Abstract
Background:
Pomegranate (Punica granatum) is currently a member of Lythraceae family which has potentially cytotoxic activities. Numerous studies have been done on cytotoxic components of pomegranate's juices, barks and leaves. The peels, which considered as a waste, contain higher antioxidant components compared with other parts of the plant.
Aim:
To investigate the potential anti-cancer activity of pomegranate peel on growth and cell death mechanisms of chronic myeloid leukemic (CML) cells, K562.
Materials and Methods:
Punica granatum peels extract (PGPE) was extracted by successive ethanol extraction, 80% (v/v), freeze dried, diluted to 20 mg/mL working concentration and was subjected to phytochemical screening. K562 cell was treated with crude PGPE for 72 h. Following IC50 concentration, the apoptosis, cell cycle and protein analysis were evaluated. Cell growth inhibition assay was performed by conventional trypan blue exclusion assay. Apoptosis and cell cycle were analyzed by flow-cytometry using BD apoptosis and cell cycle kits and protein analysis by western blotting. All the results are expressed as mean ± standard error of mean of three independent experiments. Statistical analysis was performed by nonparametric Mann-Whitney U-test.
Results:
Results demonstrated that PGPE promotes growth inhibition of K562 cells mainly via G2/M phase arrest while still conserving apoptosis induction, but at a lower rate. Apoptosis activities were proposed by the up-regulation of caspases and cytochrome c with an elevated level of p21 and p53.
Conclusion:
PGPE caused an inhibition in cell proliferation of CML cell mainly by cell cycle arrest.
Keywords: Anti-leukemic, G2/M phase arrest, p21 activation, Punica granatum peel
INTRODUCTION
Pomegranate is an ancient fruit of five to 12 cm in diameter with a rounded hexagonal shape, and has thick reddish skin, native to Iran, Afghanistan, China, Indian subcontinent.[1] In Malaysia, pomegranates are cultivated only for domestic use. Pomegranate peels (pericalps) are characterized by interior network of membrane comprising of 26-30% of fruit weight.[2] Their phytochemical analysis lead to the isolation of polyphenolic compounds, such as flavonoids (anthocyanins, catechins and other complex flavonoids), hydrolyzable tannins (punicalin, pedunculagin, punicalagin, gallic and ellagic acid).[3,4,5] It is reported that the juice and peels have anticancer properties that inhibit cell proliferation, cell cycle and angiogenesis.[2] Previous studies have shown its growth suppression effect on solid tumors, such as colon and prostate cells,[6,7] breast cancer cells[8] and in skin melanomas.[9] However, effects of pomegranate peels on blood malignancy were not reported. In the current study, crude Punica granatum peels extract (PGPE) was tested against chronic myeloid leukaemia (CML) cell line, K562 to assess their potent anti-cancer property.
Leukaemia is characterized by clonal growth of immature progenitor cells in the bone marrow. K562 cells are derived from CML patient cells that carry a BCR-ABL mutation. This mutation is associated with deregulated apoptosis, proliferation and differentiation.[10] There are various side effects in the current chemotherapies experienced by CML patients. Therefore, an alternative or complementary ailment for better treatment outcomes is required. The approach for anti-leukemic treatment relies on the intact cell death program that is targeting apoptosis pathways[11] and cell cycle analysis.[12]
The current study highlights the potential of crude pomegranate peels as apoptosis inducer or cell cycle arrestor in leukaemia. In order to identify and characterize molecules involved in regulation of cell death in leukaemia, apoptosis-related molecules, such as caspases, akt, mitochondria molecules, such as bax and cytochrome c and cell cycle related molecules, such as cyclin-dependent kinase inhibitor, p21 and transcription factor, p53 that are predominantly involved in apoptosis and cell cycle were studied.
MATERIALS AND METHODS
Imatinib mesylate
Imatinib mesylate (IM) was purchased from LC laboratories (Woburn, MA, USA) and was dissolved in sterile distilled water before use. The stock solutions were stored in 1-mm in-20°C.
Cell lines and cell culture medium
K562 (human CML cell line) was donated by Department of Hematology, Universiti Sains Malaysia, Malaysia originally purchased from American Type Cell Culture (Rockville, MD, USA). The cells were cultured in RPMI-1640 medium (Gibco®, CA, USA), supplemented with 10% (v/v) of fetal bovine serum (Sigma, MO, USA) and 1% (v/v) of penicillin-streptomycin (Invitrogen, CA, USA) in humidified temperature containing 5% carbon dioxide (CO2) at 37°C.
Extraction of Punica granatum peels
The P. granatum peels were extracted by successive ethanol extraction. Twenty grams of lyophilized powder of P. granatum peel was soaked in 200 ml of 80% ethanol (v/v) for 72 h at room temperature (28°C–34°C) with occasional shaking and filtered using Whatman filter paper.[13] The retentate was re-extracted for at least 2 times independently until it became colourless. All the filtrates were collected and evaporated at 50°C by rotary evaporator (Buchi, Lausanne, Switzerland) to remove ethanol content in the extract and subjected to freeze drying. The final extract in lyophilized powder obtained was designated as PGPE.
Phytochemical screening
Punica granatum peels extract was send to Forest Research Institute of Malaysia to screen the presence of alkaloids, saponins, flavonoids, tannins/polyphenolic compounds and triterpines/steroids, the main bio-active compounds that usually exhibit a diverse range of biological activities. The test procedures of isolated compounds were previously described by Asmaa et al.[14]
In vitro cell viability assay
The effects of PGPE on leukemic cells were determined by conventional trypan blue exclusion assay (TBEA). The cells were treated with different concentrations of complete media dissolved extracts (0, 100, 200, 400, 600, 800 μg/mL) in triplicate incubated for 72 h. The cells were harvested and counted with the haemocytometer after the treatment. Cell viability was recorded as a percentage of surviving cells following PGPE treatment. The data represent mean from three independent experiments. Inhibitory concentration to be reduced by half (IC50) was calculated from the graph.
Assessment of apoptosis by flow-cytometry
Apoptosis of K562 cells exposed to PGPE was assessed by Annexin V labeling BD Annexin-V-FITC assay kit (Becton Dickinson, NJ, USA). A total of 5 × 105 cells were collected by centrifugation at 300 gravity (g) (relative centrifugal force) for 5 min. Cells were stained with 5 μl Annexin conjugated with fluorescein isothiocyanate (FITC) and propidium iodide (PI) at room temperature in the dark for 15 min and analyzed by Flow BD Fac Canto II flow-cytometer (Becton Dickinson, NJ, USA) to measure the fluorescence intensity. The result showed relative granularity at side scattered and relative size of the cell at forward scattered with fluorescence stain. Untreated cells served as the negative control. Conventional drug in clinical use for treatment of CML, IM (Gleevec™) served as a positive control, used at IC50 concentration. A minimum of 10,000 events was collected per sample and analyzed.
Morphological observation of apoptotic features by fluorescence microscopy
Differences in cell component in apoptotic cells can be detected by using different fluorochromes observed by fluorescence microscope (AxioCam MRm, Zeiss, NY, USA) installed with inverted microscope (Axion 2 imaging, Zeiss, NY, USA). This simultaneous staining of nuclei, DNA and membrane facilitate visualization of shape of the cells. The transmitted images were collected by light detectors with different combinations of excitation and emission filter, 470/40 for PI, 365/12 for FITC and 546/12 for 4’, 6-diamidino-2-phenylindole (DAPI) for optimal signal capture. K562 cells were treated with PGPE at IC50 concentration (100 μg/mL). Untreated cells served as a negative control. 1 × 104 cells were harvested suspended in 5 μl PI/Annexin V-FITC, and mounted with DAPI. Image processing was carried out using AxioVision Rel. 4.8 computer software (Zeiss, NJ, USA).
Assessment of cell cycle progression by flow-cytometry
To study the effect of PGPE treatment on cell cycle progression of K562 cells, cell cycle distribution was studied by measuring DNA contents using flow the-cytometry (Becton Dickinson, NJ, USA). 1 × 105 cells were treated with 100 μg/ml of PGPE for 72 h. The treated cells were collected by centrifugation and resuspended in cold phosphate-buffered saline. Cells were then stained with PI solution (20 μg/ml of PI and 10 μg/ml of RNase A) at 37°C in dark for 30 min. Cell cycle distribution of nuclear DNA was determined by fluorescence activated cell sorting, fluorescence detector using Cell Quest Software (Becton Dickinson, NJ, USA). Analysis of flow-cytometric data was performed by Modfit LT 3.2 software (Verity Software House, ME, USA). All experiments were performed in triplicate.
Western blot analysis
Whole cell lysate of PGPE treated and untreated K562 were collected by centrifugation. Proteins were extracted using radio immunoprecipitation assay buffer (50 m M Tris-HCI, pH 8.0, with 150 mM sodium chloride, 1.0% Igepal CA-630 [(NP-40)], 0.5% sodium deoxycholate and 0.1% sodium dodecyl sulfate [SDS]) (Sigma-Adrich, MO, USA) and protease inhibitor cocktail (GE Healthcare, Buckinghamshire, UK) and incubated in ice for at least 10 min. Protein concentration was estimated by Bradford reagent (Biorad laboratories, CA, USA). 10 μg/ml of protein was separated on 10% SDS-PAGE, transferred onto a polyvinylidene difluoride membrane (Amersham, GE, Buckinghamshire, UK) using Transblot SD semi-dry transfer (Bio-Rad, CA, USA) at 15 V, 100 mA, for 90 min and blocked with 5% blocking agent. The membranes were immunoblotted with primary antibodies; anti-bax, anti-bcl-2, anti-cytochrome c, anti-caspase 9, anti-cleaved caspase 9, anti-caspase 3, anti-caspase 7, anti-p21Waf1/Cip1, p53, akt and anti-ß-actin) (cell signaling, MA, USA) overnight at 4°C in shaker, according to manufacturer instructions and incubated with horseradish peroxidase-linked rabbit and mouse secondary antibodies (cell signaling, MA, USA) for 1 h at room temperature. Rabbit anti ß-actin antibody (Cell signaling, MA, USA) was used as loading control. Amersham ECL Detection Agent (GE, Buckinghamshire, UK) was used to immunostain protein bands which were exposed to hyperfilm (GE, Buckinghamshire, UK) in the dark room, developed by developer machine (Fujifilm, Tokyo, Japan) to acquire image.
RESULTS
Phytochemical analysis result showed that PGPE contained moderate amount of tannins and polyphenolic compounds with small amounts of saponins and flavonoids [Table 1].
Table 1.
Preliminary phytochemical screening endorsed by FRIM

The effect of PGPE on growth of K562 cells was studied by TBEA. Results showed that PGPE inhibits growth of K562 cells in a dose-dependent manner. As the concentration of PGPE was increased, cell growth was inhibited and total cell death at 600 and 800 μg/mL [Figure 1a]. The IC50 concentration determined from the graph was 100 ± 0.05 μg/mL. In comparison, the IC50 of IM drug was 0.15 μM [Figure 1b].
Figure 1.
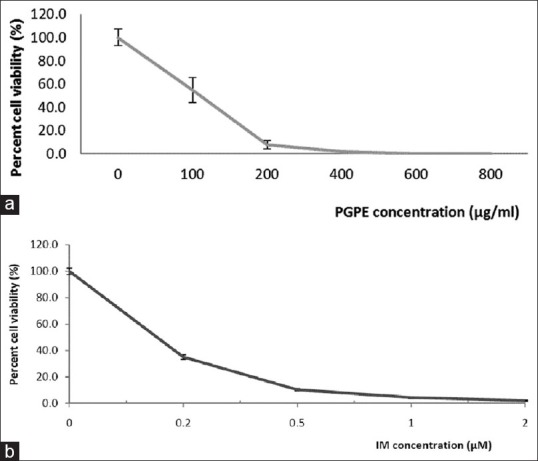
(a) Dose-dependent effect of Punica granatum peels extract on cell viability of K562 cells for 72 h (b) Cell viability assay of K562 cells treated with imatinib mesylate
The mechanism of cell death in PGPE treatment was studied by flow the-cytometry. K562 cells treated with IM (at IC50 dose) acted as a reference drug for apoptosis induction. Result indicated after 72 h treatment showed that PGPE induced apoptosis in K562 cells, but at lower extent, with total apoptosis, 8.5% compared to untreated, 2.4%. Apoptosis effects were compared in IM treated cells, which confer 26.1% of total apoptosis. In IM treatment, higher cell populations were observed in quadrant 4 which indicates early apoptosis compared to PGPE treated and untreated K562 cells [Figure 2a–c].
Figure 2.
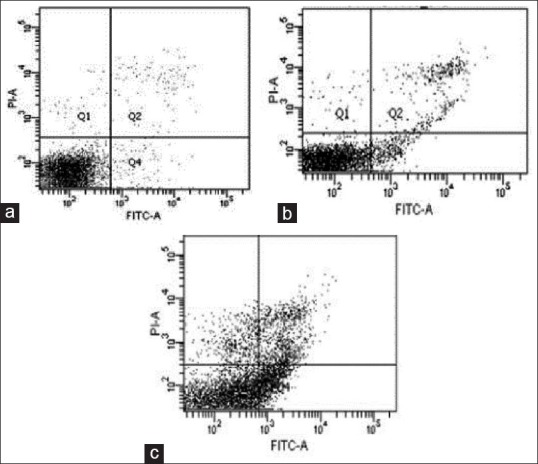
(a) Scatter plots of Annexin V/propidium iodide stained K562 cells untreated (b) Scatter plots of Annexin V/propidium iodide stained K562 cells treated Punica granatum peels extract at 100 μg/mL upon incubation for 72 h (c) Scatter plots of Annexin V/Propidium iodide stained K562 cells treated Imatinib mesylate at 0.15 ìM
To further characterize the apoptotic features of cells treated with PGPE, simultaneous staining of FITC (green emission), PI (red emission) and DAPI (blue emission) were performed. K562 treated with PGPE showed apoptotic morphological changes. High intensity of PI and DAPI emissions with several cells stained by Annexin V-FITC indicating early apoptosis characteristic, in untreated K562 cells (arrow) under 0.5 μM magnifications [Figure 3a]. In contrast, PGPE treated cells emitted low intensity of PI and DAPI, double stained with PI/Annexin-FITC thus indicating late apoptosis (arrow) [Figure 3b]. Although apoptosis occurred in untreated K562 cells, PGPE accelerated apoptotic activity.
Figure 3.
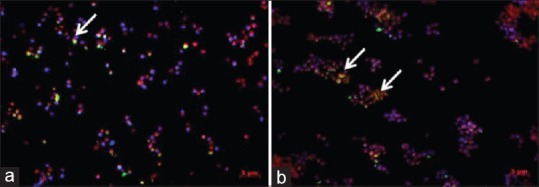
(a) Photomicrograph of the effect of Punica granatum peels extract on apoptosis in K562 cells observed under fluorescence microscope in untreated K562. Single green fluorescence is a sign of external permeabilization of phosphatidylserine (PS) whereas propidium iodide stains nucleus with ruptured PS (dead cells take this stain) and 4’, 6-diamidino-2-phenylindole stains DNA (b) Photomicrograph of the effect of Punica granatum peels extract (PGPE) on apoptosis in K562 cells observed under fluorescence microscope in treated PGPE at 100 ìg/mL. Apoptotic cells (arrows) forming clump after 72 h of PGPE treatment compared to untreated
Punica granatum peels extract induced cell cycle arrest in K562 cells. An elevation of DNA contents at G2/M phase, with subsequent decrease of DNA contents in G0/G1 phase were observed. PGPE treated cells accumulated in G2/M phase by 7.8% compared with control untreated cells 0.9% [Figure 4]. There was a slight increase in S phase fraction in PGPE treated K562 cells which demonstrated increased cells in DNA synthesizing state.
Figure 4.
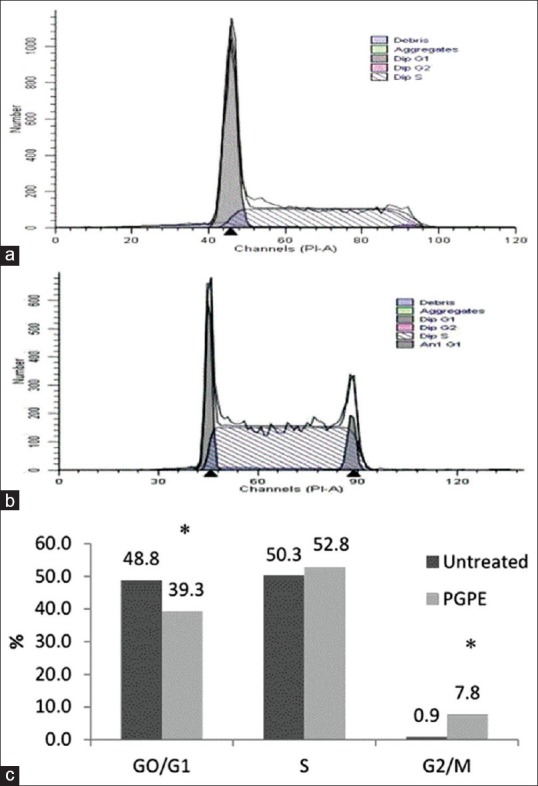
(a) Effect of Punica granatum peels extract on cell cycle progression of K562 cells for 72 h. Flow-cytometric data to score the DNA content of untreated K562 cells (b) Effect of Punica granatum peels extract (PGPE) on cell cycle progression of K562 cells for 72 h. Flow-cytometric data to score the DNA content of PGPE treated K562 cells (c) Percentage of cell fractions in G0/G1, S and G2/M phases of cell cycle populated using Modfit LT 3.2 software of treated cell compared to untreated
The regulatory mechanisms of PGPE induced apoptosis were studied by examining effect of the extract on expression of pro-apoptotic proteins, bax, cytochrome c, caspase 9, caspase 7 and caspase 3 and anti-apoptotic protein, bcl-2 and akt in K562 cells. Untreated K562 cells served as negative control and ί-actin served as internal control. Western blot analysis demonstrated that caspase 9, caspase 7 and caspase 3 proteins were up-regulated in PGPE treated K562 cells. Cytochrome c was slightly up-regulated by 1.3-fold change. Both bcl-2 and bax were down-regulated which indicates that bax activity was not dependent on anti-apoptotic activity of bcl-2. Despite a down-regulation in bcl-2 expression, the level of bax was not up-regulated. In addition, the akt expression slightly increased by 1.1-fold compared with untreated control [Figure 5]. Both p21 and p53 proteins were up-regulated by 8.3 and 1.3 times respectively in PGPE treated K562 cells compared with untreated control [Figure 5].
Figure 5.
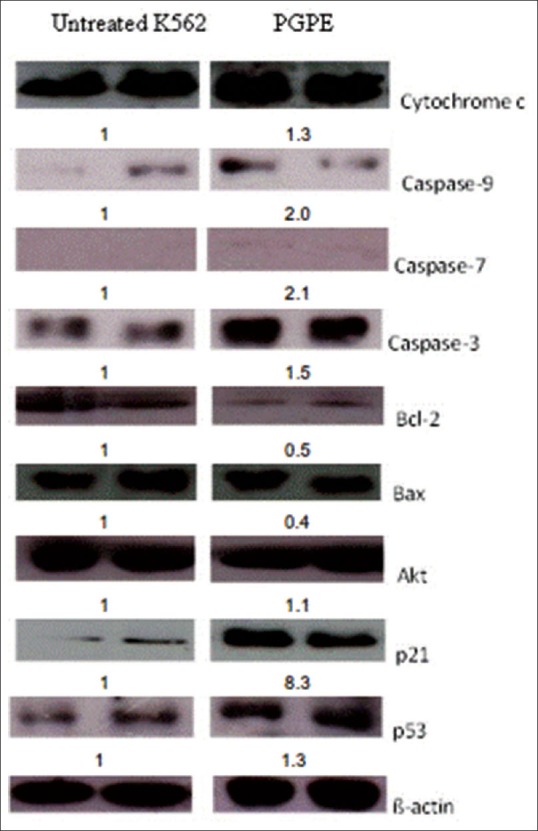
Western blot analysis showing targeted protein expression related to apoptosis and cell cycle of Punica granatum peels extract treated cells compared to untreated K562 at 72 h. The values below the figures represent the fold change (fold Δ) in protein expression of the bands normalized to ß-actin, quantified by densitometer (Adj. Vol.OD [mm2])
DISCUSSION
Anti-cancer effects of PGPE in CML were evaluated in the current study. Basic phytochemical analysis of PGPE led to the isolation of flavonoids, saponins and polyphenolic compounds which provide a good prospect for subsequent isolation of precise active constituents. Based on the current findings, PGPE inhibits growth of CML cell line, K562 cell in a dose-dependent manner. Mode of cell death was via cell cycle arrest at G2/M phase and by apoptosis induction. PGPE at IC50 concentration induced apoptotic activity by up-regulating apoptosis-related proteins, bax, bcl-2, caspase 9 and cytochrome c. In addition, PGPE also activates p21and p53 regulatory proteins associated with cell cycle arrest. However, PGPE induced apoptosis at a slower rate, compared with IM, a drug which is currently used for treatment of CML. IM is a molecular targeting drug that targets a BCR-ABL mutation in CML cells. That BCR-ABL mutation arises from the reciprocal translocation between chromosome 22 and 9 was observed in about 95% of CML cases. Therefore it has become a target in CML chemotherapy.[15] It was reported that activated BCR-ABL is able to suppress and evade apoptosis mechanism thereby promoting expansion of cells.[16]
Despite its role in suppressing apoptosis mechanism in CML cells, BCR-ABL mutation also takes part in cell cycle regulation. As observed in this study, PGPE leads to G2/M phase arrest. Previous study showed that BCR-ABL mutation in K562 cells was able to induce prolong G2/M phase activation leading to their arrest.[10] Thus, BCR-ABL mutation in K562 cells can be one of the mechanisms of cell death due to the effect of PGPE.
Cell shrinkage, membrane blebbing, nuclear condensation and DNA fragmentation are the hallmarks of apoptosis. PI and DAPI gave positive stain in the dead cells due to the loss of membrane integrity and exposed inner membrane compartment. In the current study, PGPE treated cells were likely in late apoptosis presented by double staining of PI and Annexin V-FITC. We postulated on disintegrated nuclei in PGPE treated cells based on appearance of clumped cells double stained with PI-DAPI compared with single cells stained shown in untreated K562 cells. Results also demonstrated shiny nucleus with DAPI and PI staining in untreated K562 cells while fainted emission was observed in PGPE treated cells. Previous study reported that fluorescence intensity which increased 20-folds higher occurred when DAPI is bound to double-stranded DNA.[17] This may explain the low intensity of DAPI signal in PGPE treated cells which may be due to DNA damage.
To determine regulatory proteins involved in apoptotic activity and cell cycle arrest, western blot was performed. Following PGPE treatment, cell cycle arrest was due to p21 activation and apoptosis induction was initiated by caspase 9 activation. The salient biochemical pathway was proposed as follows. Cytochrome C execution process starts after execution of mitochondrial anti-apoptotic protein, bcl-2 which is not bax dependent. Previous studies reported that cytochrome C release from mitochondria can be due to multiple mechanisms such as bax/bak activation, permeability of transition pores and loss of mitochondrial membrane potential.[18] The release of cytochrome C into intracellular spaces leads to activation of the caspase cascade by the release of caspase 9, caspase 3 and caspase 7.[19] Caspase activity causes morphological changes in the cells. Regarding cell cycle arrest in PGPE treated cells, the cyclin dependent kinase inhibitor (CDKi), p21 protein was up-regulated by 8.3-fold, which indicates that p21 is actively involved in regulation of cell cycle in PGPE treated cells. Thus, activation of p21 was an indication of cell cycle arrest. Expression of p21 was directly proportional with p53 expression. P21 transcription inhibits CDK activity, leading to cell cycle arrest while p53 has been described to be involved in gene regulation, apoptosis and cell cycle regulation. Due to external stimuli such as treatment with PGPE, the cells overcome cellular stress and DNA damage by the activation of p53.[20]
Finally, our findings indicate that the ethanol extract of pomegranate peel has growth inhibitory effects on CML cells, K562 modulated by cell cycle arrest and apoptosis induction. The main mechanism of cell inhibition is through cell cycle arrest by p21 activation. Besides, phytochemical screening led to isolation of saponins, flavonoids and tannins/polyphenolic, compounds exhibiting anti-cancer property. This preliminary data will provide an idea on potential of pomegranate peel to be used as alternative or complementary medicine for treatment in CML.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Malaysian Agricultural Research and Development, Punica granatum L. Characterization, Strategic Resources Research Centre, Serdang Selangor Malaysia. 2012 [Google Scholar]
- 2.Lansky EP, Newman RA. Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer. J Ethnopharmacol. 2007;109:177–206. doi: 10.1016/j.jep.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Afaq F, Saleem M, Krueger CG, Reed JD, Mukhtar H. Anthocyanin- and hydrolyzable tannin-rich pomegranate fruit extract modulates MAPK and NF-kappaB pathways and inhibits skin tumorigenesis in CD-1 mice. Int J Cancer. 2005;113:423–33. doi: 10.1002/ijc.20587. [DOI] [PubMed] [Google Scholar]
- 4.Viuda-Martos M, López JF, Pérez-Álvarez JA. Pomegranate and its many functional components as related to human health: A review. Compr Rev Food Sci Saf. 2010;9:635–54. doi: 10.1111/j.1541-4337.2010.00131.x. [DOI] [PubMed] [Google Scholar]
- 5.Zahin M, Aqil F, Ahmad I. Broad spectrum antimutagenic activity of antioxidant active fraction of Punica granatum L. peel extracts. Mutat Res. 2010;703:99–107. doi: 10.1016/j.mrgentox.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Malik A, Afaq F, Sarfaraz S, Adhami VM, Syed DN, Mukhtar H. Pomegranate fruit juice for chemoprevention and chemotherapy of prostate cancer. Proc Natl Acad Sci U S A. 2005;102:14813–8. doi: 10.1073/pnas.0505870102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larrosa M, Tomas-Barberan FA, Espin JC. The dietary hydrolysable tannin punicalagin releases ellagic acid that induces apoptosis in human colon adenocarcinoma Caco-2 cells by using the mitochondrial pathway. J Nutr Biochem. 2006;17:611–25. doi: 10.1016/j.jnutbio.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 8.Jeune MA, Kumi-Diaka J, Brown J. Anticancer activities of pomegranate extracts and genistein in human breast cancer cells. J Med Food. 2005;8:469–75. doi: 10.1089/jmf.2005.8.469. [DOI] [PubMed] [Google Scholar]
- 9.Yoshimura M, Watanabe Y, Kasai K, Yamakoshi J, Koga T. Inhibitory effect of an ellagic acid-rich pomegranate extract on tyrosinase activity and ultraviolet-induced pigmentation. Biosci Biotechnol Biochem. 2005;69:2368–73. doi: 10.1271/bbb.69.2368. [DOI] [PubMed] [Google Scholar]
- 10.Skorski T. Genomic instability: The cause and effect of BCR/ABL tyrosine kinase. Curr Hematol Malig Rep. 2007;2:69–74. doi: 10.1007/s11899-007-0010-6. [DOI] [PubMed] [Google Scholar]
- 11.Fulda S. Cell death in hematological tumors. Apoptosis. 2009;14:409–23. doi: 10.1007/s10495-008-0306-6. [DOI] [PubMed] [Google Scholar]
- 12.Dash BC, El-Deiry WS. Cell cycle checkpoint control mechanisms that can be disrupted in cancer. Methods Mol Biol. 2004;280:99–161. doi: 10.1385/1-59259-788-2:099. [DOI] [PubMed] [Google Scholar]
- 13.Chadarat A, Siriporn O, Songyot A. Cytotoxicity of extracts from fruit plants against leukemic cell lines. Afr J Pharm Pharmacol. 2010;4:13–21. [Google Scholar]
- 14.Asmaa MJ, Al-Jamal HA, Ang CY, Asan JM, Seeni A, Johan MF. Apoptosis induction in MV4-11 and K562 human leukemic cells by Pereskia sacharosa (Cactaceae) leaf crude extract. Asian Pac J Cancer Prev. 2014;15:475–81. doi: 10.7314/apjcp.2014.15.1.475. [DOI] [PubMed] [Google Scholar]
- 15.Talpaz M, Shah NP, Kantarjian H, Donato N, Nicoll J, Paquette R, et al. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N Engl J Med. 2006;354:2531–41. doi: 10.1056/NEJMoa055229. [DOI] [PubMed] [Google Scholar]
- 16.Patel D, Suthar MP, Patel V, Singh R. BCR ABL kinase inhibitors for cancer therapy. Int J Pharm Sci Drug Res. 2010;2:80–90. [Google Scholar]
- 17.Chazotte B. Labeling nuclear DNA using DAPI. Cold Spring Harb Protoc 2011. 2011 doi: 10.1101/pdb.prot5556. pdb.prot5556. [DOI] [PubMed] [Google Scholar]
- 18.Garrido C, Galluzzi L, Brunet M, Puig PE, Didelot C, Kroemer G. Mechanisms of cytochrome c release from mitochondria. Cell Death Differ. 2006;13:1423–33. doi: 10.1038/sj.cdd.4401950. [DOI] [PubMed] [Google Scholar]
- 19.Budihardjo I, Oliver H, Lutter M, Luo X, Wang X. Biochemical pathways of caspase activation during apoptosis. Annu Rev Cell Dev Biol. 1999;15:269–90. doi: 10.1146/annurev.cellbio.15.1.269. [DOI] [PubMed] [Google Scholar]
- 20.Brooks CL, Gu W. New insights into p53 activation. Cell Res. 2010;20:614–21. doi: 10.1038/cr.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]


