Abstract
Sinusoidal obstruction syndrome or veno-occlusive disease (SOS/VOD) is a potentially life-threatening complication of hematopoietic SCT (HSCT). This review aims to highlight, on behalf of the European Society for Blood and Marrow Transplantation, the current knowledge on SOS/VOD pathophysiology, risk factors, diagnosis and treatments. Our perspectives on SOS/VOD are (i) to accurately identify its risk factors; (ii) to define new criteria for its diagnosis; (iii) to search for SOS/VOD biomarkers and (iv) to propose prospective studies evaluating SOS/VOD prevention and treatment in adults and children.
Introduction
Sinusoidal obstruction syndrome (SOS), previously known as veno-occlusive disease (VOD; referred to as SOS/VOD hereafter), is a potentially life-threatening complication observed after hematopoietic SCT (HSCT).1 In this syndrome, sinusoidal endothelial cells and hepatocytes in the zone 3 of the hepatic acinus are damaged by toxic metabolites generated during the conditioning regimen.2 Diagnosis of SOS/VOD is based on clinical criteria including weight gain, fluid retention with ascites, tender hepatomegaly and jaundice.3, 4, 5 The condition usually develops by 30 days after HSCT, although it can occur later. Historically, its reported incidence ranges from approximately 5 to 60%, and this variation is clearly not only related to the intensity of the conditioning regimen, the type of transplant and the presence of risk factors, but also on the clinical criteria used for SOS/VOD diagnosis.3, 4, 6, 7, 8 Nowadays, SOS/VOD is more common after allogeneic HSCT (allo-HSCT) conditioned with myeloablative conditioning regimen (MAC), with an incidence around 10–15%, against <5% after allo-HSCT conditioned with reduced intensity conditioning regimen and autologous HSCT (auto-HSCT).5, 7, 8 The SOS/VOD severity varies widely from mild forms, which are resolved within a few weeks, to a severe syndrome, defined by the presence of multi-organ failure, and associated with a high mortality rate (>80%).5 For this reason, despite the relatively low incidence of this complication, a better understanding of SOS/VOD pathophysiology and risk factors is indispensable to improving prevention and treatment of potentially life-threatening severe SOS/VOD.
The aim of this work is to summarize the evidence on SOS/VOD pathophysiology, risk factors and treatment, with a special focus on current studies, and to discuss future prospects to improve our knowledge and management of SOS/VOD, on behalf of the European Society for Blood and Marrow Transplantation (EBMT).
Pathophysiology
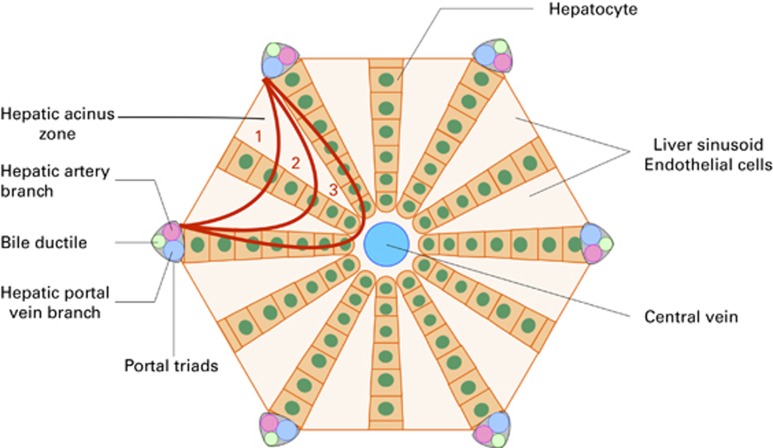
It is now clearly established that the first morphological change in SOS/VOD occurs in the sinusoidal endothelial cells, leading to the obstruction of the hepatic sinusoids in the zone 3 of the hepatic acinus (Figure 1). Endothelial cell lesions after HSCT are not limited to those lining the sinusoids and can lead to a wide range of endothelial syndromes early after transplant, including SOS/VOD, capillary leak syndrome, engraftment syndrome, transplant-associated microangiopathy or diffuse alveolar hemorrhage.2
Figure 1.
Schematic representation of the hepatic acinus. In sinusoidal obstruction syndrome, obstruction of the hepatic sinusoids occurs in the zone 3 of the hepatic acinus.
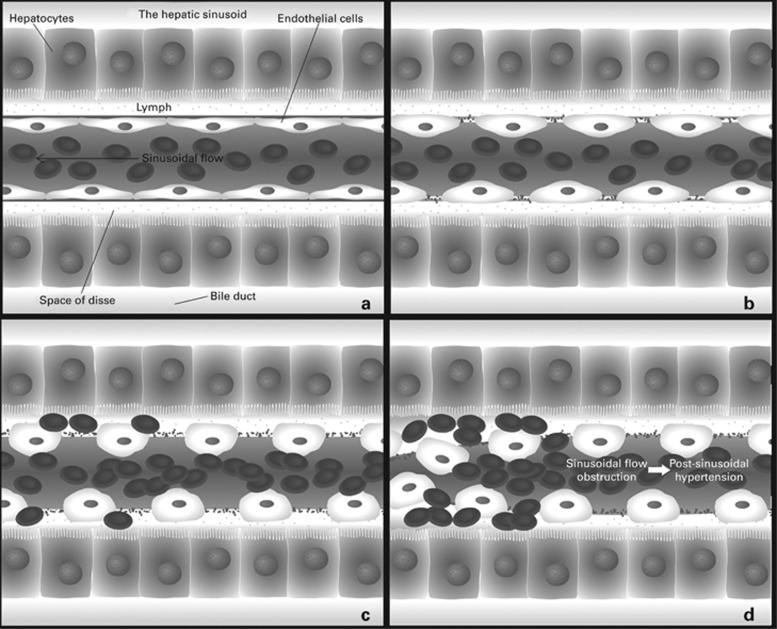
The proposed hypothesis to explain the SOS/VOD pathophysiology during HSCT is that sinusoidal endothelial cells can be activated and damaged by factors such as the chemotherapy or radiotherapy included in the conditioning regimen, cytokines produced by the injured tissues, endogenous microbial products translocated through damaged mucosal barriers,9 drugs used during the procedure (such as granulocyte colony-stimulating factors or calcineurin inhibitors)10, 11 and the complex process of engraftment. All these factors produce a physiological activation of the endothelial cells (Figure 2a); however, if they are intense and sustained, such activation can evolve to endothelial damage: sinusoidal endothelial cells round up, favoring the appearance of gaps in the sinusoidal barrier2 (Figure 2b). These changes facilitate the egress of RBCs, leucocytes and cellular debris into the space of Disse beneath the endothelial cells and dissect the endothelial lining (Figure 2c). Finally, the sloughed sinusoidal lining embolizes downstream and obstructs sinusoidal flow (Figure 2d). In these early stages, histological examinations show thickening of the subintimal zone, which leads to the narrowing of the venular lumen and an increased resistance to blood flow.12 This contributes to the post-sinusoidal portal hypertension, worsening liver dysfunction and ascites seen in the disease, eventually resulting in multi-organ failure (characterized by pulmonary and renal dysfunction, as well as encephalopathy) and death.5
Figure 2.
Sinusoidal obstruction syndrome pathogenesis. (a) Normal hepatic sinusoid; (b) sinusoidal endothelial cells damaged during conditioning round up favoring the appearance of gaps in the sinusoidal barrier; (c) RBCs, leucocytes and cellular debris penetrate into the space of Disse detaching the endothelial lining; (d) the sloughed sinusoidal lining cells embolize downstream and obstruct the sinusoidal flow (sinusoidal obstruction syndrome). Adapted from 'The role of the endothelium in the short-term complications of hematopoietic SCT' by E Carreras and M Diaz-Ricart.2
Endothelial activation after HSCT conditioning, particularly in the allogeneic setting, is associated with a prothrombotic state, demonstrated by an increase of von Willebrand factor expression and platelet adhesion.13 Furthermore, whereas pro-inflammatory and pro-apoptotic changes on epithelial cells decrease after day 14 in auto-HSCT,14 they continue to increase in the allo-HSCT setting,14 highlighting that alloreactivity could contribute to endothelial damage after conditioning.15 Vascular endothelial cells constitute a target for blood-borne executors of the immune system, antibodies and T cells, and several experimental models suggest that vascular endothelial cells are targets for alloreactive T cells in acute and chronic GVHD.16 Furthermore, the immunossuppressive therapy used after allo-HSCT has an effect on endothelial cells: CsA, compared with tacrolimus and sirolimus, has been shown to increase adhesion molecules in vitro, which could contribute to SOS/VOD.17 Overall, these observations are in accordance with the increased incidence of SOS/VOD after allo-HSCT compared with auto-HSCT.5
Finally, although the first morphological change in SOS/VOD occurs in the sinusoidal endothelial cells, hepatocyte dysfunction also contributes directly to SOS/VOD pathophysiology. Hepatocytes, through the glutathione enzymatic system, have an important role in the elimination of several drugs, such as CY.18, 19 Previous liver disease, BU, TBI, all impair this system, leading to the accumulation of CY metabolites, which will injure sinusoidal endothelial cells but also hepatocytes.18
Risk factors
Known SOS/VOD risk factors are listed Table 1. Some are directly transplant-related, such as the choice of the stem cell source, of the conditioning regimen and of the GVHD prophylaxis. As expected, because alloreactivity contributes to endothelium damage and SOS/VOD pathophysiology, the risk of SOS/VOD is higher where alloreactivity is higher: after allo-HSCT compared with auto-HSCT,5, 6 with unrelated donors and HLA-mismatched donors, and in non-T-cell depleted allo-HSCT.20, 21 The conditioning regimen intensity and drugs used also influence SOS/VOD risk: the risk is higher after conventional MAC compared with reduced intensity conditioning.5, 22, 23 The use of high dose (⩾12 Gray) or unfractionated TBI increases the risk of SOS/VOD.6 Similarly, BU, particularly in combination with CY is associated with an increased risk of SOS/VOD.6 No significant difference of SOS/VOD is reported when CY is associated with either TBI or oral BU.24 The risk of SOS/VOD is higher in patients receiving a second allo-HSCT. Regarding immunosuppressive therapy, its more controversial data suggest that sirolimus is associated with SOS/VOD after MAC TBI-based allo-SCT when used in combination with MTX.25 In contrast, preclinical data highlight the detrimental role of CsA, compared with sirolimus on endothelial cells.17 Overall, the immunosuppressive therapy effect exerted on epithelial cells probably depends on treatment association and of the conditioning regimen used.
Table 1. Traditional risk factors for SOS/VOD.
| Risk factors |
|---|
| Transplant-related |
| Allo-HSCT>auto-HSCT |
| Unrelated donor |
| HLA-mismatched donor |
| Myeloablative conditioning regimen |
| BU-based conditioning regimen |
| TBI-based conditioning regimen |
| Non-T-cell-depleted graft |
| Second HSCT |
| Patient- and disease-related |
| Older>younger (in adult patients) |
| Female receiving norethisterone |
| Karnofsky score below 90% |
| Gene polymorphism (GSTM1, GSMTT1, heparanase) |
| Advanced disease (beyond second CR or relapse) |
| Metabolic syndrome |
| Deficit of AT III, t-PA and resistance to activated protein C |
| Thalassemia |
| Hepatic related risk factors |
| Transaminase>2.5 ULN |
| Serum bilirubin>1.5 ULN |
| Cirrhosis |
| Hepatic fibrosis |
| Active viral hepatitis |
| Hepatic irradiation |
| Previous use of gemtuzumab ozogamicin |
| Use of hepatotoxic drugs |
| Iron overload |
| Pediatric specific risk factors |
| Hemophagocytic lymphohistiocytosis, adrenoleucodystrophy, osteopetrosis |
| High-dose auto-HSCT in neuroblastoma |
| Young age (under 1–2 years of age) |
| Low weight |
| Juvenile myelo-monocytic chronic leukemia |
Abbreviations: AT III=antithrombin III; HSCT=hematopoietic SCT; SOS/VOD=sinusoidal obstruction syndrome or veno-occlusive disease; t-PA=tissue plasminogen activator; ULN=upper limit of normal.
Some of the SOS/VOD risk factors are directly linked to patients' and disease characteristics. Older age, impaired Karnofsky status (<90) and advanced disease (beyond second CR or relapse/refractory disease) have been reported as SOS/VOD risk factors.6 An increased risk of SOS/VOD in women has also been reported; however, that was probably related to the use of progestin therapy to prevent gynecological bleeding, as the incidence of SOS/VOD was higher in women who received norethisterone as compared with those who did not.26 The development of reduced intensity conditioning allowed us to perform allo-HSCT in patients with co-morbidities who would otherwise be ineligible for this procedure, but this led to an increase in the number of patients presenting risk factors, such as metabolic syndrome and, particularly, obesity. Genetic polymorphism (GSTM1 and GSMTT1,27 heparanase in children28), deficit in antithrombine III29 or tissue plasminogen activator30 and resistance to the activated C protein29 are associated with increased risk of SOS/VOD. In the pediatric setting, higher incidence of SOS/VOD is seen in the primary hemophagocytic lymphohistiocytosis, adrenoleucodystrophy osteopetrosis or thalassemia major, auto-HSCT in patients with neuroblastoma, younger age (under 1–2 years of age) and low weight.31, 32, 33 Importantly, outside the transplant setting, SOS/VOD is observed in patients treated with actinomycin D and also infants in particular when treated with high-dose chemotherapy regimens.
Previous hepatic disease is one of the main risk factors of SOS/VOD. Thus, liver function abnormalities, such as serum transaminase>2.5 upper limit of normal6, 34 or serum bilirubin>1.5 upper limit of normal, active hepatic diseases such as cirrhosis, hepatic fibrosis or active viral hepatitis are SOS/VOD risk factors. However, hepatic dysfunction may be totally asymptomatic and result from previous hepatotoxic treatment including gemtuzumab ozogamicin35 and abdominal irradiation,6, 34 or from concomitant hepatotoxic drugs such as azole. Finally, iron overload has also been identified as an SOS/VOD risk factor.
Diagnosis
Given the high mortality rate (>80%) associated with severe SOS/VOD syndrome,5 a daily and strict monitoring to detect early symptoms and signs of SOS/VOD should be performed from the start of conditioning and at least up to day 14 after HSCT.8 Special attention should be paid to patients presenting one of the risk factors mentioned above. Patients must be monitored daily for weight gain, fluid retention, overt edema and ascites, hepatomegaly and jaundice.8 Nurses are indispensable in this daily monitoring, not just to weigh patients daily combined with a meticulous fluid balance, but also to monitor fluid intake and output as well as being alert to more unspecific symptoms such as abdominal discomfort and pain. For successful prevention, identification, diagnosis and treatment of SOS/VOD team work is necessary and nurses should receive specific education on SOS/VOD to understand the importance of their role. Although jaundice is usually present in adults,3 it can be absent in SOS/VOD developing late after HSCT, and is often absent in children.36 Other findings have been associated with SOS/VOD, such as symptoms related to fluid retention (pleural effusion, pulmonary infiltrate, hypoxia). New onset of transfusion-refractory thrombocytopenia with rapid consumption of transfused platelets not explained by concomitant conditions like sepsis early during HSCT can be the earliest sign of SOS/VOD reflecting the endothelial nature of the pathophysiology of SOS/VOD.8 The presence of renal and/or pulmonary dysfunction (or, less frequently, central nervous system involvement with encephalopathy) defines a multi-organ failure and severe SOS/VOD.37
To facilitate the diagnosis of SOS/VOD, two different sets of clinical criteria have been described: the revised Seattle3, 34 and the Baltimore criteria.4 These are based on the presence of clinical findings (jaundice, weight gain, hepatomegaly and ascites) not attributable to any other possible cause, in the first 3 weeks after HSCT. However, neither set of criteria considers the cases of late SOS/VOD appearing after day +21 and up to day +40 to +50. Furthermore, the use of these criteria for SOS/VOD diagnosis may be an issue when only edema and weight gain are present. Bearman et al.38 developed a model to predict the risk of developing severe SOS/VOD, based on serum bilirubin and percentage weight gain at different time points subsequent to HSCT, up to day +16. Although interesting, this model is limited to MAC (TBI CY, BU CY or CY, BCNU, VP-16), and therefore its utility is limited.
New onset of ultrasound-confirmed ascites and/or hepatomegaly and attenuated or reversed hepatic venous flow by ultrasound are more specific criteria, whereas gall bladder wall thickening despite being non-specific may be helpful for SOS/VOD diagnosis.39 Indeed, most accurate methods to confirm the diagnosis of SOS/VOD (measurement of the hepatic venous gradient pressure through the jugular vein, liver biopsy) are invasive and difficult to perform in routine practice.40
Treatment
Preventive therapy
Adoption of preventive measures that could reduce SOS/VOD incidence and/or severity is indispensable, especially because we do not have therapeutic measures with 100% efficacy in this life-threatening disease. Preventive measures combine two approaches: reversal of SOS/VOD risk factors (Table 1) and pharmacological prevention.
Most patient- and hepatic-related risk factors are impossible to reverse, and patients with such risks should be included in prophylactic programs. In those with a reversible condition (acute hepatitis, active disease), delay of the HSCT until its resolution should be discussed according to the disease status. Effort must be made to avoid any hepatotoxic concomitant drug,8 even if it is most of the case impossible. There is often no alternative to the use of gemtuzumab ozogamicin, however, splitting dose (3 mg/m2) probably allows a decrease of the SOS/VOD risk.
Transplant-related risk factors are easier to modify.8 The use of reduced intensity conditioning allo-HSCT has decreased the incidence of SOS/VOD and should be considered in elderly patients and in adult patients heavily pre-treated or with co-morbidities. It is also possible to reduce the toxicity of MAC, combining i.v. BU and fludarabine, instead of the classical oral BU and CY.23, 41, 42 Oral BU may be replaced by the equally effective i.v. BU, which has a predictable pharmacokinetic profile, is easy to monitor and reduces the incidence of SOS/VOD.19 In children, in infants, in particular, the use of BU serum level measurements can be helpful to reduce the prominent interpatient variability. Similarly, based on the pathophysiology of SOS/VOD, a change in the order of the drugs (CY/BU instead of BU/CY) may decrease the risk of SOS/VOD.43 For a TBI-based MAC regimen, hyperfractionated TBI is strongly recommended. Efforts should also be made to reduce the risk of allo-reactivity; donors with the maximum degree of compatibility or the use of T-cell-depleted grafts are recommended.20, 21
The third approach is to employ pharmacological measures to prevent SOS/VOD. The use of heparin is still very controversial. A meta-analysis evaluated patients who received either unfractionated heparin or low-molecular-weight heparin for SOS/VOD prevention.44 Twelve studies (2782 patients) were eligible. Overall, meta-analysis is negative: anticoagulation prophylaxis was associated with a non-significant decrease in the risk of SOS/VOD (pooled relative risk, 0.90; 95% confidence interval, 0.62–1.29). However, among the three randomized trials analyzed in the meta-analysis, two (one with unfractionated heparin45 and one with low-molecular-weight heparin46) showed a beneficial effect of heparin, and results of the third randomized study47 may have been affected by the delayed introduction of anticoagulation on the day of marrow infusion rather than at conditioning. Although bleeding was reported as an adverse event in 7 of the 12 studies under the meta-analysis, in none of them was it found to be more frequent in the anticoagulant group compared with the control group.44 Large randomized control studies are indispensable to properly evaluate heparin use and to enable definitive recommendation for its continuation or abandonment for SOS/VOD prevention. At present, heparin remains used for SOS/VOD prevention in some EBMT centers.
Data on the usefulness of ursodeoxycholic acid for SOS/VOD prevention are non-conclusive: some randomized trials suggest that it decreases the incidence of SOS,48, 49, 50 whereas others fail to demonstrate an advantage.51, 52 However, patients receiving this prophylaxis have less liver toxicity, less acute GVHD and better survival, strongly suggesting the beneficial effect of ursodeoxycholic acid.52 Furthermore, it has been shown that ursodeoxycholic acid use is associated with a decrease of non-relapse mortality.53 Finally, a prospective phase III study recently showed a reduced incidence of SOS/VOD in pediatric HSCT patients who received prophylaxis defibrotide (DF).
Curative therapy
The first step in the treatment of SOS/VOD is symptomatic.8 Given SOS/VOD is a life-threatening disease, therapy must be started as soon as possible. Fluid and sodium balance and careful use of diuretics (spironolactone or furosemide), should be introduced at the first suspicion, when SOS/VOD is still only probable.8 Several symptomatic measures can be used to reduce the discomfort produced by massive ascites or pleural effusions, starting with oxygen therapy.8 In particular, in infants, when massive ascites is threatening respiration via pulmonary displacement, early peritoneocentesis can be extremely helpful to avoid complications associated with assisted ventilation. When fluid accumulation and renal failure cannot be controlled, hemodialysis/hemofiltration is required.8 Severe SOS/VOD treatment requires transfer into an intensive care unit. A transjugular intrahepatic portosystemic shunt should be discussed for patients with less advanced SOS/VOD54 and hepatic transplantation in most severe diseases.55 Besides these symptomatic measures for SOS/VOD, the only proven curative treatment so far is DF.
Focus on DF
Treatment of SOS/VOD with DF
DF is a polydisperse oligonucleotide with local antithrombotic, anti-ischemic and anti-inflammatory activity,56 which has protective effects on the small vessel endothelium. Although its precise mechanism of action in SOS/VOD remains under investigation, it seemingly involves two distinct elements: the protection of endothelial cells and restoration of the thrombotic-fibrinolytic balance.
Several studies evaluating DF in SOS/VOD over the last 15 years are summarized in Table 2. In a prospective randomized multicenter dose finding phase II trial,57 adult and pediatric patients with severe SOS/VOD after HSCT were randomized to receive either a lower-dose (25 mg/kg per day, n=75) or a higher-dose (40 mg/kg per day, n=74) of DF. There were no significant differences between the two arms regarding CR rate (49 vs 43% P=0.613) and day +100 OS (44 vs 39% P=0.619). DF was generally well tolerated, but a trend towards more toxicity was seen with the 40-mg/kg per day dose, particularly among the pediatric patients. The lower dose of DF (25 mg/kg per day) was therefore used in a phase III trial for the treatment of adult and pediatric patients with severe SOS/VOD.58 Given the life-threatening nature of SOS/VOD, a trial randomizing patients to placebo or supportive care was rejected; therefore, in this phase III trial, patients receiving DF (n=102) were compared with historical controls (n=32). CR rate and day +100 OS were significantly improved in the DF arm (24% and 38%, respectively) compared with the historical control group (9% P=0.013 and 25% P=0.034, respectively). The incidence of hemorrhagic adverse events was found to be similar between patients treated with DF vs historical control (65 vs 69%).57 Additional data were obtained via a treatment investigational new drug protocol that included 425 patients with SOS/VOD after HSCT: 284 with severe and 141 with non-severe SOS/VOD.59 In the former group, the CR rate was 47% and the day +100 OS was 48%. In patients with non-severe SOS/VOD, these figures were 47% and 69%, respectively. All HSCT children (⩽16 years) had higher CR rates as compared with adults (41 vs 27% P=0.0038) and better survival (60 vs 49% P=0.0203). The overall toxicity of DF was reported to be manageable: 22% of patients experienced at least one adverse event, which primarily consisted of hemorrhage (17%) and hypotension (4%). These studies led to the approval in 2014 of DF for treatment of severe SOS/VOD after HSCT in European countries by the European Medicines Agency (EMA).
Table 2. Main studies on defibrotide in SOS/VOD.
| Reference; Phase; Number of patients | Condition | Design | Key points | Others results |
|---|---|---|---|---|
| Richardson et al.67 Retrospective CUP N=19 | Adult and pediatric Severe SOS/VOD post HSCT | Compassionate use; DF: 5–60 mg/kg per day (intra-pt dose escalation, until response/toxicity) | CR: 42% Minimal toxicity at doses tested | Day +100 survival: 32% |
| Richardson et al.68 Phase I/II N=88 | Adult and pediatric Severe SOS/VOD post HSCT | Emergency use; DF: 5–60 mg/kg per day (intra-pt dose escalation, until response/toxicity) | CR: 36% Active dose range 25–40 mg/kg per day | Day +100 survival: 35% No serious AEs attributed to DF |
| Richardson et al.57 Phase II N=149 | Adult and pediatric Severe SOS/VOD post HSCT | Randomized, dose-finding; Arm A: DF 25 mg/kg per day Arm B: DF 40 mg/kg per day For 14 days or more. | Day +100 CR: 46% Effective dose 25 mg/kg per day | Day +100 survival: 42% Overall SAE incidence: 8% (greater at 40 vs 25 mg/kg per day) |
| Richardson et al.58 Phase III N=102 | Adult and pediatric Severe SOS/VOD post HSCT | Non-randomized, comparison with historical control; DF: 6.25 mg/kg i.v. q6h (25 mg/kg per day) for 21 days or more. | Day +100 CR DF 24% HC 9% (P=0.0131) | Day +100 mortality: DF 62% HC 75% (P=0.0341) Hemorrhagic AEs: DF 65% HC 69% |
| Richardson et al.59 Prospective T-IND N=470 | Adult and pediatric SOS/VOD non-HSCT (N=45) SOS/VOD post HSCT (N=141) Severe SOS/VOD post HSCT (N=284) | Investigational new drug protocol; DF: 6.25 mg/kg i.v. q6h (25 mg/kg per day) for 21 days or more. | Day +100 CR Non-HSCT 40% SOS/VOD post HSCT 47% Severe SOS/VOD post HSCT 29% | Day +100 survival: Non-HSCT 62% SOS/VOD post HSCT 69% Severe SOS/VOD post HSCT 48% Overall hemorrhagic AEs: 18% |
| Corbacioglu et al.36 Phase III N=356 | Pediatric SOS/VOD prophylaxis post HSCT | Randomized comparison; DF: 6.25 mg/kg i.v. q6h (25 mg/kg per day) from start conditioning to 30 days post HSCT (at least 14 days if discharge before). Control: cross over to the DF arm in case of SOS/VOD onset | SOS/VOD incidence: DF 12% Control 20% P=0.0488 | Day +100 SOS/VOD related mortality: DF 2%, control 6%, P=0.10 No difference in AEs and haemorrhagic AEs |
Abbreviations: AE=adverse event; CUP=compassionate use program; DF=defibrotid; HC=historical control; HSCT=hematopoietic SCT; SAE=severe adverse event; SOS/VOD=sinusoidal obstruction syndrome or veno-occlusive disease; T-IND=treatment-investigational new drug.
Prophylaxis of SOS/VOD with DF
A recent prospective phase III study evaluated DF for prophylaxis of SOS/VOD in pediatric HSCT.36 The study population consisted of 356 patients at high risk of developing SOS/VOD after a MAC prior to HSCT, with one or more risk factors for SOS/VOD. Patients were randomized to receive prophylactic DF at 25 mg/kg per day given on the first day of conditioning until day 30 post HSCT (prophylaxis arm, n=180) or not (control arm, n=176). If patients presented SOS/VOD according to the modified Seattle criteria in the control arm, a cross over allowed those patients to received DF until SOS/VOD resolution. Reduced incidence of SOS/VOD was evident in the patients receiving DF compared with the control group (12 vs 20% P=0.0488). There was no significant difference of SOS/VOD-associated mortality at day +100 after HSCT between the DF and the control group (2 vs 6% P=0.10) most probably because of the cross-over design as the trial was not powered for this outcome. However, the mortality was four times higher in patients with SOS/VOD than in those without it (25 vs 6% P<0.0001). In total, 207 serious adverse events were reported in 108 of 180 patients (60%) of the DF group against 203 in 103 of 176 patients (59%) in the control group. Hemorrhagic adverse events were reported in nine patients from the DF group and in seven from the control group. Interestingly, the day +100 cumulative incidence of acute GVHD incidence was significantly reduced in the DF group compared with the control group (47 vs 65% P=0.0046). This result was corroborated by the significantly reduced steroid use for treatment of acute GVHD in the DF group (37 vs 48% P<0.036).
No randomized prospective study evaluating SOS/VOD prevention with DF has so far been reported in adult patients. However, given the high rates of CR and OS at day +100 in patients who received DF for treatment of SOS/VOD and the reduced incidence of SOS/VOD with DF prophylaxis in the pediatric setting, SOS/VOD prevention with DF in adult patients after HSCT appears to be an attractive approach and should be evaluated in the context of a randomized prospective trial.
Controversial issues
HSCT has undergone important changes in terms of conditioning regimens, donor and stem cell sources, patient and disease characteristics and post-transplant supportive care.60 Therefore, a more accurate identification of SOS/VOD risk factors is necessary. Should we consider all second allo-HSCT as a risk factor or only myeloablative second allo-HSCT? We must acknowledge that second transplants are often performed in more heavily pretreated patients with more advanced disease, when alternative mismatched donors are more frequently used. All these parameters are in themselves SOS/VOD risk factors, highlighting the higher probability of SOS/VOD after second allo-HSCT, whatever the conditioning. Similarly, development of haploidentical allo-HSCT with post-transplant CY61 raised a new issue. Given the high degree of mismatch and the use of CY, an increased incidence of SOS/VOD was expected in this setting; yet, so far, no center reported such an increase. One explanation might be the administration of CY after allo-HSCT, far apart in time from the conditioning. Furthermore, despite the use of haploidentical donors, there is a decreased alloreactivity in these cases, thanks to the use of post-transplant CY, as highlighted by the low incidence of GVHD.61 Therefore, data are too preliminary to draw definitive conclusion to consider or not haploidentical allo-HSCT as a SOS/VOD risk factor. We must also question the role of the so-called 'sequential' transplant approach, combining both intensive chemotherapy and transplant conditioning within the same procedure.62, 63 This procedure is increasingly used in high-risk patients such as relapse/refractory AML.62, 63 So far, no data are available regarding SOS/VOD incidence after sequential transplant. However, an increased incidence of SOS/VOD is possible, given the intensity of the chemotherapy delivered and that patients receiving a sequential approach are often high-risk heavily pretreated patients.62, 63
BU is a well-established SOS/VOD risk factor. In the last decade, reduced intensity and reduced toxicity conditioning regimens have been developed with decreased doses of BU.41, 64 Furthermore, i.v. BU has largely replaced oral BU and allow dose adjustments thanks to pharmacokinetic monitoring. It raises the question as to whether BU should be always considered as such a risk factor regardless of the dose used, or whether a threshold below which BU should no longer be considered as a risk factor should be defined.
The exact role of iron overload as a SOS/VOD risk factor is also a matter of debate. Iron overload leads to hepatocyte and not to sinusoidal endothelial cell lesion, the primary event in SOS/VOD pathophysiology. Therefore, the increased incidence of SOS/VOD in patients with iron overload is probably more related to multiple transfusions and allo-immunization. Furthermore, iron overload can lead to liver fibrosis, which is a recognized risk factor for SOS/VOD. Overall, iron overload remains as a risk factor for non-relapse mortality after HSCT, and must be avoided or decreased.65 This, in turn, raises the issue of iron chelation before HSCT: as it is a long-term treatment, its potential benefit should be carefully weighed relatively to the risk of delaying the transplant. Furthermore, it is so far difficult to define a ferritin threshold below which HSCT can be safely performed, similarly threshold are expected if liver magnetic resonance imaging scan is recommended to accurately evaluate iron overload.65, 66
Future perspectives
The majority of SOS/VOD risk factors and currently used criteria for SOS/VOD diagnosis (revised Seattle criteria and Baltimore criteria)3, 4, 34 have been defined more than 20 years ago, when only MAC were used and no, truly effective, preventive or curative drug for SOS/VOD existed. Since then, allo-HSCT has undergone profound evolution with the development of alternative donors and reduced intensity/toxicity regimen,41 and a new drug, DF, has proven to be effective for the prevention and treatment of SOS/VOD.56 These advances raise several issues, which remain to be explored.
First of all, definition of new diagnostic criteria seems indispensable. Those criteria should be different for adults and pediatric patients. In addition to the day of onset after HSCT, weight gain and hyperbilirubinemia, new parameters such as thrombocytopenia with rapid platelet consumption, or ultrasound findings of flow obstruction with Doppler evaluation should be included. Overall, the main difficulty in the definition of a new classification to allow us to diagnose and treat SOS/VOD earlier is the lack of sensitivity and specificity of the current criteria. An attractive approach to circumvent this problem would be the identification of biomarkers of SOS/VOD, an area which is currently under research. However, as allo-HSCT is very heterogeneous, the hope to identify a biomarker valid in all settings appears unlikely to be successful.
A prospective randomized trial evaluating SOS/VOD prophylaxis with DF in adult patients is warranted. Such a trial should be mainly aimed at patients with a high risk of developing SOS/VOD. Given the action of DF on endothelial cells, it would be interesting to evaluate not only the onset of SOS/VOD but also of any endothelial syndromes (capillary leak syndrome, engraftment syndrome, transplant-associated microangiopathy)2 and of acute GVHD. Similarly, a comparable randomized trial in children would be valuable given that the data from the first trial were insufficient for DF approval in SOS/VOD children prophylaxis. Such studies raise also the question of DF administration in out-patients, for whom development of an oral formulation appears to be essential. The optimal dose of DF employed for prophylaxis also remains to be determined.
In summary, our future perspectives in the setting of SOS/VOD are:
More accurate identification of SOS/VOD risk factors;
Definition of new criteria for SOS/VOD diagnosis and grading;
Identification of potential biomarkers;
Prospective trials evaluating endothelial syndrome prevention with DF.
Acknowledgments
MM thanks Professor JV Melo (Adelaide, Australia) for critical reading of the manuscript. FM was supported by educational grants from the 'Association for Training, Education and Research in Hematology, Immunology and Transplantation' (ATERHIT, Nantes, France).
All authors designed the manuscript, analyzed the literature, wrote and commented on the manuscript. All authors approved submission of the manuscript for publication purposes. All authors received honoraria and/or research support from JAZZ Pharmaceuticals whose product is discussed in this manuscript. JAZZ pharmaceuticals provided an unrestricted educational grant for support for the current study, but did not participate to conduct, data/results analyses or manuscript writing.
References
- Carreras E. Veno-occlusive disease of the liver after hemopoietic cell transplantation. Eur J Haematol. 2000;64:281–291. doi: 10.1034/j.1600-0609.2000.9r200.x. [DOI] [PubMed] [Google Scholar]
- Carreras E, Diaz-Ricart M. The role of the endothelium in the short-term complications of hematopoietic SCT. Bone Marrow Transplant. 2011;46:1495–1502. doi: 10.1038/bmt.2011.65. [DOI] [PubMed] [Google Scholar]
- McDonald GB, Sharma P, Matthews DE, Shulman HM, Thomas ED. Venocclusive disease of the liver after bone marrow transplantation: diagnosis, incidence, and predisposing factors. Hepatology. 1984;4:116–122. doi: 10.1002/hep.1840040121. [DOI] [PubMed] [Google Scholar]
- Jones RJ, Lee KS, Beschorner WE, Vogel VG, Grochow LB, Braine HG, et al. Venoocclusive disease of the liver following bone marrow transplantation. Transplantation. 1987;44:778–783. doi: 10.1097/00007890-198712000-00011. [DOI] [PubMed] [Google Scholar]
- Coppell JA, Richardson PG, Soiffer R, Martin PL, Kernan NA, Chen A, et al. Hepatic veno-occlusive disease following stem cell transplantation: incidence, clinical course, and outcome. Biol Blood Marrow Transplant. 2010;16:157–168. doi: 10.1016/j.bbmt.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreras E, Bertz H, Arcese W, Vernant JP, Tomas JF, Hagglund H, et al. Incidence and outcome of hepatic veno-occlusive disease after blood or marrow transplantation: a prospective cohort study of the European Group for Blood and Marrow Transplantation. European Group for Blood and Marrow Transplantation Chronic Leukemia Working Party. Blood. 1998;92:3599–3604. [PubMed] [Google Scholar]
- Carreras E, Diaz-Beya M, Rosinol L, Martinez C, Fernandez-Aviles F, Rovira M. The incidence of veno-occlusive disease following allogeneic hematopoietic stem cell transplantation has diminished and the outcome improved over the last decade. Biol Blood Marrow Transplant. 2011;17:1713–1720. doi: 10.1016/j.bbmt.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Carreras E. How I manage sinusoidal obstruction syndrome after haematopoietic cell transplantation. Br J Haematol. 2014;168:481–491. doi: 10.1111/bjh.13215. [DOI] [PubMed] [Google Scholar]
- Eissner G, Multhoff G, Holler E. Influence of bacterial endotoxin on the allogenicity of human endothelial cells. Bone Marrow Transplant. 1998;21:1286–1288. doi: 10.1038/sj.bmt.1701264. [DOI] [PubMed] [Google Scholar]
- Fuste B, Mazzara R, Escolar G, Merino A, Ordinas A, Diaz-Ricart M. Granulocyte colony-stimulating factor increases expression of adhesion receptors on endothelial cells through activation of p38 MAPK. Haematologica. 2004;89:578–585. [PubMed] [Google Scholar]
- Mercanoglu F, Turkmen A, Kocaman O, Pinarbasi B, Dursun M, Selcukbiricik F, et al. Endothelial dysfunction in renal transplant patients is closely related to serum cyclosporine levels. Transplant Proc. 2004;36:1357–1360. doi: 10.1016/j.transproceed.2004.05.073. [DOI] [PubMed] [Google Scholar]
- DeLeve LD, Shulman HM, McDonald GB. Toxic injury to hepatic sinusoids: sinusoidal obstruction syndrome (veno-occlusive disease) Semin Liver Dis. 2002;22:27–42. doi: 10.1055/s-2002-23204. [DOI] [PubMed] [Google Scholar]
- Palomo M, Diaz-Ricart M, Carbo C, Rovira M, Fernandez-Aviles F, Martine C, et al. Endothelial dysfunction after hematopoietic stem cell transplantation: role of the conditioning regimen and the type of transplantation. Biol Blood Marrow Transplant. 2010;16:985–993. doi: 10.1016/j.bbmt.2010.02.008. [DOI] [PubMed] [Google Scholar]
- Palomo M, Diaz-Ricart M, Carbo C, Rovira M, Fernandez-Aviles F, Escolar G, et al. The release of soluble factors contributing to endothelial activation and damage after hematopoietic stem cell transplantation is not limited to the allogeneic setting and involves several pathogenic mechanisms. Biol Blood Marrow Transplant. 2009;15:537–546. doi: 10.1016/j.bbmt.2009.01.013. [DOI] [PubMed] [Google Scholar]
- Cooke KR, Jannin A, Ho V. The contribution of endothelial activation and injury to end-organ toxicity following allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2008;14:23–32. doi: 10.1016/j.bbmt.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Biedermann BC. Vascular endothelium and graft-versus-host disease. Best Pract Res Clin Haematol. 2008;21:129–138. doi: 10.1016/j.beha.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Carmona A, Diaz-Ricart M, Palomo M, Molina P, Pino M, Rovira M, et al. Distinct deleterious effects of cyclosporine and tacrolimus and combined tacrolimus-sirolimus on endothelial cells: protective effect of defibrotide. Biol Blood Marrow Transplant. 2013;19:1439–1445. doi: 10.1016/j.bbmt.2013.07.001. [DOI] [PubMed] [Google Scholar]
- DeLeve LD. Cellular target of cyclophosphamide toxicity in the murine liver: role of glutathione and site of metabolic activation. Hepatology. 1996;24:830–837. doi: 10.1002/hep.510240414. [DOI] [PubMed] [Google Scholar]
- Almog S, Kurnik D, Shimoni A, Loebstein R, Hassoun E, Gopher A, et al. Linearity and stability of intravenous busulfan pharmacokinetics and the role of glutathione in busulfan elimination. Biol Blood Marrow Transplant. 2011;17:117–123. doi: 10.1016/j.bbmt.2010.06.017. [DOI] [PubMed] [Google Scholar]
- Soiffer RJ, Dear K, Rabinowe SN, Anderson KC, Freedman AS, Murray C, et al. Hepatic dysfunction following T-cell-depleted allogeneic bone marrow transplantation. Transplantation. 1991;52:1014–1019. doi: 10.1097/00007890-199112000-00015. [DOI] [PubMed] [Google Scholar]
- Moscardo F, Urbano-Ispizua A, Sanz GF, Brunet S, Caballero D, Vallejo C, et al. Positive selection for CD34+ reduces the incidence and severity of veno-occlusive disease of the liver after HLA-identical sibling allogeneic peripheral blood stem cell transplantation. Exp Hematol. 2003;31:545–550. doi: 10.1016/s0301-472x(03)00070-5. [DOI] [PubMed] [Google Scholar]
- Hogan WJ, Maris M, Storer B, Sandmaier BM, Maloney DG, Schoch HG, et al. Hepatic injury after nonmyeloablative conditioning followed by allogeneic hematopoietic cell transplantation: a study of 193 patients. Blood. 2004;103:78–84. doi: 10.1182/blood-2003-04-1311. [DOI] [PubMed] [Google Scholar]
- Nagler A, Labopin M, Berger R, Bunjes D, Campos A, Socie G, et al. Allogeneic hematopoietic SCT for adults AML using i.v. BU in the conditioning regimen: outcomes and risk factors for the occurrence of hepatic sinusoidal obstructive syndrome. Bone Marrow Transplant. 2014;49:628–633. doi: 10.1038/bmt.2014.7. [DOI] [PubMed] [Google Scholar]
- Nagler A, Rocha V, Labopin M, Unal A, Ben Othman T, Campos A, et al. Allogeneic hematopoietic stem-cell transplantation for acute myeloid leukemia in remission: comparison of intravenous busulfan plus cyclophosphamide (Cy) versus total-body irradiation plus Cy as conditioning regimen—a report from the acute leukemia working party of the European group for blood and marrow transplantation. J Clin Oncol. 2013;31:3549–3556. doi: 10.1200/JCO.2013.48.8114. [DOI] [PubMed] [Google Scholar]
- Cutler C, Stevenson K, Kim HT, Richardson P, Ho VT, Linden E, et al. Sirolimus is associated with veno-occlusive disease of the liver after myeloablative allogeneic stem cell transplantation. Blood. 2008;112:4425–4431. doi: 10.1182/blood-2008-07-169342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagglund H, Remberger M, Klaesson S, Lonnqvist B, Ljungman P, Ringden O. Norethisterone treatment, a major risk-factor for veno-occlusive disease in the liver after allogeneic bone marrow transplantation. Blood. 1998;92:4568–4572. [PubMed] [Google Scholar]
- Srivastava A, Poonkuzhali B, Shaji RV, George B, Mathews V, Chandy M, et al. Glutathione S-transferase M1 polymorphism: a risk factor for hepatic venoocclusive disease in bone marrow transplantation. Blood. 2004;104:1574–1577. doi: 10.1182/blood-2003-11-3778. [DOI] [PubMed] [Google Scholar]
- Seifert C, Wittig S, Arndt C, Gruhn B. Heparanase polymorphisms: influence on incidence of hepatic sinusoidal obstruction syndrome in children undergoing allogeneic hematopoietic stem cell transplantation. J Cancer Res Clin Oncol. e-pub ahead of print 22 October 2014. [DOI] [PMC free article] [PubMed]
- Lee JH, Lee KH, Kim S, Lee JS, Kim WK, Park CJ, et al. Relevance of proteins C and S, antithrombin III, von Willebrand factor, and factor VIII for the development of hepatic veno-occlusive disease in patients undergoing allogeneic bone marrow transplantation: a prospective study. Bone Marrow Transplant. 1998;22:883–888. doi: 10.1038/sj.bmt.1701445. [DOI] [PubMed] [Google Scholar]
- Bearman SI, Shuhart MC, Hinds MS, McDonald GB. Recombinant human tissue plasminogen activator for the treatment of established severe venocclusive disease of the liver after bone marrow transplantation. Blood. 1992;80:2458–2462. [PubMed] [Google Scholar]
- Corbacioglu S, Honig M, Lahr G, Stohr S, Berry G, Friedrich W, et al. Stem cell transplantation in children with infantile osteopetrosis is associated with a high incidence of VOD, which could be prevented with defibrotide. Bone Marrow Transplant. 2006;38:547–553. doi: 10.1038/sj.bmt.1705485. [DOI] [PubMed] [Google Scholar]
- Cesaro S, Pillon M, Talenti E, Toffolutti T, Calore E, Tridello G, et al. A prospective survey on incidence, risk factors and therapy of hepatic veno-occlusive disease in children after hematopoietic stem cell transplantation. Haematologica. 2005;90:1396–1404. [PubMed] [Google Scholar]
- Cheuk DK, Wang P, Lee TL, Chiang AK, Ha SY, Lau YL, et al. Risk factors and mortality predictors of hepatic veno-occlusive disease after pediatric hematopoietic stem cell transplantation. Bone Marrow Transplant. 2007;40:935–944. doi: 10.1038/sj.bmt.1705835. [DOI] [PubMed] [Google Scholar]
- McDonald GB, Hinds MS, Fisher LD, Schoch HG, Wolford JL, Banaji M, et al. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Ann Intern Med. 1993;118:255–267. doi: 10.7326/0003-4819-118-4-199302150-00003. [DOI] [PubMed] [Google Scholar]
- Wadleigh M, Richardson PG, Zahrieh D, Lee SJ, Cutler C, Ho V, et al. Prior gemtuzumab ozogamicin exposure significantly increases the risk of veno-occlusive disease in patients who undergo myeloablative allogeneic stem cell transplantation. Blood. 2003;102:1578–1582. doi: 10.1182/blood-2003-01-0255. [DOI] [PubMed] [Google Scholar]
- Corbacioglu S, Cesaro S, Faraci M, Valteau-Couanet D, Gruhn B, Rovelli A, et al. Defibrotide for prophylaxis of hepatic veno-occlusive disease in paediatric haemopoietic stem-cell transplantation: an open-label, phase 3, randomised controlled trial. Lancet. 2012;379:1301–1309. doi: 10.1016/S0140-6736(11)61938-7. [DOI] [PubMed] [Google Scholar]
- Richardson PG, Ho VT, Cutler C, Glotzbecker B, Antin JH, Soiffer R. Hepatic veno-occlusive disease after hematopoietic stem cell transplantation: novel insights to pathogenesis, current status of treatment, and future directions. Biol Blood Marrow Transplant. 2013;19:S88–S90. doi: 10.1016/j.bbmt.2012.10.023. [DOI] [PubMed] [Google Scholar]
- Bearman SI, Anderson GL, Mori M, Hinds MS, Shulman HM, McDonald GB. Venoocclusive disease of the liver: development of a model for predicting fatal outcome after marrow transplantation. J Clin Oncol. 1993;11:1729–1736. doi: 10.1200/JCO.1993.11.9.1729. [DOI] [PubMed] [Google Scholar]
- Dignan FL, Wynn RF, Hadzic N, Karani J, Quaglia A, Pagliuca A, et al. BCSH/BSBMT guideline: diagnosis and management of veno-occlusive disease (sinusoidal obstruction syndrome) following haematopoietic stem cell transplantation. Br J Haematol. 2013;163:444–457. doi: 10.1111/bjh.12558. [DOI] [PubMed] [Google Scholar]
- Carreras E, Granena A, Navasa M, Bruguera M, Marco V, Sierra J, et al. On the reliability of clinical criteria for the diagnosis of hepatic veno-occlusive disease. Ann Hematol. 1993;66:77–80. doi: 10.1007/BF01695888. [DOI] [PubMed] [Google Scholar]
- Mohty M, Malard F, Blaise D, Milpied N, Furst S, Tabrizi R, et al. Reduced-toxicity conditioning with fludarabine, once-daily intravenous busulfan, and antithymocyte globulins prior to allogeneic stem cell transplantation: Results of a multicenter prospective phase 2 trial. Cancer. 2014;121:562–569. doi: 10.1002/cncr.29087. [DOI] [PubMed] [Google Scholar]
- de Lima M, Couriel D, Thall PF, Wang X, Madden T, Jones R, et al. Once-daily intravenous busulfan and fludarabine: clinical and pharmacokinetic results of a myeloablative, reduced-toxicity conditioning regimen for allogeneic stem cell transplantation in AML and MDS. Blood. 2004;104:857–864. doi: 10.1182/blood-2004-02-0414. [DOI] [PubMed] [Google Scholar]
- Cantoni N, Gerull S, Heim D, Halter J, Bucher C, Buser A, et al. Order of application and liver toxicity in patients given BU and CY containing conditioning regimens for allogeneic hematopoietic SCT. Bone Marrow Transplant. 2011;46:344–349. doi: 10.1038/bmt.2010.137. [DOI] [PubMed] [Google Scholar]
- Imran H, Tleyjeh IM, Zirakzadeh A, Rodriguez V, Khan SP. Use of prophylactic anticoagulation and the risk of hepatic veno-occlusive disease in patients undergoing hematopoietic stem cell transplantation: a systematic review and meta-analysis. Bone Marrow Transplant. 2006;37:677–686. doi: 10.1038/sj.bmt.1705297. [DOI] [PubMed] [Google Scholar]
- Attal M, Huguet F, Rubie H, Huynh A, Charlet JP, Payen JL, et al. Prevention of hepatic veno-occlusive disease after bone marrow transplantation by continuous infusion of low-dose heparin: a prospective, randomized trial. Blood. 1992;79:2834–2840. [PubMed] [Google Scholar]
- Or R, Nagler A, Shpilberg O, Elad S, Naparstek E, Kapelushnik J, et al. Low molecular weight heparin for the prevention of veno-occlusive disease of the liver in bone marrow transplantation patients. Transplantation. 1996;61:1067–1071. doi: 10.1097/00007890-199604150-00014. [DOI] [PubMed] [Google Scholar]
- Marsa-Vila L, Gorin NC, Laporte JP, Labopin M, Dupuy-Montbrun MC, Fouillard L, et al. Prophylactic heparin does not prevent liver veno-occlusive disease following autologous bone marrow transplantation. Eur J Haematol. 1991;47:346–354. doi: 10.1111/j.1600-0609.1991.tb01859.x. [DOI] [PubMed] [Google Scholar]
- Essell JH, Schroeder MT, Harman GS, Halvorson R, Lew V, Callander N, et al. Ursodiol prophylaxis against hepatic complications of allogeneic bone marrow transplantation. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1998;128:975–981. doi: 10.7326/0003-4819-128-12_part_1-199806150-00002. [DOI] [PubMed] [Google Scholar]
- Ohashi K, Tanabe J, Watanabe R, Tanaka T, Sakamaki H, Maruta A, et al. The Japanese multicenter open randomized trial of ursodeoxycholic acid prophylaxis for hepatic veno-occlusive disease after stem cell transplantation. Am J Hematol. 2000;64:32–38. doi: 10.1002/(sici)1096-8652(200005)64:1<32::aid-ajh6>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Tay J, Tinmouth A, Fergusson D, Huebsch L, Allan DS. Systematic review of controlled clinical trials on the use of ursodeoxycholic acid for the prevention of hepatic veno-occlusive disease in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2007;13:206–217. doi: 10.1016/j.bbmt.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Park SH, Lee MH, Lee H, Kim HS, Kim K, Kim WS, et al. A randomized trial of heparin plus ursodiol vs. heparin alone to prevent hepatic veno-occlusive disease after hematopoietic stem cell transplantation. Bone Marrow Transplant. 2002;29:137–143. doi: 10.1038/sj.bmt.1703342. [DOI] [PubMed] [Google Scholar]
- Ruutu T, Eriksson B, Remes K, Juvonen E, Volin L, Remberger M, et al. Ursodeoxycholic acid for the prevention of hepatic complications in allogeneic stem cell transplantation. Blood. 2002;100:1977–1983. doi: 10.1182/blood-2001-12-0159. [DOI] [PubMed] [Google Scholar]
- Gooley TA, Chien JW, Pergam SA, Hingorani S, Sorror ML, Boeckh M, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363:2091–2101. doi: 10.1056/NEJMoa1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azoulay D, Castaing D, Lemoine A, Hargreaves GM, Bismuth H. Transjugular intrahepatic portosystemic shunt (TIPS) for severe veno-occlusive disease of the liver following bone marrow transplantation. Bone Marrow Transplant. 2000;25:987–992. doi: 10.1038/sj.bmt.1702386. [DOI] [PubMed] [Google Scholar]
- Kim ID, Egawa H, Marui Y, Kaihara S, Haga H, Lin YW, et al. A successful liver transplantation for refractory hepatic veno-occlusive disease originating from cord blood transplantation. Am J Transplant. 2002;2:796–800. doi: 10.1034/j.1600-6143.2002.20815.x. [DOI] [PubMed] [Google Scholar]
- Richardson PG, Corbacioglu S, Ho VT, Kernan NA, Lehmann L, Maguire C, et al. Drug safety evaluation of defibrotide. Expert Opin Drug Saf. 2013;12:123–136. doi: 10.1517/14740338.2012.749855. [DOI] [PubMed] [Google Scholar]
- Richardson PG, Soiffer RJ, Antin JH, Uno H, Jin Z, Kurtzberg J, et al. Defibrotide for the treatment of severe hepatic veno-occlusive disease and multiorgan failure after stem cell transplantation: a multicenter, randomized, dose-finding trial. Biol Blood Marrow Transplant. 2010;16:1005–1017. doi: 10.1016/j.bbmt.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson P, Tomblyn M, Kernan N, Brochstein JA, Mineishi S, Termuhlen A, et al. Results of a Phase 3 study utilizing a historical control. Defibrotide (DF) in the treatment of severe hepatic veno-occlusive disease (VOD) with multi-organ failure (MOF) following stem cell transplantation (SCT) ASH Annual Meeting Abstracts. 2009;114:654. [Google Scholar]
- Richardson PG, Smith AR, Triplett BM, Kernan NA, Grupp SA, Arai S, et al. Results of the large prospective study on the use of defibrotide (DF) in the treatment of hepatic veno-occlusive disease (VOD) in hematopoietic stem cell transplant (HSCT). Early intervention improves outcome - updated results of a treatment IND (T-IND) expanded access protocol. ASH Annual Meeting Abstracts. 2013;122:700–700. [Google Scholar]
- Malard F, Chevallier P, Guillaume T, Delaunay J, Rialland F, Harousseau JL, et al. Continuous reduced nonrelapse mortality after allogeneic hematopoietic stem cell transplantation: A single-institution's three decade experience. Biol Blood Marrow Transplant. 2014;20:1217–1223. doi: 10.1016/j.bbmt.2014.04.021. [DOI] [PubMed] [Google Scholar]
- Luznik L, O'Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14:641–650. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid C, Schleuning M, Ledderose G, Tischer J, Kolb HJ. Sequential regimen of chemotherapy, reduced-intensity conditioning for allogeneic stem-cell transplantation, and prophylactic donor lymphocyte transfusion in high-risk acute myeloid leukemia and myelodysplastic syndrome. J Clin Oncol. 2005;23:5675–5687. doi: 10.1200/JCO.2005.07.061. [DOI] [PubMed] [Google Scholar]
- Mohty M, Malard F, Blaise D, Milpied N, Socie G, Huynh A, et al. Sequential regimen of clofarabine, cytarabine and reduced intensity conditioning (RIC) prior to allogeneic stem cell transplantation (allo-SCT) for acute myeloid leukemia (AML) in primary treatment failure. Blood. 2014;124:1228–1228. [Google Scholar]
- Dirou S, Malard F, Chambellan A, Chevallier P, Germaud P, Guillaume T, et al. Stable long-term pulmonary function after fludarabine, antithymocyte globulin and i.v. BU for reduced-intensity conditioning allogeneic SCT. Bone Marrow Transplant. 2014;49:622–627. doi: 10.1038/bmt.2014.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majhail NS, Lazarus HM, Burns LJ. Iron overload in hematopoietic cell transplantation. Bone Marrow Transplant. 2008;41:997–1003. doi: 10.1038/bmt.2008.99. [DOI] [PubMed] [Google Scholar]
- Trottier BJ, Burns LJ, DeFor TE, Cooley S, Majhail NS. Association of iron overload with allogeneic hematopoietic cell transplantation outcomes: a prospective cohort study using R2-MRI-measured liver iron content. Blood. 2013;122:1678–1684. doi: 10.1182/blood-2013-04-499772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson PG, Elias AD, Krishnan A, Wheeler C, Nath R, Hoppensteadt D, et al. Treatment of severe veno-occlusive disease with defibrotide: compassionate use results in response without significant toxicity in a high-risk population. Blood. 1998;92:737–744. [PubMed] [Google Scholar]
- Richardson PG, Murakami C, Jin Z, Warren D, Momtaz P, Hoppensteadt D, et al. Multi-institutional use of defibrotide in 88 patients after stem cell transplantation with severe veno-occlusive disease and multisystem organ failure: response without significant toxicity in a high-risk population and factors predictive of outcome. Blood. 2002;100:4337–4343. doi: 10.1182/blood-2002-04-1216. [DOI] [PubMed] [Google Scholar]