioned molecular diagnostics has improved our abilities to recognize osteochondrodysplasias in the prenatal period.26,27 For those families with a parent affected by an autosomal dominant disorder, either molecular diagnostics via invasive techniques or by ultrasound imaging, can aid in predicting whether the fetus will be similarly affected (Figure 1). For at-risk families based on a previously affected child with an autosomal recessive disorder, the same above-mentioned approach can be utilized. However, affected neonates with skeletal dysplasias are often the first affected children in their respective families.
Figure 1.
Scheme of management in newborns with a skeletal dysplasia
Many pregnant women are offered an array of noninvasive tests to determine if their fetuses are at risk for genetic disorders.28,29 These molecular screening panels are for autosomal recessive and X-linked disorders including skeletal dysplasias such as diastrophic dysplasia; however it is important to note that many genes and mutations that can cause skeletal dysplasias are presently not included in these panels. Usually if one parent screens positive for a disorder, then the other parent is screened, determining a baseline risk for a disorder. First trimester ultrasound analysis, usually used to identify aneuploidy, is also affective in identifying severe, usually lethal skeletal dysplasias including osteogenesis imperfecta, thanatophoric dysplasia, and the short-rib polydactyly syndromes, as some examples.30-32 If a neonate with a skeletal disorder was noted to have abnormal ultrasound findings in the first trimester including a small crown-rump length for gestational age and increased nuchal fold thickness, the likelihood is that the fetus has a severe, probable lethal skeletal dysplasia.
Many prenatal skeletal dysplasias are diagnosed in the late second trimester when many pregnant women are screened by ultrasound for congenital anomalies. 33 The advantages to early detection (with and without a precise diagnosis) allows for preparation prior to delivery. This includes discussion of active resuscitation, proper assembly of consultants, collection of appropriate material for molecular diagnosis from cord blood, and smoother transition for the fetus from the prenatal to neonatal period. This is particularly important if the neonate is predicted to have a severe, but non-lethal skeletal disorder.
Experience has informed us that many skeletal disorders are not diagnosed in the late second trimester, but either in the third trimester or at birth. These disorders tend to be milder, with a less profound effect on the skeletal, including the thorax, thus severe respiratory compromise is not usually encountered. Achondroplasia, Spondyloepiphyseal Dysplasia Congenita (SEDC) and nonlethal forms of Osteogenesis Imperfecta are frequency first encountered in the newborn period. Many parents are distraught if the suggestion of a skeletal dysplasia is made at birth, and question if “something” was missed at second trimester ultrasound evaluation. It is helpful to explain that for the milder skeletal disorders, long bone measurements are frequently on the normal growth curves at 20 weeks, and fall off occurs in the third trimester since in most of these disorders the defect is in endochondral ossification (not condensation or other earlier-occurring processes) which is a process that is most active in the third trimester.34
Beyond ultrasound and enhanced carrier screening panels, noninvasive prenatal testing from maternal blood for Mendelian disorders may soon be a clinical reality. Screening for cell free fetal DNA in maternal blood during the first trimester of gestation is employed for the detection of aneuploidy, including disorders of copy number variations.35 Reports of de novo FGFR3 mutations (thanatophoric dysplasia) from the fetal cell free DNA fraction in maternal blood illustrates that this technology has the future potential to diagnose de novo autosomal dominant skeletal disorders.36
Defining Lethality
If a newborn has been diagnosed with a known lethal disorder in the fetal period, then treating physicians should offer comfort care for the newborn, but not aggressive management. Defining lethality in the prenatal period can be accomplished by two means; molecular diagnosis of a known lethal disorder, precise diagnosis by ultrasound or chest size abnormalities seen by ultrasound that correlate with lethality. If the chest to abdominal circumference ratio is less than .7, the heart to chest circumference is >50 percent or the abdomen to femur length ratio is < .16, these objective measures are highly correlated with lethality. However, these measurements can be gestational age dependent and may not apply to the infant 27with significant prematurity and a skeletal dysplasia; the presenting disorder may be a known lethal disorder, but the aforementioned criteria may not quite have been reached, or conversely a severely affected child may survive for a prolonged period of time. When lethality is suspected based only on prenatal ultrasound measurements, it is important to counsel parents that while a lethal condition is suspected that only at birth will a true assessment of lethality be possible.
Postnatally, similar criteria can apply to management of a newborn. If clinical and radiographic findings and/or molecular findings indicate a known lethal diagnosis, then supportive care is indicated. Some of the more difficult clinical management scenarios result from the clinical spectrum of disease that is associated individual gene defects. This is particularly true for the disorders listed in the categories in Table 1. For example, in type II collagen disorders, hypochonodrogenesis diagnosed by radiographic criteria is often associated with lethality due to a small chest and respiratory compromise, yet some of these infants survive the neonatal period and then are categorized as severe spondyloepiphyseal dysplasia congenita.37 Thus for each group of skeletal dysplasias, clinical judgment must be used to determine the severity of the skeletal disorder and whether aggressive management is indicated. If highly aggressive ventilation is necessary because of severe respiratory insufficiency particularly due to a small chest, respiratory failure and lethality commonly occurs.38 For all newborns that die of complications from skeletal disorders, radiographs and a source of DNA should be obtained, and families should be offered autopsy. Collection of postmortem material aids in final diagnosis, which helps to provide parents emotional closure and determine recurrence risk for families through clinical molecular diagnosis or research participation (Box 1).
Box 1. Approach to the Initial Evaluation of a Neonate with a Skeletal Dysplasia
The approach to the initial evaluation of newborn with a suspected skeletal dysplasia is outlined in Figure 1. If the newborn is known to have a uniformly lethal disorder, then supportive care is indicated. Similarly, if the diagnosis is known prior to delivery then management is predicated on the knowledge regarding the natural history of the disorder. Newborns with recognized skeletal disorders present in the newborn period with disproportion. Dependent on the skeletal disorder, there are some common findings; this includes relative macrocephaly, narrow chest appearance relative to the abdomen, rhizomelia (short upper portion extremity), mesomelia (short mid-portion extremity), and frequently brachydactyly (short hands, including phalanges). Frequently the face is normal but there are numerous disorders associated with a flat nasal bridge, frontal bossing and midface hypoplasia and include many of the lethal skeletal disorders, achondroplasia, campomelic dysplasia, chondrodysplasia punctate (all forms), type II collagen disorders, Larsen syndrome, and the mucopolysaccharidoses (most forms). Disorders with micrognathia include some of the following disorders: type II collagen disorders, acrofacial dysostoses, Robinow syndrome and again, many of the lethal skeletal dysplasias. Attention must be paid to the Robin-Pierre Robin sequence (small mandible and posterior cleft palate) associated with these disorders. Few of these newborns are delivered by EXIT procedures because of the mandibular abnormality and safety of the airway,39 but some of these children require post delivery intubation and subsequent tracheostomies until definitive surgery for jaw retraction or time, awaiting facial growth. If the newborn has Pierre-Robin sequence, timing of the repair of the cleft palate should be deferred to the craniofacial surgery team or plastic surgeons (see chapter 8).40
Care should be taken regarding the trachea in the skeletal dysplasias. The trachea is composed of fifteen to twenty incomplete C-shaped tracheal rings that contain cartilage that reinforces the front and sides of the trachea to protect and maintain the airway. 41 Pediatricians, neonatologists and anesthesiologists should be informed that many skeletal disorders have been associated with tracheal anatomic changes complicating respiratory status, intubation and any manipulations. The abnormalities include tracheal agenesis, congenital stenosis, premature cartilage calcifications, short trachea and tracheomalacia, as well as disorders associated with tracheoesphageal fistulas. 42 Caretakers should be aware of this when caring for newborns with skeletal abnormalities, and can affect prognosis in many of the nonlethal skeletal disorders.
Diagnosis of a Skeletal Dysplasia
How does one make the diagnosis of a skeletal dysplasia? If the diagnosis has been determined in the prenatal period or based on family history, then laboratory testing or radiographs should be obtained to confirm the clinical or molecular diagnosis. If the skeletal dysplasia is undiagnosed or unexpected then a similar approach should be taken. Once the newborn is stable, a thorough physical exam should be undertaken. Key measurements include head circumference (HC), birth weight (BW), birth length (BL), chest circumference if it appears small, and palm and middle finger lengths. Carefully delineation of any dysmorphic facial features should be performed and include evaluation of the fontanels, nasal bridge, midface, philtrum, mandible, palate and ears. Frequently, the neck will appear short and if the chest is small the nipples may appear widely spaced. In many disorders the hands will appear small, and the fingers will appear to have wide spaces between them because they are short. Attention should be paid to the proportion of the upper and mid sections of the arm. In most newborns they appear subjectively one to one, and in skeletal disorders, there is frequently subjective disproportion. Rhizomelia is common in achondroplasia and spondyloepiphyseal dysplasia congenita, two of the commonly seen nonlethal disorders. Very significant mesomelia relative to the rhizomelic segment suggests a group of specific disorders, the mesomelic dysplasias. Frequently, when there is rhizomelia and mesomelia, there are increased skin creases and skin folds due to the abnormal underlying bone length. A significantly curved long bone may appear substantially much shorter externally than by radiographs.
After clinical evaluation, it is critical to obtain complete anterior/posterior and lateral radiographs. This includes images of the skull, extremities (including hands and feet!) and the spine. Radiographic evaluation should start with overall assessment of epiphyseal ossification to determine if they are delayed or irregular for age, then there should be consideration for an epiphyseal dysplasia. If the metaphyses are widened, flared or irregular then the diagnosis of a metaphyseal chondrodysplasia should be entertained. If diaphyseal abnormalities are present, such as widening and/or cortical thickening, or marrow space expansion then a diaphyseal dysplasia is implied. Combination of the aforementioned abnormalities helps categorize the disorder, e.g. epimetaphyseal disorder. If the vertebral bodies are affected then there is a spondylo-component present, further categorizing the disorder. Once the extent of radiographic abnormalities are determined and placed with a category, e.g. spondylometaphyseal dysplasia, radiographic textbooks can aid in refining the diagnosis.
Organ system abnormalities beyond the skeleton are occasionally seen and can be clues to diagnosis. Congenital heart defects are commonly seen in the skeletal ciliopathies (chondroectodermal dysplasia, asphyxiating thoracic dysplasia, short rib polydactyly syndromes), abnormal formed genitalia in the skeletal ciliopathies, campomelic dysplasia, omodysplasia, Robinow dysplasia, Antley-Bixler and severe disorders of cholesterol metabolism.43-48 Immune deficiency and Hirschprung disease are seen in metaphyseal chondrodysplasia, McKusick type (Cartilage hair hypoplasia). 49,50
Commonly seen disorders
Achondroplasia Group
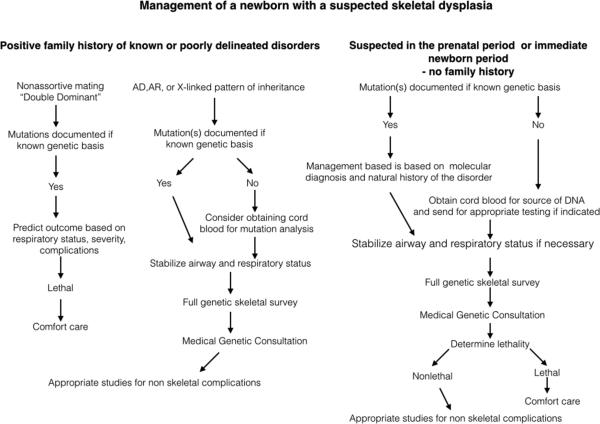
This group of disorders all result from heterozygosity for activation mutations in the gene that encodes Fibroblast Growth Factor Receptor 3 (FGFR3).51 This group of disorders includes thanatophoric dysplasia, achondroplasia, hypochondroplasia and the very rare severe achondroplasia with developmental delay and acanthosis nigricans (SADDAN). Thanatophoric dysplasia is almost uniformly lethal without aggressive management. Radiographic findings include macrocephaly with narrow skull based, long narrow trunk with handlebar clavicles, flat vertebral bodies, small flared iliac bones with very narrow sacrosciatic notches and generalized micromelia (illustrated in Figure 2A).
Figure 2.
Radiographs of FGFR3 disorders. A. Thanatophoric dysplasia in the newborn period. Note the small chest, severe rhizo-mesomelia, curved femora with proximal fadeout and distal irregularities. B. 32 week fetus with achondroplasia. Note the relatively mild narrowing of the chest, rhizomelia and fade-out of the proximal femora (arrow).
Achondroplasia is by far the most common form of nonlethal skeletal dysplasia. Most cases present at birth, though some children are diagnosed in early infancy. Newborns present with relative macrocephaly, frontal bossing, and rhiz-, meso- and acromelia, with a splaying of the fingers between digits and 3 and 4 (known as the “trident” deformity). Radiographic findings include a large skull with short, flat vertebral bodies short, narrow sciatic notch with flat acetabular roof, short thick tubular bones and flared metaphyses (Figure 2B). In the newborn period, most of the children are stable however neurologic complications including brainstem compression due to a tight foramen magnum can lead to hydrocephalous, sleep apnea and increased incidence of sudden infant death in addition to long-term neurologic sequelae.52 These complications usually present in the first few months of life and frequently present with rapidly increasing head size, bulging anterior fontanel, irritability, sweating, increased head extension when asleep, increased deep tendon reflexes and clonus. Families should be counseled that formal evaluation of the foramen magnum should be obtained in the first few months of life under the supervision of a medical geneticist, neurosurgeon or orthopaedic surgeon, rarely is this indicated in the newborn period. There are published guidelines by the American Academy of Pediatrics on health supervision in children with achondroplasia.53 Hypochondroplasia is usually a milder phenotypic presentation than achondroplasia and may not be detected in the newborn period.
Type II Collagenopathies
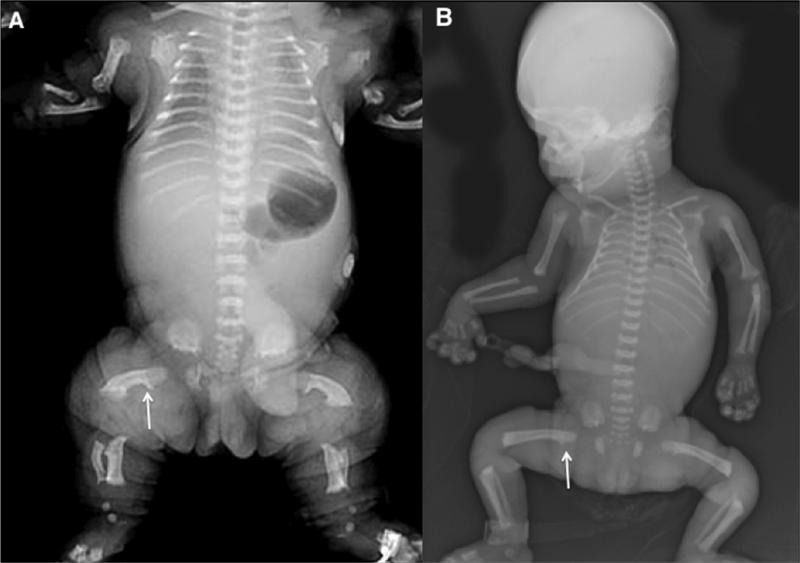
The type II collagenopathies are a heterogenous group of disorders that all show some relationship to each other clinically and radiographically.37 These disorders typically result from heterozygosity for mutations in the type II collagen gene, COL2A1. Achondrogenesis II and hychondrogenesis are lethal disorders that present with large skulls, very small and short ribs, almost lack of mineralization of most vertebral bodies. The pelvis has small iliac wings with absent ischia, pubic bones and sacral elements. The extremities show severe rhizomelia and mesomelia with relative sparing of the hands (Figure 3A and B).
Figure 3.
Radiographs of type II collagen disorders. A. Achondrogenesis II in the late second trimester. Note the severe rhizo- and mesomelia with relative sparing of the hands, lack of ossification of the vertebral bodies and absent ischia. B. Hypochondrogenesis in the late second trimester. Vertebral bodies are under ossified with dumb-bell shaped humeri and femora, and lack of ossification of the ischia.
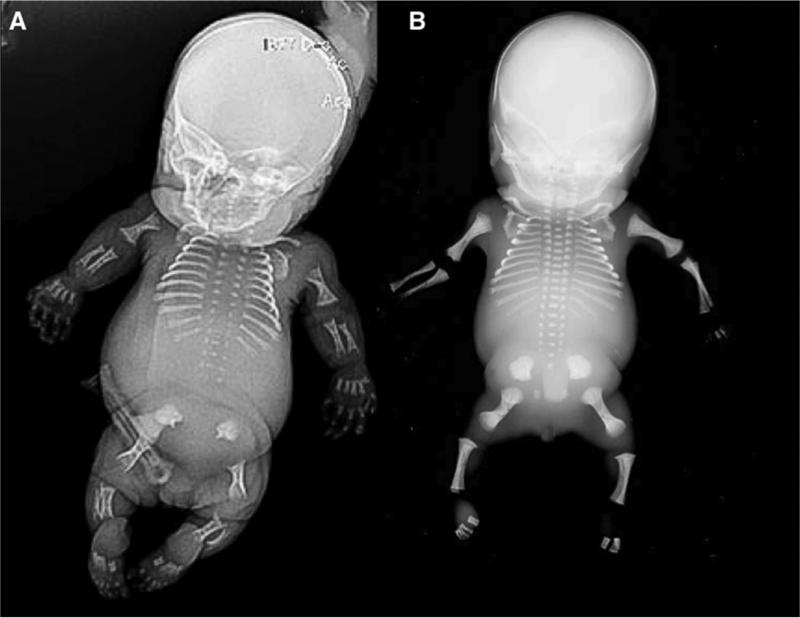
Spondyloepiphyseal dysplasia is usually nonlethal and clinical presentation includes severely short trunked, short-limbed infant with normal appearing hands, feet and skull. There may be micrognathia and a Pierre Robin sequence and attention should be paid to the upper cervical spine, which can be hypomineralized. Radiographic findings in the newborn period include short thorax with short ribs, pear shaped or oval vertebral bodies, absent pubic ossification, short long bones and unossified talus and calcaneus (which are typically ossified by about 24 weeks of gestation). 54 (Figure 4 and B ). Pediatricians should be aware that these children can have some respiratory issues based on small rib cage, feeding difficulties due to micrognathia and flat mid face and are at risk for recurrent ear infections. Families should be counseled that serial evaluation of the cervical spine by a team of specialists who can management odontoid hypoplasia in childhood as part of long-term care for these children.
Figure 4.
Radiographs of spondylo-epiphyseal dysplasia congenital (SEDC) in the newborn period. A. A/P view of the chest showing bell shaped chest with normal appearance of the rib ends, subjective platyspondyly and lack of ossification of the pubis. B. Lateral xray showing delayed ossification of the base of the skull, short ribs, and platyspondyly.
Box 2. Osteogenesis Imperfecta
There are a large group of skeletal disorders that present with decreased bone density. The most common amongst these disorders in osteogenesis imperfecta, or brittle bone disease, where severe forms may present in the newborn period with fractures sustained in-utero. This disorder has been classified in numerous forms, mild, moderate, severe and perinatal forms. These classifications are used by clinicians to manage the complications and predict some of the natural history associated with the disorder, and are somewhat arbitrary. 55 In the last decade there has been enormous progress in unwinding the molecular genetics associated with osteogenesis imperfecta (OI).56 About 90% of the cases result from mutations in the genes that encode type I collagen, COL1A1 and COL1A2. Most of the remaining cases result from gene mutations that are recessively inherited. 56 These recessively inherited cases usually present with severe forms of OI, some of them perinatal lethal.57-59 They are extremely rare, and unless there is a family history consistent with autosomal recessive inheritance (previously affected sibling or consanguinity), most OI cases result from dominantly or sporadically inherited mutations.
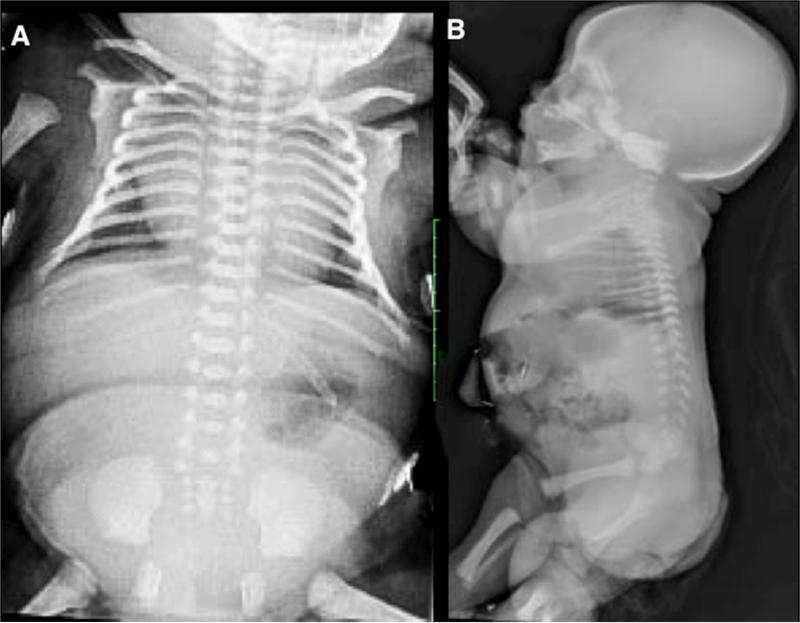
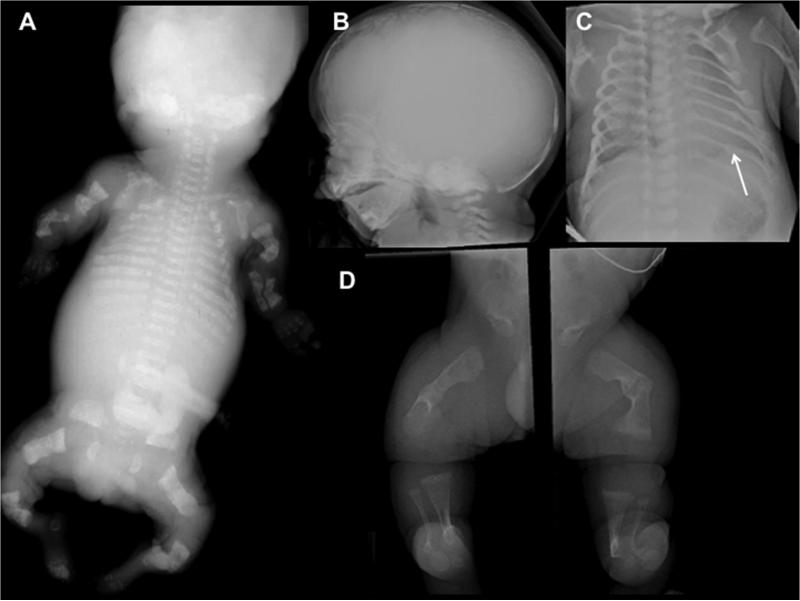
Newborns with OI present with relative macrocephaly, flat facies, short limbs, narrow thorax, deformed and fractured extremities, with normal appearing hands and feet. Radiographs show poor ossification of the skull, fractured ribs, under-mineralized bone with fractures and in very severe cases, femurs that are accordion in appearance, diffuse osteopenia including the vertebrae, flattened acetabulae and relatively normal appearing hands and feet (Figure 5A, B, C and D). Similarly to the aforementioned skeletal dysplasias, there is a broad phenotypic spectrum in OI. The perinatal lethal form usually presents with a very small chest and deformed extremities. Care should be taken if a newborn with OI and in-utero occurring fractured ribs requires ventilator assistance. There is probable underlying pulmonary insufficiency due to a small chest and ventilation may cause ongoing fractures in a probable lethal condition; thus the degree and period of active intervention should be discussed with the family.
Figure 5.
Radiographs of Osteogenesis Imperfecta (OI). A. Perinatal lethal OI showing complete under mineralization of the skeleton with crumbled appendicular bone due to recurrent fractures and poor healing. B. Lateral skull of a newborn with OI. Note poor ossification of the calvarium, visualization of the anterior fontanel and wormian bones. C. A/P of the chest showing narrowness and rib fractures (arrow). D. A/P lower extremities showing poorly modeled and under-mineralized long bones, crumbled appearance due to recurrent fractures.
For those newborns who are stable, but present with fractures, orthopaedic surgeons should evaluate the patient. In many instances, the treatment modality depends on the site of the fracture and degree of angulation at the site. Many of these fractures are managed by immobilization using strapping (e.g. femoral fractures in the subtrochanteric region are managed by strapping of the thigh to abdomen) while fractures of the shaft of bones may be managed by casting.60 In nonlethal but severe cases, consultation should be obtained from medical genetics or pediatric endocrinology for consideration of treatment with intravenous bisphosphonates while in the nursery. Studies have shown that early treatment with bisphosphonates decreases the number of subsequent fractures. 61,62 Individuals with OI also have fragile skin and blood vessels because type I collagen is an important component of connective tissue. Care in handling of these children should be discussed with staff.
Long-term follow-up
Children with skeletal disorders are best managed by a multidisciplinary approach that includes obstetricians (prior to delivery), pediatricians, neonatologists, medical geneticists, endocrinologists, neurosurgeons, otolaryngologists and orthopaedic surgeons. Sub-speciality care should be directed toward the individual presenting issue. Families should be prepared for ongoing visits to supervise health management. For most children with nonlethal skeletal dysplasias, their quality of life is good. Families should be encourage to seek out support from organizations dedicated to the well being of individuals with forms of dwarfism that include Little People of America (www.lpaonline.org) and the Osteogenesis Imperfecta Foundation (www.oif.org). Pediatricians should be aware that these children with normal immune systems should receive standard doses of vaccines on routine schedules, as well as recognizing the potential for increased ear infections in disorders associated with midface hypoplasia, hearing loss due to increased infection and abnormalities of the stapes, as well as hydrocephalous in disorders associated with foramen magnum stenosis (achondroplasia, hypochondroplasia, diastrophic dysplasia and metatropic dysplasia).
Best Practices.
Determine the diagnosis either through clinical, radiographic and molecular testing to determine the mode of inheritance and recurrence risk.
Be aware of nonassortive mating in the short stature community and that outcomes for children with compound heterozyogosity for autosomal disorders can be guarded and variable.
Ideally, the diagnosis of the skeletal dysplasia should be made in the prenatal period to improve implementation of a plan of management.
Discussion should ensue with families before and immediately after delivery regarding management of disorders associated with high incidences of lethality.
Lethality should ideally be determined in the prenatal period based on ultrasound parameter and/or by molecular diagnosis.
Infants with skeletal dysplasias with small chests and need for aggressive ventilation are likely to have a lethal skeletal dysplasia and counseling of families is necessary.
Newborns should be evaluated by clinical examination and radiographs.
Initial care should be highly focused on respiratory status.
Close attention should be paid to the status of the trachea/larynx.
Awareness of the high incidence of cervical abnormalities in the skeletal dysplasias should be communicated to all caregivers.
Radiographs and molecular testing can differentiate severe achondroplasia from thanatophoric dysplasia, particularly if there is respiratory compromise.
Caregivers should be aware of the need to evaluate the foramen magnum in the first few months of life and special attention should be paid to rapidly increasing head size and bulging anterior fontanel.
Caregivers should be aware of the high incidence of odontoid hypoplasia and cervical vertebrae 1 and 2 hypoplasia.
Club feet can present in these disorders and these children should be evaluated by pediatric orthopaedic surgeons with experience in caring for children with skeletal dysplasias
Osteogenesis imperfecta (OI) can present with a broad phenotypic and genetic basis of disease, thus, molecular diagnosis can be helpful in predicting severity and recurrence risk.
Bisphosphonates can be employed in the newborn period in severe cases of OI after consultation with medical genetics or another pediatric specialist experienced in the use of IV bisphosphonates.
Caregivers should be counseled on how to handle a newborn with OI.
Pediatric orthopaedic surgeons should be consulted regarding the management of appendicular skeletal fractures.
Key Points.
Approximately 5% of child with congenital birth defects have skeletal dysplasias
Lethality usually results from small chest, pulmonary hypoplasia and respiratory compromise
Diagnosis is made based on clinical and radiographic findings, including molecular diagnosis when possible
Nonlethal skeletal dysplasias are associated with short stature but overall affected individuals are cognitively normal and have a good quality of life
Best Practices Box.
What is the current practice?
[Center Disease/Condition(s)]
The Skeletal Dysplasias Best Practice/Guideline/Care Path Objective(s)
What changes in current practice are likely to improve
outcomes?
Advances in Molecular Diagnosis through gene discovery will lead to improved counseling of families with affected children.
Knowing the precise diagnosis of type of skeletal dysplasia will also allow clinicians to tailor the treatment of children based on objective natural history data.
Improvement in imaging of the skeleton through MRI and CT will aid in identify children at risk for cervical vertebrae abnormalities.
Is there a Clinical Algorithm?
Major Recommendations
Clinical Algorithm(s)
Rating for the Strength of the Evidence
Bibliographic Source(s)
In skeletal disorders it is important to identify those affected individuals with lethal disorders.
Evaluation of lethality is outlined in Figure 1.
Children with skeletal dysplasias are ideally be managed by a multidisciplinary team.
Cervical vertebral abnormalities occur commonly in skeletal disorders and merits close evaluation by orthopaedic and/or neurosurgeons in childhood.
Krakow D, Rimoin DL. The skeletal dysplasias. Genetics in medicine : official journal of the American College of Medical Genetics. Jun 2010;12(6):327-341.
Krakow D, Lachman RS, Rimoin DL. Guidelines for the prenatal diagnosis of fetal skeletal dysplasias. Genetics in medicine : official journal of the American College of Medical Genetics. Feb 2009;11(2):127-133.
Summary Statement
Children with skeletal disorders are ideally care for by a multidisciplinary approach that includes obstetricians (prior to delivery), pediatricians, neonatologists, medical geneticists, endocrinologists, neurosurgeons, otolaryngologists and orthopaedic surgeons.
There are more than 450 skeletal disorders, some primarily affecting cartilage, some primarily bone, and some both. Knowledge of the precise diagnosis determined by clinical and radiographic data, confirmed by available molecular testing provides clinicians with the basis to care for a newborns with a skeletal disorder.
Acknowledgements
DK is supported by NIH grants RO1 AR066124, the March of Dimes Birth Defects Foundation, the Joseph Drown Foundation and the Orthopaedic Institute for Children/Orthopaedic Hospital Research Center. Support was also provided by NIH/National Center for Advancing Translational Science (NCATS) UCLA CTSI Grant Number UL1TR000124.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
References
- 1.Warman ML, Cormier-Daire V, Hall C, et al. Nosology and classification of genetic skeletal disorders: 2010 revision. American journal of medical genetics. Part A. 2011 May;155A(5):943–968. doi: 10.1002/ajmg.a.33909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krakow D, Rimoin DL. The skeletal dysplasias. Genetics in medicine : official journal of the American College of Medical Genetics. 2010 Jun;12(6):327–341. doi: 10.1097/GIM.0b013e3181daae9b. [DOI] [PubMed] [Google Scholar]
- 3.Provot S, Schipani E. Molecular mechanisms of endochondral bone development. Biochemical and biophysical research communications. 2005;328(3):658–665. doi: 10.1016/j.bbrc.2004.11.068. [DOI] [PubMed] [Google Scholar]
- 4.Hall BK, Miyake T. All for one and one for all: condensations and the initiation of skeletal development. Bioessays. 2000;22(2):138–147. doi: 10.1002/(SICI)1521-1878(200002)22:2<138::AID-BIES5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 5.Ballock RT, O'Keefe RJ. The biology of the growth plate. The Journal of Bone & Joint Surgery. 2003;85(4):715–726. [PubMed] [Google Scholar]
- 6.Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423(6937):332–336. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]
- 7.Gedeon ÁK, Colley A, Jamieson R, et al. Identification of the gene (SEDL) causing X-linked spondyloepiphyseal dysplasia tarda. Nature genetics. 1999;22(4):400–404. doi: 10.1038/11976. [DOI] [PubMed] [Google Scholar]
- 8.Edwards MJ, Wenstrup RJ, Byers PH, Cohn DH. Recurrence of lethal osteogenesis imperfecta due to parental mosaicism for a mutation in the COL1A2 gene of type I collagen. The mosiac parent exhibits phenotypic features of a mild form of the disease. Human mutation. 1992;1(1):47–54. doi: 10.1002/humu.1380010108. [DOI] [PubMed] [Google Scholar]
- 9.Cohn D, Starman B, Blumberg B, Byers P. Recurrence of lethal osteogenesis imperfecta due to parental mosaicism for a dominant mutation in a human type I collagen gene (COL1A1). American journal of human genetics. 1990;46(3):591. [PMC free article] [PubMed] [Google Scholar]
- 10.Pauli RM, Conroy MM, Langer LO, et al. Homozygous achondroplasia with survival beyond infancy. American journal of medical genetics. 1983;16(4):459–473. doi: 10.1002/ajmg.1320160404. [DOI] [PubMed] [Google Scholar]
- 11.Flynn MA, Pauli RM. Double heterozygosity in bone growth disorders: Four new observations and review. American Journal of Medical Genetics Part A. 2003;121(3):193–208. doi: 10.1002/ajmg.a.20143. [DOI] [PubMed] [Google Scholar]
- 12.Young I, Ruggins N, Somers J, Zuccollo J, Rutter N. Lethal skeletal dysplasia owing to double heterozygosity for achondroplasia and spondyloepiphyseal dysplasia congenita. Journal of medical genetics. 1992;29(11):831–833. doi: 10.1136/jmg.29.11.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Günthard J, Fliegel C, Ohnacker H, Rutishauser M, Bühler E. Lung hypoplasia and severe pulmonary hypertension in an infant with double heterozygosity for spondyloepiphyseal dysplasia congenita and achondroplasia. Clinical genetics. 1995;48(1):35–40. doi: 10.1111/j.1399-0004.1995.tb04051.x. [DOI] [PubMed] [Google Scholar]
- 14.Chitty LS, Tan AW, Nesbit DL, Hall CM, Rodeck CH. Sonographic diagnosis of SEDC and double heterozygote of SEDC and achondroplasia—a report of six pregnancies. Prenatal diagnosis. 2006;26(9):861–865. doi: 10.1002/pd.1525. [DOI] [PubMed] [Google Scholar]
- 15.Langer LO, Schaefer GB, Wadsworth DT. Patient with double heterozygosity for achondroplasia and pseudoachondroplasia, with comments on these conditions and the relationship between pseudoachondroplasia and multiple epiphyseal dysplasia, Fairbank type. American journal of medical genetics. 1993;47(5):772–781. doi: 10.1002/ajmg.1320470535. [DOI] [PubMed] [Google Scholar]
- 16.Woods C, Rogers J, Mayne V. Two sibs who are double heterozygotes for achondroplasia and pseudoachondroplastic dysplasia. Journal of medical genetics. 1994;31(7):565–569. doi: 10.1136/jmg.31.7.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Unger S, Korkko J, Krakow D, Lachman RS, Rimoin DL, Cohn DH. Double heterozygosity for pseudoachondroplasia and spondyloepiphyseal dysplasia congenita. American journal of medical genetics. 2001;104(2):140–146. doi: 10.1002/ajmg.10062. [DOI] [PubMed] [Google Scholar]
- 18.Ross JL, Bellus G, Scott CI, Abboudi J, Grigelioniene G, Zinn AR. Mesomelic and rhizomelic short stature: The phenotype of combined Leri - Weill dyschondrosteosis and achondroplasia or hypochondroplasia. American Journal of Medical Genetics Part A. 2003;116(1):61–65. doi: 10.1002/ajmg.a.10807. [DOI] [PubMed] [Google Scholar]
- 19.Kitoh H, Oki T, Arao K, Nogami H. Bone dysplasia in a child born to parents with osteogenesis imperfecta and pseudoachondroplasia. American journal of medical genetics. 1994;51(3):187–190. doi: 10.1002/ajmg.1320510302. [DOI] [PubMed] [Google Scholar]
- 20.Cohn D, Apone S, Eyre D, et al. Substitution of cysteine for glycine within the carboxyl-terminal telopeptide of the alpha 1 chain of type I collagen produces mild osteogenesis imperfecta. Journal of Biological Chemistry. 1988;263(29):14605–14607. [PubMed] [Google Scholar]
- 21.Marini JC, Forlino A, Cabral WA, et al. Consortium for osteogenesis imperfecta mutations in the helical domain of type I collagen: regions rich in lethal mutations align with collagen binding sites for integrins and proteoglycans. Human mutation. 2007;28(3):209–221. doi: 10.1002/humu.20429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Byers PH, Pyott SM. Recessively inherited forms of osteogenesis imperfecta. Annual review of genetics. 2012;46:475–497. doi: 10.1146/annurev-genet-110711-155608. [DOI] [PubMed] [Google Scholar]
- 23.Forlino A, Cabral WA, Barnes AM, Marini JC. New perspectives on osteogenesis imperfecta. Nature Reviews Endocrinology. 2011;7(9):540–557. doi: 10.1038/nrendo.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laine CM, Joeng KS, Campeau PM, et al. WNT1 mutations in early-onset osteoporosis and osteogenesis imperfecta. New England Journal of Medicine. 2013;368(19):1809–1816. doi: 10.1056/NEJMoa1215458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pyott SM, Tran TT, Leistritz DF, et al. < i> WNT1</i> Mutations in Families Affected by Moderately Severe and Progressive Recessive Osteogenesis Imperfecta. The American Journal of Human Genetics. 2013;92(4):590–597. doi: 10.1016/j.ajhg.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krakow D, Alanay Y, Rimoin LP, et al. Evaluation of prenatal-onset osteochondrodysplasias by ultrasonography: a retrospective and prospective analysis. American journal of medical genetics. Part A. 2008 Aug 1;146A(15):1917–1924. doi: 10.1002/ajmg.a.32269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krakow D, Lachman RS, Rimoin DL. Guidelines for the prenatal diagnosis of fetal skeletal dysplasias. Genetics in medicine : official journal of the American College of Medical Genetics. 2009 Feb;11(2):127–133. doi: 10.1097/GIM.0b013e3181971ccb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levenson D. New test could make carrier screening more accessible. American Journal of Medical Genetics Part A. 2010;152(4):fm vii–fm viii. doi: 10.1002/ajmg.a.33290. [DOI] [PubMed] [Google Scholar]
- 29.Carmichael M. One hundred tests. Scientific American. 2010;303(6):50–50. [PubMed] [Google Scholar]
- 30.Parilla BV, Leeth EA, Kambich MP, Chilis P, MacGregor SN. Antenatal detection of skeletal dysplasias. Journal of Ultrasound in Medicine. 2003;22(3):255–258. doi: 10.7863/jum.2003.22.3.255. [DOI] [PubMed] [Google Scholar]
- 31.Makrydimas G, Souka A, Skentou H, Lolis D, Nicolaides K. Osteogenesis imperfecta and other skeletal dysplasias presenting with increased nuchal translucency in the first trimester. American journal of medical genetics. 2001;98(2):117–120. [PubMed] [Google Scholar]
- 32.Dugoff L, Thieme G, Hobbins J. First trimester prenatal diagnosis of chondroectodermal dysplasia (Ellis–van Creveld syndrome) with ultrasound. Ultrasound in obstetrics & gynecology. 2001;17(1):86–88. doi: 10.1046/j.1469-0705.2001.00255.x. [DOI] [PubMed] [Google Scholar]
- 33.Schramm T, Gloning K, Minderer S, et al. Prenatal sonographic diagnosis of skeletal dysplasias. Ultrasound in Obstetrics & Gynecology. 2009;34(2):160–170. doi: 10.1002/uog.6359. [DOI] [PubMed] [Google Scholar]
- 34.Krakow D, Williams J, Poehl M, Rimoin D, Platt L. Use of three - dimensional ultrasound imaging in the diagnosis of prenatal - onset skeletal dysplasias. Ultrasound in obstetrics & gynecology. 2003;21(5):467–472. doi: 10.1002/uog.111. [DOI] [PubMed] [Google Scholar]
- 35.Fan HC, Blumenfeld YJ, Chitkara U, Hudgins L, Quake SR. Noninvasive diagnosis of fetal aneuploidy by shotgun sequencing DNA from maternal blood. Proceedings of the National Academy of Sciences. 2008;105(42):16266–16271. doi: 10.1073/pnas.0808319105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saito H, Sekizawa A, Morimoto T, Suzuki M, Yanaihara T. Prenatal DNA diagnosis of a single-gene disorder from maternal plasma. The Lancet. 2000;356(9236):1170. doi: 10.1016/S0140-6736(00)02767-7. [DOI] [PubMed] [Google Scholar]
- 37.Kannu P, Bateman J, Savarirayan R. Clinical phenotypes associated with type II collagen mutations. Journal of paediatrics and child health. 2012;48(2):E38–E43. doi: 10.1111/j.1440-1754.2010.01979.x. [DOI] [PubMed] [Google Scholar]
- 38.Harding CO, Green CG, Perloff WH, Pauli RM. Respiratory complications in children with spondyloepiphyseal dysplasia congenita. Pediatric pulmonology. 1990;9(1):49–54. doi: 10.1002/ppul.1950090112. [DOI] [PubMed] [Google Scholar]
- 39.Costello BJ, Edwards SP, Clemens M. Fetal diagnosis and treatment of craniomaxillofacial anomalies. Journal of Oral and Maxillofacial Surgery. 2008;66(10):1985–1995. doi: 10.1016/j.joms.2008.01.042. [DOI] [PubMed] [Google Scholar]
- 40.Schaefer RB, Gosain AK. Airway management in patients with isolated Pierre Robin sequence during the first year of life. Journal of Craniofacial Surgery. 2003;14(4):462–467. doi: 10.1097/00001665-200307000-00011. [DOI] [PubMed] [Google Scholar]
- 41.Minnich DJ, Mathisen DJ. Anatomy of the trachea, carina, and bronchi. Thoracic surgery clinics. 2007;17(4):571–585. doi: 10.1016/j.thorsurg.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 42.Wells AL, Wells TR, Landing BH, Cruz B, Galvis DA. Short trachea, a hazard in tracheal intubation of neonates and infants: syndromal associations. Anesthesiology. 1989;71(3):367–373. doi: 10.1097/00000542-198909000-00009. [DOI] [PubMed] [Google Scholar]
- 43.Mansour S, Hall C, Pembrey M, Young I. A clinical and genetic study of campomelic dysplasia. Journal of medical genetics. 1995;32(6):415–420. doi: 10.1136/jmg.32.6.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kelley RI, Kratz LE, Glaser RL, Netzloff ML, Miller Wolf L, Jabs EW. Abnormal sterol metabolism in a patient with Antley - Bixler syndrome and ambiguous genitalia. American journal of medical genetics. 2002;110(2):95–102. doi: 10.1002/ajmg.10510. [DOI] [PubMed] [Google Scholar]
- 45.Patton M, Afzal A. Robinow syndrome. Journal of medical genetics. 2002;39(5):305–310. doi: 10.1136/jmg.39.5.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ho NC, Francomano CA, van Allen M. Jeune asphyxiating thoracic dystrophy and short - rib polydactyly type III (Verma - Naumoff) are variants of the same disorder. American journal of medical genetics. 2000;90(4):310–314. doi: 10.1002/(sici)1096-8628(20000214)90:4<310::aid-ajmg9>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 47.Maroteaux P, Sauvegrain J, Chrispin A, Farriaux J. Omodysplasia. American journal of medical genetics. 1989;32(3):371–375. doi: 10.1002/ajmg.1320320321. [DOI] [PubMed] [Google Scholar]
- 48.Baujat G, Le Merrer M. Ellis-van Creveld syndrome. Orphanet J Rare Dis. 2007;2(6):27. doi: 10.1186/1750-1172-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lux SE, Johnston Jr RB, August CS, et al. Chronic neutropenia and abnormal cellular immunity in cartilage-hair hypoplasia. New England Journal of Medicine. 1970;282(5):231–236. doi: 10.1056/NEJM197001292820501. [DOI] [PubMed] [Google Scholar]
- 50.Mäkitie O, Kaitila I. Cartilage-hair hypoplasia—clinical manifestations in 108 Finnish patients. European journal of pediatrics. 1993;152(3):211–217. doi: 10.1007/BF01956147. [DOI] [PubMed] [Google Scholar]
- 51.Vajo Z, Francomano CA, Wilkin DJ. The Molecular and Genetic Basis of Fibroblast Growth Factor Receptor 3 Disorders: The Achondroplasia Family of Skeletal Dysplasias, Muenke Craniosynostosis, and Crouzon Syndrome with Acanthosis Nigricans 1. Endocrine reviews. 2000;21(1):23–39. doi: 10.1210/edrv.21.1.0387. [DOI] [PubMed] [Google Scholar]
- 52.Hecht JT, Butler IJ, Scott Jr CI. Long-term neurological sequelae in achondroplasia. European journal of pediatrics. 1984;143(1):58–60. doi: 10.1007/BF00442750. [DOI] [PubMed] [Google Scholar]
- 53.Trotter TL, Hall JG. Health supervision for children with achondroplasia. Pediatrics. 2005;116(3):771–783. doi: 10.1542/peds.2005-1440. [DOI] [PubMed] [Google Scholar]
- 54.Unger S. A genetic approach to the diagnosis of skeletal dysplasia. Clinical orthopaedics and related research. 2002;401:32–38. doi: 10.1097/00003086-200208000-00006. [DOI] [PubMed] [Google Scholar]
- 55.Sillence D. Osteogenesis imperfecta: an expanding panorama of variants. Clinical Orthopaedics and Related Research. 1981;159:11–25. [PubMed] [Google Scholar]
- 56.Marini JC, Blissett AR. New genes in bone development: what's new in osteogenesis imperfecta. The Journal of Clinical Endocrinology & Metabolism. 2013;98(8):3095–3103. doi: 10.1210/jc.2013-1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cabral WA, Chang W, Barnes AM, et al. Prolyl 3-hydroxylase 1 deficiency causes a recessive metabolic bone disorder resembling lethal/severe osteogenesis imperfecta. Nature genetics. 2007;39(3):359–365. doi: 10.1038/ng1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barnes AM, Chang W, Morello R, et al. Deficiency of cartilage-associated protein in recessive lethal osteogenesis imperfecta. New England Journal of Medicine. 2006;355(26):2757–2764. doi: 10.1056/NEJMoa063804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baldridge D, Schwarze U, Morello R, et al. CRTAP and LEPRE1 mutations in recessive osteogenesis imperfecta. Human mutation. 2008;29(12):1435–1442. doi: 10.1002/humu.20799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kancherla R, Sankineani SR, Naranje S, et al. Birth-related femoral fracture in newborns: risk factors and management. Journal of children's orthopaedics. 2012;6(3):177–180. doi: 10.1007/s11832-012-0412-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chien Y-H, Chu S-Y, Hsu C-C, Hwu W-L. Pamidronate treatment of severe osteogenesis imperfecta in a newborn infant. Journal of inherited metabolic disease. 2002;25(7):593–595. doi: 10.1023/a:1022099425316. [DOI] [PubMed] [Google Scholar]
- 62.Cheung MS, Glorieux FH. Osteogenesis imperfecta: update on presentation and management. Reviews in Endocrine and Metabolic Disorders. 2008;9(2):153–160. doi: 10.1007/s11154-008-9074-4. [DOI] [PubMed] [Google Scholar]