Abstract
Background
Preterm birth (PTB), or birth before 37 weeks of gestation, is the leading cause of newborn death worldwide. PTB is a critical area of scientific study not only due to its worldwide toll on human lives and economies, but also due to our limited understanding of its pathogenesis and, therefore, its prevention. This systematic review and meta-analysis synthesizes the landscape of PTB transcriptomics research to further our understanding of the genes and pathways involved in PTB subtypes.
Methods
We evaluated published genome-wide pregnancy studies across gestational tissues and pathologies, including those that focus on PTB, by performing a targeted PubMed MeSH search and systematically reviewing all relevant studies.
Results
Our search yielded 2,361 studies on gestational tissues including placenta, decidua, myometrium, maternal blood, cervix, fetal membranes (chorion and amnion), umbilical cord, fetal blood, and basal plate. Selecting only those original research studies that measured transcription on a genome-wide scale and reported lists of expressed genetic elements identified 93 gene expression, 21 microRNA, and 20 methylation studies. Although 30 % of all PTB cases are due to medical indications, 76 % of the preterm studies focused on them. In contrast, only 18 % of the preterm studies focused on spontaneous onset of labor, which is responsible for 45 % of all PTB cases. Furthermore, only 23 of the 10,993 unique genetic elements reported to be transcriptionally active were recovered 10 or more times in these 134 studies. Meta-analysis of the 93 gene expression studies across 9 distinct gestational tissues and 29 clinical phenotypes showed limited overlap of genes identified as differentially expressed across studies.
Conclusions
Overall, profiles of differentially expressed genes were highly heterogeneous both between as well as within clinical subtypes and tissues as well as between studies of the same clinical subtype and tissue. These results suggest that large gaps still exist in the transcriptomic study of specific clinical subtypes as well in the generation of the transcriptional profile of well-studied clinical subtypes; understanding the complex landscape of prematurity will require large-scale, systematic genome-wide analyses of human gestational tissues on both understudied and well-studied subtypes alike.
Electronic supplementary material
The online version of this article (doi:10.1186/s12920-015-0099-8) contains supplementary material, which is available to authorized users.
Keywords: Preterm birth, Gestational tissues, Transcriptomics, Gene expression, microRNA, Methylation, Preeclampsia, Idiopathic preterm birth, Meta-analysis
Background
In humans, gestation typically lasts 40 weeks; preterm birth (PTB) is defined as birth before 37 completed weeks of gestation and is the leading cause of newborn death worldwide. More than 15 million babies are born too soon every year and rates of PTB had been increasing until 2006 when changes in obstetrical practices regarding early cesarean sections led to a recent decrease in deliveries before term [1]. Nevertheless, 10 % of pregnancies still end before 37 weeks across the world and this high incidence of PTB is problematic because premature babies are at higher risk for lifelong health and developmental problems [2, 3]. For example, almost half of all children born premature suffer from vision or hearing loss and learning disabilities at some point in their life [4, 5]. The combined medical costs stemming from care during the labor and delivery process as well as from care later in life are estimated to be near $26 billion annually [6].
PTB is a complex, multifactorial syndrome comprised of multiple clinical subtypes, which often occur at different gestational ages and can be defined as either ‘spontaneous’ or ‘medically indicated’ [7]. Medically indicated preterm deliveries account for 30 % of PTB cases and are often preceded by complications including preeclampsia (PE), intrauterine growth restriction (IUGR), gestational diabetes mellitus (GDM), and chorioamnionitis [8]. The remaining 70 % of PTB cases are idiopathic; 45 % is due to the spontaneous onset of labor (sPTB) and the remaining 25 % is due to the preterm premature rupture of membranes (PPROM) [9–11]. Regardless of PTB subtype, however, current therapies are not successful in prolonging time to birth once labor has been initiated and the most effective therapy, progesterone supplementation, is only effective in a small number of high-risk cases [12]. It is critical that we gain greater insight into the genes and pathways that regulate birth timing in humans in order to develop effective prevention and treatment strategies, including for cases of sPTB.
A number of environmental risk factors have been associated with sPTB including infection, nutrition, socioeconomic status, and stress but the pathways through which these risk factors act remain unclear [13]. Recent evidence from family, twin, and case–control studies suggests that genetics also plays an important role in birth timing, and the heritability of PTB is estimated to be approximately 30 % [1, 6, 8]. Thus, PTB tends to run in families and women who were born preterm are also more likely to deliver preterm themselves. Interestingly, however, fathers born prematurely do not appear to pass on this risk to offspring [1]. Furthermore, one of the strongest predictors of PTB is previous preterm birth and, in subsequent pregnancies from the same woman, birth timing tends to occur around the same gestational age for each pregnancy [9, 14]. Candidate gene studies have targeted genes with known biological roles potentially related to processes occurring during pregnancy but, in general, teasing apart the complex genetic architecture of pregnancy and PTB has proved challenging.
Further complicating our understanding of PTB genetic architecture are the numerous maternal and fetal gestational tissues that must interact to facilitate parturition [12, 15]. These tissues include decidua, myometrium, cervix and maternal blood originating from the mother and villous placenta, fetal membranes (chorion and amnion), umbilical cord, and fetal blood originating from the fetus (Fig. 1). Furthermore, the basal plate is a region at the maternofetal interface that is commonly biopsied for the study of PTB and includes cells from both the decidua and villous placenta. The decidua, myometrium, and cervix act to house the fetus as well as expel it during labor and delivery, the chorion and amnion act as membranes separating the fetus from the mother, and the umbilical cord allows for efficient nutrient transfer. Together, these tissues share a general functionality in the efficient maternofetal exchange of nutrients, gas, and waste.
Fig. 1.
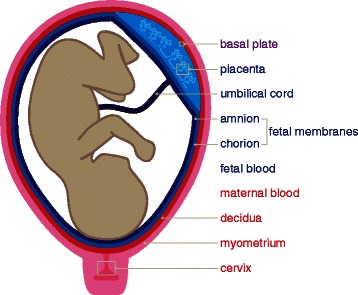
The tissues of pregnancy. Our systematic literature review surveyed a total of 9 distinct gestational tissue types including 4 of maternal origin (cervix, myometrium, decidua, and maternal blood; shown in red), 4 of fetal origin (fetal blood, fetal membranes, umbilical cord, and placenta; shown in blue), and 1 of mixed maternal and fetal origin (basal plate; shown in purple)
Although little is known about the complex etiology of PTB, many studies have generated pregnancy-related transcriptomes in various tissue types and pathologies. Because of the diversity of tissues and clinical subtypes involved as well as the large number of questions examined, few studies have attempted to synthesize any dimension of the admittedly complex transcriptional landscape of this multifactorial syndrome. To synthesize what is known about PTB transcriptomics, we analyzed all published genome-wide studies of gestational tissues (placenta, decidua, myometrium, maternal blood, cervix, basal plate, fetal membranes, umbilical cord, and fetal blood) in both healthy and diseased human pregnancies to identify all statistically supported candidate genetic elements in PTB subtypes.
Our meta-analysis identified 134 genome-wide studies of pregnancy and PTB. The majority of PTB research focused on PE; very few studies were focused on sPTB (18 %) even though sPTB accounts for 45 % of all PTB cases. Moreover, there was limited overlap in the identity of candidate genes across studies. In placenta (n = 53), for example, 6,444 differentially expressed unique genes were identified but only 2, LEP and FLT1, were present in more than 10 gene expression studies. Similarly, in PE studies (n = 27), 5,329 differentially expressed unique genes were identified but only 13 were found in 5 or more gene expression studies. The limited overlap of differentially expressed genes across studies of the same tissue or clinical subtype as well as the highly uneven coverage of studies targeting highly prevalent clinical subtypes suggest that larger-scale, systematic studies aimed at understanding the transcriptional profiles of the diverse clinical PTB subtypes and characterizing their disease-relevant transcriptional differences will be necessary to identify genes whose dysregulation contributes to this complex, multifactorial syndrome.
Results
A systematic review identified 134 transcriptomic studies on 9 gestational tissues and 29 different phenotypes
Of the 2,361 studies identified in our PubMed search, 134 genome-wide transcriptomic studies in human gestational tissue samples were, based on a number of selection criteria, deemed eligible for systematic review (Additional file 1) [16–133]. These 134 studies were identified from a total of 116 distinct publications; this is so because 14 publications reported multiple comparisons that were separated into 33 distinct studies for the purpose of this analysis. Platform-wise, 127/134 (95 %) were microarray studies, 4/134 (3 %) were bisulfite-sequencing studies, and 3/134 (2 %) were RNA-sequencing studies. All studies were published between 1999 and 2014, primarily in the journals Placenta and The American Journal of Obstetrics and Gynecology. The phenotypes examined in these studies were quite diverse; 14/134 (10 %) studies examined preterm pregnancies, 80/134 (60 %) term pregnancies, and 40/134 (30 %) both preterm and term pregnancies. One non-clinical phenotype (healthy pregnancies) and 28 distinct clinical phenotypes were represented. Finally, 21/134 (16 %) were microRNA studies, 20/134 (15 %) were methylation studies, and the remaining 93/134 (69 %) were gene expression studies. A total of 10,993 unique genetic elements were reported to be transcriptionally active across all 134 studies (Additional file 2), but only 23/10,993 (0.2 %) were reported in 10 or more studies.
The 134 studies analyzed 9 distinct gestational tissues, namely placenta, decidua, myometrium, maternal blood, cervix, fetal membranes (chorion and amnion), umbilical cord, fetal blood, and basal plate. The three most common tissues studied were placenta (82/134; 61 %), fetal membranes (16/134; 12 %), and myometrium (17/134; 12 %), whereas each of the other six tissues was sampled in 7 or fewer studies (Fig. 2).
Fig. 2.
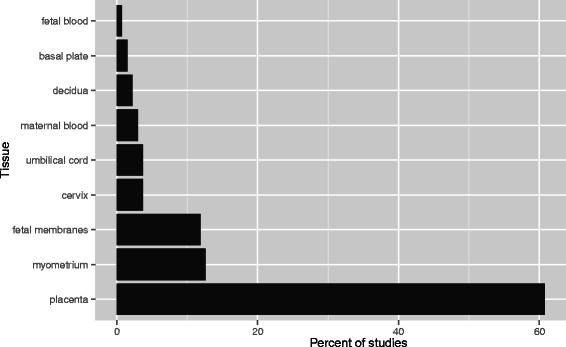
The vast majority of genome-wide transcriptomic studies on gestational tissues have focused on the placenta. A targeted PubMed search for genome-wide transcriptomic studies yielded a total of 134 studies focusing on 9 distinct gestational tissue types. Placental research accounted for 61 % of all studies in the meta-analysis, followed by fetal membranes (12 %) and myometrium (12 %)
The 134 studies analyzed 29 distinct phenotypes (Fig. 3). 11/134 (8 %) studies focused on healthy pregnancies, while the remaining 123/134 (92 %) studies focused on clinical phenotypes. The most common phenotypes studied were PE (40/134; 30 %), labor (16/134; 12 %), and sPTB (10/134; 7 %). Definitions for all phenotypes are provided in Additional file 3.
Fig. 3.
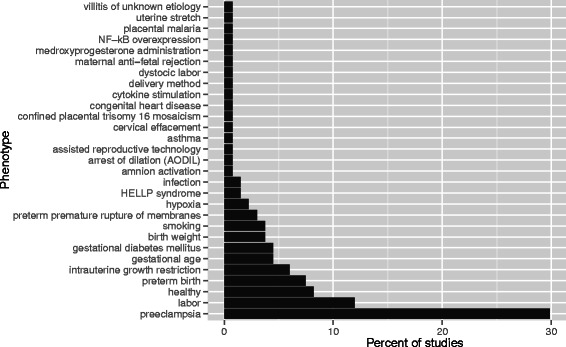
Gestational tissue transcriptomic studies in term and preterm human pregnancies organized by phenotype. A targeted PubMed search for genome-wide transcriptomic studies yielded a total of 134 studies focusing on 29 distinct phenotypes. PE research accounted for 30 % of all studies in the meta-analysis, followed by labor (12 %) and sPTB (7 %). Phenotype definitions are provided in Supplementary Table S2
PTB research focus does not reflect PTB subtype epidemiological prevalence
To evaluate whether the proportion of transcriptomic studies devoted on different PTB subtypes reflects their clinical prevalence, we compared the frequencies of the three major clinical etiologies (sPTB at 45 %, PPROM at 25 %, and medically indicated PTB at 30 %) to the frequency of transcriptomic studies devoted to these etiologies (Fig. 4). We found that although only 30 % of all PTB cases are due to medical indications, such as PE, IUGR, or GDM, 41/54 (76 %) of the studies categorized as preterm in our systematic review focused on them; 21/54 (39 %) of the preterm studies focused on PE alone. In contrast, although sPTB is responsible for 45 % of all cases, only 10/54 (18 %) of the preterm studies in our systematic review studied this clinical subtype.
Fig. 4.
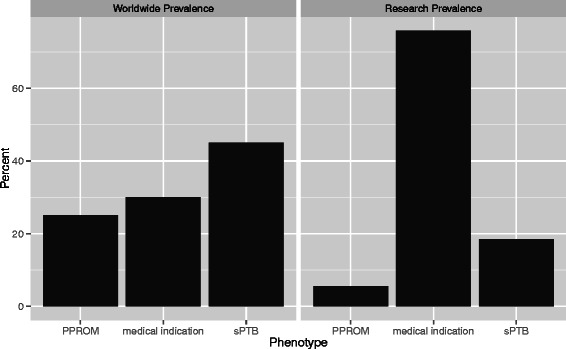
Proportion of transcriptomic research does not correspond to PTB subtype prevalence. Although only 30 % of all PTB cases are due to medical indications, such as PE, IUGR, or GDM, 76 % of the preterm studies in our systematic review focused on them. In contrast, only 18 % of the studies focused on sPTB, even though this clinical subtype accounts for the majority (45 %) of PTB cases
A meta-analysis of 93 gene expression studies across 9 distinct gestational tissues showed limited overlap of candidate genes
To perform an aggregated meta-analysis, we focused on the 93/134 gene expression studies. These 93 gene expression studies analyzed all 9 distinct gestational tissues, namely placenta, decidua, myometrium, maternal blood, cervix, fetal membranes (chorion and amnion), umbilical cord, fetal blood, and basal plate. The three most common tissues studied for differential gene expression were placenta (53/93; 57 %), myometrium (17/93; 18 %), and fetal membranes (11/93; 12 %), whereas each of the other six tissues was sampled in 4 or fewer studies. Genome-wide gene expression profiling studies of the three most commonly studied gestational tissues, i.e., placenta, myometrium, and fetal membranes, identified a total of 8,437 unique differentially expressed genes, of which only 2,123 (25 %) were found in two or more studies (Fig. 5, Additional file 4). This examination also showed that only 23 candidate genes were differentially expressed two or more times in studies of all three tissues (Additional file 5). Among the genes present in this overlap were interleukin 1 beta, a proinflammatory cytokine shown to be involved in infection-related PTB and PE, and superoxide dismutase 2, an antioxidant enzyme shown to be involved in oxidative stress associated with PTB [18, 23, 34, 49, 65, 134–138].
Fig. 5.
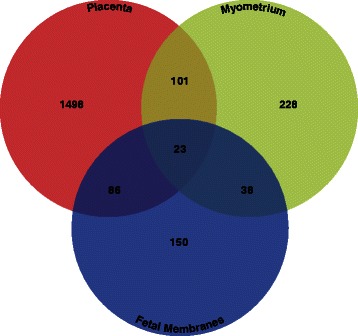
Overlap of differentially expressed genes across tissues. Differentially expressed genes present in two or more gene expression studies categorized by tissue were compared across the three most commonly studied (placenta, myometrium, and fetal membranes). Out of 2,123 genes identified to be differentially expressed in at least two studies, 23 genes were shared across all three tissues
Although gene expression profiles are available for 29 distinct phenotypes, PTB research is dominated by studies focused on select phenotypes of PTB
The 93 gene expression studies analyzed 29 distinct phenotypes. From these studies, 5/93 (5 %) focused on a non-clinical phenotype (healthy pregnancies), with the remaining 88/93 (95 %) focused on clinical phenotypes. Among studies focused on clinical phenotypes, the three most common phenotypes investigated were PE (27/93; 29 %), labor (15/93; 16 %), and IUGR (8/93; 9 %); each of the other 26 clinical phenotypes was studied in 5 or fewer studies. Genome-wide gene expression studies of the three most commonly studied clinical phenotypes identified a total of 7,730 unique genes, of which only 1,336 (15 %) were present in two or more studies (Fig. 6, Additional file 6). No candidate genes were found two or more times in studies of all three phenotypes. Generally, overlap of differentially expressed genes was more limited across clinical phenotypes than across gestational tissues.
Fig. 6.
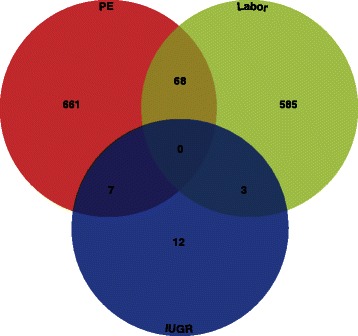
Overlap of differentially expressed genes across phenotypes. Differentially expressed genes identified in two or more gene expression studies categorized by phenotype were compared across the most commonly studied (PE, labor, and IUGR). Out of 1,336 genes identified to be differentially expressed in at least two studies, none were shared across all three phenotypes
Overlap of differentially expressed genes identified across PTB studies is limited
Studies of placenta, myometrium, and fetal membranes, the three most commonly studied tissues, focused on a total of 25 distinct phenotypes (Fig. 7a, Additional file 7). The clinical phenotype studied, however, differed between tissues, with PE dominating placental research (23/53 placental studies or 43 %), labor dominating myometrial research (9/17 myometrial studies or 53 %), and PPROM dominating fetal membrane research (4/13 fetal membrane studies or 31 %). Likewise, the range of tissues studied differed between phenotypes. PE was studied across 4 distinct gestational tissues (placenta, decidua, basal plate, and maternal blood), labor was studied across 4 distinct gestational tissues (myometrium, fetal membranes, placenta, and cervix), and PPROM was studied across only 1 distinct gestational tissue (fetal membranes) (Fig. 7b, Additional file 8).
Fig. 7.
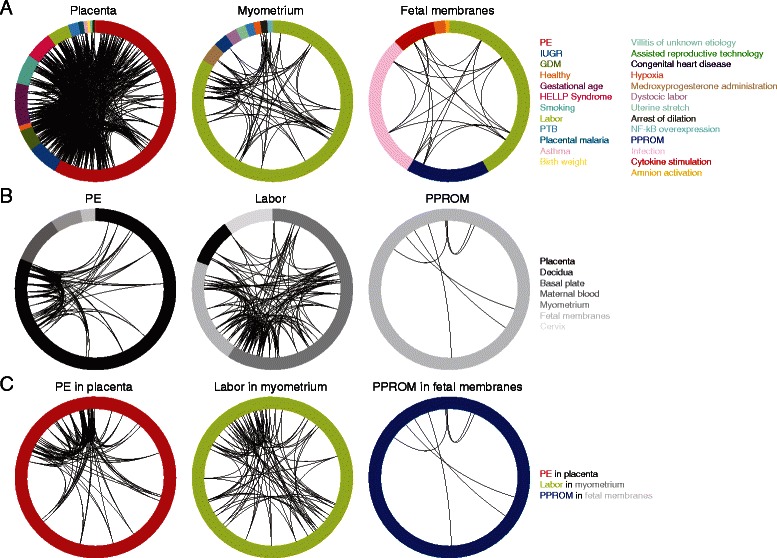
Representation of overlap in differentially expressed genes across the most commonly studied tissues, phenotypes, and tissues & phenotypes. Studies are represented as distinct wedges in the outermost track, colored by phenotype and sized by number of genes reported. Genes that show a high degree of overlap across studies (4 or more placenta, PE, or PE in placenta studies; 4 or more myometrium, labor, or labor in myometrium studies; 4 or more fetal membranes studies; or 2 or more PPROM or PPROM in fetal membranes studies) appear as black links connecting each study reporting the gene. In general, the scarcity of links illustrates the considerable lack of overlap in the genes identified as differentially expressed across PTB studies. a Representation of overlap in differentially expressed genes across the most commonly studied tissues. Studies of placenta, myometrium, and fetal membranes, the three most commonly studied tissues, focused on a total of 25 distinct phenotypes with PE dominating placental research, labor dominating myometrial research, and PPROM dominating fetal membranes research. b Representation of overlap in differentially expressed genes across the most commonly studied phenotypes. PE was studied across 4 distinct gestational tissues (placenta, decidua, basal plate, and maternal blood), labor was studied across 4 distinct gestational tissues (myometrium, fetal membranes, placenta, and cervix), and PPROM was studied across only 1 distinct gestational tissue (fetal membranes). c Representation of overlap in differentially expressed genes across the most commonly studied tissue and phenotype combinations. The most studied combinations were PE in placenta (n = 23), labor in myometrium (n = 9), and PPROM in fetal membranes (n = 3). Examination of PE in placenta studies identified 16 genes that were present in 4 or more studies, examination of labor in myometrium studies identified 15 genes that were present in 4 or more studies, and examination of PPROM in fetal membranes studies identified 6 genes that were present in 2 or more studies
To identify common differential gene expression signatures, we looked for overlap between differentially expressed genes reported in studies of the same phenotype and tissue. The most studied phenotype-tissue combinations were PE in placenta (n = 23), labor in myometrium (n = 9), and PPROM in fetal membranes (n = 4) (Fig. 7c, Table 1). Examination of PE in placenta studies identified 16 genes that were present in 4 or more studies including LEP, a fat-regulating hormone commonly shown to be differentially expressed in gestational tissues of women with PE and HELLP Syndrome, and FLT1, a growth factor known to be highly expressed in preeclamptic placental trophoblast cells [21, 32, 44, 48, 53, 75, 80, 88, 94]. Examination of labor in myometrium studies identified 15 genes that were present in 4 or more studies including PTGS2, a cyclooxygenase involved in inflammation and commonly upregulated in myometrium during labor [18, 26, 40, 64, 66, 136, 139]. Finally, 6 genes were present in 2 or more PPROM in fetal membranes studies including IL8, a proinflammatory chemokine often associated with PTB [36, 37, 55, 87, 92, 140].
Table 1.
The most often recovered differentially expressed genes in PE in placenta, labor in myometrium, and PPROM in fetal membranes
| PE in placenta | ||
| Entrez gene ID | Official gene symbol | # studies |
| 3952 | LEP | 7 |
| 2321 | FLT1 | 6 |
| 3623 | INHA | 6 |
| 3624 | INHBA | 6 |
| 2022 | ENG | 5 |
| 6647 | SOD1 | 5 |
| 10148 | EBI3 | 5 |
| 604 | BCL6 | 4 |
| 1082 | CGB | 4 |
| 3972 | LHB | 4 |
| 10272 | FSTL3 | 4 |
| 10544 | PROCR | 4 |
| 54210 | TREM1 | 4 |
| 60676 | PAPPA2 | 4 |
| 93659 | CGB5 | 4 |
| 94115 | CGB8 | 4 |
| Labor in myometrium | ||
| Entrez gene ID | Official gene symbol | # studies |
| 165 | AEBP1 | 4 |
| 366 | AQP9 | 4 |
| 861 | RUNX1 | 4 |
| 2354 | FOSB | 4 |
| 3164 | NR4A1 | 4 |
| 3576 | IL8 | 4 |
| 3976 | LIF | 4 |
| 5054 | SERPINE1 | 4 |
| 5292 | PIM1 | 4 |
| 5334 | PLCL1 | 4 |
| 5743 | PTGS2 | 4 |
| 6401 | SELE | 4 |
| 9123 | SLC16A3 | 4 |
| 51129 | ANGPTL4 | 4 |
| 117247 | SLC16A10 | 4 |
| PPROM in fetal membranes | ||
| Entrez gene ID | Official gene symbol | # studies |
| 972 | CD74 | 2 |
| 1117 | CHI3L2 | 2 |
| 3576 | IL8 | 2 |
| 7805 | LAPTM5 | 2 |
| 6280 | S100A9 | 2 |
| 23574 | PRG1 | 2 |
To examine whether the sets of genes that were most prevalent in each of the three tissue and phenotype pairs (PE in placenta, labor in myometrium, and PPROM in fetal membranes) disproportionally represented particular functions, we examined whether any Gene Ontology functional category was statistically significantly enriched (p < 0.0001) in each of the three gene sets (Additional file 9). Candidate genes identified in PE in placenta studies were enriched for regulation of cell death (GO:0010941) and apoptosis (GO:0042981), candidate genes identified in labor in myometrium were enriched for wounding (GO:0009611) and inflammatory response (GO:0006954), and candidate genes identified in PPROM in fetal membranes were enriched for immune system process (GO:0002376) and immune response (GO:0006955).
Discussion
PTB is a complex, multifactorial syndrome with high prevalence worldwide, whose pathogenesis remains poorly understood, especially for cases of early spontaneous labor. To provide an overview as well as a synthesis of the current landscape of PTB transcriptomics, we conducted an in-depth systematic review of the literature as well as a meta-analysis of 93 gene expression studies on a wide diversity of gestational tissues and clinical phenotypes. Examination of our results identifies two key findings. First, the correspondence between PTB subtype prevalence and proportion of transcriptomic research devoted to these subtypes is weak. Second, the overlap between differentially expressed genes identified in different studies is quite small, even on studies aimed on the same phenotypes and tissues. Below, we discuss the possible factors that underlie these two key findings and their implications for research on PTB.
In general, transcriptomic studies on placental tissue samples from women with preeclampsia dominate PTB research. Furthermore, there are very few studies focusing on sPTB, a subtype responsible for 45 % of all PTB cases. Although genes commonly associated with PTB clinical subtypes (i.e., LEP and FLT1) are identified in many of the gene expression studies to be differentially expressed, the overlap between the differentially expressed genes identified across studies is generally very limited. This is not surprising in comparisons between tissues (Fig. 5) because these often involve examinations of different clinical subtypes, although it does suggest that there is little overlap in tissue-specific transcriptional profiles of different clinical subtypes. Similarly, it is not surprising that comparisons between clinical subtypes do not show a high degree of overlap (Fig. 6) because these often involve examinations of different tissues. Nevertheless, it should be noted that differentially expressed genes with substantial overlap across studies appear to be biologically meaningful. For example, genes involved in hormone regulation (i.e., CGB, CRH, INHA, and GH2), which have been previously shown to be key in the maintenance of pregnancy, show substantial overlap in preeclampsia studies. Genes involved in inflammation (i.e., IL8), which have been previously shown to be dysregulated in PPROM and other clinical PTB subtypes, are also identified to be differentially expressed in multiple studies.
The observed minimal overlap between the differentially expressed genes identified across studies focused on the same tissue and clinical phenotype (Fig. 7) is possibly more serious. One potential explanation may be the difficulty in obtaining appropriate controls important in pregnancy research; comparing studies that differ with respect to the presence of labor, gestational age, and fetal sex is challenging, since all of these factors are thought to influence the gene expression landscape in gestational tissues. Even though matching of samples with respect to all these factors is very challenging, the reporting of a standard list of such factors as required metadata in transcriptomic studies would facilitate further examination of their importance and likely influence on transcriptomic profiles.
In addition to transcriptomics, several other systematic reviews and meta-analyses have focused on identifying biomarkers, usually proteins, that are associated with PTB [141–143]. Overlapping 19 previously identified common PTB biomarkers with the studies in our meta-analysis indicates that most (12/19; 63 %) are replicated in 4 or more studies (Table 2). Therefore, our comparison shows evidence of considerable overlap between transcriptomic and proteomic studies in PTB. Further research from both approaches is necessary, however, because our comparison also indicates that transcriptomics and proteomics can target unique candidate genes and proteins as well.
Table 2.
Overlap of meta-analysis with previously identified PTB biomarkers
| Entrez gene ID | Official gene symbol | # studies |
|---|---|---|
| 1392 | CRH | 11 |
| 6279 | S100A8 | 10 |
| 3569 | IL6 | 9 |
| 1082 | CGB | 9 |
| 2335 | FN1 | 9 |
| 3553 | IL1B | 9 |
| 7124 | TNF | 6 |
| 5617 | PRL | 5 |
| 2810 | SFN | 4 |
| 6283 | S100A12 | 4 |
| 2512 | FTL | 4 |
| 4318 | MMP9 | 4 |
| 5443 | POMC | 2 |
| 174 | AFP | 1 |
| 4317 | MMP8 | 0 |
| 308 | ANXA5 | 0 |
| 3700 | ITIH4 | 0 |
| 3558 | IL2 | 0 |
| 6013 | RLN1 | 0 |
Furthermore, the recent publication of comprehensive phenotyping tools necessitates the connection of evidence-based phenotype knowledge with genomic data collection in order to make more targeted conclusions [144]. It’s challenging to compare and contrast gene expression signatures between distinct subtypes without knowing whether the transcriptomes came from cases of sPTB due to maternal stress, uterine distention, or another subtype. Therefore, a greater focus needs to be placed on collecting the most detailed meta-data available regarding sPTB diagnosis as well as performing genome-wide studies of these newly described sPTB subtypes.
Finally, it is important to note that different studies follow different guidelines with respect to data availability. For example, some studies do not report the full list of differentially expressed genes identified or do not make them easily available for subsequent analysis (e.g., reporting tables that contain differential expression data on hundreds or thousands of genes in PDF format), therefore limiting and biasing the data available for subsequent analyses. The publishing of the data for all genes with differential expression above an explicit significance threshold in an easily accessible format is crucial in order to carefully analyze aggregated results and draw meaningful conclusions.
Conclusions
This study synthesizes all high-quality transcriptomic studies on gestational tissues to examine the landscape of PTB as well as to identify genes and genomic elements associated with it. We found that highly prevalent PTB subtypes, such as sPTB, are not well studied and that differentially expressed genes identified in different studies are often non-overlapping. Thus, the identification of the genes whose dysregulation contributes to this complex and multifactorial syndrome will require many more large-scale, systematic studies aimed at understanding the transcriptional profiles of these diverse clinical PTB subtypes across gestational tissues and characterizing their disease-relevant transcriptional differences.
Note Added in Proof
While this manuscript was in review, by studying the variation in the placental transcriptome of healthy humans, Hughes and coworkers estimated that more than 90 % of the observed transcriptomic variation is explained by variation within and between individuals [145]. These results provide an alternative, yet complementary, explanation for our finding that profiles of differentially expressed genes were highly heterogeneous both between and within clinical subtypes and tissues as well as between studies of the same clinical subtype and tissue.
Methods
Search strategy
This systematic review and meta-analysis followed guidelines set by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Additional files 10, 11 and 12) [146]. The electronic search was performed on August 16, 2014 in PubMed with no restrictions to identify all articles relating to differentially expressed or methylated genes and microRNAs in human gestational tissues. The search strategy was constructed based on related MeSH terms:
“Pregnancy”[mh] AND “Humans”[mh] AND (“Gene Expression Profiling”[mh] OR “Gene Expression Regulation”[mh]) AND (“Placenta”[mh] OR “Decidua”[mh] OR “Myometrium”[mh] OR “Cervix Uteri”[mh] OR “Extraembryonic Membranes”[mh] OR “Blood”[mh] OR “Plasma”[mh] OR “Umbilical Cord”[mh])
Systematic review
We collected abstracts for all 2,361 studies identified from this search and annotated eligibility based on 6 inclusion criteria:
Published in English
Full text available
Original research
Human gestational tissue samples
Genome-wide analysis
Candidate gene list assembled
134 studies met all 6 criteria and were included in the systematic review. Furthermore, studies were excluded when the study data was not accessible (the number of gene candidates was reported but the list of candidate genes was not), the study data was not reported (the number of candidate genes was not reported and a list of candidate genes was not provided), the data was unclear, there were no significant gene candidates, the study was not genome-wide, the study was not human-specific, the study was not relevant, the study was not single-gene based (i.e., was focused on pathways or gene sets), the study used data from proteomics, the study was performed on cell line rather than in an in-vivo tissue, the study’s supplement was not available, or when the study’s tissue was collected before the third trimester (Additional file 12).
Meta-analysis
Studies were included in our meta-analysis if they met an additional 3 inclusion criteria:
Studied differential gene expression
Provided candidate gene list
DAVID ID conversion successful
116 references met all inclusion criteria and, due to multiple comparisons or analyses in 14 of these references, a total of 134 distinct studies were summarized (Additional file 1). Of the 134 studies included in our systematic literature review, 93 gene expression studies met these criteria and were further analyzed. All differentially expressed genes reported in these studies were first extracted and then converted to Entrez ID format using the DAVID online tool, selecting the smallest Entrez ID number if multiple IDs mapped to single genes. We extracted all reported significantly differentially expressed genes based on each study’s significance threshold for differential expression. Overlap was determined simply by the presence of the same gene in the gene lists from different studies. DAVID was used to assay functional enrichment according to Gene Ontology categories. All analyses were performed using Python and visualizations were performed using ggplot2 and Circos [147, 148].
Acknowledgements
We thank Louis J. Muglia for offering invaluable advice on experimental design at an early stage of this experiment. This work was conducted in part using the resources of the Advanced Computing Center for Research and Education at Vanderbilt University. HRE was supported by the Graduate Program in Biological Sciences at Vanderbilt University. Research on this project was supported by the March of Dimes through the March of Dimes Prematurity Research Center Ohio Collaborative.
Abbreviations
- PTB
Preterm birth
- sPTB
Spontaneous idiopathic preterm birth
- PE
Preeclampsia
- IUGR
Intrauterine growth restriction
- GDM
Gestational diabetes mellitus
- PPROM
Preterm premature rupture of membranes
- MeSH
medical subject headings
Additional files
Summary of studies in systematic review.
All reported candidate genomic elements.
Phenotype definitions.
Duplicated genes in well-studied gestational tissues. Genes in 2 or more placenta studies or 2 or more myometrium studies or 2 or more fetal membranes studies.
Well-replicated genes in well-studied gestational tissues. 22 genes in 2 or more placenta studies and 2 or more myometrium studies and 2 or more fetal membranes studies.
Duplicated genes in well-studied clinical phenotypes. Genes in 2 or more PE studies or 2 or more labor studies or 2 or more IUGR studies.
Well-replicated genes in placenta, myometrium, and fetal membranes. Genes in 5 or more placenta studies or 5 or more myometrium studies or 5 or more fetal membranes studies.
Well-replicated genes in PE, labor, and PPROM. Genes in 5 or more PE studies or 5 or more labor studies or 2 or more PPROM studies.
GO enrichment. Enriched GO functional categories for replicated genes in PE in placenta, labor in myometrium, and PPROM in fetal membranes.
PRISMA checklist.
PRISMA flow chart.
Excluded studies. All studies excluded from systematic review.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
HRE and AR designed the study with input from WEA, KLM, and PA. HRE carried out the study and drafted the manuscript with subsequent contributions and revisions from AR. All authors read and approved the final manuscript.
Contributor Information
Haley R. Eidem, Email: haley.eidem@vanderbilt.edu
William E. Ackerman, IV, Email: william.ackerman@osumc.edu.
Kriston L. McGary, Email: kris.mcgary@vanderbilt.edu
Patrick Abbot, Email: patrick.abbot@vanderbilt.edu.
Antonis Rokas, Email: antonis.rokas@vanderbilt.edu.
References
- 1.Muglia LJ, Katz M. The enigma of spontaneous preterm birth. N Engl J Med. 2010;362:529–35. doi: 10.1056/NEJMra0904308. [DOI] [PubMed] [Google Scholar]
- 2.Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller A-B, Narwal R, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. The Lancet. 2012;379:2162–72. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- 3.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Esplin MS. Overview of spontaneous preterm birth: a complex and multifactorial phenotype. Clin Obstet Gynecol. 2014;57:518–30. doi: 10.1097/GRF.0000000000000037. [DOI] [PubMed] [Google Scholar]
- 5.Chang HH, Larson J, Blencowe H, Spong CY, Howson CP, Cairns-Smith S, et al. Preventing preterm births: analysis of trends and potential reductions with interventions in 39 countries with very high human development index. The Lancet. 2013;381:223–34. doi: 10.1016/S0140-6736(12)61856-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Plunkett J, Muglia LJ. Genetic contributions to preterm birth: implications from epidemiological and genetic association studies. Ann Med. 2008;40:167–95. doi: 10.1080/07853890701806181. [DOI] [PubMed] [Google Scholar]
- 7.Myatt L, Eschenbach DA, Lye SJ, Mesiano S, Murtha AP, Williams SM, et al. A standardized template for clinical studies in preterm birth. Reprod Sci. 2012;19:474–82. doi: 10.1177/1933719111426602. [DOI] [PubMed] [Google Scholar]
- 8.Menon R. Spontaneous preterm birth, a clinical dilemma: etiologic, pathophysiologic and genetic heterogeneities and racial disparity. Acta Obstet Gynecol Scand. 2008;87:590–600. doi: 10.1080/00016340802005126. [DOI] [PubMed] [Google Scholar]
- 9.Ananth CV, Vintzileos AM. Epidemiology of preterm birth and its clinical subtypes. J Matern-Fetal Neonatal Med Off J Eur Assoc Perinat Med Fed Asia Ocean Perinat Soc Int Soc Perinat Obstet. 2006;19:773–82. doi: 10.1080/14767050600965882. [DOI] [PubMed] [Google Scholar]
- 10.Henderson JJ, McWilliam OA, Newnham JP, Pennell CE. Preterm birth aetiology 2004–2008. Maternal factors associated with three phenotypes: spontaneous preterm labour, preterm pre-labour rupture of membranes and medically indicated preterm birth. J Matern Fetal Neonatal Med. 2012;25:642–7. doi: 10.3109/14767058.2011.597899. [DOI] [PubMed] [Google Scholar]
- 11.Moutquin J. Classification and heterogeneity of preterm birth. BJOG Int J Obstet Gynaecol. 2003;110:30–3. doi: 10.1016/S1470-0328(03)00021-1. [DOI] [PubMed] [Google Scholar]
- 12.Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science. 2014;345:760–5. doi: 10.1126/science.1251816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bezold KY, Karjalainen MK, Hallman M, Teramo K, Muglia LJ. The genomics of preterm birth: from animal models to human studies. Genome Med. 2013;5:34. doi: 10.1186/gm438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lengyel C, Muglia LJ, Pavlicev M: Genetics of Preterm Birth. eLS. 2014:1–13.
- 15.Iams JD. Preterm birth categories-labels with consequences. Am J Obstet Gynecol. 2014;210:97–8. doi: 10.1016/j.ajog.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 16.Arcelli D, Farina A, Cappuzzello C, Bresin A, De Sanctis P, Perolo A, Prandstraller D, Valentini D, Zucchini C, Priori S, Rizzo N: Identification of circulating placental mRNA in maternal blood of pregnancies affected with fetal congenital heart diseases at the second trimester of pregnancy: implications for early molecular screening. Prenat Diagn. 2010;30(3):229-34. [DOI] [PubMed]
- 17.Bethin KE, Nagai Y, Sladek R, Asada M, Sadovsky Y, Hudson TJ, et al. Microarray analysis of uterine gene expression in mouse and human pregnancy. Mol Endocrinol Baltim Md. 2003;17:1454–69. doi: 10.1210/me.2003-0007. [DOI] [PubMed] [Google Scholar]
- 18.Bollapragada S, Bollopragada S, Youssef R, Jordan F, Greer I, Norman J, et al. Term labor is associated with a core inflammatory response in human fetal membranes, myometrium, and cervix. Am J Obstet Gynecol. 2009;200:104. doi: 10.1016/j.ajog.2008.08.032. [DOI] [PubMed] [Google Scholar]
- 19.Brennan DJ, McGee SF, Rexhepaj E, O’Connor DP, Robson M, O’Herlihy C. Identification of a myometrial molecular profile for dystocic labor. BMC Pregnancy Childbirth. 2011;11:74. doi: 10.1186/1471-2393-11-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bruchova H, Vasikova a, Merkerova M, Milcova A: Effect of maternal tobacco smoke exposure on the placental transcriptome. Placenta. 2010;31(3):186-91. [DOI] [PubMed]
- 21.Buimer M, Keijser R, Jebbink JM, Wehkamp D, van Kampen AHC, Boer K, et al. Seven placental transcripts characterize HELLP-syndrome. Placenta. 2008;29:444–53. doi: 10.1016/j.placenta.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 22.Bukowski R, Hankins GDV, Saade GR, Anderson GD, Thornton S. Labor-associated gene expression in the human uterine fundus, lower segment, and cervix. PLoS Med. 2006;3:e169. doi: 10.1371/journal.pmed.0030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Centlow M, Wingren C, Borrebaeck C, Brownstein MJ, Hansson SR. Differential gene expression analysis of placentas with increased vascular resistance and pre-eclampsia using whole-genome microarrays. J Pregnancy. 2011;2011:472354–12. doi: 10.1155/2011/472354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaemsaithong P, Madan I, Romero R, Than NG, Tarca AL, Draghici S, et al. Characterization of the myometrial transcriptome in women with an arrest of dilatation during labor. J Perinat Med. 2013;41:665–81. doi: 10.1515/jpm-2013-0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan EC, Fraser S, Yin S, Yeo G, Kwek K, Fairclough RJ, et al. Human myometrial genes are differentially expressed in labor: a suppression subtractive hybridization study. J Clin Endocrinol Metab. 2002;87:2435–41. doi: 10.1210/jcem.87.6.8439. [DOI] [PubMed] [Google Scholar]
- 26.Chan Y-W, van den Berg HA, Moore JD, Quenby S, Blanks AM. Assessment of myometrial transcriptome changes associated with spontaneous human labour by high throughput RNA-seq. Exp Physiol. 2014;0:1–15. doi: 10.1113/expphysiol.2013.072868. [DOI] [PubMed] [Google Scholar]
- 27.Chang S-D, Chao A-S, Peng H-H, Chang Y-L, Wang C-N, Cheng P-J, et al. Analyses of placental gene expression in pregnancy-related hypertensive disorders. Taiwan J Obstet Gynecol. 2011;50:283–91. doi: 10.1016/j.tjog.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 28.Charpigny G, Leroy M-J, Breuiller-Fouché M, Tanfin Z, Mhaouty-Kodja S, Robin P, et al. A functional genomic study to identify differential gene expression in the preterm and term human myometrium. Biol Reprod. 2003;68:2289–96. doi: 10.1095/biolreprod.102.013763. [DOI] [PubMed] [Google Scholar]
- 29.Chim SSC, Lee WS, Ting YH, Chan OK, Lee SWY, Leung TY. Systematic identification of spontaneous preterm birth-associated RNA transcripts in maternal plasma. PLoS ONE. 2012;7:e34328. doi: 10.1371/journal.pone.0034328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cordeaux Y, Tattersall M, Charnock-Jones DS, Smith GCS. Effects of medroxyprogesterone acetate on gene expression in myometrial explants from pregnant women. J Clin Endocrinol Metab. 2010;95:E437–47. doi: 10.1210/jc.2010-1541. [DOI] [PubMed] [Google Scholar]
- 31.Dunk CE, Roggensack AM, Cox B, Perkins JE, AAsenius F, Keating S, et al. A distinct microvascular endothelial gene expression pro. Placenta. 2012;33:285–93. doi: 10.1016/j.placenta.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 32.Enquobahrie DA, Meller M, Rice K, Psaty BM, Siscovick DS, Williams MA. Differential placental gene expression in preeclampsia. Am J Obstet Gynecol. 2008;199:566. doi: 10.1016/j.ajog.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Enquobahrie DA, Williams MA, Qiu C, Meller M, Sorensen TK. Global placental gene expression in gestational diabetes mellitus. Am J Obstet Gynecol. 2009;200:206. doi: 10.1016/j.ajog.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 34.Esplin MS, Fausett MB, Peltier MR, Hamblin S, Silver RM, Branch DW, et al. The use of cDNA microarray to identify differentially expressed labor-associated genes within the human myometrium during labor. Am J Obstet Gynecol. 2005;193:404–13. doi: 10.1016/j.ajog.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 35.Gack S, Marme A, Marme F, Wrobel G, Vonderstrass B, Bastert G, et al. Preeclampsia: increased expression of soluble ADAM 12. J Mol Med Berl Ger. 2005;83:887–96. doi: 10.1007/s00109-005-0714-9. [DOI] [PubMed] [Google Scholar]
- 36.Haddad R, Tromp G, Kuivaniemi H. Human spontaneous labor without histologic chorioamnionitis is characterized by an acute inflammation gene expression signature. Am J Ldots. 2006;195:394–405. doi: 10.1016/j.ajog.2005.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Han YM, Romero R, Kim J-S, Tarca AL, Kim SK, Draghici S, et al. Region-specific gene expression profiling: novel evidence for biological heterogeneity of the human amnion. Biol Reprod. 2008;79:954–61. doi: 10.1095/biolreprod.108.069260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hansson SR, Chen Y, Brodszki J, Chen M, Hernandez-Andrade E, Inman JM, et al. Gene expression profiling of human placentas from preeclamptic and normotensive pregnancies. Mol Hum Reprod. 2006;12:169–79. doi: 10.1093/molehr/gal011. [DOI] [PubMed] [Google Scholar]
- 39.Hassan SS, Romero R, Tarca AL, Nhan-Chang C-L, Vaisbuch E, Erez O, et al. The transcriptome of cervical ripening in human pregnancy before the onset of labor at term: Identification of novel molecular functions involved in this process. J Matern Fetal Neonatal Med. 2009;22:1183–93. doi: 10.3109/14767050903353216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Havelock OC, Keller P, Muleba N, Mayhew BA, Casey BM, Rainey WE, et al. Human myometrial gene expression before and during parturition. Biol Reprod. 2005;72:707–19. doi: 10.1095/biolreprod.104.032979. [DOI] [PubMed] [Google Scholar]
- 41.Heikkilä A, Tuomisto T, Häkkinen S-K, Keski-Nisula L, Heinonen S, Ylä-Herttuala S. Tumor suppressor and growth regulatory genes are overexpressed in severe early-onset preeclampsia–an array study on case-specific human preeclamptic placental tissue. Acta Obstet Gynecol Scand. 2005;84:679–89. doi: 10.1111/j.0001-6349.2005.00814.x. [DOI] [PubMed] [Google Scholar]
- 42.Heng YJ, Pennell CE, Chua HN, Perkins JE, Lye SJ. Whole blood gene expression profile associated with spontaneous preterm birth in women with threatened preterm labor. PLoS ONE. 2014;9:e96901. doi: 10.1371/journal.pone.0096901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hiden U, Maier A, Bilban M, Ghaffari-Tabrizi N, Wadsack C, Lang I, et al. Insulin control of placental gene expression shifts from mother to foetus over the course of pregnancy. Diabetologia. 2006;49:123–31. doi: 10.1007/s00125-005-0054-x. [DOI] [PubMed] [Google Scholar]
- 44.Hoegh AM, Borup R, Nielsen FC, Sørensen S, Hviid TVF. Gene expression profiling of placentas affected by pre-eclampsia. J Biomed Biotechnol. 2010;2010:787545–11. doi: 10.1155/2010/787545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jarvenpaa J, Vuoristo JT, Savolainen E, Ukkola O, Vaskivuo T, Ryynanen M. Altered expression of angiogenesis-related placental genes in pre-eclampsia associated with intrauterine growth restriction. Gynecol Endocrinol Off J Int Soc Gynecol Endocrinol. 2007;23:351–5. doi: 10.1080/09513590701350291. [DOI] [PubMed] [Google Scholar]
- 46.Junus K, Centlow M, Wikstrom AK, Larsson I, Hansson SR, Olovsson M. Gene expression profiling of placentae from women with early- and late-onset pre-eclampsia: down-regulation of the angiogenesis-related genes ACVRL1 and EGFL7 in early-onset disease. Mol Hum Reprod. 2012;18:146–55. doi: 10.1093/molehr/gar067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kang BY, Tsoi S, Zhu S, Su S, Kay HH. Differential gene expression profiling in HELLP syndrome placentas. Reprod Sci. 2008;15:285–94. doi: 10.1177/1933719108314626. [DOI] [PubMed] [Google Scholar]
- 48.Kang JH, Song H, Yoon JA, Park DY, Kim SH, Lee KJ, et al. Preeclampsia leads to dysregulation of various signaling pathways in placenta. J Hypertens. 2011;29:928–36. doi: 10.1097/HJH.0b013e328344a82c. [DOI] [PubMed] [Google Scholar]
- 49.Khanjani S, Kandola MK, Lindstrom TM, Sooranna SR, Melchionda M, Lee YS, et al. NF-κB regulates a cassette of immune/inflammatory genes in human pregnant myometrium at term. J Cell Mol Med. 2011;15:809–24. doi: 10.1111/j.1582-4934.2010.01069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim MJ, Romero R, Kim CJ, Tarca AL, Chhauy S, LaJeunesse C, et al. Villitis of unknown etiology is associated with a distinct pattern of Chemokine Up-regulation in the feto-maternal and placental compartments: implications for conjoint maternal allograft rejection and maternal anti-fetal graft-versus-host disease. J Immunol. 2009;182:3919–27. doi: 10.4049/jimmunol.0803834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim J, Zhao K, Jiang P, Lu Z, Wang J, Murray JC, et al. Transcriptome landscape of the human placenta. BMC Genomics. 2012;13:115. doi: 10.1186/1471-2164-13-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lapaire O, Grill S, Lalevee S, Kolla V, Hösli I, Hahn S. Microarray screening for novel preeclampsia biomarker candidates. Fetal Diagn Ther. 2012;31:147–53. doi: 10.1159/000337325. [DOI] [PubMed] [Google Scholar]
- 53.Lee GSR, Joe YS, Kim SJ, Shin JC. Cytokine-related genes and oxidation-related genes detected in preeclamptic placentas. Arch Gynecol Obstet. 2010;282:363–9. doi: 10.1007/s00404-009-1222-x. [DOI] [PubMed] [Google Scholar]
- 54.Lee J, Romero R, Chaiworapongsa T, Dong Z, Tarca AL, Xu Y, et al. Characterization of the fetal blood transcriptome and proteome in maternal anti-fetal rejection: evidence of a distinct and novel type of human fetal systemic inflammatory response. Am J Reprod Immunol. 2013;70:265–84. doi: 10.1111/aji.12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li R, Ackerman WE, Summerfield TL, Yu L, Gulati P, Zhang J, et al. Inflammatory gene regulatory networks in amnion cells following cytokine stimulation: translational systems approach to modeling human parturition. PLoS ONE. 2011;6:e20560. doi: 10.1371/journal.pone.0020560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lian IA, Toft JH, Olsen GD, Langaas M, Bjørge L, Eide IP, et al. Matrix metalloproteinase 1 in pre-eclampsia and fetal growth restriction: reduced gene expression in decidual tissue and protein expression in extravillous trophoblasts. Placenta. 2010;31:615–20. doi: 10.1016/j.placenta.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 57.Lim S, MacIntyre DA, Lee YS, Khanjani S, Terzidou V, Teoh TG, et al. Nuclear factor kappa B activation occurs in the amnion prior to labour onset and modulates the expression of numerous labour associated genes. PLoS ONE. 2012;7:e34707. doi: 10.1371/journal.pone.0034707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu Y, Li N, You L, Liu X, Li H, Wang X. HSP70 is associated with endothelial activation in placental vascular diseases. Mol Med Camb Mass. 2008;14:561–6. doi: 10.2119/2008-00009.Liu. [DOI] [PubMed] [Google Scholar]
- 59.Løset M, Mundal SB, Johnson MP, Fenstad MH, Freed KA, Lian IA, et al. A transcriptional profile of the decidua in preeclampsia. Am J Obstet Gynecol. 2011;204:84. doi: 10.1016/j.ajog.2010.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mayor-Lynn K, Toloubeydokhti T, Cruz AC, Chegini N. Expression profile of microRNAs and mRNAs in human placentas from pregnancies complicated by preeclampsia and preterm labor. Reprod Sci Thousand Oaks Calif. 2011;18:46–56. doi: 10.1177/1933719110374115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McCarthy C, Cotter FE, McElwaine S, Twomey A, Mooney EE, Ryan F, et al. Altered gene expression patterns in intrauterine growth restriction: potential role of hypoxia. Am J Obstet Gynecol. 2007;196:70. doi: 10.1016/j.ajog.2006.08.027. [DOI] [PubMed] [Google Scholar]
- 62.McMinn J, Wei M, Schupf N, Cusmai J, Johnson EB, Smith AC, et al. Unbalanced placental expression of imprinted genes in human intrauterine growth restriction. Placenta. 2006;27:540–9. doi: 10.1016/j.placenta.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 63.Meng T, Chen H, Sun M, Wang H, Zhao G, Wang X. Identification of differential gene expression profiles in placentas from preeclamptic pregnancies versus normal pregnancies by DNA microarrays. Omics J Integr Biol. 2012;16:301–11. doi: 10.1089/omi.2011.0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mittal P, Romero R, Tarca AL, Gonzalez J, Draghici S, Xu Y, et al. Characterization of the myometrial transcriptome and biological pathways of spontaneous human labor at term. J Perinat Med. 2010;38:617–43. doi: 10.1515/jpm.2010.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Muehlenbachs A, Fried M, Lachowitzer J, Mutabingwa TK, Duffy PE. Genome-wide expression analysis of placental malaria reveals features of lymphoid neogenesis during chronic infection. J Immunol Baltim Md 1950. 2007;179:557–65. doi: 10.4049/jimmunol.179.1.557. [DOI] [PubMed] [Google Scholar]
- 66.Nhan-Chang CL, Romero R, Tarca AL, Mittal P, Kusanovic JP, Erez O, et al. Characterization of the transcriptome of chorioamniotic membranes at the site of rupture in spontaneous labor at term. Am J Obstet Gynecol. 2010;202:462. doi: 10.1016/j.ajog.2010.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nishizawa H, Pryor-Koishi K, Kato T, Kowa H, Kurahashi H, Udagawa Y. Microarray analysis of differentially expressed fetal genes in placental tissue derived from early and late onset severe pre-eclampsia. Placenta. 2007;28:487–97. doi: 10.1016/j.placenta.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 68.Nishizawa H, Ota S, Suzuki M, Kato T, Sekiya T, Kurahashi H, et al. Comparative gene expression profiling of placentas from patients with severe pre-eclampsia and unexplained fetal growth restriction. Reprod Biol Endocrinol RBE. 2011;9:107. doi: 10.1186/1477-7827-9-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ogita K, Kimura T, Nakamura H, Koyama S, Tsujie T, Tomiie M, et al. Differential expression and localization of decorin in human choriodecidual membrane during preterm and term pregnancy. Am J Reprod Immunol. 2004;51:204–10. doi: 10.1111/j.1600-0897.2004.00143.x. [DOI] [PubMed] [Google Scholar]
- 70.Okamoto A, Endo H, Kalionis B, Shinya M, Saito M, Nikaido T, et al. IGFBP1 and follistatin-like 3 genes are significantly up-regulated in expression profiles of the IUGR placenta. Placenta. 2006;27:317–21. doi: 10.1016/j.placenta.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 71.Osei-Kumah A, Smith R, Jurisica I, Caniggia I, Clifton VL. Differences in placental global gene expression in pregnancies complicated by asthma. Placenta. 2011;32:570–8. doi: 10.1016/j.placenta.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 72.Peng H-H, Kao C-C, Chang S-D, Chao A-S, Chang Y-L, Wang C-N, et al. The effects of labor on differential gene expression in parturient women, placentas, and fetuses at term pregnancy. Kaohsiung J Med Sci. 2011;27:494–502. doi: 10.1016/j.kjms.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Radaelli T, Varastehpour A, Catalano P, Haugeul-de Mouzon S. Gestational diabetes induces placental genes for chronic stress and inflammatory pathways. Diabetes. 2003;52:2951–8. doi: 10.2337/diabetes.52.12.2951. [DOI] [PubMed] [Google Scholar]
- 74.Rehman KS, Yin S, Mayhew BA. Human myometrial adaptation to pregnancy: cDNA microarray gene expression profiling of myometrium from non-pregnant and pregnant women. Mol Hum Reprod. 2003;9(11):681-700. [DOI] [PubMed]
- 75.Reimer T, Koczan D, Gerber B, Richter D, Thiesen HJ, Friese K. Microarray analysis of differentially expressed genes in placental tissue of pre-eclampsia: up-regulation of obesity-related genes. Mol Hum Reprod. 2002;8:674–80. doi: 10.1093/molehr/8.7.674. [DOI] [PubMed] [Google Scholar]
- 76.Roh CR, Budhraja V, Kim HS, Nelson DM, Sadovsky Y. Microarray-based identification of differentially expressed genes in hypoxic term human trophoblasts and in placental villi of pregnancies with growth restricted fetuses. Placenta. 2005;26:319–28. doi: 10.1016/j.placenta.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 77.Saben J, Zhong Y, McKelvey S, Dajani NK, Andres A, Badger TM, et al. A comprehensive analysis of the human placenta transcriptome. Placenta. 2014;35:125–31. doi: 10.1016/j.placenta.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shankar R, Johnson MP, Williamson NA, Cullinane F, Purcell AW, Moses EK, et al. Molecular markers of preterm labor in the choriodecidua. Reprod Sci Thousand Oaks Calif. 2010;17:297–310. doi: 10.1177/1933719109353454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sitras V, Paulssen R, Leirvik J, Vårtun A, Acharya G. Placental gene expression profile in intrauterine growth restriction due to placental insufficiency. Reprod Sci. 2009;16:701–11. doi: 10.1177/1933719109334256. [DOI] [PubMed] [Google Scholar]
- 80.Sitras V, Paulssen RH, Grønaas H, Leirvik J, Hanssen TA, Vårtun A, et al. Differential placental gene expression in severe preeclampsia. Placenta. 2009;30:424–33. doi: 10.1016/j.placenta.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 81.Sitras V, Fenton C, Paulssen R, Vårtun Å, Acharya G. Differences in gene expression between first and third trimester human placenta: a microarray study. PLoS ONE. 2012;7:e33294. doi: 10.1371/journal.pone.0033294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Soleymanlou N, Jurisica I, Nevo O, Ietta F, Zhang X, Zamudio S, et al. Molecular evidence of placental hypoxia in preeclampsia. J Clin Endocrinol Metab. 2005;90:4299–308. doi: 10.1210/jc.2005-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sood R, Zehnder JL, Druzin ML, Brown PO. Gene expression patterns in human placenta. Proc Natl Acad Sci U S A. 2006;103:5478–83. doi: 10.1073/pnas.0508035103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tashima LS, Millar LK: Genes upregulated in human fetal membranes by infection or labor. Obstet Gynecol. 1999;94(3):441-9. [DOI] [PubMed]
- 85.Tattersall M, Cordeaux Y, Charnock-Jones DS, Smith GCS. Expression of gastrin-releasing peptide is increased by prolonged stretch of human myometrium, and antagonists of its receptor inhibit contractility. J Physiol. 2012;590:2081–93. doi: 10.1113/jphysiol.2012.228239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Textoris J, Ivorra D, Ben Amara A, Sabatier F, Ménard J-P, Heckenroth H, et al. Evaluation of current and New biomarkers in severe preeclampsia: a microarray approach reveals theVSIG4 gene as a potential blood biomarker. PLoS ONE. 2013;8:e82638. doi: 10.1371/journal.pone.0082638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tromp G, Kuivaniemi H, Romero R, Chaiworapongsa T, Kim YM, Kim MR, et al. Genome-wide expression profiling of fetal membranes reveals a deficient expression of proteinase inhibitor 3 in premature rupture of membranes. Am J Obstet Gynecol. 2004;191:1331–8. doi: 10.1016/j.ajog.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 88.Tsai S, Hardison NE, James AH, Motsinger-Reif AA, Bischoff SR, Thames BH, et al. Transcriptional profiling of human placentas from pregnancies complicated by preeclampsia reveals disregulation of sialic acid acetylesterase and immune signalling pathways. Placenta. 2011;32:175–82. doi: 10.1016/j.placenta.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Uusküla L, Männik J, Rull K, Minajeva A, Kõks S, Vaas P, et al. Mid-gestational gene expression profile in placenta and link to pregnancy complications. PLoS ONE. 2012;7:e49248. doi: 10.1371/journal.pone.0049248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Várkonyi T, Nagy B, Füle T, Tarca AL, Karászi K, Schönléber J, et al. Microarray profiling reveals that placental transcriptomes of early-onset HELLP syndrome and preeclampsia are similar. Placenta. 2011;32(Suppl):S21–9. doi: 10.1016/j.placenta.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Votavova H, Dostalova Merkerova M, Fejglova K, Vasikova A, Krejcik Z, Pastorkova A, et al. Transcriptome alterations in maternal and fetal cells induced by tobacco smoke. Placenta. 2011;32:763–70. doi: 10.1016/j.placenta.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 92.Weiner CP, Mason CW, Dong Y, Buhimschi IA, Swaan PW, Buhimschi CS. Human effector/initiator gene sets that regulate myometrial contractility during term and preterm labor. Am J Obstet Gynecol. 2010;202:474. doi: 10.1016/j.ajog.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Winn VD, Haimov-Kochman R, Paquet AC, Yang YJ, Madhusudhan MS, Gormley M, et al. Gene expression profiling of the human maternal-fetal interface reveals dramatic changes between midgestation and term. Endocrinology. 2007;148:1059–79. doi: 10.1210/en.2006-0683. [DOI] [PubMed] [Google Scholar]
- 94.Winn VD, Gormley M, Paquet AC, Kjaer-Sorensen K, Kramer A, Rumer KK, et al. Severe preeclampsia-related changes in gene expression at the maternal-fetal interface include sialic acid-binding immunoglobulin-like lectin-6 and pappalysin-2. Endocrinology. 2009;150:452–62. doi: 10.1210/en.2008-0990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yan YH, Yi P, Zheng YR, Yu LL, Han J, Han X-M, et al. Screening for preeclampsia pathogenesis related genes. Eur Rev Med Pharmacol Sci. 2013;17:3083–94. [PubMed] [Google Scholar]
- 96.Zhang Y, Cui Y, Zhou Z, Sha J, Li Y, Liu J. Altered global gene expressions of human placentae subjected to assisted reproductive technology treatments. Placenta. 2010;31:251–8. doi: 10.1016/j.placenta.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 97.Zhao YH, Wang DP, Zhang LL, Zhang F, Wang DM, Zhang WY. Genomic expression profiles of blood and placenta reveal significant immune-related pathways and categories in Chinese women with gestational diabetes mellitus. Diabet Med J Br Diabet Assoc. 2011;28:237–46. doi: 10.1111/j.1464-5491.2010.03140.x. [DOI] [PubMed] [Google Scholar]
- 98.Zhou R, Zhu Q, Wang Y, Ren Y, Zhang L, Zhou Y. Genomewide oligonucleotide microarray analysis on placentae of pre-eclamptic pregnancies. Gynecol Obstet Invest. 2006;62:108–14. doi: 10.1159/000092857. [DOI] [PubMed] [Google Scholar]
- 99.Zhou Y, Gormley MJ, Hunkapiller NM, Kapidzic M, Stolyarov Y, Feng V, et al. Reversal of gene dysregulation in cultured cytotrophoblasts reveals possible causes of preeclampsia. J Clin Invest. 2013;123:2862–72. doi: 10.1172/JCI66966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Barbaux S, Gascoin-Lachambre G, Buffat C, Monnier P, Mondon F, Tonanny M-B, et al. A genome-wide approach reveals novel imprinted genes expressed in the human placenta. Epigenetics Off J DNA Methylation Soc. 2012;7:1079–90. doi: 10.4161/epi.21495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Blair JD, Yuen RKC, Lim BK, McFadden DE, von Dadelszen P, Robinson WP. Widespread DNA hypomethylation at gene enhancer regions in placentas associated with early-onset pre-eclampsia. Mol Hum Reprod. 2013;19:697–708. doi: 10.1093/molehr/gat044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Blair JD, Langlois S, McFadden DE, Robinson WP. Overlapping DNA methylation profile between placentas with trisomy 16 and early-onset preeclampsia. Placenta. 2014;35:216–22. doi: 10.1016/j.placenta.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 103.Chu T, Bunce K, Shaw P, Shridhar V, Althouse A, Hubel C, et al. Comprehensive analysis of preeclampsia-associated DNA methylation in the placenta. PLoS ONE. 2014;9:e107318. doi: 10.1371/journal.pone.0107318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cruickshank MN, Oshlack A, Theda C, Davis PG, Martino D, Sheehan P, Dai Y, Saffery R, Doyle LW, Craig JM: Analysis of epigenetic changes in survivors of preterm birth reveals the effect of gestational age and evidence for a long term legacy. Gen Med. 2013;5:1–1. [DOI] [PMC free article] [PubMed]
- 105.Das R, Lee YK, Strogantsev R, Jin S, Lim YC, Ng PY, et al. DNMT1 and AIM1 imprinting in human placenta revealed through a genome-wide screen for allele-specific DNA methylation. BMC Genomics. 2013;14:1–1. doi: 10.1186/1471-2164-14-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gordon L, Joo JE, Powell JE, Ollikainen M, Novakovic B, Li X, et al. Neonatal DNA methylation profile in human twins is specified by a complex interplay between intrauterine environmental and genetic factors, subject to tissue-specific influence. Genome Res. 2012;22:1395–406. doi: 10.1101/gr.136598.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kim SY, Romero R, Tarca AL, Bhatti G, Kim CJ, Lee J, et al. Methylome of fetal and maternal monocytes and macrophages at the feto-maternal interface. Am J Reprod Immunol. 2012;68:8–27. doi: 10.1111/j.1600-0897.2012.01108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kim J, Pitlick MM, Christine PJ, Schaefer AR, Saleme C, Comas B, et al. Genome-wide analysis of DNA methylation in human amnion. ScientificWorldJournal. 2013;2013:678156–11. doi: 10.1155/2013/678156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lun FMF, Chiu RWK, Sun K, Leung TY, Jiang P, Chan KCA, et al. Noninvasive prenatal methylomic analysis by genomewide bisulfite sequencing of maternal plasma DNA. Clin Chem. 2013;59:1583–94. doi: 10.1373/clinchem.2013.212274. [DOI] [PubMed] [Google Scholar]
- 110.Ruchat S-M, Houde A-A, Voisin G, St-Pierre J, Perron P, Baillargeon J-P, et al. Gestational diabetes mellitus epigenetically affects genes predominantly involved in metabolic diseases. Epigenetics Off J DNA Methylation Soc. 2013;8:935–43. doi: 10.4161/epi.25578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schroeder DI, Blair JD, Lott P, Yu HOK, Hong D, Crary F, et al. The human placenta methylome. PNAS. 2013;110:6037–42. doi: 10.1073/pnas.1215145110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Suter M, Ma J, Harris A, Patterson L, Brown KA, Shope C, et al. Maternal tobacco use modestly alters correlated epigenome-wide placental DNA methylation and gene expression. Epigenetics Off J DNA Methylation Soc. 2011;6:1284–94. doi: 10.4161/epi.6.11.17819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Turan N, Ghalwash MF, Katari S, Coutifaris C, Obradovic Z, Sapienza C. DNA methylation differences at growth related genes correlate with birth weight: a molecular signature linked to developmental origins of adult disease? BMC Med Genomics. 2012;5:10. doi: 10.1186/1755-8794-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yuen RKC, Chen B, Blair JD, Robinson WP, Nelson DM. Hypoxia alters the epigenetic profile in cultured human placental trophoblasts. Epigenetics Off J DNA Methylation Soc. 2013;8:192–202. doi: 10.4161/epi.23400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Choi SY, Yun J, Lee OJ, Han HS, Yeo MK, Lee MA, et al. MicroRNA expression profiles in placenta with severe preeclampsia using a PNA-based microarray. Placenta. 2013;34:799–804. doi: 10.1016/j.placenta.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 116.Elovitz MA, Brown AG, Anton L, Gilstrop M, Heiser L, Bastek J. Distinct cervical microRNA profiles are present in women destined to have a preterm birth. Am J Obstet Gynecol. 2014;210:221. doi: 10.1016/j.ajog.2013.12.043. [DOI] [PubMed] [Google Scholar]
- 117.Enquobahrie DA, Abetew DF, Sorensen TK, Willoughby D, Chidambaram K, Williams MA. Placental microRNA expression in pregnancies complicated by preeclampsia. Am J Obstet Gynecol. 2011;204:178. doi: 10.1016/j.ajog.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gu Y, Sun J, Groome LJ, Wang Y. Differential miRNA expression profiles between the first and third trimester human placentas. Am J Physiol Endocrinol Metab. 2013;304:E836–43. doi: 10.1152/ajpendo.00660.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Guo L, Yang Q, Lu J, Li H, Ge Q, Gu W, et al. A comprehensive survey of miRNA repertoire and 3′ addition events in the placentas of patients with pre-eclampsia from high-throughput sequencing. PLoS ONE. 2011;6:e21072. doi: 10.1371/journal.pone.0021072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hassan SS, Romero R, Pineles B, Tarca AL, Montenegro D, Erez O, et al. MicroRNA expression profiling of the human uterine cervix after term labor and delivery. Am J Obstet Gynecol. 2010;202:80. doi: 10.1016/j.ajog.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 121.Higashijima A, Miura K, Mishima H, Kinoshita A, Jo O, Abe S, et al. Characterization of placenta-specific microRNAs in fetal growth restriction pregnancy. Prenat Diagn. 2013;33:214–22. doi: 10.1002/pd.4045. [DOI] [PubMed] [Google Scholar]
- 122.Hu Y, Li P, Hao S, Liu L, Zhao J, Hou Y. Differential expression of microRNAs in the placentae of Chinese patients with severe pre-eclampsia. Clin Chem Lab Med CCLM FESCC. 2009;47:923–9. doi: 10.1515/CCLM.2009.228. [DOI] [PubMed] [Google Scholar]
- 123.Ishibashi O, Ohkuchi A, Ali MM, Kurashina R, Luo S-S, Ishikawa T, et al. Hydroxysteroid (17-β) dehydrogenase 1 is dysregulated by miR-210 and miR-518c that are aberrantly expressed in preeclamptic placentas: a novel marker for predicting preeclampsia. Hypertension. 2012;59:265–73. doi: 10.1161/HYPERTENSIONAHA.111.180232. [DOI] [PubMed] [Google Scholar]
- 124.Luo SS, Ishibashi O, Ishikawa G, Ishikawa T, Katayama A, Mishima T, et al. Human villous trophoblasts express and secrete placenta-specific MicroRNAs into maternal circulation via exosomes. Biol Reprod. 2009;81:717–29. doi: 10.1095/biolreprod.108.075481. [DOI] [PubMed] [Google Scholar]
- 125.Montenegro D, Romero R, Pineles BL, Tarca AL, Kim YM, Draghici S, et al. Differential expression of microRNAs with progression of gestation and inflammation in the human chorioamniotic membranes. Am J Obstet Gynecol. 2007;197:289. doi: 10.1016/j.ajog.2007.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Montenegro D, Romero R, Kim SS, Tarca AL, Draghici S, Kusanovic JP, et al. Expression patterns of microRNAs in the chorioamniotic membranes: a role for microRNAs in human pregnancy and parturition. J Pathol. 2009;217:113–21. doi: 10.1002/path.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Morales-Prieto DM, Chaiwangyen W, Ospina-Prieto S, Schneider U, Herrmann J, Gruhn B, et al. MicroRNA expression profiles of trophoblastic cells. Placenta. 2012;33:725–34. doi: 10.1016/j.placenta.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 128.Mouillet JF, Chu T, Nelson DM, Mishima T, Sadovsky Y. MiR-205 silences MED1 in hypoxic primary human trophoblasts. FASEB J. 2010;24:2030–9. doi: 10.1096/fj.09-149724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Noack F, Ribbat-Idel J, Thorns C, Chiriac A, Axt-Fliedner R, Diedrich K, et al. miRNA expression profiling in formalin-fixed and paraffin-embedded placental tissue samples from pregnancies with severe preeclampsia. J Perinat Med. 2011;39:267–71. doi: 10.1515/jpm.2011.012. [DOI] [PubMed] [Google Scholar]
- 130.Pineles BL, Romero R, Montenegro D, Tarca AL, Han YM, Kim YM, et al. Distinct subsets of microRNAs are expressed differentially in the human placentas of patients with preeclampsia. Am J Obstet Gynecol. 2007;196:261. doi: 10.1016/j.ajog.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 131.Wang W, Feng L, Zhang H, Hachy S, Satohisa S, Laurent LC, et al. Preeclampsia up-regulates angiogenesis-associated MicroRNA ( i.e., miR-17, −20a, and -20b) that target Ephrin-B2 and EPHB4 in human placenta. J Clin Endocrinol Metab. 2012;97:E1051–9. doi: 10.1210/jc.2011-3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Xu P, Zhao Y, Liu M, Wang Y, Wang H, Li YX, et al. Variations of MicroRNAs in human placentas and plasma from preeclamptic pregnancy. Hypertension. 2014;63:1276–84. doi: 10.1161/HYPERTENSIONAHA.113.02647. [DOI] [PubMed] [Google Scholar]
- 133.Zhu X, Han T, Sargent IL, Yin G, Yao Y. Differential expression profile of microRNAs in human placentas from preeclamptic pregnancies vs normal pregnancies. Am J Obstet Gynecol. 2009;200:661. doi: 10.1016/j.ajog.2008.12.045. [DOI] [PubMed] [Google Scholar]
- 134.Burleigh DW, Kendziorski CM, Choi YJ, Grindle KM, Grendell RL, Magness RR, et al. Microarray analysis of BeWo and JEG3 trophoblast cell lines: identification of differentially expressed transcripts. Placenta. 2007;28:383–9. doi: 10.1016/j.placenta.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 135.Chen H-W, Chen JJW, Tzeng C-R, Li H-N, Chang S-J, Cheng Y-F, et al. Global analysis of differentially expressed genes in early gestational decidua and chorionic villi using a 9600 human cDNA microarray. Mol Hum Reprod. 2002;8:475–84. doi: 10.1093/molehr/8.5.475. [DOI] [PubMed] [Google Scholar]
- 136.Chevillard G, Derjuga A, Devost D, Zingg HH, Blank V. Identification of interleukin-1 regulated genes in uterine smooth muscle cells. Reproduction. 2007;134:811–22. doi: 10.1530/REP-07-0289. [DOI] [PubMed] [Google Scholar]
- 137.Fukushima K, Murata M, Hachisuga M, Tsukimori K, Seki H, Takeda S, et al. Gene expression profiles by microarray analysis during Matrigel-induced tube formation in a human extravillous trophoblast cell line: comparison with endothelial cells. Placenta. 2008;29:898–904. doi: 10.1016/j.placenta.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 138.Popovici RM, Betzler NK, Krause MS, Luo M, Jauckus J, Germeyer A, et al. Gene expression profiling of human endometrial-trophoblast interaction in a coculture model. Endocrinology. 2006;147:5662–75. doi: 10.1210/en.2006-0916. [DOI] [PubMed] [Google Scholar]
- 139.Soloff MS, Jeng YJ, Izban MG, Sinha M, Luxon BA, Stamnes SJ, et al. Effects of progesterone treatment on expression of genes involved in uterine quiescence. Reprod Sci Thousand Oaks Calif. 2011;18:781–97. doi: 10.1177/1933719111398150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Brou L, Almli LM, Pearce BD, Bhat G, Drobek CO, Fortunato S, et al. Dysregulated biomarkers induce distinct pathways in preterm birth. BJOG Int J Obstet Gynaecol. 2012;119:458–73. doi: 10.1111/j.1471-0528.2011.03266.x. [DOI] [PubMed] [Google Scholar]
- 141.Conde-Agudelo A, Papageorghiou A, Kennedy S, Villar J. Novel biomarkers for the prediction of the spontaneous preterm birth phenotype: a systematic review and meta-analysis: novel biomarkers to predict spontaneous preterm birth. BJOG Int J Obstet Gynaecol. 2011;118:1042–54. doi: 10.1111/j.1471-0528.2011.02923.x. [DOI] [PubMed] [Google Scholar]
- 142.Kacerovsky M, Lenco J, Musilova I, Tambor V, Lamont R, Torloni MR, et al. Proteomic biomarkers for spontaneous preterm birth: a systematic review of the literature. Reprod Sci. 2014;2014(21):283–95. doi: 10.1177/1933719113503415. [DOI] [PubMed] [Google Scholar]
- 143.Menon R, Torloni MR, Voltolini C, Torricelli M, Merialdi M, Betran AP, et al. Biomarkers of spontaneous preterm birth: an overview of the literature in the last four decades. Reprod Sci. 2011;18:1046–70. doi: 10.1177/1933719111415548. [DOI] [PubMed] [Google Scholar]
- 144.Manuck TA, Esplin MS, Biggio J, Bukowski R, Parry S, Zhang H, et al. The phenotype of spontaneous preterm birth: application of a clinical phenotyping tool. Am J Obstet Gynecol. 2015;212:487. doi: 10.1016/j.ajog.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hughes DA, Kircher M, He Z, Guo S, Fairbrother GL, Moreno CS, Khaitovich P, Stoneking M. Evaluating intra- and inter-individual variation in the human placental transcriptome. Genome Biol. 2015;16:54. doi: 10.1186/s13059-015-0627-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Krzywinski M, Schein J, Birol I, Connors J, Gascoyne R, Horsman D, et al. Circos: an information aesthetic for comparative genomics. Genome Res. 2009;19:1639–45. doi: 10.1101/gr.092759.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wickham H. Ggplot2: Elegant Graphics for Data Analysis. New York: Springer; 2009. [Google Scholar]


