Abstract
Background:
The aim of this study was to evaluate the antibacterial activity of pure green coffee bean extract on periodonto pathogenic bacteria Porphyromonas gingivalis (Pg), Prevotella intermedia (Pi), Fusobacterium nucleatum (Fn) and Aggregatibacter actinomycetemcomitans (Aa).
Materials and Methods:
Minimum inhibitory concentrations (MICs) and minimum bactericidal concentrations (MBC) were used to assess the antibacterial effect of pure green coffee bean extract against periodonto pathogenic bacteria by micro dilution method and culture method, respectively.
Results:
MIC values of Pg, Pi and Aa were 0.2 μg/ml whereas Fn showed sensitive at concentration of 3.125 μg/ml. MBC values mirrors the values same as that of MIC.
Conclusion:
Antimicrobial activity of pure green coffee bean extract against Pg, Pi, Fn and Aa suggests that it could be recommended as an adjunct to mechanical therapy in the management of periodontal disease.
Keywords: Antibacterial activity, green coffee bean extract, minimum bactericidal concentration, minimum inhibitory concentration, periodontogenic bacteria
Introduction
Chronic periodontitis (CP) is one of the most common oral health problems. It is microbially induced chronic inflammatory disease. The incidence and progression of this disease are related to a substantial increase in Gram-negative anaerobic rods. Among them, Porphyromonas gingivalis (Pg), Prevotella intermedia (Pi), Fusobacterium nucleatum (Fn), and Aggregatibacter actinomycetemcomitans (Aa) are strongly implicated in the etiology of CP.[1] When these pathogens habitat the local periodontal tissues, an immune response is initiated by fibroblasts and macrophages by producing several cytokines (interleukins 1 and 6 and tumor necrosis factor-a), as mediators of the inflammatory response and immune reaction.[2]
Routine practice of antimicrobial adjunct to conventional periodontal therapy was well-established owing to tissue penetrable nature of pathogenic bacteria.[3] Hence, the development of natural form of therapies for the treatment of periodontal diseases is of great relevance, as the administration of systemic antimicrobials has been reported to cause the development of multi resistant microorganisms, inter bacterial transfer of resistance determinants and various side effects.[4]
Currently there are various popular therapeutic antimicrobial products in the market, but the search and screening for the development of herbal remedies with wide range of pharmaceutical properties without the side effects of synthetic medications for the treatment of periodontal diseases is still ongoing.[4] Among the various herbal products, green coffee bean extract has received greater attention due to its antimicrobial effect against both Gram-positive and Gram-negative bacteria.[5] Some components in coffee such as caffeine, volatile and nonvolatile organic acids, phenols and aromatic compounds are reported to have antimicrobial activity. Chlorogenic acid (CGA) and caffeic acid, which are nonvolatile organic acids found in coffee, inhibit the growth of some Gram-positive microorganisms such as Staphylococcus aureus, Bacillus cereus, Lactobacillus bulgaricus, Streptococus lactis and Streptococcus faecalis and Gram-negative bacteria like Escherichia coli, Salmonella typhi and Pseudomonas auerginosa.[5]
Chlorogenic acid is a polyphenolic natural compound. Structurally, it is an ester of caffeic acid with the 3-hydroxyl group of a quinic acid. It has been reported to possess many health benefits including antibacterial, antifungal, antiviral, antiphlogistic, antioxidant, chemopreventive, and other biological activities.[6] Green (or raw) coffee is a major source of CGA in nature (5–12 g/100 g).[7] However, 30–50% of CGA decomposes during roasting.[5] Recent studies demonstrated that the consumption of green coffee extracts produced antihypertensive effect in rats and humans, improvement in human vasoreactivity, inhibitory effect on fat accumulation and body weight in mice and humans, and modulation of glucose metabolism in humans. Such biological effects have been attributed to CGA present in green coffee.[7]
To the best of our knowledge, no study has been conducted to assess the antibacterial effect of green coffee bean extract on the most common periodontal pathogens so far. Hence, the aim of the present study was to find out the minimum inhibitory concentrations (MICs) and minimum bactericidal concentration (MBC) of pure green coffee bean extract that can be safely and effectively administered as local drug delivery system on specific periodontal pathogens like Pg, Pi, Fn and Aa.
Materials and Methods
Green coffee bean extract
About 100% pure green coffee (Coffea robusta) extract with 50% CGA was obtained from Top Secret Nutrition supplements, New York, USA, in January 2014. It was certified to be free from any form of bacteria, yeast, or mold by the manufacturer after microbial analysis.
Bacterial strains
Bacterial strains used in this study were American type culture collection, Manassas, VA, USA. The tested bacterial strains in this study were Pg, ATCC 33277, Pi ATCC 25611, Fn, ATCC 25586 and Aa, ATCC 29523.
Antibacterial activity
Minimum inhibitory concentrations
Stock solution of the antimicrobial agent was prepared by adding 100 μg of green coffee bean extract to 1 ml of thioglycollate (TG) broth medium (100 μg/1 ml). For MIC, nine dilutions of the drug were prepared with TG broth medium using microdilution method by means of standard protocols given by Schwalbe et al.[8] Further, 20 μl of drug from the stock solution was added into the initial tube which contains 380 μl of TG broth. For dilutions, 200 μl of TG broth was added into the next nine tubes separately. Then from the initial tube, 200 μl was transferred to the first tube containing 200 μl of TG broth. This was considered as 10-1 dilution. From 10-1 diluted tube, 200 microliter was transferred to the second tube to make 10-2 dilution. The serial dilution was repeated up to 10-9 dilution for each drug. From the maintained stock cultures of required organisms (Pg, Pi, Aa, Fn), 10 μl was taken and added to 2 ml of TG broth. In each serially diluted tube, 200 μl of above culture suspension was added and then tubes were sealed airtightly and incubated for ≥48 h in an anaerobic jar/chamber and observed for turbidity. The minimum concentration of the drug in the tube which does not show any turbidity is considered as the MIC of the drug.
Minimum bactericidal concentration
After the MIC procedure, four dilution tubes which were showing sensitivity to antibacterial agent at lower concentrations were taken and inoculated into respective culture medium to check the growth of microorganisms. Formerly plates were incubated in anaerobic jar/chamber for ≥48 h and then colonies were counted.
Results
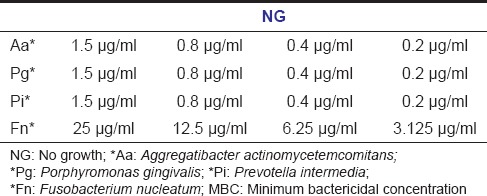
The results showed that green coffee bean extract was effective at a very low concentration against four periodonto pathogenic bacteria. MIC of Pg, Pi and Aa was 0.2 μg/ml whereas Fn shows resistance till the concentration of 1.5 μg/ml, but it was sensitive at a concentration of 3.125 μg/ml [Table 1]. There was no growth of bacteria after inoculation into the culture media at all the four concentrations, for which specifies MBC values were same as of MIC [Table 2].
Table 1.
MIC values
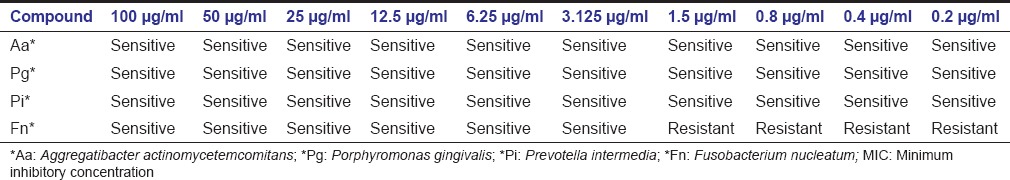
Table 2.
MBC values
Discussion
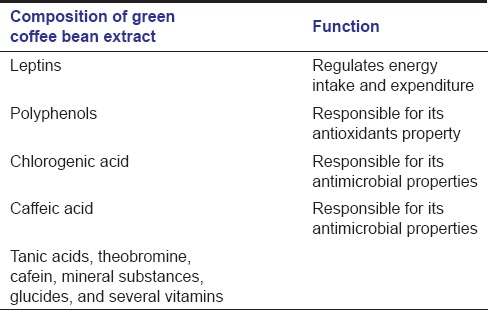
The use of plant extracts with medicinal properties represents an essence alternative for the treatment of different diseases. This includes the use of natural products as antimicrobial agents,[9] even though in the absence of scientific basis, such practices may generate serious adverse effects. Regarding the use of coffee extracts, it could not be disapproved. Coffee is the beverage of choice for many people, who love its rich taste and aroma and its stimulating effect. Until recently, coffee wasn’t considered to be a health food unlike tea and other leading hot beverages. Now researchers are finding that coffee seems to have a range of health benefits, including reducing the risk of type 2 diabetes, gallstones, liver cancer, Parkinson's disease and Alzheimer's disease. In addition, green coffee beans have been found to have antibacterial effects against pathogenic microorganisms.[7] Green coffee bean extract is a natural supplement whose primary ingredients [Table 3] help people be well and healthy.[10] CGA, as the active ingredient present in the unroasted green coffee beans, is responsible for its antimicrobial property. Polyphenol is an organic compound found in this extract is responsible for its antioxidants property. Leptin is another compound found in this extract is responsible for regulating the energy intake and expenditure.[11]
Table 3.
Composition of green coffee bean
Studies on the antimicrobial properties of coffee species are limited in the literature even though it is considered the best known and one of the most appreciated drink in the world. According to Fardiaz[5] CGA and caffeic acids, are nonvolatile organic acids found in coffee, inhibit the growth of some Gram-positive bacteria like (S. aureus, B. cereus, L. bulgaricus, S. lactis and S. faecal) and Gram-negative bacteria viz. (E. coli, S. typhi and P. aeruginosa), but not molds and yeast. Pruthviraj et al.[12] demonstrated that the caffeine extracted from the leaves and leaf buds of Camellia sinensis (green tea), and beans of Coffea arabica (coffee) inhibits the growth of gram-negative bacteria like E. coli, Proteus mirabilis, Klebsiella pneumonia, P. aeruginosa. Brandao et al.[10] explored that coffee solutions of different origin that is, Coffea arabica and Coffea canephora, known as “arabic coffee” and “robust coffee”, respectively cultivated in Brazil had no antimicrobial activity against Strep mutans, but the tested coffee solutions reduced significantly the adherence of Strep mutans to glass surface which suggest potential anticariogenic activity of coffee solutions. Toda et al.[13] related the effects of coffee on microbial species such as S. aureus, S. thiphi, Shigella dysenteriae, Vibrio cholerae, Vibrio parahaemolyticus and Yersinia enterocolitica and attributed this bactericide effect to the tanic acid.
After extensive literature search and to the best our knowledge, this is the first study that determines the in vitro antibacterial activity of pure green coffee bean extract against four periodonto pathogenic bacteria Pg, Pi, Fn and Aa by MIC microdilution method and MBC. The MIC and MBC are measures to identify the potency of an antibacterial drug and are consider as the important issues in diagnostic laboratories to confirm the resistance of antimicrobial agent and also to monitor the activity of new antimicrobials. Different bacterial species have varying MICs and MBCs. Sensitive strains will have relatively low values whereas resistant strains have relatively high values.[8] As a general rule of thumb, the concentration of antimicrobial drug in the blood should exceed the MIC by a factor of 2–8 times to offset the tissue barriers that restrict the access to the infected site.[14] In the present study, the MIC and MBC values of pure green coffee bean extract obtained for Pg, Pi and Aa, were found to be very much lower but for Fn, it was found to be relatively higher [Tables 1 and 2].
The present in vitro antibacterial assessment study helps us to focus on an intervention approach to design and conduct a clinical trial to detect the beneficial effect of pure green coffee bean extract on patients at risk for periodontitis. But in vitro values of MIC and MBC may not hold good for in vivo studies due to their inherent limitations. The growth of micro-organisms in vitro is exponential whereas the growth in vivo can be very slow to none.[15] Though MIC and MBC do not indicate the true activity of the drug at the locus of infection, the in vitro MIC and MBC serve as surrogate markers attempting to quantify the drug activity.[16]
Scientifically based understanding of the etiopathogenesis of periodontal disease has laid a new responsibility on dentists not only to care for the present dental health of their patients but also to chalk out an comprehensive plan for the future periodontitis patients. Within limitations of the present study, the lowest concentration of pure green coffee bean extract was proven to be effective on four periodontogenic bacteria Pg, Pi, Fn and Aa. However, since periodontitis is a polymicrobial disease, the susceptibility of various other periodontal pathogens to this extract must to be evaluated. Though the long-term safety profiles of herbal drugs like green coffee bean extract are well documented, further studies are required to: (a) Assess the in vivo efficacy of pure green coffee bean extract with other traditionally prescribed antimicrobials used for periodontal therapy. (b) Evaluate the in vivo effect of pure green coffee bean extract in different formulations (gel, chips, strips, fibres etc.) with variable concentrations. The in vitro determination of their concentration in GCF and serum samples might help us to know the ideal dosage and formulation required for antimicrobial and regenerative activity of pure green coffee bean extract to treat periodontitis.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Wolff L, Dahlén G, Aeppli D. Bacteria as risk markers for periodontitis. J Periodontol. 1994;65(5 Suppl):498–510. doi: 10.1902/jop.1994.65.5s.498. [DOI] [PubMed] [Google Scholar]
- 2.Preshaw PM, Taylor JJ. How has research into cytokine interactions and their role in driving immune responses impacted our understanding of periodontitis? J Clin Periodontol. 2011;38(Suppl 11):60–84. doi: 10.1111/j.1600-051X.2010.01671.x. [DOI] [PubMed] [Google Scholar]
- 3.Academic report by AAP Systemic antibiotics in periodontics. J Periodontol. 1996;67:831–8. [PubMed] [Google Scholar]
- 4.Walker CB. The acquisition of antibiotic resistance in the periodontal microflora. Periodontol 2000. 1996;10:79–88. doi: 10.1111/j.1600-0757.1996.tb00069.x. [DOI] [PubMed] [Google Scholar]
- 5.Fardiaz S. Antimicrobial activity of coffee (Coffea robusta) extract. ASEA Food J. 1995;10:103–6. [Google Scholar]
- 6.Karunanidhi A, Thomas R, van Belkum A, Neela V. In vitro antibacterial and antibiofilm activities of chlorogenic acid against clinical isolates of Stenotrophomonas maltophilia including the trimethoprim/sulfamethoxazole resistant strain. Biomed Res Int. 2013;2013:392058. doi: 10.1155/2013/392058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farah A, Monteiro M, Donangelo CM, Lafay S. Chlorogenic acids from green coffee extract are highly bioavailable in humans. J Nutr. 2008;138:2309–15. doi: 10.3945/jn.108.095554. [DOI] [PubMed] [Google Scholar]
- 8.Schwalbe R, Steele-Moore L, Goodwin AC. Macro-and microdilution methods of antimicrobial susceptibility testing. In: Schwalbe R, editor. Antimicrobial Susceptibility Testing Protocols. Florida, Fla, USA: AC: CRC Press Taylor and Francis Group; 2007. pp. 76–9. [Google Scholar]
- 9.Alviano WS, Alviano DS, Diniz CG, Antoniolli AR, Alviano CS, Farias LM, et al. In vitro antioxidant potential of medicinal plant extracts and their activites against oral bacteria based on Brazilian folk medicine. Arch Oral Biol. 2008;53:545–52. doi: 10.1016/j.archoralbio.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Brandao F, Oliveira L, Landucci L, Koga-Ito C. Antimicrobial activity of coffee-based solutions and their effects on Streptococcus mutans adherence. Braz J Oral Sci. 2007;6:1274–7. [Google Scholar]
- 11.Pure green coffee. Where the secrets of green coffee bean extract is hiding? [Last updated on 2013 Aug 14; Last cited on 2013 Sep 19]. Available from: http://www.puregreencoffee.com/articles/green.coffee.bean.extract/
- 12.Pruthviraj P, Suchita B, Shital K, Shilpa K. Evaluation of antibacterial activity of caffeine. Int J Res Ayurveda Pharm. 2011;2:1354–7. [Google Scholar]
- 13.Toda M, Okubo S, Hiyoshi R, Shimamurat T. The bactericidal activity of tea and coffee. Lett Appl Microbiol. 1989;8:123–5. [Google Scholar]
- 14.Neu HC. Current practices in antimicrobial dosing. Rev Infect Dis. 1981;3:12–8. doi: 10.1093/clinids/3.1.12. [DOI] [PubMed] [Google Scholar]
- 15.Levison ME. Pharmacodynamics of antibacterial drugs. Infect Dis Clin North Am. 2000;14:281–91, vii. doi: 10.1016/s0891-5520(05)70248-8. [DOI] [PubMed] [Google Scholar]
- 16.Briethaupt H. The new antibiotics: Can nivel antibacterial treatment combat the rising tide of drug resistant infections? Nat Biotechnol. 1999;17:1165–9. doi: 10.1038/70705. [DOI] [PubMed] [Google Scholar]


