Abstract
Objective: To evaluate long-term efficacy and safety of bimatoprost for treatment of chemotherapy-induced eyelash hypotrichosis. Design: One-year, multicenter, double-masked, parallel-group study. Setting: Twenty-one centers in the United States and one center in the United Kingdom. Participants: This study randomized (3:1) 130 subjects to bimatoprost 0.03% or vehicle applied topically to upper eyelid margins for six months. All subjects used bimatoprost for a second six months. Measurements: Responders for the primary composite end point achieved ≥1-grade improvement in Global Eyelash Assessment score and ≥3-point improvement in Confidence, Attractiveness, and Professionalism domain score of the Eyelash Satisfaction Questionnaire at Month 4. Secondary assessments included eyelash length, thickness, and darkness, using digital image analysis. Results: The responder rate was significantly higher with bimatoprost versus vehicle at Month 4 (37.5% vs. 18.2%; p=0.041) and Month 6 (46.9% vs. 18.2%; p=0.004). Significant improvements favoring bimatoprost occurred in eyelash length (p=0.008), thickness (p<0.001), or darkness (p=0.029) at Month 4, with similar results at Month 6 (p<0.001, length; p<0.001, thickness; p=0.002, darkness). Responder rates reached 61.5 percent at Month 12 for subjects continuing bimatoprost and 67.6 percent for those switched from vehicle to bimatoprost. Conjunctival hyperemia (16.7%) and punctate keratitis (9.4%) were the most common adverse events. Conclusion: Bimatoprost provides rapid eyelash recovery, whether started shortly after chemotherapy (4 to 12 weeks) or delayed for six months, with minimal adverse events. Clinical trial registry: NCT00907426
Eyelashes are important for self-image1,2 and to protect the ocular surface from particulate matter.3,4 Hypotrichosis of the eyelashes is characterized by reduced eyelash growth.2 Although its etiology is often unknown, eyelash hypotrichosis or complete eyelash loss may develop as an adverse effect of cytotoxic chemotherapy. For many, hair loss including eyelash loss can negatively affect self-image and can lead to impairments in psychosocial functioning.5,6 Following chemotherapy completion, new hair growth is generally finer and thinner; it may take several hair cycles before hair characteristics are restored to prechemotherapy levels.7 Mascara, used to darken, thicken, and lengthen eyelashes, may not be a viable option when eyelashes are sparse or missing.8
Bimatoprost is a synthetic prostamide F2α analog approved for treatment of eyelash hypotrichosis.9 Once-daily application of bimatoprost 0.03% to the upper eyelid margins significantly increases eyelash prominence, length, thickness, and darkness compared with vehicle in subjects with idiopathic hypotrichosis.10 The 0.03% concentration is the same as that found in bimatoprost ophthalmic solution used for treating other ophthalmic conditions.11-13 Mechanistically, bimatoprost is thought to induce eyelash growth through prostamide-sensitive receptors in hair follicles, which promote transition of the follicle from the telogen (resting) to anagen (growth) phase of the hair cycle and prolong the duration spent in anagen.14 The present study was designed to evaluate the long-term safety and efficacy of bimatoprost 0.03% in subjects with chemotherapy-induced eyelash hypotrichosis.
METHODS
Study design. This one-year, multicenter, double-masked, parallel-group study enrolled subjects with chemotherapy-induced eyelash hypotrichosis at 21 centers in the United States and one center in the United Kingdom. The study protocol was approved by an institutional review board or independent ethics committee at each site, and the study was conducted in compliance with Good Clinical Practice. All subjects provided written informed consent and privacy-related documentation.
The study comprised two six-month treatment periods. At baseline, subjects were stratified by etiology of hypotrichosis (postchemotherapy or idiopathic). The postchemotherapy subgroup was randomized (3:1) using an automatic interactive voice or Web response system to bimatoprost 0.03% or vehicle during the first treatment period. All subjects allocated to vehicle were switched to bimatoprost for the second six-month period, whereas those allocated to bimatoprost continued on the same treatment. Subjects were instructed to apply one drop of study treatment to a sterile, single-use-per-eye applicator, then brush it along the upper eyelid margins once every evening. Investigators, their staff, and subjects were masked to treatment assignment during the entire one-year treatment period. There were nine study site visits (baseline and Months 1, 2, 4, 6, 7, 8, 10, and 12).
Study subjects. Adults 18 years of age or older who reported chemotherapy-induced eyelash hypotrichosis were enrolled if they had been treated for Stage I to IIIA solid tumors with curative intent or received front-line therapy for Stage I to II diffuse large-cell lymphoma or Hodgkin’s lymphoma; had resolution of all chemotherapy-related side effects except for hair loss; had completed their chemotherapy 4 to 16 weeks before study baseline; had Eastern Cooperative Oncology Group performance status of 0 or 1; and had no evidence of metastases or anticipated need for further chemotherapy during the study period.
Eligible subjects also had a Global Eyelash Assessment (GEA) score of 1 or 2, an Eyelash Satisfaction Questionnaire (ESQ) score of 1 or 2 on each of the three items in the Confidence, Attractiveness, and Professionalism domain (hereafter referred to as Domain 2), and, in each eye, a best-corrected visual acuity (BCVA) score equivalent to a Snellen acuity of 20/100 and intraocular pressure (IOP) ≤20mm Hg. Subjects with unequal GEA scores for the right and left eyelashes, uncontrolled systemic disease, or known ocular disease or abnormality were excluded.
GEA and ESQ assessment tools. The GEA is a validated 4-point scale with photonumeric guide (1=none or minimal eyelash prominence; 2=moderate eyelash prominence; 3=marked eyelash prominence; 4=very marked eyelash prominence). The ESQ, a validated patient-reported outcome measure, comprises 23 questions, all measured on a 5-point Likert-type scale (1=very much disagree, 2=disagree, 3=neutral, 4=agree, 5=very much agree). Of the 23 questions, nine are distributed across three domains (three questions each). The Length, Fullness, and Overall Satisfaction Domain (Domain 1) assesses satisfaction with physical attributes of the eyelashes, Domain 2 assesses satisfaction with subjective attributes of eyelashes, and the Daily Routine Domain (Domain 3) assesses satisfaction with daily routines in making eyelashes presentable. Each domain is scored separately, with domain scores ranging from 3 to 15; higher scores indicate greater satisfaction.
The ESQ Domain 2 has been shown to be an appropriate measure of psychological impact. A 3-point change in ESQ Domain 2 is the minimal important difference that reflects a clinically meaningful and relevant benefit to subjects. Including both GEA and ESQ in a composite measure provides a more robust efficacy assessment than either measure alone, requiring enhanced eyelash prominence combined with increased subject satisfaction.
Efficacy analyses. At each visit, investigators assessed eyelash prominence using the GEA scale, and subjects completed the ESQ. The primary composite analysis was the proportion of responders who achieved both a ≥1-grade improvement from baseline in GEA score and a ≥3-point improvement in total score on ESQ Domain 2 at Month 4. Additional analyses included each component of the primary composite (i.e., proportion of subjects with ≥1-grade improvement in GEA score and proportion of subjects with ≥3-point improvement on ESQ Domain 2).
Key secondary analyses included changes from baseline in digital image analysis (DIA) measures of upper eyelash length (in mm), thickness (in mm2), and darkness (in intensity units [IU]). Increased length and thickness were shown by positive changes from baseline, whereas increased darkness was shown by a negative change in IU from baseline (i.e., larger negative numbers indicate darker lashes). Digital eyelash photographs using standard photographic equipment were taken at each visit.
Safety assessments. Ophthalmic examinations conducted throughout the study included BCVA, IOP, iris color, and biomicroscopy, assessed at baseline and at Months 1, 2, 4, 6, 8, and 12, and dilated ophthalmoscopy, performed at baseline and at Months 6 and 12. Iris color was assessed by an ophthalmologist or trained designee using 10 color categories, including “other.” Broader categories of “light” and “dark” iris colors considered the categories brown and dark brown as “dark” and all other categories as “light,” unless the “other” category contained “black” in the description, in which case it was considered “dark.” Additionally, adverse events (AEs) were recorded at each visit and physical examinations with vital signs were conducted at baseline and at Months 6 and 12.
Data analysis and statistics. Sample size calculations were made for the overall population, but not specifically for the subgroup with postchemotherapy hypotrich-osis reported herein. Efficacy variables were evaluated in the intent-to-treat population, which comprised all randomized subjects; safety parameters were evaluated in all subjects who received one or more doses of study treatment. Missing efficacy data were imputed using a last-observation-carried-forward approach. For
RESULTS
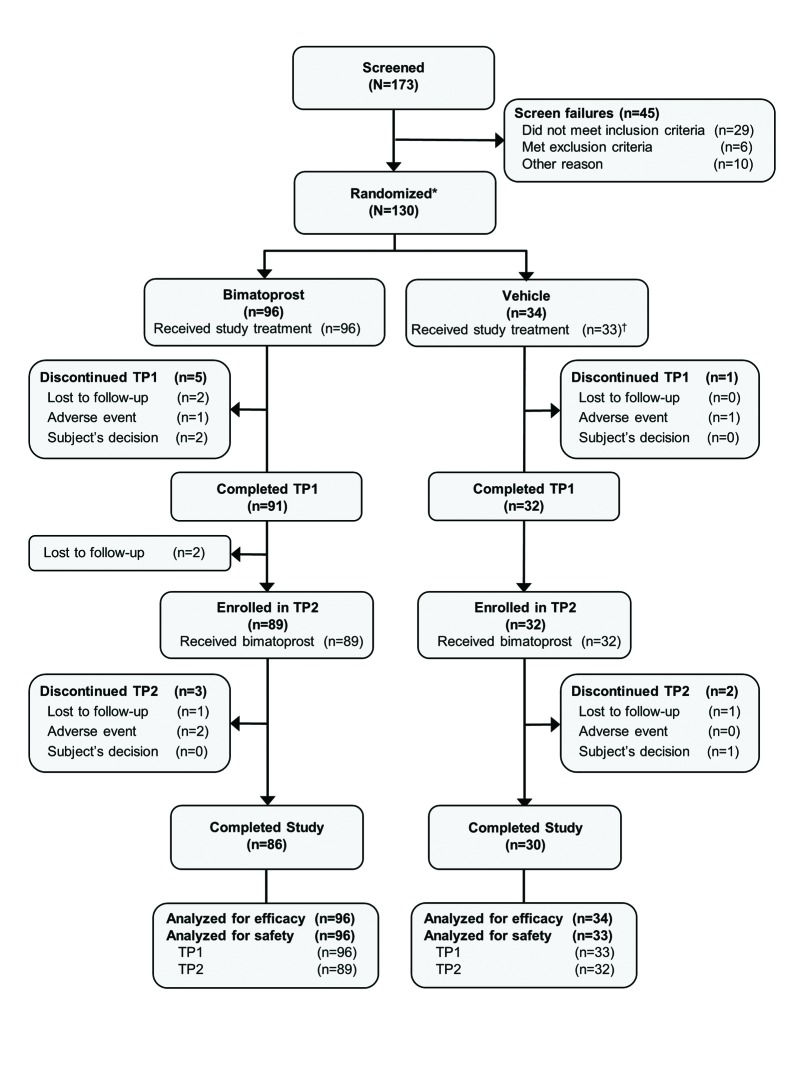
Subject demographics and disposition. Of the 130 subjects with chemotherapy-induced hypotrichosis, 123 (94.6%) completed the first six-month treatment period and 116 (89.2%) completed the entire 12-month study (Figure 1). The most common reasons for early discontinuation during the entire 12-month period were lost to follow-up (3.1%), AEs (3.1%), and personal reasons (2.3%).
Figure 1.
Patient disposition. TP=treatment period; TP1=Months 1 to 6; TP2=months 7 to 12. *Includes two subjects randomized in error. †One subject randomized in error did not receive treatment.
The groups initially allocated to bimatoprost and vehicle were well matched with respect to baseline characteristics (Table 1). At baseline, most (71.5%) had a GEA score of 1, indicative of no or minimal eyelash prominence; the mean baseline ESQ Domain 2 score was 3.9, indicative of the subject’s dissatisfaction with the subjective attributes of their eyelashes (i.e., subjects disagreed that their eyelashes made them feel confident, attractive, or professional).
TABLE 1.
Baseline characteristics
| TREATMENT IN TP1/TP2 | |||
|---|---|---|---|
| CHARACTERISTIC | BIMATOPROST/BIMATOPROST (n=96) | VEHICLE/BIMATOPROST (n=34) | TOTAL (n=130) |
| Age, mean (range), y | 50.3 (28–76) | 51.6 (26–71) | 50.7 (26–76) |
| Female, n (%) | 95 (99.0) | 34 (100) | 129 (99.2) |
| Race, n (%) | |||
| Caucasian | 78 (81.3) | 25 (73.5) | 103 (79.2) |
| African American | 10 (10.4) | 5 (14.7) | 15 (11.5) |
| Asian | 2 (2.1) | 2 (5.9) | 4 (3.1) |
| Hispanic | 6 (6.3) | 2 (5.9) | 8 (6.2) |
| Iris color*, n (%) | |||
| Dark | 42 (43.8) | 17 (50.0) | 59 (45.4) |
| Light | 54 (56.3) | 17 (50.0) | 71 (54.6) |
| GEA score | |||
| 1 (none/minimal) | 69 (71.9) | 24 (70.6) | 93 (71.5) |
| 2 (moderate) | 27 (28.1) | 10 (29.4) | 37 (28.5) |
| ESQ Domain 2 total score, mean (range) | 3.9 (3–6) | 3.9 (3–6) | 3.9 (3–6) |
| Type of cancer | |||
| Breast | 94 (97.9) | 31 (91.2) | 125 (96.2) |
| Colorectal | 1 (1.0) | 1 (2.9) | 2 (1.5) |
| Diffuse large cell lymphoma | 1 (1.0) | 1 (2.9) | 2 (1.5) |
| Unknown | 0 | 1 (2.9) | 1 (0.8) |
TP=treatment period; TP1=Months 0 to 6; TP2=Months 6 to 12
Dark iris color included brown, dark brown, and black; light iris color included blue, blue-gray, blue/gray-brown, green, green-brown, grey, and hazel
Responder rates. The proportion of subjects who responded to bimatoprost with both a >1-grade improvement from baseline in GEA score and a >3-point improvement from baseline in total score on Domain 2 of the ESQ was significantly greater with bimatoprost compared with vehicle at the Month 4 primary analysis time point (37.5% vs. 18.2%; p=0.041) and at the end of the first six-month treatment period (46.9% vs. 18.2%; p=0.004) (Figure 2A). The responder rate increased steadily in the group that continued on bimatoprost, reaching 61.5 percent at Month 12. There was no vehicle group during the second treatment period; rather, patients initially allocated to vehicle were switched to bimatoprost. In this latter group, the responder rate reached 67.6 percent at Month 12 (i.e., after bimatoprost use for 6 months). The increase in responder rates over time for subjects switched to bimatoprost was similar to that observed during the first treatment period for subjects initially allocated to bimatoprost. Some subjects responded to bimatoprost as soon as 1 to 2 months. Comparable results were observed for each component of the primary composite analysis (Figures 2B and Figures 2C). In addition, the proportion with ≥2-grade improvement in GEA was also significantly greater in the bimatoprost group compared with vehicle at Month 4 (36.5% vs. 6.1%; p<0.001) and Month 6 (45.8% vs. 9.1%; p<0.001) and reached 57.3 percent at Months 8 to 12 in subjects initially allocated to bimatoprost. Again, the time course for response after the switch to bimatoprost was similar to that observed in the group initially allocated to bimatoprost.
Figure 2.
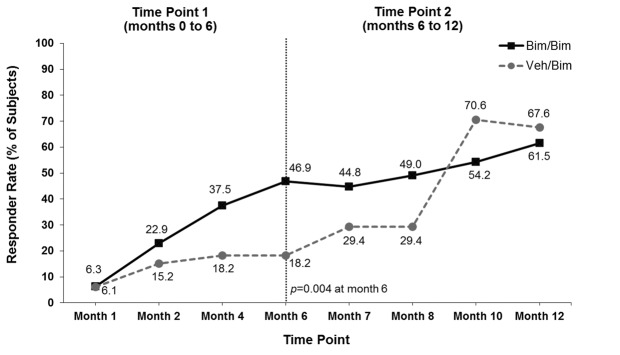
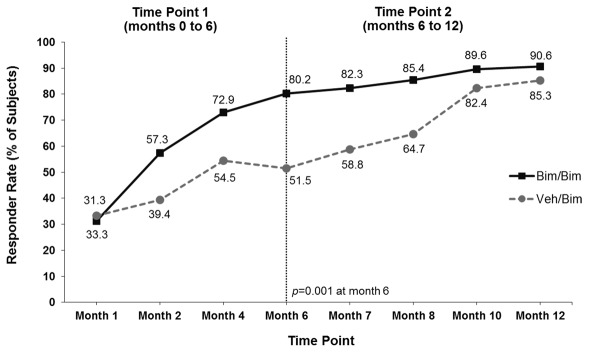
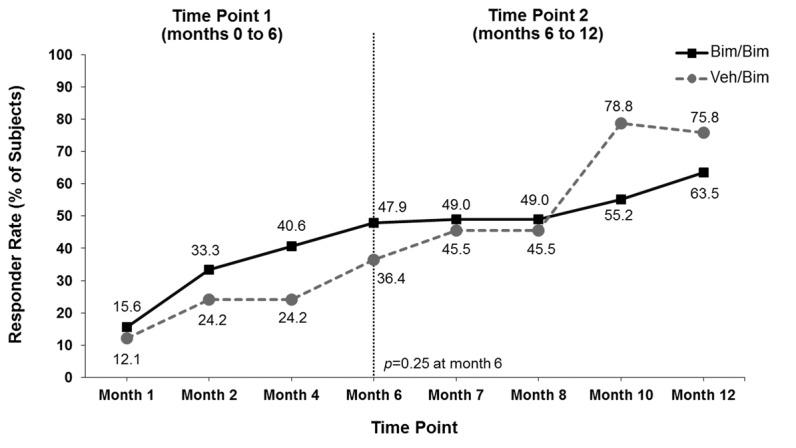
Responders for the composite primary analysis (both a ≥1-grade improvement from baseline in Global Eyelash Assessment (GEA) score and a ≥3-point improvement from baseline in total score on Domain 2 (Confidence, Attractiveness, and Professionalism) of the Eyelash Satisfaction Questionnaire (A) and for each component of the primary analysis separately (B and C). Bim=bimatoprost; veh=vehicle
Digital image analysis. Bimatoprost significantly increased mean (±standard deviation [SD]) eyelash length compared with vehicle at Month 6 (1.99±1.56 vs. 1.01±1.28mm; p<0.001) (Figure 3A). The increase in eyelash length in the vehicle group reflected natural regrowth following chemotherapy. Comparable results favoring bimatoprost over vehicle were observed at Month 6 in the analyses of mean eyelash thickness (0.83±0.58 vs. 0.04±1.01 mm2; p<0.001) and darkness (-26.46±21.96 vs. -10.19±15.98 IU; p=0.002) (Figures 3B and 3C). Subjects initially allocated to vehicle achieved similar increases in eyelash length and thickness by four months after switching to bimatoprost (i.e., Month 10) and greater increases in eyelash darkness at Months 10 and 12 as those who continued to receive bimatoprost. Examples of improvements in eyelash length, thickness, and darkness for bimatoprost-treated subjects at Month 6 and Month 12 are illustrated in Figure 4.
Figure 3.
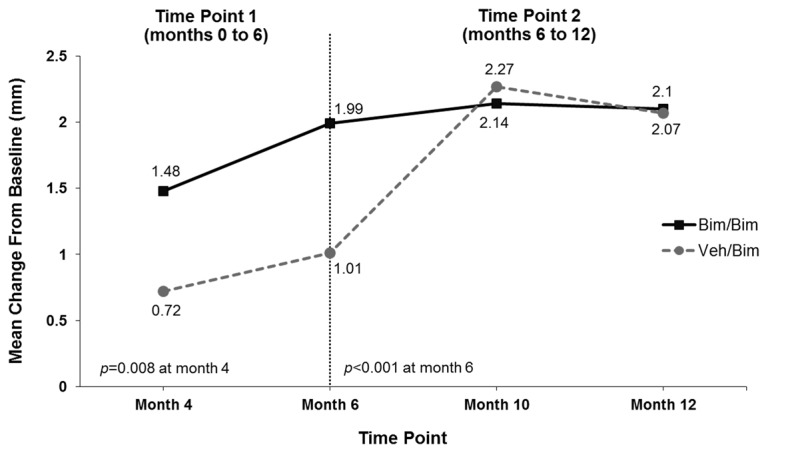
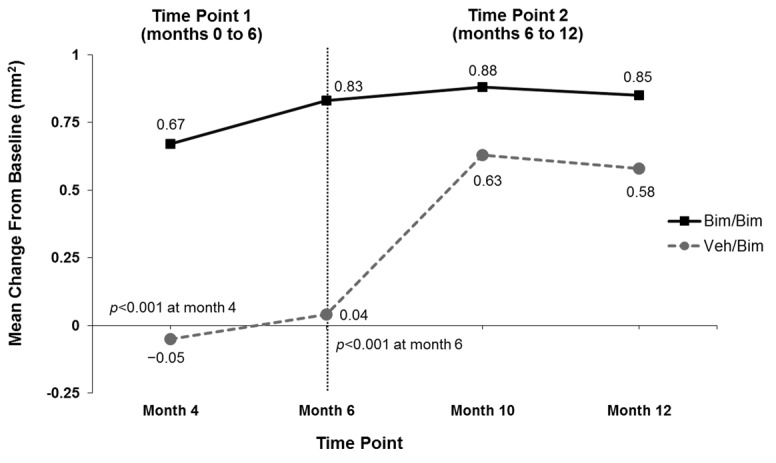
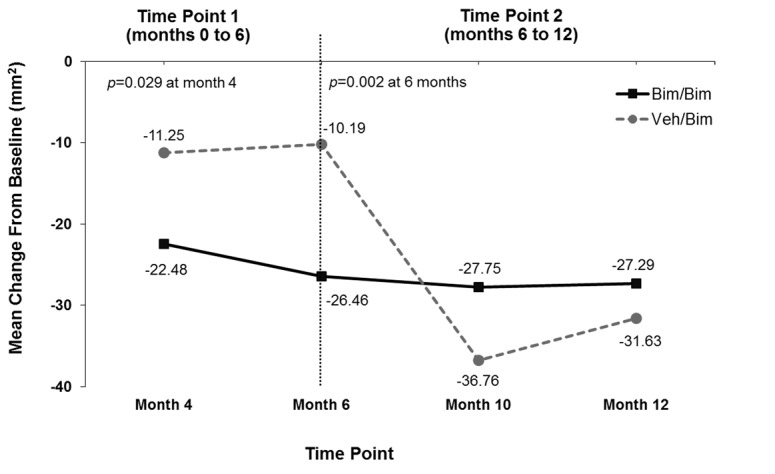
Mean change from baseline in eyelash length (A), eyelash thickness (B), and eyelash darkness (C); increased darkness is depicted by a negative change in intensity units (IU) from baseline. Bim=bimatoprost; veh=vehicle
Figure 4.
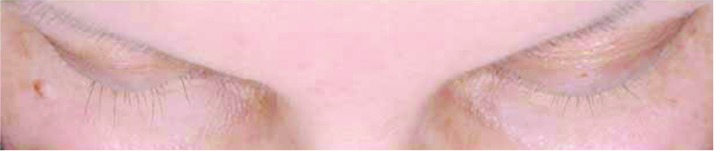

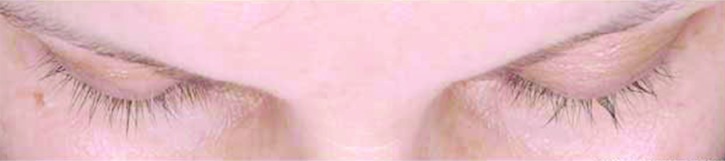
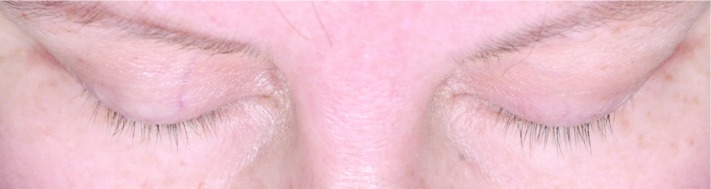
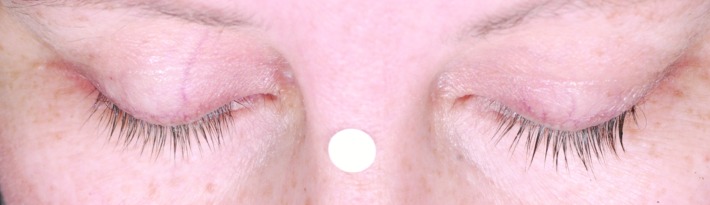
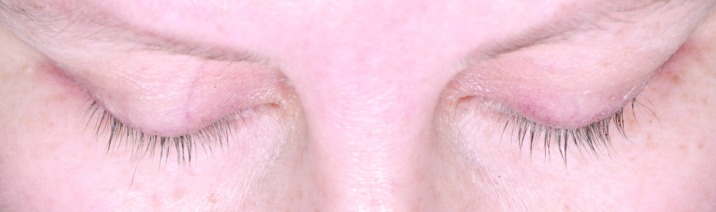
Subjects treated for 12 months with bimatoprost (Bim), whose changes in eyelash length and eyelash thickness approximated the median percentage changes for the population with chemotherapy-induced eyelash hypotrichosis receiving bimatoprost during both study periods.
A) Eyelash length in a subject from the Bim/Bim group at baseline (4.5mm), 6 months (36.7% increase from baseline), and 12 months (36.7% increase from baseline). Top: Baseline length = 4.5mm. Median length at baseline for Bim/Bim postchemotherapy group = 4.80mm. Middle: Month 6 percent increase from baseline length = 36.67%. Median percent change from baseline to Month 6 for Bim/Bim postchemotherapy group = 37.84%. Bottom: Month 12 percent increase from baseline length = 36.67%. Median percent change from baseline to Month 12 for Bim/Bim postchemotherapy group = 39.08%.
B) Eyelash thickness in a subject from the Bim/Bim group at baseline (0.30 mm2), 6 months (266.7% increase from baseline), and 12 months (200.0% increase from baseline). Top: Baseline thickness = 0.30mm2. Median thickness at baseline for Bim/Bim postchemotherapy group = 0.3 mm2. Middle: Month 6 percent increase from baseline thickness = 266.7%. Median percent change from baseline to Month 6 for Bim/Bim postchemotherapy group = 245.0%. Bottom: Month 12 percent increase from baseline thickness = 200.0%. Median percent change from baseline to Month 12 for Bim/Bim postchemotherapy group = 211.8%.
Patient-reported outcomes. Mean scores for each question and domain of the ESQ improved from baseline to Month 12. The degree of improvement on many ESQ questions was greater in the bimatoprost group versus the vehicle group through Month 6. However, subjects who were switched to bimatoprost had generally similar improvements at Month 12 as those who continued on bimatoprost. Mean (±SD) ESQ Domain 2 scores increased from 3.9±1.2 at baseline to 6.7±3.4 at Month 6 and 7.7±3.4 at Month 12 in the group treated with bimatoprost during both treatment periods (Figure 5). Mean ESQ Domain 2 scores increased more gradually with vehicle from 3.9±1.3 at baseline to 5.6±2.0 at Month 6, and then more rapidly following the switch from vehicle to bimatoprost, reaching 8.4±2.9 at Month 12.
Figure 5.
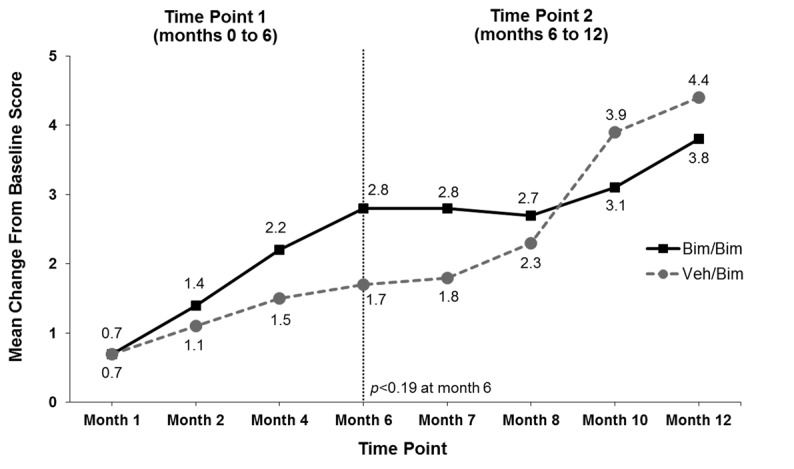
Mean change from baseline in Eyelash Satisfaction Questionnaire Domain 2 (Confidence, Attractiveness, and Professionalism) over time. Bim=bimatoprost; veh=vehicle
Safety. Eighty-eight (68.8%) of the 128 subjects who received bimatoprost reported one or more AEs during a bimatoprost treatment period, including 71 of 96 subjects (74.0%) who received bimatoprost during both periods and 17 of 32 subjects (53.1%) who received bimatoprost only during the second six-month period (Table 2). In comparison, 15 of 33 subjects (45.5%) reported AEs while receiving vehicle. The vast majority of AEs were described as mild. The most frequently reported AEs during the bimatoprost treatments (i.e., 12 months of bimatoprost or 6 months of bimatoprost following 6 months of vehicle) were conjunctival hyperemia (14.8%), punctate keratitis (7.8%), upper respiratory tract infection (6.3%), and eye pruritus (5.5%). Skin hyperpigmentation was reported by five subjects (3.9%) who received bimatoprost for 12 months. A large majority of AEs coded to the eye system organ class occurred during the first six months of bimatoprost treatment; only one case each of conjunctival hyperemia, punctate keratitis, and eye pruritus was reported from Months 6 to 12 in subjects receiving bimatoprost for 12 months.
TABLE 2.
Summary of adverse events with an incidence of at least 5% in any group by treatment period
| BIMATOPROST (TP1)/BIMATOPROST (TP2) | VEHICLE (TP1)/BIMATOPROST (TP2) | ||||
|---|---|---|---|---|---|
| SYSTEM ORGAN CLASS PREFERRED TERM | 0 TO 12 MONTHS (n=96) | 0 TO 6 MONTHS (n=96) | 6 TO 12 MONTHS (n=89) | 0 TO 6 MONTHS (n=33) | 6 TO 12 MONTHS (n=32) |
| Any adverse event | 71 (74.0) | 55 (57.3) | 46 (51.7) | 15 (45.5) | 17 (53.1) |
| Eye disorders | |||||
| Conjunctival hyperemia | 16 (16.7) | 15 (15.6) | 1 (1.1) | 1 (3.0) | 3 (9.4) |
| Punctate keratitis | 9 (9.4) | 8 (8.3) | 1 (1.1) | 2 (6.1) | 1 (3.1) |
| Eye pruritus | 6 (6.3) | 5 (5.2) | 1 (1.1) | 0 (0) | 1 (3.1) |
| Skin and subcutaneous disorders | |||||
| Skin hyperpigmentation | 5 (5.2) | 3 (3.1) | 2 (2.2) | 0 (0) | 0 (0) |
| Infections and infestations | |||||
| Upper respiratory tract infection | 5 (5.2) | 2 (2.1) | 3 (3.4) | 1 (3.0) | 3 (9.4) |
| Sinusitis | 5 (5.2) | 1 (1.0) | 5 (5.6) | 0 (0) | 0 (0) |
| Nasopharyngitis | 3 (3.1) | 0 (0) | 3 (3.4) | 1 (3.0) | 2 (6.3) |
| Bronchitis | 2 (2.1) | 2 (2.1) | 0 (0) | 0 (0) | 2 (6.3) |
| Endocrine disorders | |||||
| Hypothyroidism | 1 (1.0) | 1 (1.0) | 0 (0) | 0 (0) | 2 (6.3) |
TP=treatment period
One death occurred; a 63-year-old Caucasian woman died 55 days after receiving her first dose of bimatoprost. The death occurred one day after a planned elective breast reconstruction surgery and was attributed to pulmonary embolism and cardiac arrest considered not related to study medication. Seventeen subjects reported serious AEs, with none considered by investigators to be related to treatment. Four subjects discontinued study treatment due to AEs, including three subjects with cancer recurrence and one subject with increased lacrimation. There were no clinically meaningful changes in vital signs or physical examination findings.
Ophthalmic examinations. There were no clinically meaningful changes in mean iOP during bimatoprost therapy (e.g., mean change in iOP at Month 6 was -1.2mm Hg for bimatoprost and -1.1mm Hg for vehicle). Mean iOP at baseline was 15.0mm Hg among subjects who received bimatoprost in both treatment periods; mean changes in iOP from baseline ranged from -1.0 to -1.4mm Hg across study visits. For comparison, mean changes in IOP in the vehicle group through the Month 6 study visit were -0.1 and -1.1mm Hg, respectively. Only one subject had an IOP measurement ≤6mm Hg in either eye. This subject had a baseline IOP of 8mm Hg in both eyes, and measurements of 6 and 8mm Hg in the right and left eyes, respectively, at Month 6. No relevant AEs or changes to BCVA, biomicroscopy, or ophthalmoscopy findings were reported in association with the decrease in IOP.
During the one-year study, 41 subjects (32.0%) had biomicroscopy and ophthalmoscopy findings of a ≥1-grade severity increase from baseline at one or more study visits during bimatoprost therapy, which was most commonly due to conjunctival hyperemia (14.1%), corneal erosion (5.5%), and eyelid erythema (3.9%). Of these, five subjects (3.9%) receiving bimatoprost for 12 months had a ≥2-grade severity increase. Through the Month 6 study visit, three subjects from the vehicle group had biomicroscopy and ophthalmoscopy findings of a ≥1-grade severity increase from baseline at one or more study visits due to conjunctival hyperemia, corneal erosion, and posterior capsule opacification. The majority (96.5%) of subjects receiving bimatoprost for 12 months had no change in BCVA (defined as a ≤2-line change). No subject had a change in iris color from light to dark, but two subjects had changes from dark to light during bimatoprost treatment (brown to hazel at Month 4 and returning to brown at Month 6 in 1 subject; brown to green-brown at Month 12 in the other subject). These represented 1 and 2 category changes on a 10-category scale, respectively; neither change was considered clinically relevant, and both were likely due to variability in observer assessment, especially since a darker to lighter category shift is inconsistent with the mechanism of a drug with potential for hyperpigmentation.
DISCUSSION
Cytotoxic chemotherapy damages hair follicles, resulting in hair loss and altered hair regrowth following treatment completion.15 Patients may lose their eyelashes in a manner similar to scalp hair loss. Hair loss can have substantial psychological impact on patients already dealing with the challenges of undergoing chemotherapy. Hair growth recovers slowly after chemotherapy; the key for hair growth recovery is for the hair follicle to enter a new growth cycle from the dormant anagenarrest state caused by chemotherapy. Bimatoprost stimulates resting follicles to enter the next growth phase.14 Once the new hair cycle is established, it should continue in a manner similar to natural, pre-chemotherapy growth.
Little is known about natural eyelash regrowth following chemotherapy and whether it can be enhanced through pharmacologic treatment. This study is the first to document the natural course of eyelash recovery in the postchemotherapy setting. Subjects entered this study 4 to 16 weeks after completing chemotherapy and, in the vehicle group, were followed for six months without active treatment. Therefore, the vehicle group provides valuable information about natural eyelash growth following completion of chemotherapy. Comparisons of the vehicle and bimatoprost groups showed that eyelash prominence may be enhanced through pharmacologic treatment. Notably, bimatoprost restored eyelash prominence rapidly relative to the slow, natural regrowth process.
The vehicle group showed gradual improvement in efficacy measures, reflecting the natural recovery following chemotherapy. After six months, 18.2 percent achieved the primary composite measure. Other analyses, including GEA score, ESQ Domain 2 score, and DiA measures also improved gradually from baseline to Month 6. Despite these improvements, most subjects would still be considered to have eyelash hypotrichosis inasmuch as the efficacy measures were similar to baseline data for cohorts with idiopathic hypotrichosis. For example, subjects with idiopathic hypotrichosis in this study had median eyelash length of 5.70mm and 5.88mm at baseline (bimatoprost vs. vehicle groups, respectively), whereas the post-chemotherapy subjects had median eyelash length of 5.95mm after six months of vehicle. Similarly, median eyelash thickness was 0.78mm2 and 0.93mm2 in the idiopathic group at baseline (bimatoprost vs. vehicle groups, respectively) and 0.65 mm2 in the postchemotherapy cohort after six months of vehicle. Notably, the responder rate of 18.2 percent with vehicle in the postchemotherapy cohort was still higher than the corresponding rate of 3.4 percent with vehicle in the idiopathic hypotrichosis cohort enrolled in this study. This difference in responder rates suggests some natural eyelash regrowth in the postchemotherapy cohort compared with a continued state of eyelash hypotrichosis in the idiopathic cohort. By contrast, after six months, 46.9 percent of postchemotherapy subjects in the bimatoprost group achieved the primary composite analysis. Similar results (47.5%) were seen in subjects with idiopathic hypotrichosis enrolled in this study after six months of bimatoprost.
The patient-reported outcome analyses demonstrated that the postchemotherapy cohort at baseline was negatively impacted psychologically by their eyelash loss, as evidenced by low scores on ESQ Domain 2 and relatively small improvements with vehicle over a six-month period. These findings indicate that eyelash hypotrichosis is likely associated with psychological variables, such as loss of self-esteem, confidence, and acceptance by others.16
Eyelash prominence increased steadily with continued bimatoprost treatment through Month 12. The lack of a vehicle group during the second six-month treatment period precludes an assessment of whether this further improvement reflects a treatment-induced increase in eyelash growth rate or whether the improvements realized over the first six months allowed natural hair growth to occur at a faster rate. The vehicle group was switched to bimatoprost at Month 6 and subsequently had rapid recovery of eyelashes comparable to that seen in the group initially allocated to bimatoprost. By Month 12, efficacy analyses were generally comparable for subjects started on vehicle and switched to bimatoprost and those who received bimatoprost for the entire 12-month treatment period.
No unexpected safety findings were obtained in this study. The safety profile of bimatoprost 0.03% has been established in patients with open-angle glaucoma and ocular hypertension, in which one drop is instilled daily in the affected eye.13,17 For eyelash hypotrichosis, the total dose applied to the upper eyelid margins was only about five percent of the dose compared with an eyedrop.3 Conjunctival hyperemia was the most common AE, consistent with a previous study of bimatoprost in subjects with idiopathic hypotrichosis.10 Other ocular and skin AEs were also consistent with the previous study. It should be noted that these AEs may also be related to an enduring effect of chemotherapeutic agents on the eyes.18 During bimatoprost treatment, mean changes in iOP compared with baseline were similar to mean changes in the vehicle group. These changes were well within the range of normal daily iOP fluctuations of 3 to 7mm Hg,19,20 and mean iOP remained well within the normal range of 10 to 20mm Hg.21
The lack of a control group from Month 6 to 12 did not allow collection of information on the percentage of subjects who do not naturally recover normal eyelash growth. It was operationally impractical to have a vehicle group for one year due to expected recruitment challenges and high discontinuation rates. instead, a six-month parallel-arm vehicle control was deemed appropriate in this population for fully characterizing the effect of bimatoprost on post-chemotherapy eyelash growth. A limitation of this study was that the study cohort may not be representative of the broader postchemotherapy population; rather, they may be representative of subjects who are more likely to request treatment in that they sought improvement by enrolling in a clinical trial.
In summary, natural eyelash regrowth following chemotherapy occurs at a relatively slow rate, with most subjects still exhibiting eyelash hypotrichosis and its associated negative psychological impact at six months (corresponding to 7 to 10 months after chemotherapy completion). Treatment with bimatoprost provides rapid recovery of eyelashes with minimal AEs, whether started shortly after chemotherapy (4 to 16 weeks) or delayed for an additional six-month period. The improvements in eyelash prominence, length, thickness, and darkness with bimatoprost are accompanied by increased subject satisfaction, as reflected by improvements in ESQ Domain 2.
CONCLUSION
Bimatoprost topical dermal application, once daily, to the eyelid margin provides a clinically meaningful benefit of rapid eyelash recovery in patients with chemotherapy-induced eyelash loss or hypotrichosis. The treatment was well-tolerated with minimal adverse events.
ACKNOWLEDGMENT
The authors thank the subjects, study coordinators, clinical staff, and operations staff who participated in this study.
Footnotes
DISCLOSURE:Dr. Wirta is a consultant for and has received research grants from Allergan, Inc. Dr. Baumann has no disclosures to report. Dr. Bruce is a consultant for Allergan, Inc., Lithera, Lumenis, and Ulthera, Inc. She is also an investigator for AbGenomics, Actavis, Inc., Allergan, Inc., Anacor Pharmaceuticals, Inc., Braintree Laboratories, Inc., Cipher Pharmaceuticals, Inc., DUSA Pharmaceuticals, Inc., Galderma R&D Inc., G & E Herbal Biotechnology Co. Ltd., G & W Laboratories, Inc., Health Outcomes Solutions, LEO Pharma, Inc., Lithera, Maruho Co. Ltd., Novartis Pharmaceuticals Corp., Obagi Medical Products, Inc., Pfizer, Inc., Promius Pharma, LLC, Ranbaxy Laboratories, Ltd., Revance Therapeutics, Inc., Stiefel Laboratories, Inc., Suneva Medical, Inc., Taro Pharmaceutical Industries, Ltd., Tigercat Industries Inc., Tolmar, Inc., and Watson Pharmaceuticals, Inc. Drs. Ahluwalia and Weng are employees of Allergan, Inc. Dr. Daniels was an employee of Allergan, Inc., at the time of this study. This study was sponsored by Allergan, Inc., Irvine, California. Writing and editorial assistance were provided to the authors by Linda Romagnano, PhD, of Peloton Advantage, Parsippany, New Jersey, and was funded by Allergan Inc. All authors met the ICMJE authorship criteria. Neither honoraria nor other form of payments were made for authorship.
REFERENCES
- 1.Shaikh MY, Bodla AA. Hypertrichosis of the eyelashes from prostaglandin analog use: a blessing or a bother to the patient? J Ocul Pharmacol Ther. 2006;22:76–77. doi: 10.1089/jop.2006.22.76. [DOI] [PubMed] [Google Scholar]
- 2.Law SK. Bimatoprost in the treatment of eyelash hypotrichosis. Clin Ophthalmol. 2010;4:349–358. doi: 10.2147/opth.s6480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fagien S. Management of hypotrichosis of the eyelashes: focus on bimatoprost. Clin Cosrnet Investig Dermatol. 2010;3:39–48. doi: 10.2147/ccid.s5488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones D. Enhanced eyelashes: prescription and over-the-counter options. Aesthetic Plast.Surg. 2011;35:116–121. doi: 10.1007/s00266-010-9561-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lemieux J, Maunsell E, Provencher L. Chemotherapy-induced alopecia and effects on quality of life among women with breast cancer: a literature review. Psychooncology. 2008;17:317–328. doi: 10.1002/pon.1245. [DOI] [PubMed] [Google Scholar]
- 6.Hesketh PJ, Batchelor D, Golant M, et al. Chemotherapy-induced alopecia: psychosocial impact and therapeutic approaches. Support Care Cancer. 2004;12:543–549. doi: 10.1007/s00520-003-0562-5. [DOI] [PubMed] [Google Scholar]
- 7.Trueb RM. Chemotherapy-induced alopecia. Semin Cutan Med Surg. 2009;28:11–14. doi: 10.1016/j.sder.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Draelos ZD. Special considerations in eye cosmetics. Clin Dermatol. 2001;19:424–430. doi: 10.1016/s0738-081x(01)00204-8. [DOI] [PubMed] [Google Scholar]
- 9.Woodward DF, Liang Y, Krauss AH. Prostamides (prostaglandin-ethanolamides) and their pharmacology. Br J Pharmacol. 2008;153:410–419. doi: 10.1038/sj.bjp.0707434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith S, Fagien S, Whitcup SM, et al. Eyelash growth in subjects treated with bimatoprost: a multicenter, randomized, double-masked, vehicle-controlled, parallel-group study. J Am Acad Dermatol. 2012;66:801–806. doi: 10.1016/j.jaad.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Higginbotham EJ, Schuman JS, Goldberg I, et al. One-year, randomized study comparing bimatoprost and timolol in glaucoma and ocular hypertension. Arch Ophthalmol. 2002;120:1286–1293. doi: 10.1001/archopht.120.10.1286. [DOI] [PubMed] [Google Scholar]
- 12.Whitcup SM, Cantor LB, VanDenburgh AM, Chen K. A randomised, double masked, multicentre clinical trial comparing bimatoprost and timolol for the treatment of glaucoma and ocular hypertension. Br J Ophthalmol. 2003;87:57–62. doi: 10.1136/bjo.87.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams RD, Cohen JS, Gross RL, et al. Long-term efficacy and safety of bimatoprost for intraocular pressure lowering in glaucoma and ocular hypertension: year 4. Br J Ophthalmol. 2008;92:1387–1392. doi: 10.1136/bjo.2007.128454. [DOI] [PubMed] [Google Scholar]
- 14.Cohen JL. Enhancing the growth of natural eyelashes: the mechanism of bimatoprost-induced eyelash growth. Dermatol Surg. 2010;36:1361–1371. doi: 10.1111/j.1524-4725.2010.01522.x. [DOI] [PubMed] [Google Scholar]
- 15.Paus R, Haslam IS, Sharov AA, Botchkarev VA. Pathobiology of chemotherapy-induced hair loss. Lancet Oncol. 2013;14:e50–e59. doi: 10.1016/S1470-2045(12)70553-3. [DOI] [PubMed] [Google Scholar]
- 16.Sadick NS. The impact of cosmetic interventions on quality of life. Dermatol Online J. 2008;14:2. [PubMed] [Google Scholar]
- 17.Wirta D, VanDenburgh AM, Weng E, et al. Long-term safety evaluation of bimatoprost ophthalmic solution 0.03%: a pooled analysis of six double-masked, randomized, active-controlled clinical trials. Clin Ophthalmol. 2011;5:759–765. doi: 10.2147/OPTH.S17457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hazin R, Abuzetun JY, Daoud YJ, Abu-Khalaf MM. Ocular complications of cancer therapy: a primer for the ophthalmologist treating cancer patients. Curr Opin Ophthalmol. 2009;20:308–317. doi: 10.1097/ICU.0b013e32832c9007. [DOI] [PubMed] [Google Scholar]
- 19.Martin XD. Normal intraocular pressure in man. Ophthalmologica. 1992;205:57–63. doi: 10.1159/000310313. [DOI] [PubMed] [Google Scholar]
- 20.Fogagnolo P, Orzalesi N, Ferreras A, Rossetti L. The circadian curve of intraocular pressure: can we estimate its characteristics during office hours? Invest Ophthalmol Vis Sci. 2009;50:2209–2215. doi: 10.1167/iovs.08-2889. [DOI] [PubMed] [Google Scholar]
- 21.Murgatroyd H, Bembridge J. Intraocular pressure. Cont Educ Anaesth Crit Care Pain. 2008;8:100–103. [Google Scholar]