Abstract
Background:
The diabetic condition is influenced by several factors, some of which can accelerate the disease's progression to various complications that aggravate the morbidity.
Aims:
This study aimed at determining the prevalence of metabolic syndrome (MetS) and its individual components and the most critical predictive risk factors of MetS in type 2 diabetic patients.
Materials and Methods:
This cross-sectional study involved 150 type 2 diabetes mellitus patients and was conducted at the Diabetes Centre of the Komfo Anokye Teaching Hospital in Kumasi, the Ashanti Region of Ghana, from February, 2013 to April, 2013. The study involved the use of a questionnaire to obtain some information on the diabetics, undertaking anthropometric measurements, as well as collecting blood samples for the measurement of some biochemical parameters; fasting blood glucose and lipid profile. MetS was defined according to the National Cholesterol Education Program/Adult Treatment Panel III criteria.
Results:
The prevalence of MetS was 58% in the studied Ghanaian population. Hypertension was the commonest risk factor (60%), followed by central obesity (48.67%) and dyslipidemia (37%). Female type 2 diabetics had a higher prevalence of MetS, and carried more components than their male counterparts. Regression analysis showed three factors; femininity, high body mass index and low educational status were the most critical predictive risk factors of MetS, according to this study.
Conclusion:
With hypertension being the commonest component, future cardiovascular disease prevention strategies should focus attention on its management and prevention, through education.
Keywords: Metabolic syndrome, National Cholesterol Education Program/Adult Treatment Panel III criteria, predictive risk factors, prevention strategies, type 2 diabetes
INTRODUCTION
The hyperglycemic condition in diabetics makes them prone to developing some complications, which contribute to the morbidity. The susceptibility of diabetics to complications is driven by both modifiable and nonmodifiable risk factors. Currently, there is heightened interest in a cluster of some risk factors called metabolic syndrome (MetS) that predicts cardiovascular disease and type 2 diabetes mellitus.[1] It comprises the following major characteristics: Hypertriglyceridemia, low levels of high-density lipoprotein-cholesterol (HDL-C), central obesity (abdominal) obesity, hypertension and concomitant insulin resistance/glucose intolerance (hyperinsulinemia).[2] It is associated with a three to five fold increased risk for the development of type 2 diabetes mellitus[3] which has now reached high proportions in many countries.[4] The worldwide prevalence of MetS is between 7.9% and 43% in males and 7% and 56% in females.[5] The prevalence of diabetes mellitus worldwide in the adult population is assumed to be 4%.[6] In urban Ghana, according to the Guidelines of the National Cholesterol Education Program/Adult Treatment Panel (NCEP/ATP III), the prevalence of the MetS is between 23 and 38%,[7] whereas type 2 diabetes mellitus affects at least 6% of the adult population in urban Ghana.[6] In 2008, the prevalence of MetS in type 2 diabetes patients at the Komfo Anokye Teaching Hospital was found to be 55.9%.[8] The syndrome may progress to type 2 diabetes,[9] a condition that is also becoming increasingly common.
The MetS is known to be caused by insulin resistance or insulin resistance-linked obesity, a condition whereby the body's cells are incapable of taking up glucose from the blood. Insulin resistance-linked obesity is caused by poor dieting and lack of regular exercise. Other genetic or lifestyle risk factors/predictor variables equally lead to the metabolic MetS. They are increasing age (greater than 40 years), smoking of cigarette, alcohol intake, overweight, sedentary life-style and family history of type 2 diabetes.[9]
Over the years, there has been increasing deaths from type 2 diabetes mellitus. Locally, however, there is little information on its causes, due to few published data on the prevalence of MetS and its association with type 2 diabetes mellitus. This study aims at identifying the most critical risk factors leading to MetS which predispose these populations to prediabetes and type 2 diabetes mellitus, as well as the impact of MetS on the progression of diabetes.
MATERIALS AND METHODS
Subjects
This study was conducted between February and April, 2013. One hundred and fifty (150) participants of the Diabetic Centre of the Komfo Anokye Teaching Hospital (50 males and 100 females) were involved. The study participants were of ages between 20 and 86 years. Participants fasted overnight before blood sampling. Excluded from the study were type 1 diabetics and pregnant women. The participants who were all indigenes of Ghana consented to take part in the study after a thorough explanation of the aim of the study. This study was approved by the Committee on Human Research, Publication and Ethics at the School of Medical Sciences of the Kwame Nkrumah University of Science and Technology and Komfo Anokye Teaching Hospital, Kumasi.
BLOOD SAMPLE COLLECTION AND PROCESSING
About 5 ml venous blood samples were collected after an overnight fast; 4 ml was dispensed into vacutainer® plain tubes and 1 ml into fluoride-oxalate tubes. After centrifugation at 500 g for 15 min, the serum and plasma were stored at −80°C until assayed. Parameters that were determined included; fasting blood glucose (FBG), triglycerides (TG) and HDL-C. The protocol for the determination of the parameters was as indicated in the manufacturer's instructions (Fortress Diagnostics Limited, Unit 2C Antrim Technology Park, Antrim BT41 1QS, United Kingdom).
Anthropometric variables
Using a questionnaire and patients medical records, information on demographic and clinical characteristics, such as age, sex, age of onset of diabetes, family history of diabetes were extracted. Blood pressure was measured using a sphygmomanometer. Blood pressure was recorded in the sitting position in the right arm. Two readings were taken 5 min apart, and the mean of the two was taken as blood pressure. Height was measured to the nearest 0.1 cm without shoes, using a stadiometer and weight to the nearest 0.1 kg in light clothing, using a bathroom scale (Zhongshan Camry Electronic Co. Ltd, Guangdong, China). Body mass index (BMI) was calculated by dividing weight (kg) by height squared (m2). Waist circumference was measured to the nearest 0.1 cm, using a Gulick II spring-loaded measuring tape midway between the inferior angle of the ribs and the suprailiac crest.
METABOLIC SYNDROME DEFINITION
Metabolic syndrome was defined according to the criteria of the NCEP/ATP III to include individuals with any three or more of the following five components:[1] Abdominal obesity (waist circumference >102 cm for men or >88 cm for women); (2) high TG ≥ 1.7 mmol/L (150 mg/dl); (3) low HDL-C: Men < 0.9 mmol/L (<40 mg/dl) or women <1.0 mmol/L (<50 mg/dl); (4) high blood pressure (systolic BP ≥ 130 mm Hg or diastolic BP ≥ 85 mm Hg or treatment of hypertension) and high FBG ≥ 6.1 mmol/L.[10]
Statistical analysis
The results are expressed as means ± standard error of mean (SEM), using GraphPad Prism 5.01 for windows (GraphPad Software, San Diego California USA, www.graphpad.com). Student's t-test was used to ascertain the significance of the difference between two means of continuous variables and ANOVA for difference in mean values of grouped data. A level of P < 0.05 was considered as statistically significant. Using STATA (version 16.0, Inc., Chicago, IL) statistical software, multiple logistic regression was performed to estimate the relationship between MetS and some risk factors, in order to find the most significant observed predictor of MetS by estimating the odds ratio (OR) of the predictors (i.e. the measure of association between a predictor and an outcome, which in this case is MetS). The coefficients or ORs, give an estimate of the magnitude of each predictor. Without adjusting for these predictors, the ORs were calculated at 95% confidence interval. Next, the adjusted ORs were calculated, using the variables that showed statistical significance, with P < 0.05.
RESULTS
General characteristics of the population
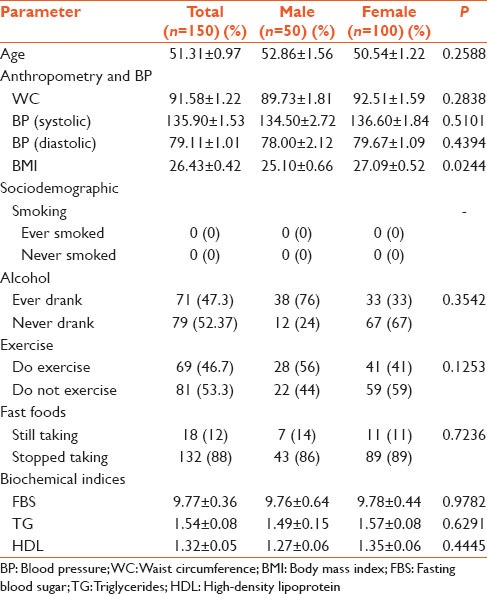
The study population comprised 150 type 2 diabetes mellitus patients, made up of 50 males (33.3%) and 100 females (66.67%). The overall mean age of the population was 51.31 (SEM = 0.97) years, whereas the ages of the males and females were 52.86 (SEM = 1.56) and 50.54 (SEM = 1.22), respectively [Table 1]. The overall mean value of BMI was 26.43 kg/m2, and the mean BMI of females was significantly higher (P < 0.0244) than that of males [Table 1]. Though the waist circumference of females was higher, there was no statistical significance. None of the subjects were smokers or had ever smoked. On the whole, 46.7% of the subjects said they exercised, 56% being males and 42% being females while 12.7% of the subjects were still taking fast foods. With respect to the biochemical parameters, none showed statistical significance between the males and females.
Table 1.
General characteristics of the studied population
Prevalence of metabolic syndrome and its individual components
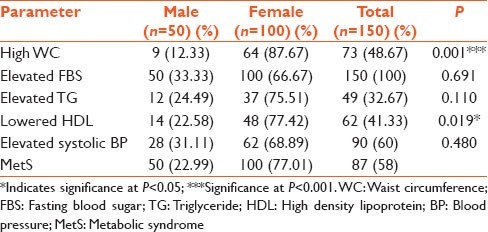
The overall percentage prevalence of MetS was 58% [Table 2]. Males had a lower percentage prevalence of 22.99%, compared to a higher percentage prevalence of 77.01% for the females. For the overall population, hypertension was the commonest component (60%) of the MetS, followed by high waist circumference, or central obesity. In females, central obesity was the most common component (87.67%), followed by lowered HDL (77.42%). In males, hypertension was the most common component (31.11%), followed by hypertriglyceridemia (24.49%). Central obesity and low HDL were the only components that showed statistically significant difference between males and females.
Table 2.
Prevalence of the individual components of MetS
Risk factors and their extent of influence or association with metabolic syndrome
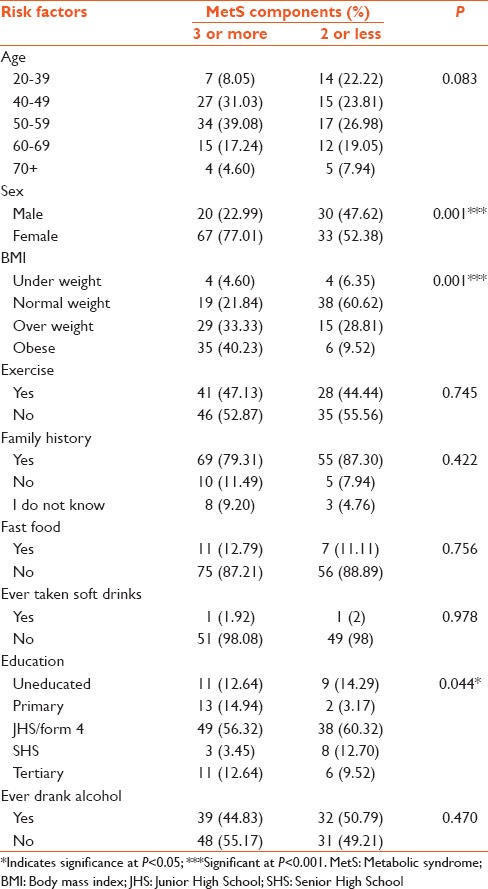
Individuals having three or more of the risk factors were reckoned as having the MetS. With respect to age, individuals within the age group of 50-59 had the highest prevalence of the MetS (39.08%), whereas individuals of age 70 years and above, had the lowest prevalence (4.60%) [Table 3]. The prevalence of MetS in the females was about 3 times higher (77.01%), compared to males. Obese people had more of the metabolic components (40.23%), compared to normal weight individuals (21.84%). Based on the level of education, majority of the junior high school or form four leavers had the highest prevalence of the MetS (56.32%). Factors like sex, BMI and educational status caused statistically significant differences, unlike the other independent variables.
Table 3.
A comparative analysis of the influence of the risk factors on MetS
Predictive effects of risk factors and their contribution to increased chances of acquiring metabolic syndrome using the National Cholesterol Education Program/Adult Treatment Panel III Criteria
The predictive effects of the risk factors (ie those that proved to be statistically significant in Table 3 and their contribution to an individual's increased chances or likelihood of meeting the criteria as defined by the NCEP/ATP III definition are shown in Table 4.
Table 4.
Extent of influence of selected risk factors on MetS (multiple logistic regression)
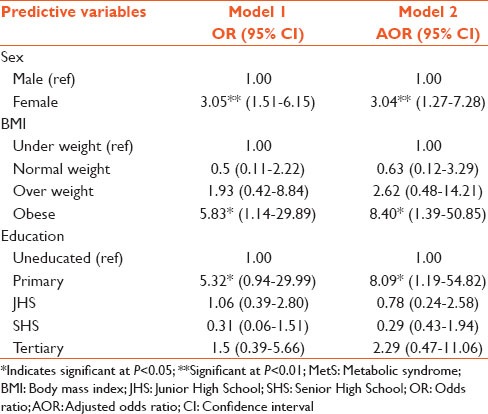
With respect to sex, the likelihood for females to develop MetS was 3 times (3.05 [1.51-6.15]) that for males. Obesity increased one's probability of having MetS, as obese individuals were almost 6 times [5.83 (1.14–29.89)] more likely to develop the MetS, as compared with underweight and normal weight individuals. Being a primary school leaver increased the odds of one having the MetS, as it showed one to be 5 times [5.32 (0.94–29.99)] more likely to having the MetS, as compared to the uneducated class.
DISCUSSION
Metabolic syndrome has been on the rise, contributing to the increasing prevalence of noncommunicable disorders such as cardiovascular diseases and type 2 diabetes mellitus. Despite the increasing prevalence, few studies have been done on the condition in Ghana, in the West African sub-region. Felix-Val et al.[8] undertook a study in the Diabetes Centre, and our study is a follow-up of that study. The NCEP/ATP III criteria was chosen to assess MetS prevalence because the indicators used are easily and readily measureable.
In contrast to the study of Felix-Val et al.,[8] the present study, apart from assessing the prevalence of MetS in type 2 diabetic patients, also used logistic regression analysis to determine the most critical risk factors that need to be monitored in order to control, prevent and treat diabetes. The main finding of this study was a high prevalence (58%) of MetS in type 2 diabetics.
Females showed higher prevalence of MetS (77.01%) as they had more of the risk factors contributing to MetS, compared to males (22.99%), which was consistent with a previous study by Felix-Val et al.[8] and Ford et al.[11] From the logistic regression analysis, females were 3 times more likely to have MetS than males [Table 4]. The reason may be due to a relatively sedentary lifestyle of women in this part of the world as most of them are traders or unemployed or it could be due to genetic factors.
There was a high prevalence of obesity, contributing to 40.23% of the entire diabetes study population. Obese persons were 5 times more likely to have MetS, compared to normal weight persons [Table 4]. Obesity worsens insulin resistance which then leads to increased hepatic production of very low density lipoprotein and the consequent release of high levels of TG in the bloodstream.
Persons with impaired glucose tolerance and type 2 diabetes, have hypertriglyceridemia as well as increased HDL catabolism, leading to lowered HDL levels.[12] A number of potential mechanisms could explain the inverse relationship between the hypertriglyceridemia of insulin resistant states and increased HDL catabolism, leading to low plasma HDL concentrations. One possibility is a reduction in lipoprotein lipase (LPL) activity, which would have the effect of impairing the maturation of HDL particles. The normal insulin-mediated stimulation of LPL activity has been shown to be blunted in insulin resistance.[13] In type 2 diabetes, particularly when glycemic control is poor and in patients who are relatively insulin deficient, LPL activity is reduced.[14] Thus, obesity is a major risk factor that really needs to be controlled in order to prevent type 2 diabetes mellitus or to stop or slow down the development of some complications.
There was a significantly higher prevalence of MetS amongst the diabetics with low educational status [Table 4], a finding similar to a study by Moebus et al. in 2007.[15] Diabetics with primary school education (14.94%) and those who were junior high school or form four leavers (56.32%) had higher prevalence, compared to senior high school (3.45%) diabetics with tertiary level education (12.64%). This could be due to their ignorance of good dietary habits, like eating too much saturated fatty foods, high carbohydrate diet, as well as irregular exercising and physical inactivity. In this study population, fast foods (12.79%) and soft drinks (1.92%) did not contribute significantly to the acquisition of MetS as most of the diabetics avoided them prior to developing the disease and also during their diseased state.
Family history has been reported to contribute to the speeding up of the acquisition of MetS.[16] In this study, 79.31% of the respondents who had a family history of diabetes, had three or more components of the MetS.
In the present study, hypertension was found to be the commonest component in the entire type 2 diabetes study population, followed by central obesity and lowered HDL-C. In the males, the most prevalent component was hypertension, followed by hypertriglyceridemia and then lowered HDL [Table 2]. This result is in fair agreement with Felix-Val et al. in 2008,[8] who, using the NCEP/ATP III, found hypertension to be the commonest in males, followed by hypertriglyceridemia. Most of the men (76%) were involved in heavy alcohol drinking prior to being diagnosed of diabetes [Table 1] and alcohol is known to induce hypertension.[17] Hypertensive diabetic patients have a greater risk of micro and macrovascular complications than normotensive patients.
In the females, the commonest component was central obesity, followed by lowered HDL, elevated triglyceride and hypertension [Table 2]. This could be due to less frequent participation of women in physical exercise, their sedentary lifestyle, probably attributable to their trading activity and regular intake of starchy foods, refined carbohydrates and late night eating. Moreover, it was found out that central obesity and lowered HDL-C prevalence were markedly higher in females than in males. It is well documented that lowered HDL-C levels are associated with an increased risk of coronary heart diseases or cardiovascular diseases.[18]
From Table 3, 27 (31.03%) of the 40–49 age group and 34 (39.08%) of the 50–59 age group had three or more components of the MetS. This indicates that between the ages of 40 and 59, more people are likely to have a high prevalence of the MetS components, irrespective of sex. Thus, serious preventive and control measures should be taken as one nears these age groups since the prevalence of MetS increases with age.[19] Individuals should be advised to exercise more, watch their diet by eating food containing little amounts of saturated fats and cholesterol, and foods containing refined sugars and rather take in more fiber-rich foods. High fiber diets have been shown to have good glycemic index, having the potential to lower fasting plasma glucose, total cholesterol and triglyceride, plasma concentrations. They simply decrease gastrointestinal absorption of cholesterol and carbohydrates.[20]
On the whole, there was a high prevalence of MetS among the type 2 diabetics studied, similar to the study of Felix-Val et al.[8] Through logistic regression analysis, our study has shown that three factors; sex, obesity and primary education have odd ratios of 3.05, 5.83 and 5.32, respectively in the causation of MetS. The respective adjusted odd ratios were 3.04, 8.40 and 8.09. BMI and low educational status are two modifiable factors. Knowing the commonest component (s) or risk factor (s) will give a guide to prevention and treatment of type 2 diabetes mellitus.
CONCLUSION
This study has shown an increased prevalence of MetS (58%), and through logistic regression analysis, has delineated the key risk factors driving morbidity. Most of the individual risk factors were more prevalent in women, compared to men; women were 3 times more likely to have MetS. The most prevalent component was hypertension, followed by central obesity, low HDL-C and hypertriglyceridemia. Low educational status and obesity also have great predictive effects on MetS in the type 2 diabetics.
LIMITATIONS AND STRENGTHS OF STUDY
The small sample size, makes it less representative of the general diabetic population of the country. The cross-sectional nature would not allow the cause-effect relationship to be established, making generalization of the findings difficult. Being a hospital-based study also introduces some bias factor.
The strengths of the study are the assessment of multiple parameters in one study, and the use of multiple logistic regression analyses to identify risk factors towards MetS in type 2 diabetics, and the quantification of the contributions due to the selected risk factors.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Lorenzo C, Okoloise M, Williams K, Stern MP, Haffner SM. San Antonio Heart Study. The metabolic syndrome as predictor of type 2 diabetes: The San Antonio heart study. Diabetes Care. 2003;26:3153–9. doi: 10.2337/diacare.26.11.3153. [DOI] [PubMed] [Google Scholar]
- 2.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 3.Wilson PW, D’Agostino RB, Parise H, Sullivan L, Meigs JB. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation. 2005;112:3066–72. doi: 10.1161/CIRCULATIONAHA.105.539528. [DOI] [PubMed] [Google Scholar]
- 4.Ford ES. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: A summary of the evidence. Diabetes Care. 2005;28:1769–78. doi: 10.2337/diacare.28.7.1769. [DOI] [PubMed] [Google Scholar]
- 5.Balkau B. Smoking, type 2 diabetes and metabolic syndrome. Diabetes Metab. 2004;30:110–1. doi: 10.1016/s1262-3636(07)70096-4. [DOI] [PubMed] [Google Scholar]
- 6.Danquah I, Bedu-Addo G, Terpe KJ, Micah F, Amoako YA, Awuku YA, et al. Diabetes mellitus type 2 in urban Ghana: Characteristics and associated factors. BMC Public Health. 2012;12:210. doi: 10.1186/1471-2458-12-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nyarko A, Adubofour K, Ofei F, Kpodonu J, Owusu S. Serum lipid and lipoprotein levels in Ghanaians with diabetes mellitus and hypertension. J Natl Med Assoc. 1997;89:191–6. [PMC free article] [PubMed] [Google Scholar]
- 8.Felix-Val K, Titty WK, Owiredu WK, Agyei-Frimpong MT. Prevalence of metabolic syndrome and its components among diabetes patients in Ghana. J Biol Sci. 2008;8:1057–61. [Google Scholar]
- 9.Ferrannini E. Physiological and metabolic sequences of obesity. Metabolism. 1995;44(Suppl 3):15–7. doi: 10.1016/0026-0495(95)90313-5. [DOI] [PubMed] [Google Scholar]
- 10.RidkerRidker PM, Buring JE, Cook NR, Rifai N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: An 8-year follow-up of 14 719 initially healthy American women. Circulation. 2003;107:391–7. doi: 10.1161/01.cir.0000055014.62083.05. [DOI] [PubMed] [Google Scholar]
- 11.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: Findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–9. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 12.Pietzsch J, Julius U, Nitzsche S, Hanefeld M. In vivo evidence for increased apolipoprotein A-I catabolism in subjects with impaired glucose tolerance. Diabetes. 1998;47:1928–34. doi: 10.2337/diabetes.47.12.1928. [DOI] [PubMed] [Google Scholar]
- 13.Moebus S, Hanisch JU, Aidelsburger P, Bramlage P, Wasem J, Jöckel KH. Impact of 4 different definitions used for the assessment of the prevalence of the Metabolic Syndrome in primary healthcare: The German Metabolic and Cardiovascular Risk Project (GEMCAS) Cardiovasc Diabetol. 2007;6:22. doi: 10.1186/1475-2840-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 15.Fan AZ, Zhou YB. 1st ed. New York: Humana Press; 2013. Alcohol, Nutrition and Health Consequences: Alcohol Intake and High Blood Pressure; pp. 321–7. [Google Scholar]
- 16.Lewis GF, Rader DJ. New insights into the regulation of HDL metabolism and reverse cholesterol transport. Circ Res. 2005;96:1221–32. doi: 10.1161/01.RES.0000170946.56981.5c. [DOI] [PubMed] [Google Scholar]
- 17.Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–7. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- 18.Chandalia M, Garg A, Lutjohann D, von Bergmann K, Grundy SM, Brinkley LJ. Beneficial effects of high dietary fiber intake in patients with type 2 diabetes mellitus. N Engl J Med. 2000;342:1392–8. doi: 10.1056/NEJM200005113421903. [DOI] [PubMed] [Google Scholar]
- 19.Eckel RH, Yost TJ, Jensen DR. Alterations in lipoprotein lipase in insulin resistance. Int J Obes Relat Metab Disord. 1995;19(Suppl 1):S16–21. [PubMed] [Google Scholar]
- 20.Yost TJ, Froyd KK, Jensen DR, Eckel RH. Change in skeletal muscle lipoprotein lipase activity in response to insulin/glucose in non-insulin-dependent diabetes mellitus. Metabolism. 1995;44:786–90. doi: 10.1016/0026-0495(95)90193-0. [DOI] [PubMed] [Google Scholar]


