Abstract
Objectives:
Oral cancer ranks third among all cancers in the Indian population. Human papilloma virus (HPV) plays a significant role in oral carcinogenesis. Population-based subtype variations are present in the HPV prevalence. This study gives an emphasis on the parameters to be considered in formalin fixed paraffin embedded tissues for polymerase chain reaction (PCR)-based research work.
Materials and Methods:
Cross-sectional study on archival paraffin-embedded tissue samples of oral squamous cell carcinoma (OSCC), epithelial dysplasia, and normal oral mucosa surrounding impacted tooth was amplified by PCR for the E6 gene of HPV type 16 and E1 gene of HPV type 18.
Results:
HPV 18 was positive in three OSCC cases. There was no statistically significant association of the positivity of HPV with the age, gender or habit. The HPV positive patients had a tobacco habit and were of a younger age group.
Conclusion:
The presence of HPV in carcinomatous tissue highlights the possible role of HPV in carcinogenesis and archival paraffin embedded tissue specimen can be used for this analysis. Recent studies on genomic analyses have highlighted that the HPV positive tumors are a separate subgroup based on genomic sequencing. The results of a larger retrospective study will help further in our understanding of the role of HPV in carcinogenesis, this study could form the baseline for such follow-up studies.
Keywords: High-risk human papilloma virus, oral epithelial dysplasia, oral squamous cell carcinoma
Introduction
Papilloma virus (PV) was first established as a carcinogenic virus by Rous and Beard (1934) in cotton rabbit.[1] Human papilloma virus (HPV) was identified from the cervical smears by Meisels and Fortins (1976).[2] Durst (1983) isolated HPV from cervical cancers.[3] Syrjanen K (1983) demonstrated the presence of HPV in oral cancer tissue by immunohistochemical markers.[4] HPV has also shown to immortalize epithelial cells and has a synergistic effect with chemicals, like tobacco. This combined mutagenic effect plays a key role in HPV-induced carcinogenesis.[5,6] HPV detection rate varies based on the technique identification. Polymerase chain reaction (PCR)-based technique has a higher detection rate.[7] This article highlights the role of HPV in oral cancer, dysplasia, and the presence in normal oral mucosa and the technical difficulties commonly encountered in PCR-based amplification of paraffin-embedded tissue sections.
Materials and Methods
The archival samples from the cases reported to the Dental College within the duration of 2 years were selected. The samples were grouped into three groups, Group I-patients with oral squamous cell carcinoma (OSCC), Group II-patients with dysplasia (oral epithelial dysplasia), and Group III-control group included patients who had reported for impaction, the surrounding tissue was used in the study. The ethical clearance was obtained from the college ethical board. The samples were fixed in 4% formalin and paraffin embedded. A section of 40 μ thick was utilized for DNA extraction for each specimen. Care was taken to prevent contamination during tissue sectioning. New blades were used for each block. The area was cleaned with xylene between each block.
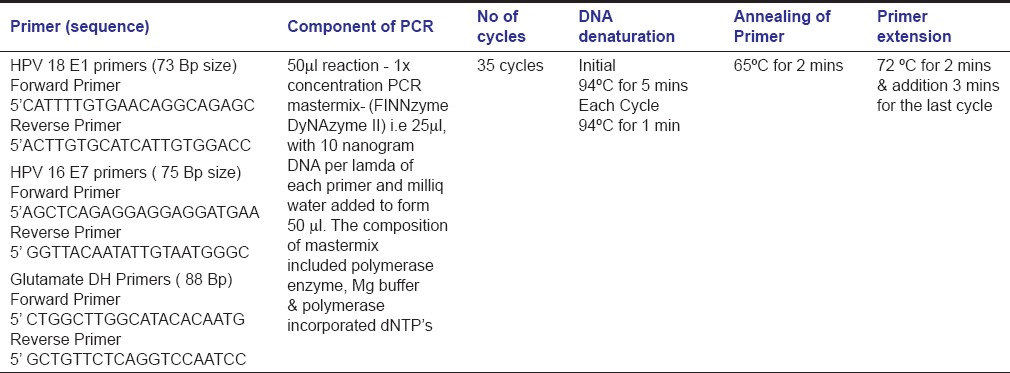
DNA was extracted with the Qiagen Mini-AMP DNA kit. The proteinase K incubation was done overnight at 56°C after discussion with the technical team to increase the yield from paraffin sections. The samples with adequate quantity of DNA were then amplified with a house keeping gene glutamate dehydrogenase (GluDH) the primer was selected such that the final amplicon size was small. The samples which were positive for the PCR amplification of GluDH was amplified for the E7 and E1 genes of HPV 16 and 18, respectively. The PCR amplifications were carried out in separate labs to prevent contamination [Table 1].
Table 1.
PCR setting for three primers
Results
In the 23 cases which were included in our study, 3 patients with OSCC were positive for HPV 18. The patients with HPV positivity had a history of tobacco habits. Histopathologically, two patients had a well-differentiated and one moderately differentiated squamous cell carcinoma. There was no statistically significant association of the positivity of HPV with the age, gender or habit [Figure 1 and Tables 2 and 3].
Figure 1.
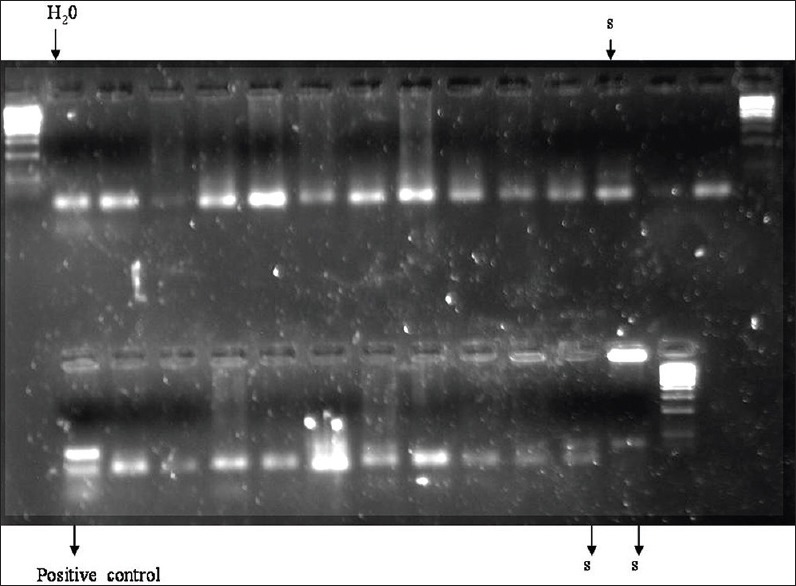
Samples positive for human papilloma virus 18 (s), positive control from Hela cell line and water blank
Table 2.
Demographics and Histopathologic grade of the Oral epithelial dysplasia cases
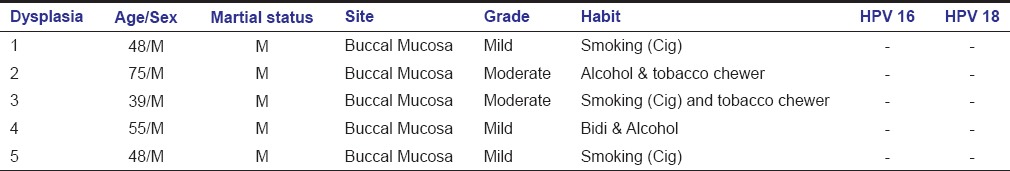
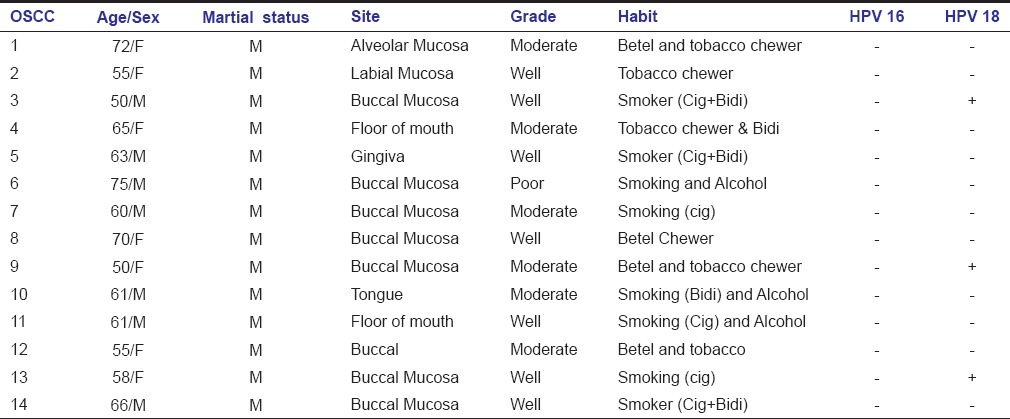
Table 3.
Demographics and Histopathologic grade of the oral squamous cell carcinoma cases
Discussion
Viruses have been identified as etiological agents in several types of human cancers. High-risk types of HPV's (16 and 18) have been established as the cause of invasive cervical cancer and anogenital carcinomas. There is an increasing evidence implicating viruses as etiological agents in the development of a subgroup of upper aerodigestive tract cancers.[8] The role of HPV as an etiological agent in oral cancer is supported by the fact that HPV DNA is present in oral cancer tissue and the observation that high-risk HPV virus can also immortalize and transform normal oral epithelial cells. In this background, the present study was conducted to detect the presence of HPV type 16 and 18 in OSCC, potentially premalignant lesions and normal mucosa.
Totally, 60 cases were selected after the histopathological diagnosis, 23 samples were used for the study, since the housekeeping gene could be amplified only in these samples. PCR inhibitors, e.g. proteinase K, detergents, ions (used for DNA extraction) may prevent the PCR amplification by inhibiting the activity of the thermostable enzymes.[9] DNA integrity is altered due to the cross-linking with proteins induced by formaldehyde.[10] These two limitations should be considered when PCR technique is used in archival formalin fixed paraffin embedded tissue.
Better DNA amplication is possible when primers of amplicon size lesser than 200 base pair are used for PCR amplification.[11] A study on cancer tissues which were formalin fixed and paraffin embedded, DNA was extracted in only 160 of 228 cancer specimens.[12] In our investigative study, initial housekeeping gene primers, β– globin (220 bp size), and 18 S genes (300 bp) were amplified. However, due to the lack of DNA integrity, the efficiency of the PCR-based amplifications of the initial housekeeping genes was not effective for primers >200 bp products.[11] GluDh primers were chosen as the housekeeping gene with the amplicon product size of 88 bp, better amplification when primers of lesser than or equal to 200 bp of amplicon product size were used for these samples. The amplicon product size for HPV 18 and HPV 16 selected were 73 bp and 75 bp, respectively. The final sample size of 23 samples, which were GluDh positive, were selected for the identification of HPV type 16 and 18.
The relation of habit and demographics in HPV-induced oral carcinogenesis is controversial. Some authors state that the presence of HPV has no relation to the habits, site of the tumor, age and gender.[13,14]
In contrast, the HPV prevalence has been reported in tobacco chewers and smokers but a lower rate.[15] Some studies have not found any association between HPV and tobacco associated habits suggesting that these acted as independent factors in the pathogenesis of cancer.[16] Tobacco had an additive effect and that alcohol consumption had a synergistic effect with HPV positive cancers.[16] In our study, all the HPV positive cases were habitual users of tobacco indicating a possible correlation with tobacco, though there was no significant association.
In our study, oral cancer cases were predominantly from buccal mucosa; this finding was consistent with the data of site prevalence of oral cancer in South Asia.[17] HPV has also been detected in gingival biopsies, the gingiva could possibly considered a reservoir of the virus.[18] The presence of HPV in normal oral mucosa could be due to a latent HPV infection.[19]
Latent infection can be as a result of two important factors, that is, low immune clearance of the virus and other factor is probably the site viral genome integration or lack integration.[20,21] This immune response is based on genetic predisposition of the individual, human leukocyte antigen haplotype shows a characteristic pattern in HPV positive cancers.[22] The genetic markers in HPV positive cancers differ from negative cancers, commonly expressed gene include certain cell cycle regulators and transcription factors.[23] These parameters could probably explain the absence of progression of HPV positive normal oral mucosa to precancerous or cancerous lesions.
Human papilloma virus was predominantly detected only in a younger age group below 60 years in our study, though there was no significant association. Some authors have hypothesized that combined effects of the habits and HPV infection may lead to the early occurrence of OSCC in younger age group.[24] HPV 18 was detected in cases within a mean age being 52 years, that is, below the age group of 60 years in our study.
In this study, among the HPV positive cases, two were female patients and one case was a male patient. A higher HPV prevalence in males than females is noted in OSCC.[24]
The prevalence rate of different subtypes of HPV is varied in geographical locations, few studies there is a predominance of HPV type 18. HPV 18 was the only subtype detected by real time PCR in OSCC.[13] In patients of OSCC and other mucosal lesions in an Italian population detected HPV 18 in 85%, of their cases and in contrast had a very low prevalence of HPV 16, 31 and 33.[25] HPV type 18 was detected in 47% of cases (higher percentage) and HPV 16 in 42% of OSCC cases in the South Indian population.[26] In a North Indian population, only HPV 16 was present with an absence of HPV 18 in OSCC and potentially malignant lesions.[19] In our study of South Indian individuals, HPV type 18 was detected in 3 cases of OSCC. HPV 16 was not detected in any of the groups in our study. We could probably explain the absence of HPV 16 in our study due to the geographical variation as documented in other studies among different population.[19,26,27,28]
In this study, the cases which were positive for HPV 18, two were well-differentiated carcinomas and one was moderately differentiated carcinoma. This finding was similar to other studies which did not find an association between the presence of HPV and the grade of the tumor.[29] PCR of paraffin embedded tissue increases the sensitivity of diagnosis in diseases, like tuberculosis.[30] DNA quality in this study was also checked by amplification of a housekeeping gene.
The incidence of HPV normal oral mucosa has always been the topic of controversy about the role of HPV in oral carcinogenesis. The presence of HPV in oral tissues observed was 15 cases (15%) of OSCC, 27 cases (34%) of potentially malignant lesions and 15 cases (31%) of the corresponding normal mucosa the subtype HPV 16 only was present. No correlation was observed in their study between the presence of HPV and gender, age and tumor stage and differentiation.[19] HPV in the normal tissues can exist in latent form without induction of oral cancer, which may be depended on the genotypic predisposition.[19,22] Genomic sequencing in HPV positive and HPV negative tumors has revealed difference in mutation and the p16 expression (surrogate marker for HPV positive cancers).[31] HPV positive can be considered a separate subset of cancers.
In the present study, a relation of HPV positive cases of OSCC with habit and age group of individual was evident. Scope of the study was to use type specific primers of high-risk subtypes of HPV, HPV 16, and HPV 18 which are the most common subtypes associated with oral cancer and dysplasia. The short comings of this study were that the sample size was reduced due to failure of amplification of the house keeping gene. During genomic studies, the samples for DNA extraction should not be exposed to any PCR inhibitors or formalin for long periods. This DNA can be checked with a housekeeping gene. This is an exploratory study to test the feasibility, as this could potentially enable archival material to be used in a larger retrospective study.
Conclusion
This study also highlights that HPV-induced cancers can be a separate subgroup of cancers, in those individuals who are lesser than 60 years of age. This could be due to the dual mutagenic effects of HPV and the combined effect of habits. When archival samples of formalin fixed sections are for such studies, few alterations can be undertaken, like overnight incubation with proteinase K for maximum DNA yield and primers should be selected with a small amplicon size. The results of a larger retrospective study will help further in our understanding of the role of HPV in carcinogenesis, this study could form the baseline for such follow-up studies.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Rous P, Beard JW. The progression to carcinoma of virus-induced rabbit papillomas (SHOPE) J Exp Med. 1935;62:523–48. doi: 10.1084/jem.62.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meisels A, Fortin R. Condylomatous lesions of the cervix and vagina. I. Cytologic patterns. Acta Cytol. 1976;20:505–9. [PubMed] [Google Scholar]
- 3.Dürst M, Gissmann L, Ikenberg H, zur Hausen H. A papillomavirus DNA from a cervical carcinoma and its prevalence in cancer biopsy samples from different geographic regions. Proc Natl Acad Sci U S A. 1983;80:3812–5. doi: 10.1073/pnas.80.12.3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Syrjänen K, Syrjänen S, Lamberg M, Pyrhönen S, Nuutinen J. Morphological and immunohistochemical evidence suggesting human papillomavirus (HPV) involvement in oral squamous cell carcinogenesis. Int J Oral Surg. 1983;12:418–24. doi: 10.1016/s0300-9785(83)80033-7. [DOI] [PubMed] [Google Scholar]
- 5.Subramanyam D, Krishna S. c-Myc substitutes for Notch1-CBF1 functions in cooperative transformation with papillomavirus oncogenes. Virology. 2006;347:191–8. doi: 10.1016/j.virol.2005.11.028. [DOI] [PubMed] [Google Scholar]
- 6.Fan X, Liu Y, Chen JJ. Down-regulation of p21 contributes to apoptosis induced by HPV E6 in human mammary epithelial cells. Apoptosis. 2005;10:63–73. doi: 10.1007/s10495-005-6062-y. [DOI] [PubMed] [Google Scholar]
- 7.Miller CS, Johnstone BM. Human papillomavirus as a risk factor for oral squamous cell carcinoma: A meta-analysis, 1982-1997. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91:622–35. doi: 10.1067/moe.2001.115392. [DOI] [PubMed] [Google Scholar]
- 8.Soares RC, Oliveira MC, Souza LB, Costa AL, Medeiros SR, Pinto LP. Human papillomavirus in oral squamous cells carcinoma in a population of 75 Brazilian patients. Am J Otolaryngol. 2007;28:397–400. doi: 10.1016/j.amjoto.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 9.Abu Al-Soud W, Râdström P. Capacity of nine thermostable DNA polymerases To mediate DNA amplification in the presence of PCR-inhibiting samples. Appl Environ Microbiol. 1998;64:3748–53. doi: 10.1128/aem.64.10.3748-3753.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shedlock AM, Haygood MG, Pietsch TW, Bentzen P. Enhanced DNA extraction and PCR amplification of mitochondrial genes from formalin-fixed museum specimens. Biotechniques. 1997;22:394–6. doi: 10.2144/97223bm03. 398, 400. [DOI] [PubMed] [Google Scholar]
- 11.Baay MF, Quint WG, Koudstaal J, Hollema H, Duk JM, Burger MP, et al. Comprehensive study of several general and type-specific primer pairs for detection of human papillomavirus DNA by PCR in paraffin-embedded cervical carcinomas. J Clin Microbiol. 1996;34:745–7. doi: 10.1128/jcm.34.3.745-747.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mork J, Lie AK, Glattre E, Hallmans G, Jellum E, Koskela P, et al. Human papillomavirus infection as a risk factor for squamous-cell carcinoma of the head and neck. N Engl J Med. 2001;344:1125–31. doi: 10.1056/NEJM200104123441503. [DOI] [PubMed] [Google Scholar]
- 13.Boy S, Van Rensburg EJ, Engelbrecht S, Dreyer L, van Heerden M, van Heerden W. HPV detection in primary intra-oral squamous cell carcinomas – Commensal, aetiological agent or contamination? J Oral Pathol Med. 2006;35:86–90. doi: 10.1111/j.1600-0714.2006.00385.x. [DOI] [PubMed] [Google Scholar]
- 14.Chen PC, Kuo C, Pan CC, Chou MY. Risk of oral cancer associated with human papillomavirus infection, betel quid chewing, and cigarette smoking in Taiwan – An integrated molecular and epidemiological study of 58 cases. J Oral Pathol Med. 2002;31:317–22. doi: 10.1034/j.1600-0714.2002.00129.x. [DOI] [PubMed] [Google Scholar]
- 15.Herrero R, Castellsagué X, Pawlita M, Lissowska J, Kee F, Balaram P, et al. Human papillomavirus and oral cancer: The International Agency for Research on Cancer multicenter study. J Natl Cancer Inst. 2003;95:1772–83. doi: 10.1093/jnci/djg107. [DOI] [PubMed] [Google Scholar]
- 16.Smith EM, Ritchie JM, Summersgill KF, Hoffman HT, Wang DH, Haugen TH, et al. Human papillomavirus in oral exfoliated cells and risk of head and neck cancer. J Natl Cancer Inst. 2004;96:449–55. doi: 10.1093/jnci/djh074. [DOI] [PubMed] [Google Scholar]
- 17.Siriwardena BS, Tilakaratne A, Amaratunga EA, Tilakaratne WM. Demographic, aetiological and survival differences of oral squamous cell carcinoma in the young and the old in Sri Lanka. Oral Oncol. 2006;42:831–6. doi: 10.1016/j.oraloncology.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Hormia M, Willberg J, Ruokonen H, Syrjänen S. Marginal periodontium as a potential reservoir of human papillomavirus in oral mucosa. J Periodontol. 2005;76:358–63. doi: 10.1902/jop.2005.76.3.358. [DOI] [PubMed] [Google Scholar]
- 19.D’Costa J, Saranath D, Dedhia P, Sanghvi V, Mehta AR. Detection of HPV-16 genome in human oral cancers and potentially malignant lesions from India. Oral Oncol. 1998;34:413–20. doi: 10.1016/s1368-8375(98)00028-1. [DOI] [PubMed] [Google Scholar]
- 20.Tindle RW. Immune evasion in human papillomavirus-associated cervical cancer. Nat Rev Cancer. 2002;2:59–65. doi: 10.1038/nrc700. [DOI] [PubMed] [Google Scholar]
- 21.Wentzensen N, Vinokurova S, von Knebel Doeberitz M. Systematic review of genomic integration sites of human papillomavirus genomes in epithelial dysplasia and invasive cancer of the female lower genital tract. Cancer Res. 2004;64:3878–84. doi: 10.1158/0008-5472.CAN-04-0009. [DOI] [PubMed] [Google Scholar]
- 22.Hernández-Hernández DM, Cerda-Flores RM, Juárez-Cedillo T, Granados-Arriola J, Vargas-Alarcón G, Apresa-García T, et al. Human leukocyte antigens I and II haplotypes associated with human papillomavirus 16-positive invasive cervical cancer in Mexican women. Int J Gynecol Cancer. 2009;19:1099–106. doi: 10.1111/IGC.0b013e3181a83cf4. [DOI] [PubMed] [Google Scholar]
- 23.Slebos RJ, Yi Y, Ely K, Carter J, Evjen A, Zhang X, et al. Gene expression differences associated with human papillomavirus status in head and neck squamous cell carcinoma. Clin Cancer Res. 2006;12(3 Pt 1):701–9. doi: 10.1158/1078-0432.CCR-05-2017. [DOI] [PubMed] [Google Scholar]
- 24.Cruz IB, Snijders PJ, Steenbergen RD, Meijer CJ, Snow GB, Walboomers JM, et al. Age-dependence of human papillomavirus DNA presence in oral squamous cell carcinomas. Eur J Cancer B Oral Oncol. 1996;32B:55–62. doi: 10.1016/0964-1955(95)00060-7. [DOI] [PubMed] [Google Scholar]
- 25.Giovannelli L, Campisi G, Lama A, Giambalvo O, Osborn J, Margiotta V, et al. Human papillomavirus DNA in oral mucosal lesions. J Infect Dis. 2002;185:833–6. doi: 10.1086/339193. [DOI] [PubMed] [Google Scholar]
- 26.Balaram P, Nalinakumari KR, Abraham E, Balan A, Hareendran NK, Bernard HU, et al. Human papillomaviruses in 91 oral cancers from Indian betel quid chewers – High prevalence and multiplicity of infections. Int J Cancer. 1995;61:450–4. doi: 10.1002/ijc.2910610403. [DOI] [PubMed] [Google Scholar]
- 27.Tang X, Jia L, Ouyang J, Takagi M. Comparative study of HPV prevalence in Japanese and North-east Chinese oral carcinoma. J Oral Pathol Med. 2003;32:393–8. doi: 10.1034/j.1600-0714.2003.00122.x. [DOI] [PubMed] [Google Scholar]
- 28.Tsuhako K, Nakazato I, Miyagi J, Iwamasa T, Arasaki A, Hiratsuka H, et al. Comparative study of oral squamous cell carcinoma in Okinawa, Southern Japan and Sapporo in Hokkaido, Northern Japan; with special reference to human papillomavirus and Epstein-Barr virus infection. J Oral Pathol Med. 2000;29:70–9. doi: 10.1034/j.1600-0714.2000.290204.x. [DOI] [PubMed] [Google Scholar]
- 29.Elamin F, Steingrimsdottir H, Wanakulasuriya S, Johnson N, Tavassoli M. Prevalence of human papillomavirus infection in premalignant and malignant lesions of the oral cavity in UK subjects: A novel method of detection. Oral Oncol. 1998;34:191–7. doi: 10.1016/s1368-8375(97)00081-x. [DOI] [PubMed] [Google Scholar]
- 30.Hofman V, Selva E, Landraud L, Sicard D, Vénissac N, Castillo L, et al. Value of PCR amplification from formalin-fixed paraffin-embedded tissues in the diagnosis of Mycobacterium tuberculosis infection. Ann Pathol. 2003;23:206–15. [PubMed] [Google Scholar]
- 31.Nichols AC, Chan-Seng-Yue M, Yoo J, Xu W, Dhaliwal S, Basmaji J, et al. A pilot study comparing HPV-positive and HPV-negative head and neck squamous cell carcinomas by whole exome sequencing. ISRN Oncol. 2012;2012:809370. doi: 10.5402/2012/809370. [DOI] [PMC free article] [PubMed] [Google Scholar]


