Abstract
Background:
The aim of this study was to compare the clinical efficacy of coronally advanced flap (CAF) alone and in combination with autologous platelet rich fibrin membrane (PRF) in Miller's class I and II gingival recessions.
Materials and Method:
Thirty isolated Miller class I or II sites in 26 subjects were randomly divided into test (15 sites- CAF+PRF) and control (15 sites- CAF alone). Parameters probing pocket depth (PPD), Recession depth (RD), Clinical attachment loss (CAL), Keratinised tissue width (KTW) and Gingival tissue thickness (GTH) were evaluated at baseline, 3 months and 6 months postoperatively. Data was subjected to statistical analysis. P< 0.05 was considered statistically significant.
Results:
Mean percentage root coverage was 91.00±19.98% and 86.60±23.83% for test and control group respectively. Difference between the groups in all parameters at baseline, 3 months and 6 months was non significant. Complete root coverage was obtained in 12 (80%) and 11 (73.3%) subjects in test and control group respectively. The difference was found to be non-significant. Both groups showed significant differences in all parameters at 3 and 6 months respectively except difference in gingival tissue thickness which was non-significant in control group at 3 months.
Conclusion:
Combination of PRF to CAF procedure did not provide any added advantage in term of recession coverage in Miller class I and II recessions. Long term trials with more sample size are needed to validate these findings.
Keywords: Coronally advanced flap, Miller recession, platelet rich fibrin membrane
Introduction
Gingival recession has been associated with dentinal hypersensitivity, root caries and esthetic compromise.[1] Root coverage procedures aim at providing both tangible (resolution of dentinal hypersensitivity and esthetic dilemma) and intangible benefits (clinical attachment gain, recession coverage, increased keratinized tissue height and gingival thickness [GTH]) to the patients. Various procedures have been tried to obtain root coverage of single rooted teeth. Miller Class I and II gingival recessions hold out the best promise for root coverage as there is no interdental bone and soft tissue loss associated with these recessions.[2]
There are mainly three different types of approaches to achieve root coverage; the free gingival graft, the coronally advanced flap (CAF) and combined procedures involving CAF with tissue/material interposed between the flap and root surface.[3] CAF has been tried with varying degrees of success to cover the recession defects. Histologically, this technique leads to reformation of junctional epithelium and the connective tissue attachment with minimal bone repair. The connective tissue attachment achieved by CAF is not stable over long periods, and various adjunctive agents have been used to promote healing and to further enhance the clinical outcomes.[4] These include the use of root biomodification agents, connective tissue grafts (CTG), barrier membranes, enamel matrix derivatives (EMD), acellular dermal matrix (ADM), platelet rich plasma (PRP), living tissue engineered human fibroblast-derived dermal substitute and platelet-rich fibrin (PRF), etc.[5]
Platelet-rich fibrin was developed in France by Choukroun et al.[6] It is a second-generation platelet concentrate. Its advantages over the better known PRP include an ease of preparation/application, minimal expense and lack of biochemical modification as no bovine thrombin or anticoagulant is required for its preparation. PRF is a fibrin matrix in which platelet cytokines (growth factors) and cells are trapped and are released over time. It can also serve as a resorbable interpositional membrane.[7] The PRF layer avoids early invagination of the gingival epithelium, thereby serving as a barrier to epithelium migration.[8]
This has been used successfully in combination with CAF for root coverage in isolated and multiple gingival recessions. Within limits of our knowledge, literature is deficient in randomized controlled trials comparing CAF alone and in combination with PRF as barrier membrane. Therefore, this study was conceived with an aim to clinically evaluate and compare the efficacy of CAF alone and in combination with PRF membrane in the treatment of Miller Class I and II gingival recessions.
Materials and Methods
This study was conducted in the Department of Periodontics, Modern Dental College and Research Centre, Indore. Twenty-six systemically healthy patients with isolated Miller Class I and II gingival recessions in Maxillary anterior and premolar teeth were included in the study. Informed consent was obtained from all the patients. Ethical clearance was obtained from Institutional Ethical Committee.
Systemically and periodontally healthy patients of 20–50 years of age having isolated Miller's Class I or Class II buccal recession defects at least at one site and who were willing for the study were included as study subjects.
Subjects suffering from any systemic disease, nonambulatory or severely ill patients, Miller's Class III and Class IV recession defects, teeth with cervical abrasion, with sulcular probing depth more than 2 mm, with inadequate width keratinized gingiva and indistinguishable cemento-enamel junction (CEJ), nonvital, rotated or malposed teeth and sites with history of any prior mucogingival surgery and trauma from occlusion were excluded from the study. Patients using tobacco in any form (chewable and smoking), drug addicts and suffering from aggressive periodontitis were also excluded.
The following clinical measurements were taken immediately before surgery (baseline) and at 3 and 6 months follow-up visits. Presence and absence of plaque and bleeding on probing were noted. Recession depth (RD) was measured from the CEJ to the apical extension of the gingival margin (gingival zenith). Probing pocket depth (PPD) was measured from the gingival margin to base of the gingival sulcus. Clinical attachment level (CAL) was measured from CEJ to the base of the gingival sulcus. Keratinized tissue height (width of keratinized gingiva) was measured from the gingival margin to the mucogingival line. All these measurements were performed with the help of William's periodontal probe.
Thickness of attached gingiva (GTH) was measured to assess gingival biotype. GTH was measured 3 mm below the gingival margin at attached gingiva with a number 15 endodontic reamer with a silicon disk stop. The gingival surface was pierced at a right angle with slight pressure until hard tissue was reached. The silicon stop on the reamer was slid until it was in close contact with the gingiva. After the removal of the reamer, the distance between the tip of the reamer and the outer border of silicon stop was measured to nearest 0.1 mm with digital Vernier calipers.[9] The width of the stop was subtracted from the reading to obtain GTH.
Percentage of root coverage was calculated by the following formula:[10]
Perecentage of root coverage =

Methods
Full mouth supra and subgingival scaling were completed 2 weeks before the surgery, and oral hygiene instructions were given. Patients were randomly divided (by coin toss method) into two groups - Group I (control) and Group II (test); each comprised of 15 recession defects. In Group I, gingival recession was treated by CAF and in Group II, gingival recession was treated by CAF in combination with PRF membrane.
Ten milliliter of blood was drawn in test tubes without an anti-coagulant and centrifuged immediately. Blood was centrifuged using a table top centrifuge (REMI Laboratories) for 12 min at 2700 rpm.[11]
Because of the absence of an anticoagulant, blood begins to coagulate as soon as it comes in contact with the glass surface. Therefore, for successful preparation of PRF, speedy blood collection and immediate centrifugation before the initiation of clotting cascade was practiced. The uppermost platelet poor plasma layer was discarded, and PRF clot was separated from bottom red blood cell (RBC) layer with the help of scissors. Care was taken to retain at least 1 mm area of RBC layer as the leucocytes and platelets are found to be concentrated at the junction of PRF clot and RBC layer.[12] PRF membrane was obtained by squeezing out the fluids from the fibrin clot by soft compression method in between palms.[13]
All the procedures were performed by the operators who were specifically trained to perform these procedures. Patients were blinded for allocation to particular group and treatment. A coin toss (done by a person other than the examiner) was used to determine the group (test/control) in which patient was enrolled. Assessor was also blinded about the group allocation and treatment rendered.
Surgical technique of test case
The operative site was anaesthetized using 2% xylocaine with adrenaline (1:200,000). A modified coronally positioned flap technique consisting of split-full-split thickness flap as devised by de Sanctis and Zucchelli (2007) was performed at the surgical site [Figures 1-5].[14]
Figure 1.
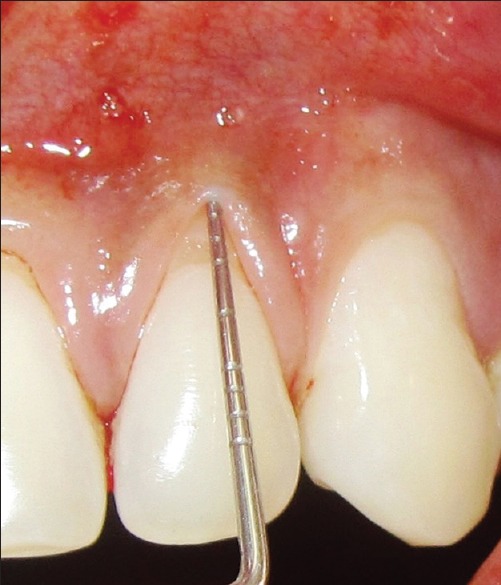
Preoperative recession defect of test site
Figure 5.

Six months postoperative view of test site
Figure 2.
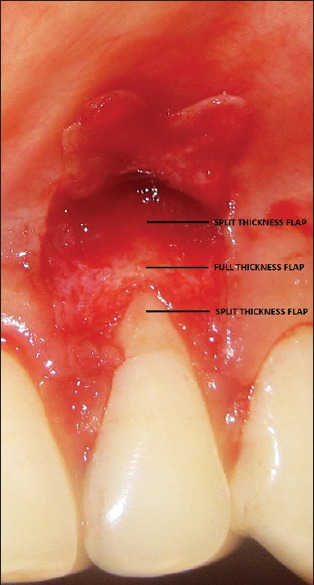
Reflection of flap showing split-full-split design
Figure 3.
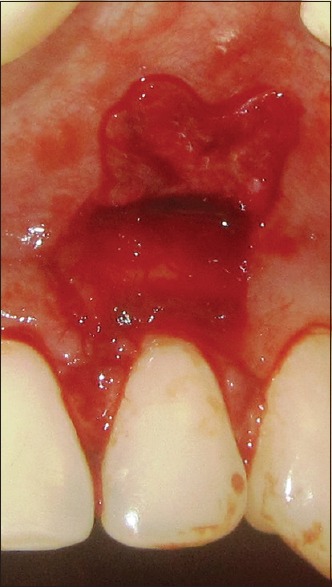
Placement of platelet rich fibrin membrane
Figure 4.
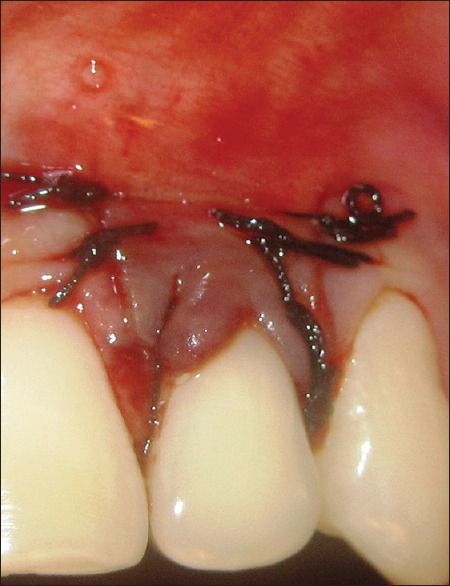
Sutured flap
The prepared PRF membrane was positioned over the recession defects, just below the CEJ.[15]
The flap was coronally moved to reach the tip of the dis-epithelized anatomical papillae, and the vestibular soft tissue was positioned 1 mm coronal to CEJ to account for soft tissue shrinkage.
The suturing of the flap started with coronal sling suture. Interrupted sutures, given around vertical releasing incision were directed apico-coronal from the flap to adjacent buccal soft tissue. This was done to facilitate the coronal displacement of the flap and to reduce the tension on the flap. In some cases, the flap stabilization was further enhanced by placing an interdental tag suture on each papilla 1 mm apical to the site of sling suture.[16]
The periodontal dressing was applied to the surgical site on the buccal and lingual aspects without application of excessive pressure interdentally.
In control group, same surgical protocol was performed without the placement of PRF membrane [Figures 6 and 7].
Figure 6.

Preoperative recession defect of control site
Figure 7.
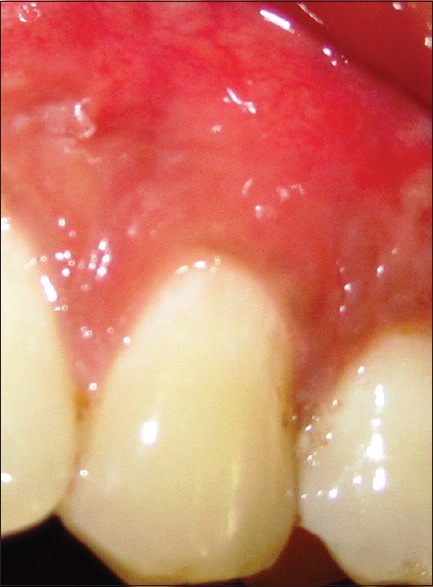
Six months postoperative view of control site
All patients were given antibiotics, analgesics and anti-inflammatory drugs for 5 days. They were instructed to rinse their mouth with a 0.12% chlorhexidine solution, 3 times a day for 1 min, for 4 weeks. Periodontal dressing and sutures were removed after 14 days. All patients were reviewed and instructed to refrain from brushing for a period of another 2 weeks. The restitution of mechanical tooth cleaning using an ultrasoft toothbrush and a roll technique was instructed at the end of first postoperative month. All patients were recalled at 1, 3 and 6 months for evaluation and prophylaxis. Clinical measurements were recorded at 3 and 6 months.
The clinical data obtained were fed into the computer using Microsoft Excel 2000 package, and statistical analysis was carried out usingSPSS software (SPSS windows version 16, Chicago, SPSS Inc).
The means and standard deviations of all parameters were calculated. Student's paired t-test was used for intragroup comparisons while the Student's unpaired t-test was used for intergroup comparison. Mann–Whitney U-test was used to compare the distribution of males and females in both groups.
Association between two variables was evaluated by correlation coefficient. Multiple linear regression analysis was performed to evaluate the impact of individual factors. P < 0.05 was considered significant.
Results
Data have been depicted in Tables 1-5. A total of 30 sites in 26 patients (mean age 37.17 ± 8.81 years; 16 males and 10 females) were selected for the study. The distribution of these 30 sites was as follows: Three maxillary right and one maxillary left lateral incisor, ten maxillary right and 15 maxillary left canines and one maxillary right first premolar. These sites were randomly divided into control (15 sites) and test (15 sites).
Table 1.
Comparison of various parameters of control and test group using unpaired t-test
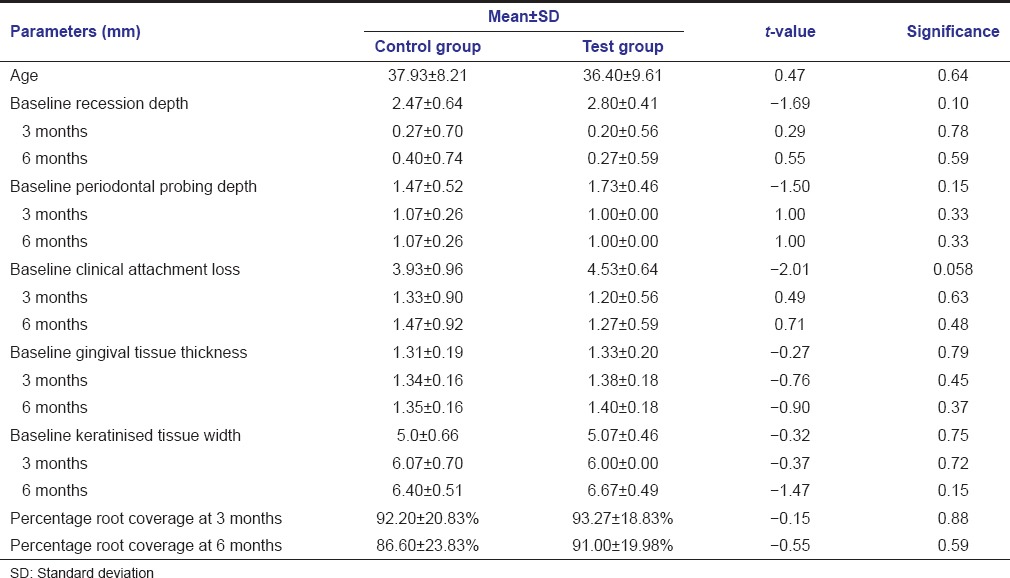
Mean age of control and test group was 37.93 ± 8.21 and 36.40 ± 9.61 years. The difference of mean age between both the groups was found to be statistically nonsignificant t = 0.47, P = 0.64 [Table 1].
The difference between the distribution of males and females between control and test group was found to be nonsignificant (Mann–Whitney U value 90.0, P = 0.367).
The differences between all parameters of test and control group at baseline, 3 and 6 months postoperatively were found to be statistically nonsignificant [Table 1].
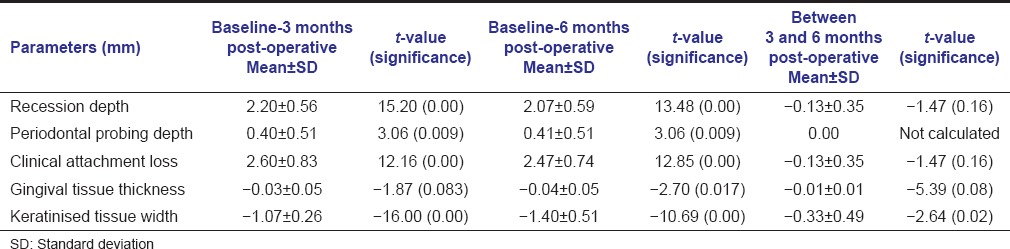
In control group, statistically significant improvement has been observed with all parameters at 3 and 6 months postoperatively except gingival tissue thickness which was found to be improved significantly at 6 months postoperatively. No difference was observed between all parameters between 3 and 6 months [Table 2].
Table 2.
Comparison of various parameters of control group at baseline, 3 and 6 months post-operative using paired t-test
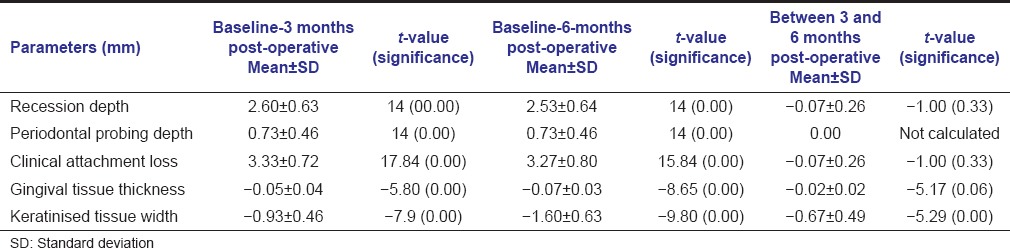
In test group, all parameters showed statistically significant difference at both 3 and 6 months follow-up but no significant change has been observed between 3 and 6 months postoperatively except keratinized tissue width which showed statistically significant gain at 6 months as compared to 3 months follow-up [Table 3].
Table 3.
Comparison of various parameters of test group at baseline, 3 and 6 months post-operative using paired t-test
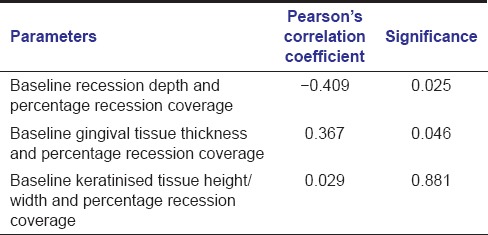
Pearson's correlation coefficients between baseline RD and percentage recession coverage and between baseline gingival tissue thickness and percentage recession coverage were statistically significant. Pearson's correlation coefficient between baseline keratinized tissue width and percentage recession coverage was found to be statistically nonsignificant [Table 4].
Table 4.
Pearson's correlation coefficients between various parameters
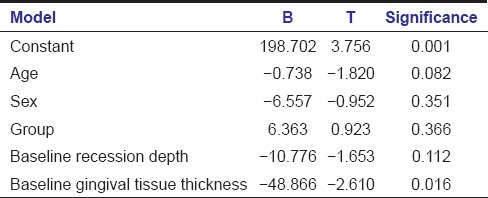
Multiple linear regression analysis demonstrated statistically significant effect of baseline gingival tissue thickness on percentage root coverage at 6 months independent of other covariates [Table 5].
Table 5.
Multiple linear regression analysis (dependent variable: percentage root coverage at 6 months)
Discussion
This randomized controlled study was performed to evaluate and compare the clinical efficacy of CAF alone and in combination with PRF as membrane to cover the exposed root surfaces of Miller Class I and II recessions.
Systemically healthy patients were selected for this study to avoid the altered host response caused by various systemic illnesses.[17] Smokers and tobacco chewers were excluded from this study as they have an altered tissue response and smoking also interferes with the initial healing.[18]
Unidentifiable CEJ would have led to errors in the measurements of clinical parameters. Presence of various restorations could have altered the mode of attachment of the overlying tissues, thus creating a bias in the outcome of the therapy.[17] Hence, these teeth were excluded.
Though a 3 months postoperative protocol had been followed in various studies for evaluating the success of root coverage procedures; a 6 months protocol has been followed in this study as this period has been considered necessary to evaluate the stability of gingival margin after root coverage procedures such as CAF.[19]
All baseline parameters were found to be similar in the test and control groups. This homogeneity in the baseline criteria and randomization protocol led to the elimination of bias in case selection.
Presence or absence of plaque and bleeding on probing was recorded. This indicated the level of oral hygiene maintenance and consequent inflammation. All the patients maintained their oral hygiene as none of the sites showed presence of plaque and bleeding on probing at all follow-ups. This elucidates successful patient motivation and compliance to the instructions rendered.
No untoward reaction such as pain, swelling, hypersensitivity and redness was reported by any of the patients. Soft tissue showed excellent healing in terms of achievement of color, contour and texture integration as that of clinically healthy gingiva. The tissue merging with the adjacent areas was satisfactory at the sites of vertical incisions in both the groups.
Platelet-rich fibrin membranes were sturdy (did not undergo disintegration while placement) and were easily manipulated.
In control group, statistically significant achievement in a recession reduction was reported at both 3 and 6 months. Though a mild reduction in recession coverage was reported between 3 and 6 months, the difference was statistically nonsignificant. 13 out of 15 (86.6%) patients showed complete root coverage (100%) at 3 months whereas only 11 out of 15 (73.3%) retained this complete root coverage at 6 months. Similar results were reported by Baldi et al.,[20] Pini Prato et al.,[21] Del Pizzo et al.,[22] Pini Prato et al.,[23] Huang et al.[10] de Sanctis and Zuchhelli,[14] Silva et al.[18] and Huang and Wang.[24]
Statistically significant reductions in clinical parameters in control group could be attributed to stringent inclusion and exclusion criteria, adequate gingival tissue thickness (1.31 ± 0.19 mm), adequate baseline width of keratinized gingiva, split-full-split design of flap, passive tension free placement of flap at least 1 mm coronal to the CEJ, increased stability due to application of both Sling and Tag sutures and rigorous maintenance regimen. All the precautions were taken to avoid any trauma to the surgical site during initial healing period. Patients were refrained from brushing for the first 4 weeks postoperatively after which they were instructed to maintain the area with roll technique of brushing.
Our results were similar to various other studies where a mild nonsignificant reduction in root coverage was reported at subsequent follow-ups. This can be explained by the reversal of patients’ behavior toward traumatic tooth brushing technique even under strict supervision. These results are in contrast with the study of Padma et al.[25] who reported recession coverage of 31.15 ± 20.53% at 1-month which increased to 61.46 ± 19.56% at 3 months and further increased to 68.44 ± 17.42% at 6 months.
In test group, statistically significant achievement in a recession reduction was reported at both 3 and 6 months. Though a mild loss of recession coverage was reported between 3 and 6 months, the difference was statistically nonsignificant. 13 out of 15 (86.6%) patients showed complete root coverage (100%) at 3 months whereas only 12 out of 15(80%) retained this complete root coverage at 6 months. Only 1 patient showed an increase in a recession reduction from 3 months to 6 months which can be attributed to creeping attachment and the healing potential provided by PRF.[26]
The maintenance of the gingival margin without much change for 6 months can be attributed to the placement of PRF membrane slightly hanging over the edge of the gingival collar as proposed by Del Corso et al.[27] which in turn separates and stimulates the interface between gingival tissue and root surface along the whole length of the flap.
Jankovic et al.[16] reported almost similar recession reduction in patients treated with CAF in combination with PRF. Padma et al.[25] reported a 34.58 ± 15.84% reduction in RD 1-month postoperatively in sites treated with CAF and PRF membrane which progressively increased at 3 and 6 months.
Significant reductions have been achieved in PPD and CAL at 3 and 6 months postoperatively in both groups as compared to baseline. The gain in clinical attachment can be attributed to the decrease in PPDs and increased recession coverage. This might also be a result of the formation of new connective tissue attachment, though in the absence of histological evidence, it is not possible to determine the type of attachment. In test group, the growth factors secreted by PRF might have improved the attachment of cells in the overlying flap to membrane and of the membrane to the underlying root surface resulting in prevention of the flap shrinkage.[28]
In control group, the gain in mean gingival tissue thickness was nonsignificant at 3 months but was significant at 6 months. The split-full-split thickness design of the flap might explain this increase of thickness as the thick portion (mucoperisoteal flap) was placed at avascular root surface. The value increased significantly from 3 to 6 months.
In test group, the gain in mean gingival tissue thickness were significant at all follow-up intervals. Similar results were reported by Aroca et al.[15] This increase in soft tissue thickness may be the result of proliferation of the gingival and periodontal ligament fibroblasts which, in turn, may be due to influence of growth factors released from PRF or to a spacing effect provided by PRF membrane.[15] PRF has been shown to modulate cell proliferation in a cell type specific manner as PRF was found to increase the proliferation of the periodontal ligament cells, gingival fibroblasts and osteoblasts while acted as the inhibitor of epithelial cells.[29]
The increase in the keratinized tissue height in both groups can be explained by the fact that mucogingival junction is genetically determined as it demarcates the junction between the basal bone and alveolar process. This mucogingival junction has the tendency to re-establish itself to the original position leading to the gain in keratinized tissue height. This can also be explained by the ability of the connective tissue proliferation from the periodontal ligament. Since the stimulation of keratinization of surface epithelium is provided by underlying connective tissue, it can be construed that the newly formed connective tissue had the ability to induce keratinization of the overlying epithelium.[30]
The differences between various parameters between test and control parameters, that is, RD reduction, decrease in PPD and CAL, increased width of keratinized tissue and increased gingival tissue thickness were found to be nonsignificant at 3 months and 6 months. Padma et al.[25] have found significant differences between test (CAF in combination with PRF) and control group (CAF alone). They reported significant differences between test and control group in width of keratinized gingiva at both 1 and 3 months. All other parameters did not show any statistical difference between both groups at 1 and 3 months follow-up. This can be attributed to the differences in baseline width of keratinized gingiva in two groups. These differences between Padma et al.[25] and our study could be attributed to the fact that our study included similar sites at baseline.
Statistically, significant Pearson's correlation coefficients found between baseline RD and percentage of recession coverage (−0.409) indicates an inverse relationship between the two. Significant correlation between baseline gingival tissue thickness and percentage root coverage indicates a direct association between these two parameters. No significant association has been detected between baseline width of attached gingiva and percentage root coverage. The inclusion of primarily Miller Class I recessions might have led to this nonsignificant association.
Multiple linear regression analysis showed statistically significant relationship of percentage root coverage at 6 months with baseline gingival tissue thickness. This indicated that the initial flap thickness is an important predictive variable for long-term maintenance of achieved root coverage. Similar facts have been reported by Baldi et al.[20] and Huang et al. (2005).[17]
Comparable results were achieved in our study in test group (CAF + PRF) as reported with CTG,[16] PRP,[10] EMD[22] and ADM.[31] This suggested that this autologous material in combination with CAF can be considered as a predictable root coverage procedure for Miller Class I and Class II recessions.
Small sample size, lack of histological evidence due to ethical concerns, and failure to incorporate split-mouth design can be considered as potential limitations. The results achieved by PRF might be more pronounced in compromised types of recession.
In the light of above findings, it can be safely concluded that CAF alone and in combination with PRF membrane is a highly predictable procedure for the treatment of Miller Class I and Class II gingival recessions. However, PRF provided an additional advantage of earlier healing and quicker attainment of optimal gingival tissue thickness which was maintained throughout the follow-up period. As adequate gingival tissue thickness is a known predictive factor for long-term stability of soft tissue recession coverage; it can be inferred that the use of PRF in conjunction with CAF can prove to be a superior choice for the treatment of such defects. PRF being an autologous material might possess both regenerative capacities as well as resorption potential. Hence, long-term studies with a larger sample size are required to authenticate the use of PRF as an adjunct with CAF in more severe recessions.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Thomson WM, Hashim R, Pack AR. The prevalence and intraoral distribution of periodontal attachment loss in a birth cohort of 26-year-olds. J Periodontol. 2000;71:1840–5. doi: 10.1902/jop.2000.71.12.1840. [DOI] [PubMed] [Google Scholar]
- 2.Cairo F, Pagliaro U, Nieri M. Treatment of gingival recession with coronally advanced flap procedures: A systematic review. J Clin Periodontol. 2008;35:136–62. doi: 10.1111/j.1600-051X.2008.01267.x. [DOI] [PubMed] [Google Scholar]
- 3.Allen EP, Miller PD., Jr Coronal positioning of existing gingiva: Short term results in the treatment of shallow marginal tissue recession. J Periodontol. 1989;60:316–9. doi: 10.1902/jop.1989.60.6.316. [DOI] [PubMed] [Google Scholar]
- 4.Lee EJ, Meraw SJ, Oh TJ, Giannobile WV, Wang HL. Comparative histologic analysis of coronally advanced flap with and without collagen membrane for root coverage. J Periodontol. 2002;73:779–88. doi: 10.1902/jop.2002.73.7.779. [DOI] [PubMed] [Google Scholar]
- 5.Cortellini P, Pini Prato G. Coronally advanced flap and combination therapy for root coverage. Clinical strategies based on scientific evidence and clinical experience. Periodontol 2000. 2012;59:158–84. doi: 10.1111/j.1600-0757.2011.00434.x. [DOI] [PubMed] [Google Scholar]
- 6.Choukroun J, Adda F, Schoeffler C, Vervelle A. Une opportunité en paro-implantologie: Le PRF. Implantodontie. 2001;42:55–62. [Google Scholar]
- 7.Mosesson MW. Fibrinogen and fibrin structure and functions. J Thromb Haemost. 2005;3:1894–904. doi: 10.1111/j.1538-7836.2005.01365.x. [DOI] [PubMed] [Google Scholar]
- 8.Tanya J, Thomas BS. Platelet rich fibrin membrane for recession coverage. EJ Dent. 2012;2:223–7. [Google Scholar]
- 9.Paolantonio M. Treatment of gingival recessions by combined periodontal regenerative technique, guided tissue regeneration, and subpedicle connective tissue graft. A comparative clinical study. J Periodontol. 2002;73:53–62. doi: 10.1902/jop.2002.73.1.53. [DOI] [PubMed] [Google Scholar]
- 10.Huang LH, Neiva RE, Soehren SE, Giannobile WV, Wang HL. The effect of platelet-rich plasma on the coronally advanced flap root coverage procedure: A pilot human trial. J Periodontol. 2005;76:1768–77. doi: 10.1902/jop.2005.76.10.1768. [DOI] [PubMed] [Google Scholar]
- 11.Sunitha Raja V, Munirathnam Naidu E. Platelet-rich fibrin: Evolution of a second-generation platelet concentrate. Indian J Dent Res. 2008;19:42–6. doi: 10.4103/0970-9290.38931. [DOI] [PubMed] [Google Scholar]
- 12.Dohan Ehrenfest DM, Del Corso M, Diss A, Mouhyi J, Charrier JB. Three-dimensional architecture and cell composition of a Choukroun's platelet-rich fibrin clot and membrane. J Periodontol. 2010;81:546–55. doi: 10.1902/jop.2009.090531. [DOI] [PubMed] [Google Scholar]
- 13.Sharma A, Pradeep AR. Autologous platelet-rich fibrin in the treatment of mandibular degree II furcation defects: A randomized clinical trial. J Periodontol. 2011;82:1396–403. doi: 10.1902/jop.2011.100731. [DOI] [PubMed] [Google Scholar]
- 14.de Sanctis M, Zucchelli G. Coronally advanced flap: A modified surgical approach for isolated recession-type defects: Three-year results. J Clin Periodontol. 2007;34:262–8. doi: 10.1111/j.1600-051X.2006.01039.x. [DOI] [PubMed] [Google Scholar]
- 15.Aroca S, Keglevich T, Barbieri B, Istavan G, Daniel E. Clinical evaluation of a modified coronally advanced flap alone or in combination with a platelet rich fibrin membrane for the treatment of adjacent multiple gingival recession: A 6 month study. J Periodontol. 2009;80:244–52. doi: 10.1902/jop.2009.080253. [DOI] [PubMed] [Google Scholar]
- 16.Jankovic S, Aleksic Z, Klokkevold P, Lekovic V, Dimitrijevic B, Kenney EB, et al. Use of platelet-rich fibrin membrane following treatment of gingival recession: A randomized clinical trial. Int J Periodontics Restorative Dent. 2012;32:e41–50. [PubMed] [Google Scholar]
- 17.Huang LH, Neiva RE, Wang HL. Factors affecting the outcomes of coronally advanced flap root coverage procedure. J Periodontol. 2005;76:1729–34. doi: 10.1902/jop.2005.76.10.1729. [DOI] [PubMed] [Google Scholar]
- 18.Silva CO, de Lima AF, Sallum AW, Tatakis DN. Coronally positioned flap for root coverage in smokers and non-smokers: Stability of outcomes between 6 months and 2 years. J Periodontol. 2007;78:1702–7. doi: 10.1902/jop.2007.070068. [DOI] [PubMed] [Google Scholar]
- 19.Cheng YF, Chen JW, Lin SJ, Lu HK. Is coronally positioned flap procedure adjunct with enamel matrix derivative or root conditioning a relevant predictor for achieving root coverage. A systemic review? J Periodontal Res. 2007;42:474–85. doi: 10.1111/j.1600-0765.2007.00971.x. [DOI] [PubMed] [Google Scholar]
- 20.Baldi C, Pini-Prato G, Pagliaro U, Nieri M, Saletta D, Muzzi L, et al. Coronally advanced flap procedure for root coverage Is flap thickness a relevant predictor to achieve root coverage? A 19-case series. J Periodontol. 1999;70:1077–84. doi: 10.1902/jop.1999.70.9.1077. [DOI] [PubMed] [Google Scholar]
- 21.Pini Prato G, Pagliaro U, Baldi C, Nieri M, Saletta D, Cairo F, et al. Coronally advanced flap procedure for root coverage. Flap with tension versus flap without tension: A randomized controlled clinical study. J Periodontol. 2000;71:188–201. doi: 10.1902/jop.2000.71.2.188. [DOI] [PubMed] [Google Scholar]
- 22.Del Pizzo M, Zucchelli G, Modica F, Villa R, Debernardi C. Coronally advanced flap with or without enamel matrix derivative for root coverage: A 2-year study. J Clin Periodontol. 2005;32:1181–7. doi: 10.1111/j.1600-051X.2005.00831.x. [DOI] [PubMed] [Google Scholar]
- 23.Pini Prato GP, Baldi C, Nieri M, Franseschi D, Cortellini P, Clauser C, et al. Coronally advanced flap: The post-surgical position of the gingival margin is an important factor for achieving complete root coverage. J Periodontol. 2005;76:713–22. doi: 10.1902/jop.2005.76.5.713. [DOI] [PubMed] [Google Scholar]
- 24.Huang LH, Wang HL. Sling and tag suturing technique for coronally advanced flap. Int J Periodontics Restorative Dent. 2007;27:379–85. [PubMed] [Google Scholar]
- 25.Padma R, Shilpa A, Kumar PA, Nagasri M, Kumar C, Sreedhar A. A split mouth randomized controlled study to evaluate the adjunctive effect of platelet-rich fibrin to coronally advanced flap in Miller's class-I and II recession defects. J Indian Soc Periodontol. 2013;17:631–6. doi: 10.4103/0972-124X.119281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kochar D, Narula S, Sharma RK, Tewari S, Chopra D. Creeping attachment in Miller class III recessions: A report of five cases. Clin Adv Periodontics. 2012;2:217–22. [Google Scholar]
- 27.Del Corso M, Sammartino G, Dohan Ehrenfest DM. Re: Clinical evaluation of a modified coronally advanced flap alone or in combination with a platelet-rich fibrin membrane for the treatment of adjacent multiple gingival recessions: A 6-month study. J Periodontol. 2009;80:1694–7. doi: 10.1902/jop.2009.090253. [DOI] [PubMed] [Google Scholar]
- 28.Matter J. Creeping attachment of free gingival grafts. A five-year follow-up study. J Periodontol. 1980;51:681–5. doi: 10.1902/jop.1980.51.12.681. [DOI] [PubMed] [Google Scholar]
- 29.Tsai CH, Shen SY, Zhao JH, Chag YC. Platelet rich fibrin modulates cell proliferation of human periodontally related cells in vitro. J Dent Sci. 2009;4:130–5. [Google Scholar]
- 30.Lundberg M, Wennström JL. Development of gingiva following surgical exposure of a facially positioned unerupted incisor. J Periodontol. 1988;59:652–5. doi: 10.1902/jop.1988.59.10.652. [DOI] [PubMed] [Google Scholar]
- 31.Woodyard JG, Greenwell H, Hill M, Drisko C, Iasella JM, Scheetz J. The Clinical effect of acellular dermal matrix on gingival thickness and root coverage compared to coronally positioned flap alone. J Periodontol. 2004;75:44–56. doi: 10.1902/jop.2004.75.1.44. [DOI] [PubMed] [Google Scholar]


