Abstract
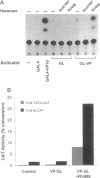
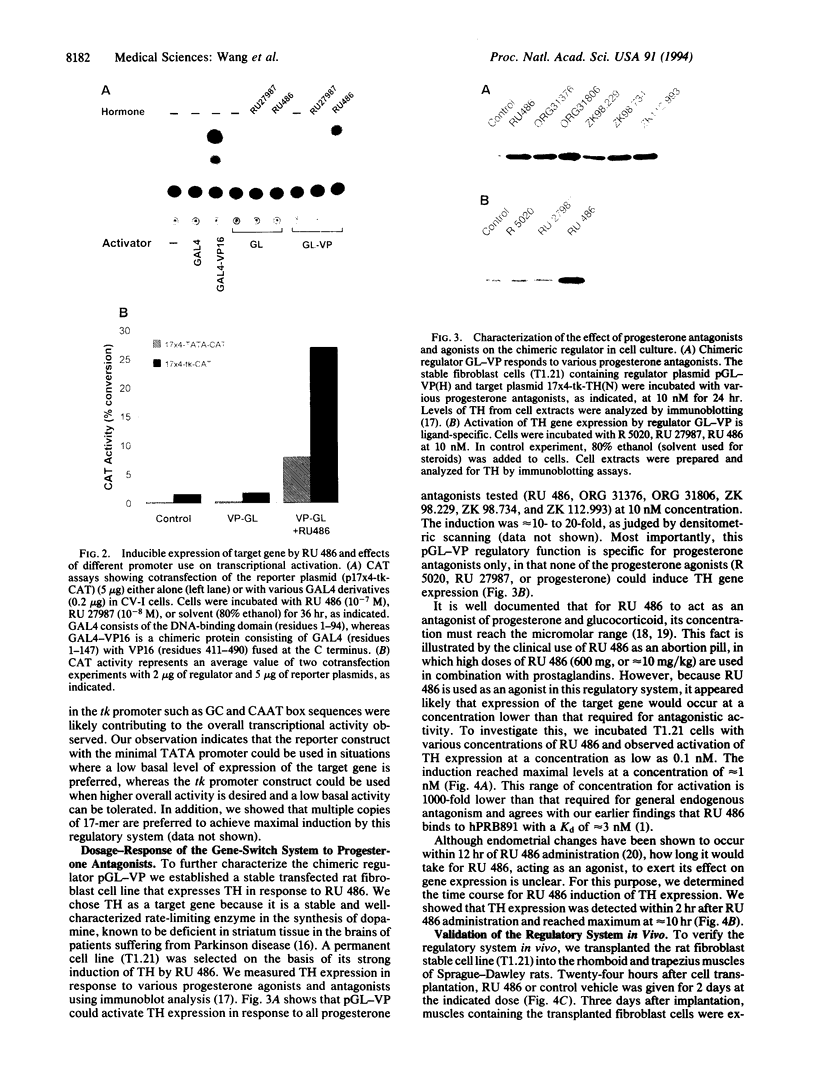
We recently have demonstrated that a C-terminal deletion mutant of the human progesterone receptor (hPRB891) fails to bind to progesterone but can bind RU 486 (Mifepristone) and other progesterone antagonists. Most significantly, this mutant receptor activates transcription of a reporter gene containing the progesterone response element in the presence of these antagonists. Taking advantage of this finding and the modular nature of functional domains of steroid receptors, we constructed a chimeric regulator (pGL-VP) by fusing the ligand-binding domain of human progesterone receptor hPRB891 to the yeast transcriptional activator GAL4 DNA-binding domain and the herpes simplex virus protein VP16 activation domain. We demonstrated that this chimeric regulator activates target genes containing the GAL4-binding sites in transient transfection assays in response to RU 486. In addition, this regulatory system has been validated by ex vivo transplantation of a stable cell line containing both the regulator and a reporter gene into rats. The dosage of RU 486 used is significantly lower than that required for antagonizing progesterone action. The gene-switch system reported here represents a regulatory system, which could be applicable for gene-transfer studies involving animals, as well as humans, in which the delivered gene(s) can be specifically turned on/off in response to an exogenous compound.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baim S. B., Labow M. A., Levine A. J., Shenk T. A chimeric mammalian transactivator based on the lac repressor that is regulated by temperature and isopropyl beta-D-thiogalactopyranoside. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5072–5076. doi: 10.1073/pnas.88.12.5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baniahmad A., Köhne A. C., Renkawitz R. A transferable silencing domain is present in the thyroid hormone receptor, in the v-erbA oncogene product and in the retinoic acid receptor. EMBO J. 1992 Mar;11(3):1015–1023. doi: 10.1002/j.1460-2075.1992.tb05140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baulieu E. E. Contragestion and other clinical applications of RU 486, an antiprogesterone at the receptor. Science. 1989 Sep 22;245(4924):1351–1357. doi: 10.1126/science.2781282. [DOI] [PubMed] [Google Scholar]
- Braselmann S., Graninger P., Busslinger M. A selective transcriptional induction system for mammalian cells based on Gal4-estrogen receptor fusion proteins. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1657–1661. doi: 10.1073/pnas.90.5.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brogden R. N., Goa K. L., Faulds D. Mifepristone. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential. Drugs. 1993 Mar;45(3):384–409. doi: 10.2165/00003495-199345030-00007. [DOI] [PubMed] [Google Scholar]
- Carey M., Kakidani H., Leatherwood J., Mostashari F., Ptashne M. An amino-terminal fragment of GAL4 binds DNA as a dimer. J Mol Biol. 1989 Oct 5;209(3):423–432. doi: 10.1016/0022-2836(89)90007-7. [DOI] [PubMed] [Google Scholar]
- Epner D. E., Herschman H. R. Heavy metals induce expression of the TPA-inducible sequence (TIS) genes. J Cell Physiol. 1991 Jul;148(1):68–74. doi: 10.1002/jcp.1041480109. [DOI] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich J. A., Hoey T., Thut C. J., Admon A., Tjian R. Drosophila TAFII40 interacts with both a VP16 activation domain and the basal transcription factor TFIIB. Cell. 1993 Nov 5;75(3):519–530. doi: 10.1016/0092-8674(93)90386-5. [DOI] [PubMed] [Google Scholar]
- Gossen M., Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunberg S. M., Weiss M. H., Spitz I. M., Ahmadi J., Sadun A., Russell C. A., Lucci L., Stevenson L. L. Treatment of unresectable meningiomas with the antiprogesterone agent mifepristone. J Neurosurg. 1991 Jun;74(6):861–866. doi: 10.3171/jns.1991.74.6.0861. [DOI] [PubMed] [Google Scholar]
- Heikinheimo O., Haukkamaa M., Lähteenmäki P. Distribution of RU 486 and its demethylated metabolites in humans. J Clin Endocrinol Metab. 1989 Feb;68(2):270–275. doi: 10.1210/jcem-68-2-270. [DOI] [PubMed] [Google Scholar]
- Hornykiewicz O. Parkinson's disease: from brain homogenate to treatment. Fed Proc. 1973 Feb;32(2):183–190. [PubMed] [Google Scholar]
- Ingles C. J., Shales M., Cress W. D., Triezenberg S. J., Greenblatt J. Reduced binding of TFIID to transcriptionally compromised mutants of VP16. Nature. 1991 Jun 13;351(6327):588–590. doi: 10.1038/351588a0. [DOI] [PubMed] [Google Scholar]
- Keegan L., Gill G., Ptashne M. Separation of DNA binding from the transcription-activating function of a eukaryotic regulatory protein. Science. 1986 Feb 14;231(4739):699–704. doi: 10.1126/science.3080805. [DOI] [PubMed] [Google Scholar]
- Lee F., Mulligan R., Berg P., Ringold G. Glucocorticoids regulate expression of dihydrofolate reductase cDNA in mouse mammary tumour virus chimaeric plasmids. Nature. 1981 Nov 19;294(5838):228–232. doi: 10.1038/294228a0. [DOI] [PubMed] [Google Scholar]
- Lillie J. W., Green M. R. Transcription activation by the adenovirus E1a protein. Nature. 1989 Mar 2;338(6210):39–44. doi: 10.1038/338039a0. [DOI] [PubMed] [Google Scholar]
- Lin Y. S., Ha I., Maldonado E., Reinberg D., Green M. R. Binding of general transcription factor TFIIB to an acidic activating region. Nature. 1991 Oct 10;353(6344):569–571. doi: 10.1038/353569a0. [DOI] [PubMed] [Google Scholar]
- Mayo K. E., Warren R., Palmiter R. D. The mouse metallothionein-I gene is transcriptionally regulated by cadmium following transfection into human or mouse cells. Cell. 1982 May;29(1):99–108. doi: 10.1016/0092-8674(82)90094-0. [DOI] [PubMed] [Google Scholar]
- Nelson M., Silver P. Context affects nuclear protein localization in Saccharomyces cerevisiae. Mol Cell Biol. 1989 Feb;9(2):384–389. doi: 10.1128/mcb.9.2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Malley B. The steroid receptor superfamily: more excitement predicted for the future. Mol Endocrinol. 1990 Mar;4(3):363–369. doi: 10.1210/mend-4-3-363. [DOI] [PubMed] [Google Scholar]
- Picard D., Salser S. J., Yamamoto K. R. A movable and regulable inactivation function within the steroid binding domain of the glucocorticoid receptor. Cell. 1988 Sep 23;54(7):1073–1080. doi: 10.1016/0092-8674(88)90122-5. [DOI] [PubMed] [Google Scholar]
- Sadowski I., Ma J., Triezenberg S., Ptashne M. GAL4-VP16 is an unusually potent transcriptional activator. Nature. 1988 Oct 6;335(6190):563–564. doi: 10.1038/335563a0. [DOI] [PubMed] [Google Scholar]
- Spencer D. M., Wandless T. J., Schreiber S. L., Crabtree G. R. Controlling signal transduction with synthetic ligands. Science. 1993 Nov 12;262(5136):1019–1024. doi: 10.1126/science.7694365. [DOI] [PubMed] [Google Scholar]
- Spitz I. M., Bardin C. W. Mifepristone (RU 486)--a modulator of progestin and glucocorticoid action. N Engl J Med. 1993 Aug 5;329(6):404–412. doi: 10.1056/NEJM199308053290607. [DOI] [PubMed] [Google Scholar]
- Swahn M. L., Bygdeman M., Cekan S., Xing S., Masironi B., Johannisson E. The effect of RU 486 administered during the early luteal phase on bleeding pattern, hormonal parameters and endometrium. Hum Reprod. 1990 May;5(4):402–408. doi: 10.1093/oxfordjournals.humrep.a137111. [DOI] [PubMed] [Google Scholar]
- Vegeto E., Allan G. F., Schrader W. T., Tsai M. J., McDonnell D. P., O'Malley B. W. The mechanism of RU486 antagonism is dependent on the conformation of the carboxy-terminal tail of the human progesterone receptor. Cell. 1992 May 15;69(4):703–713. doi: 10.1016/0092-8674(92)90234-4. [DOI] [PubMed] [Google Scholar]
- Wang Y., Miksicek R. J. Identification of a dominant negative form of the human estrogen receptor. Mol Endocrinol. 1991 Nov;5(11):1707–1715. doi: 10.1210/mend-5-11-1707. [DOI] [PubMed] [Google Scholar]