An Arabidopsis thaliana-specific isoform of a brassinosteroid-related transcription factor contains an additional N-terminal nuclear localization signal that affects its localization and function.
Abstract
Brassinosteroids (BRs) are essential steroid hormones that regulate plant growth and development. The transcription factor BRI1-EMS-SUPPRESSOR1 (BES1) regulates the expression of thousands of target genes in response to BRs. Here, we report an Arabidopsis thaliana-specific long isoform of BES1, BES1-L, which has stronger activity in promoting BR signaling than the canonical and widely used short BES1-S. The BES1-L isoform contains an additional N-terminal bipartite nuclear localization signal, which strongly promotes its nuclear localization. BES1-L also promotes the nuclear localization of BES1-S and BRASSINAZOLE-RESISTANT1 via dimerization. The transcription of BES1-L and BES1-S is differentially regulated by BRs due to the presence of G-box element in the BES1-S promoter. Moreover, BES1-L uniquely exists in the majority of A. thaliana ecotypes, but not in other species, even its Brassicaceae relatives, including Arabidopsis lyrata. The phenotypes of the BES1-L overexpression lines and plants with truncated BES1-L indicate that BES1-L is a more important isoform of BES1 in Arabidopsis and may have contributed to the evolution and expansion of A. thaliana.
INTRODUCTION
Brassinosteroids (BRs) are a class of plant-specific steroid hormones essential for plant normal development and responses to various environmental cues (Clouse and Sasse, 1998; Yang et al., 2011). BRs are perceived at the plasma membrane by the BRASSINOSTEROID INSENSITIVE1 (BRI1) and BRI1-ASSOCIATED KINASE1 (BAK1) receptor-coreceptor complex (Li and Chory, 1997; Li et al., 2002; Nam and Li, 2002). In the absence of BRs, BRI1 activity is cis- and trans-inhibited by its C terminus and BRI1 KINASE INHIBITOR1 (BKI1), respectively (Wang et al., 2005; Wang and Chory, 2006). Downstream of BR signaling, the GSK3-like kinase BRASSINOSTEROID INSENSITIVE2 (BIN2) phosphorylates and inhibits the activity of a class of plant-specific transcription factors, BRI1-EMS-SUPPRESSOR1 (BES1) and/or BRASSINAZOLE RESISTANT1 (BZR1) (Li and Nam, 2002; Wang et al., 2002; Yin et al., 2002; Zhao et al., 2002). When BRs bind to BRI1, the BRI1-BAK1 ectodomain dimerization is induced or stabilized, leading to the transphosphorylation and activation of their cytoplasmic kinase domain and phosphorylation of BKI1 and several receptor-like cytoplasmic kinases (Jiang et al., 2013; Santiago et al., 2013; Sun et al., 2013; J. Wang et al., 2014). The receptor-like cytoplasmic kinases activate the kelch-repeat phosphatase BRI1 SUPPRESSOR1, which dephosphorylates BIN2 to release its inhibition on BES1/BZR1 (Kim et al., 2009, 2011). Moreover, Protein Phosphatase 2A (PP2A) can dephosphorylate and activate BES1/BZR1 (Tang et al., 2011).
BES1/BZR1 were identified through several forward genetic screens. The gain-of-function mutant bes1-D was found among bri1-119, bri1-5, and bri1-9 suppressors (Yin et al., 2002; Zhao et al., 2002). BZR1, a close homolog of BES1, was found through identification of the dominant mutant bzr1-1D, which has long hypocotyls on medium containing the BR biosynthesis inhibitor brassinazole under darkness (Wang et al., 2002). Both BES1 and BZR1 consist of an N-terminal domain including a bipartite nuclear localization signal (NLS) and a DNA binding domain, a BIN2 phosphorylation domain, a PEST motif (rich in proline, glutamic acid, serine, and threonine), and a C-terminal transcriptional activation domain (Yin et al., 2005). Phosphorylation of BES1/BZR1 by BIN2 results in a reduction in protein stability (He et al., 2002; Yin et al., 2002), DNA binding, and transcriptional activity (Vert and Chory, 2006; Gampala et al., 2007), as well as enhanced interaction with 14-3-3 proteins for cytoplasmic accumulation (Gampala et al., 2007; Ryu et al., 2007). The unphosphorylated BES1 and BZR1 prefer to bind E-boxes (CANNTG) and/or BR response elements (CGTGT/CG) to regulate target gene expression (He et al., 2005; Yin et al., 2005). Furthermore, BES1 interacts with basic helix-loop-helix (bHLH) transcription factors BIM1-3, histone demethylases ELF6 and REF6, histone methyltransferase SDG8, MYB family transcription factor AtMYB30, corepressors MYBL2, HAT1, and TPL-HDA19, and transcription elongation factor IWS1 to regulate gene expression (He et al., 2005; Yin et al., 2005; Yu et al., 2008; Li et al., 2009, 2010; Ye et al., 2012; Ryu et al., 2014; X. Wang et al., 2014; Zhang et al., 2014). Genome-wide studies have revealed that the BES1/BZR1 family regulates a transcription network involving thousands of genes (Sun et al., 2010; Yu et al., 2011).
However, several major questions about BES1 remain unclear. First, the BES1 locus may encode different isoforms of proteins, but whether other isoforms exist is unknown. Almost all previous studies have focused on a short isoform of this protein. Second, overexpression of the canonical BES1 in wild-type Arabidopsis thaliana did not lead to an obvious BR-enhanced phenotype, and the mechanisms of its function are unknown (Yin et al., 2002; Ryu et al., 2010). Third, the nuclear localization of BES1/BZR1 in response to BRs is controversial. Some studies suggested that BES1 and BZR1 are distributed in the nucleus and cytoplasm, and their nuclear accumulation is enhanced by BR treatment (Wang et al., 2002; Yin et al., 2002). In contrast, some other studies reported that BES1 and BZR1 are constitutively localized in the nucleus (Zhao et al., 2002; Vert and Chory, 2006).
In this study, we verified that the A. thaliana BES1 locus encodes at least two types of transcripts, including the canonical BES1 (BES1-S) and a novel long isoform, BES1-L. The two transcripts are constitutively and widely expressed in multiple tissues. In contrast to BES1-S, BES1-L overexpression in the wild type led to a bes1-D-like phenotype with slim and curled leaves, as well as accelerated flowering time. The A. thaliana plants lacking BES1-L were small and dwarfed with delayed flowering. BES1-L was constitutively localized in the nucleus due to an additional NLS in the N terminus. Furthermore, BES1-L can promote nuclear accumulation of BES1-S and BZR1, and BIN2 negatively regulated this process. Interestingly, BES1-L was specifically present in A. thaliana, not in other examined plant species, including its Brassicaceae relatives. Therefore, BES1-L is a novel isoform of BES1, which plays important roles in plant growth and development and may have contributed to the evolution and expansion of A. thaliana.
RESULTS
Identification of BES1-L in A. thaliana
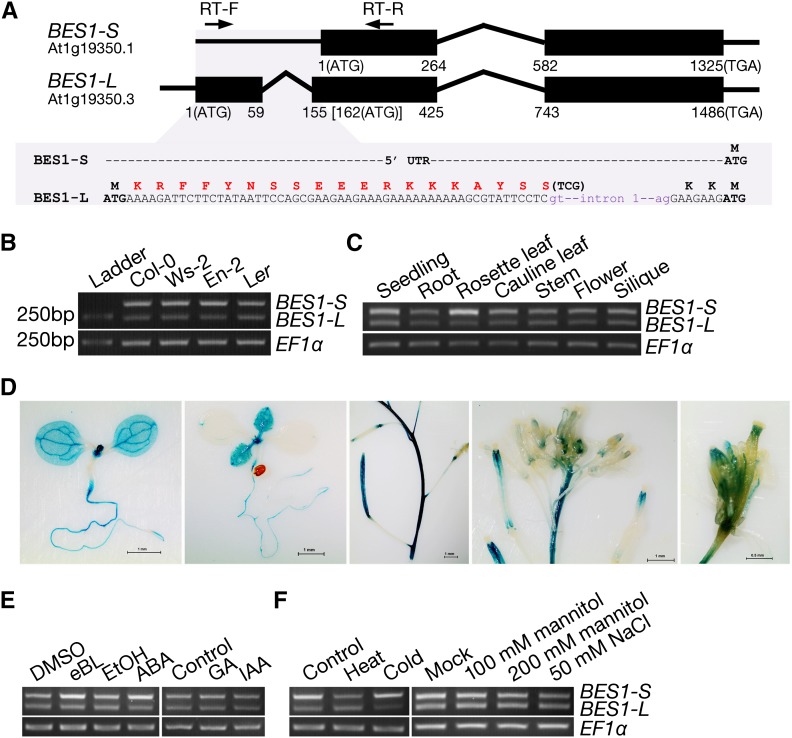
The canonical transcript produced by the BES1 locus (At1g19350) in A. thaliana consists of two exons and one intron, encoding a protein with 335 residues (Yin et al., 2002) (Figure 1A). However, BES1 has five predicted gene models encoding two possible proteins based on The Arabidopsis Information Resource (Lamesch et al., 2012; Lachowiec et al., 2013). Sequence analysis indicated that At1G19350.3 arises from an alternative transcription initiation (ATI) at another upstream transcription start site (TSS) and an alternative splicing (AS), thus encoding a longer protein with additional 22 amino acid residues at the N terminus (Figure 1A). We designated the widely studied BES1 as BES1-S and the longer BES1 identified in this study as BES1-L. The 22 extra residues are rich in positively charged amino acids (Arg and Lys) and were predicted to consist of a bipartite NLS with a higher activity score than the previously reported NLS of the canonical BES1 (Supplemental Figures 1A to 1C).
Figure 1.
Identification of BES1-L in A. thaliana.
(A) Schematic overview of the transcript variants from the A. thaliana BES1 locus. Introns (lines) and exons (boxes) are shown. The peptide sequence corresponding to the bipartite NLS is highlighted with red font.
(B) Gel electrophoresis results of RT-PCR. The BES1 specific primers (RT-F and RT-R) are shown in (A). An EF1α was used as a control.
(C) The expression pattern of BES1 isoforms revealed by RT-PCR in the 9-d-old seedlings and in different tissues from adult plants.
(D) GUS staining of the pBES1(L):GUS reporter line. The pBES1(L) promoter contains the 1.56-kb region upstream of the BES1-L start codon. GUS activity was monitored in 6-d-old seedlings, 9-d-old seedlings, and siliques, inflorescences, and flowers of adult plants. Bars = 1 mm (the 1st to 4th) or 0.5 mm (the 5th).
(E) The expression of BES1 isoforms after various plant hormone treatments. Seedlings were soaked in half-strength MS liquid medium containing 5 μM eBL or 50 μM ABA for 1 h or grown on half-strength MS medium containing 100 μM GA3 or 50 μM IAA for 9 d.
(F) The expression of BES1 isoforms after several abiotic stress treatments. Seedlings on plates were treated with heat (38°C) or cold (4°C) for 1 h or grown on half-strength MS medium with 100 and 200 mM mannitol or 50 mM NaCl for 9 d.
To confirm the existence of the BES1-L transcript in plants, we performed RT-PCR using total RNAs from A. thaliana seedlings. A pair of primers (RT-F/RT-R) was designed to amplify the two possible variants with a single PCR reaction (Figure 1A). DNA gel electrophoresis revealed that there are two PCR products with different sizes, which are present in several ecotypes, including Columbia-0 (Col-0), Wassilewskija-2, Enkheim-2 (En-2), and Landsberg erecta (Figure 1B). Sequencing the two bands demonstrated that the larger one consists of the 5′-untranslated region (UTR) and a portion of the first exon of BES1-S, while the smaller one contains the extra exon and a portion of the second exon of BES1-L with the first intron spliced.
Distribution and Regulation of BES1-L Expression
To explore the expression pattern of BES1-L, we conducted RT-PCR and quantitative RT-PCR (qRT-PCR) analysis using RNAs from various tissues. We found that the BES1-L was expressed in roots, rosette leaves, cauline leaves, stems, flowers, and siliques, which was similar to the expression pattern of BES1-S except that there was a higher ratio of BES1-S expression in rosette leaves (Figure 1C; Supplemental Figures 2A and 2B). Furthermore, we generated transgenic plants with a β-glucuronidase (GUS) reporter driven by a BES1-L promoter. Histochemical analysis indicated that pBES1(L):GUS was expressed in cotyledons, leaves, roots, stems, flowers, and siliques (Figure 1D). In addition, its expression in vascular tissues, root-hypocotyl junction, and shoot meristem appeared to be much stronger than in other tissues (Figure 1D). We also detected the expression of the two BES1 isoforms after treatment with various phytohormones and several abiotic stresses. Epibrassinolide (eBL) and abscisic acid (ABA) induced the expression of BES1-S, as revealed in both the qRT-PCR and RT-PCR assays, while the other hormones gibberellin (GA3) and auxin (indole-3-acetic acid [IAA]) had no obvious effect on the ratio of BES1-S/BES1-L expression (Figure 1E; Supplemental Figure 2C). Mannitol and NaCl treatments did not strongly alter the expression of BES1-S or BES1-L, while heat (38°C) treatment significantly repressed both BES1-L and BES1-S expression, and cold (4°C) treatment only inhibited the BES1-L expression (Figure 1F; Supplemental Figure 2D).
The Biological Functions of BES1-L
To explore the function of BES1-L in plants, we overexpressed the BES1-L cDNA fused with a C-terminal fluorescent protein (green fluorescent protein [GFP] or mCherry) driven by the 35S promoter in the BR-insensitive mutant bin2-1 and the BR-deficient mutant det2-1. The BES1-L overexpression (P35S:BES1-L-GFP) suppressed the dwarfism of bin2-1+/− (Supplemental Figures 3A and 3B). Furthermore, overexpression of BES1-L in det2-1 (P35S:BES1-L-mCherry) not only rescued its dwarfism, but also resulted in curly leaves, a phenotype observed in bes1-D (Supplemental Figures 3C and 3D). These data suggest that BES1-L functions in BR signaling.
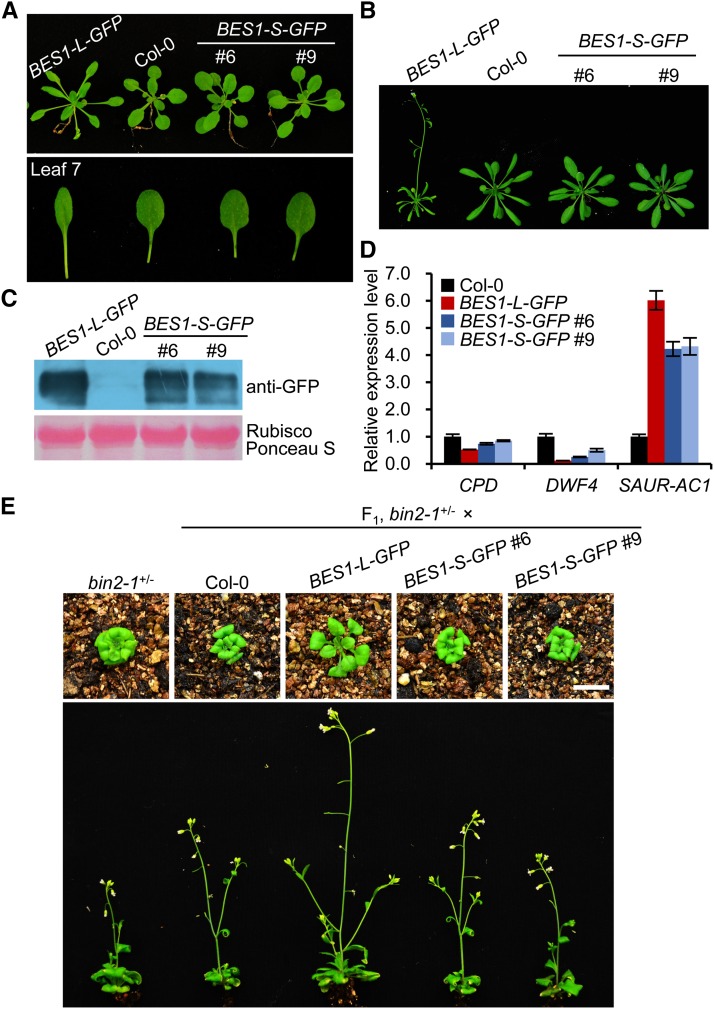
In previous studies, overexpression of BES1-S in the wild-type background led to no obviously altered phenotypes (Yin et al., 2002; Ryu et al., 2010). To compare the function of the two isoforms, we analyzed the phenotypes of the BES1-L-GFP (P35S:BES1-L-GFP) and the BES1-S-GFP (P35S:BES1-S-GFP) transgenic Col-0 plants. Interestingly, BES1-L-GFP overexpression led to plants with longer petioles and slightly curled leaves (Figures 2A to 2C; Supplemental Figures 3E and 3F), which were similar to the phenotypes of the moderate bes1-D (the dominant mutant of BES1-S) overexpression line (Yin et al., 2002). The BES1-L-GFP plants also showed accelerated flowering at the reproductive stage (Figure 2B). In contrast, the BES1-S-GFP overexpression lines did not display obviously different phenotypes from the wild type (Figures 2A to 2C; Supplemental Figures 3E and 3F). In addition, the expression of the BR-suppressed genes CONSTITUTIVE PHOTOMORPHOGENIC DWARF (CPD) and DWARF4 (DWF4) and the BR-induced small-auxin-up-RNA gene SAUR-AC1 in the BES1-L-GFP plants was inhibited or promoted more than that in the BES1-S-GFP plants (Figure 2D). Furthermore, we crossed the BES1-L-GFP or BES1-S-GFP overexpression lines with the bin2-1+/− mutant. In F1 plants, BES1-L but not BES1-S largely suppressed the dwarfism of bin2-1+/− (Figure 2E). These data suggest that BES1-L may possess a stronger function than BES1-S in promoting plant growth and BR signaling.
Figure 2.
The Phenotypes of BES1-L-GFP Transgenic Plants.
(A) Three-week-old BES1-L-GFP and BES1-S-GFP plants grown on soil. Col-0 was served as a control. The lower panel shows the seventh leaves.
(B) The 4-week-old plants shown in (A).
(C) Protein levels in corresponding plants shown in (A) and (B). Proteins were detected with anti-GFP antibody. Ponceau S-stained Rubisco large subunit serves as a loading control.
(D) The relative expression levels of some BR-responsive marker genes. CPD, DWF4, and SAUR-AC1 were detected by qRT-PCR in the corresponding plants shown in (A) and (B) (means ± sd). Three technical replicates were conducted.
(E) The phenotypes of the F1 plants of bin2-1+/− crossed with Col-0, BES1-L-GFP, or BES1-S-GFP, respectively. Upper panels, 3-week-old plants; lower panels, 5-week-old plants. Bar = 1 cm.
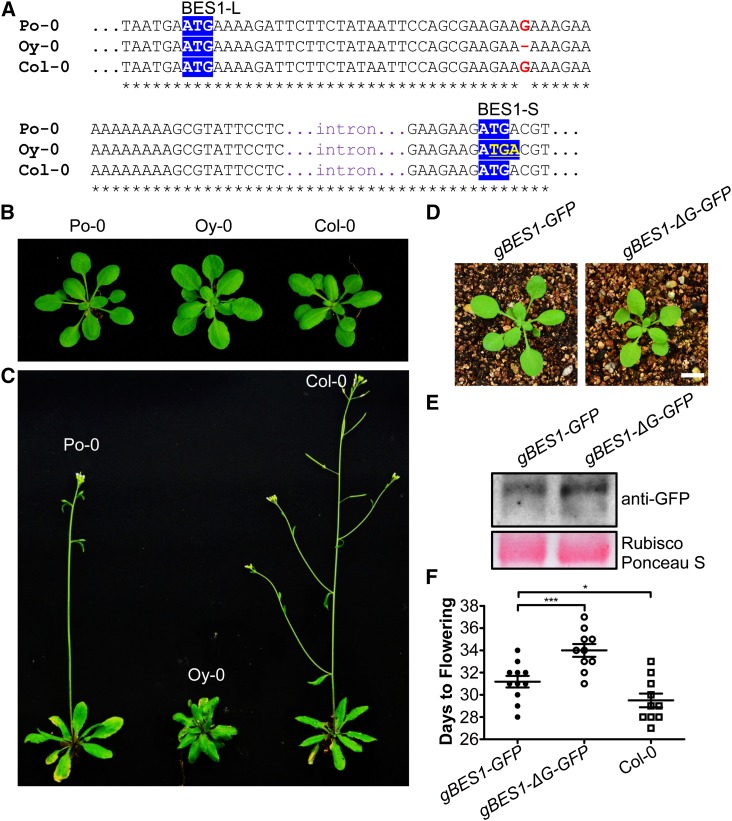
To further investigate the function of BES1-L, we tried to delete BES1-L using a CRISPR/Cas9 system, which was reported to be an efficient tool to edit plant or animal genomes (Cong et al., 2013; Mao et al., 2013). We designed two target positions on the BES1 locus, aiming to delete BES1-L alone or both BES1-L and BES1-S (Supplemental Figure 4A). We successfully obtained a mutant plant with a truncated BES1 (BES1Δ) but failed to obtain a mutant only knocking out the BES1-L. In the BES1Δ mutant (#4-3), the sequence of BES1 was changed by a 1-bp insertion in the 49th bp after the start codon of BES1-S, resulting in frame shift and a premature stop codon (Supplemental Figure 4B). The BES1Δ mutant displayed a dwarf phenotype with short petioles and round leaves (Supplemental Figure 4C). The BES1 protein level was reduced in the BES1Δ mutant (Supplemental Figure 4D). Alternatively, we checked the DNA sequences of BES1 locus among natural accessions to search for possible ecotypes lacking BES1-L protein, as many A. thaliana ecotypes had been sequenced by the 1001 Genomes Project and its cooperative projects (Cao et al., 2011; Gan et al., 2011). From the 19 Genomes Project (Gan et al., 2011), we found two ecotypes, Poppelsdorf-0 (Po-0; from Germany) and Oystese-0 (Oy-0; from Norway), carrying a 1-bp (G34) deletion in the 22-residual coding region, which presumably leads to a premature stop codon in BES1-L without affecting BES1-S. Resequencing the BES1 locus of the two ecotypes demonstrated that only Oy-0 carried this mutation (Figure 3A). Compared with Po-0 and Col-0, Oy-0 showed phenotypes with short petioles, round leaves, and delayed flowering (Figures 3B and 3C). Furthermore, overexpression of the genomic sequence of BES1 from Col-0 (gBES1-GFP) in Oy-0 resulted in larger plants and significantly accelerated flowering compared with Oy-0 (gBES1-ΔG-GFP) (Figures 3D to 3F). These data suggest that BES1-L plays an important role in promoting plant growth and development.
Figure 3.
The Phenotypes of A. thaliana Natural Accession Oy-0 Lacking BES1-L.
(A) The DNA sequence alignment of BES1 locus from Po-0, Oy-0, and Col-0. Red letters show the deletion of G34 in Oy-0. Yellow letters in blue background indicate the premature stop codon for BES1-L in Oy-0. White letters in blue show the start codon of BES1-L or BES1-S.
(B) The morphologic phenotype of the 3-week-old Po-0, Oy-0, and Col-0 ecotypes, respectively.
(C) The morphologic phenotype of the 4-week-old plants corresponding to that in (B).
(D) The phenotype of the 3-week-old transgenic Oy-0 with gBES1-GFP (corresponding to Col-0) and gBES1-ΔG-GFP (corresponding to Oy-0). Typical individuals are shown. Bar = 1 cm.
(E) Protein gel blotting detection of BES1-GFP in the rosette leaves of plants shown in (D).
(F) The flowering time of T1 gBES1-GFP/Oy-0 (n = 11) and gBES1-ΔG-GFP/Oy-0 (n = 10) transgenic plants and Col-0. Data are original data (plots) and means ± se. Student's t tests were used to determine the significant levels. ***P < 0.001 and *P < 0.05.
Subcellular Localization of BES1-L
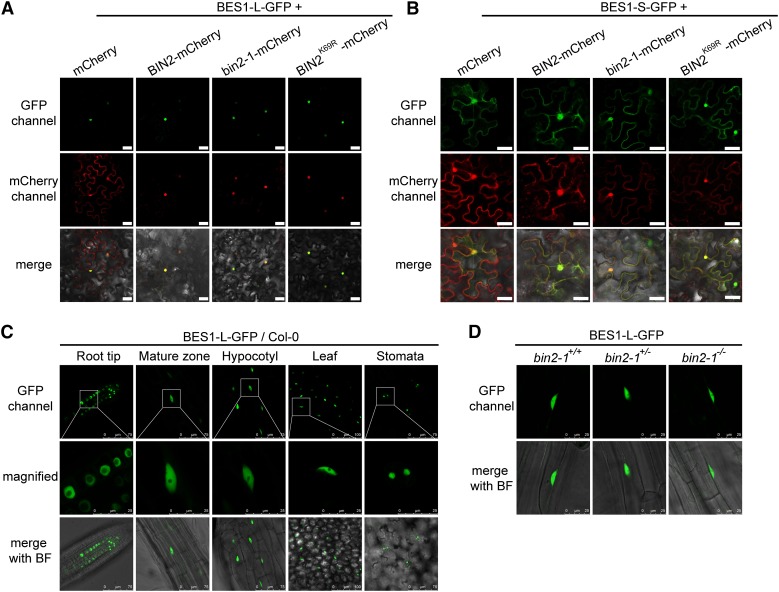
To determine whether the predicted additional NLS of BES1-L is functional, we examined the subcellular localization of BES1-L-GFP and found that it was almost entirely localized in the nucleus in wild tobacco (Nicotiana benthamiana) pavement cells (Figures 4A). In contrast, BES1-S-GFP was localized in both the nucleus and cytoplasm (Figures 4B). These data suggest that this NLS enhances the nuclear localization of BES1-L. We also found that BES1-L-GFP was constitutively localized in the nucleus in A. thaliana (Figure 4C). To further test whether BIN2 regulates the subcellular localization of BES1-L, we coexpressed BES1-L-GFP or BES1-S-GFP with a mCherry-tagged wild-type BIN2, a gain-of-function mutation bin2-1 (E263K), or a kinase-dead BIN2K69R in N. benthamiana. The subcellular localization of the BES1-S-GFP and BES1-L-GFP was not regulated by coexpression of BIN2-, bin2-1-, or BIN2K69R-mCherry (Figures 4A and 4B). Similarly, BES1-L was also solely localized in the nucleus of the bin2-1 mutant (Figure 4D). In contrast, the BES1-L-GFP apparently enhanced the nuclear accumulation of BIN2, bin2-1, and BIN2K69R (Figure 4A), implying that the BES1-L can promote the nuclear localization of its interacting proteins.
Figure 4.
The Subcellular Localization of BES1-L in N. benthamiana and A. thaliana.
(A) and (B) The subcellular localization of BES1-L-GFP (A) or BES1-S-GFP (B) coexpressed with mCherry, BIN2-, bin2-1-, or BIN2K69R-mCherry. The indicated constructs were transformed into N. benthamiana. Bars = 40 μm.
(C) The localization of BES1-L-GFP in various tissues of A. thaliana. The 4-d-old plants were used. The middle row shows the magnified images with visible nucleolus of the corresponding regions in the upper panels. Bars are labeled in the figure.
(D) The subcellular localization of BES1-L-GFP in root mature zones of the bin2-1 gain-of-function mutant. bin2-1+/+, +/− and −/− stands for wild type, heterozygous, and homozygous mutants, respectively. Bars = 25 μm.
BES1-L Promotes the Nuclear Localization of BES1-S and BZR1
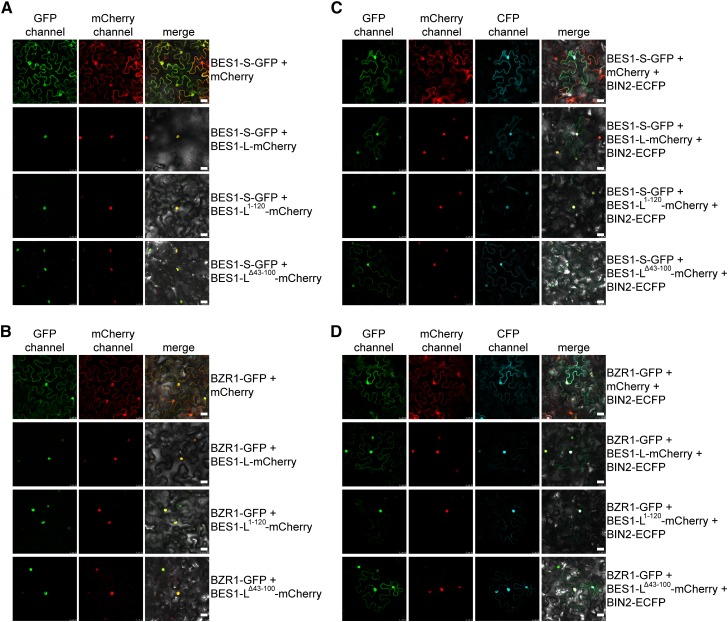
It was reported that BES1 undergoes homo-oligomerization (Vert and Chory, 2006), which was also demonstrated by our pull-down experiments (Supplemental Figure 5). Therefore, we hypothesized that BES1-L may promote the nuclear localization of BES1-S and BZR1 through dimerization. Coexpression of BES1-L-mCherry with BES1-S-GFP or BZR1-GFP in N. benthamiana showed that BES1-L-mCherry triggered the nuclear localization of both proteins (Figures 5A and 5B). To determine which domain mediates their interactions, we first predicted the secondary structure of BES1 and found that it contains a basic helix 1, a loop, and a helix 2 (bHLH) in the DNA binding domain (Supplemental Figure 6A). A previous study also suggested that BES1/BZR1 contain atypical bHLH DNA binding motifs (Yu et al., 2008). The bHLH motifs usually mediate homo- or heterodimerization to bind specific DNA elements (Ma et al., 1994; Toledo-Ortiz et al., 2003; Liu et al., 2013). To investigate whether the BES1-L’s bHLH domain mediates its interaction with BES1-S or BZR1 to promote their nuclear localization, we made a truncated BES1-L1-120 containing the NLS and the bHLH domain, and a truncated BES1-LΔ43-100 without the bHLH. Microscopic observation revealed that the coexpressed BES1-L1-120-mCherry promoted the nuclear accumulation of BES1-S-GFP and BZR1-GFP (Figures 5A and 5B). The BES1-LΔ43-100-mCherry also promoted their nuclear accumulation (Figures 5A and 5B), suggesting that the bHLH domain and other unknown domains can mediate the homo/heterointeraction between BES1-L and BES1-S/BZR1.
Figure 5.
BES1-L Promotes the Nuclear Localization of BES1-S and BZR1 in N. benthamiana.
(A) and (B) The localization of BES1-S-GFP (A) and BZR1-GFP (B) coexpressed with mCherry, BES1-L-, BES1-L1-120-, or BES1-LΔ43-100-mCherry, respectively. Bars = 25 μm.
(C) and (D) Coexpression of three fluorescence proteins. BIN2-ECFP was coexpressed with BES1-L-mCherry, BES1-L1-120-mCherry, or BES1-LΔ43-100-mCherry and BES1-S-GFP (C) or BZR1-GFP (D), respectively. mCherry coexpression serves as control. The indicated constructs were injected into leaves of N. benthamiana. Bars = 25 μm.
It was reported that BES1 phosphorylation by BIN2 kinase is able to disrupt the dimerization and DNA binding of BES1 (Vert and Chory, 2006; Gampala et al., 2007). To investigate whether BIN2 phosphorylation disrupts BES1-L’s effect on the subcellular localization of BES1-S and BZR1, we coexpressed three fluorescent proteins including BES1-S- or BZR1-GFP, BES1-L-, BES1-L1-120- or BES1-LΔ43-100-mCherry, and the enhanced cyan fluorescent protein (ECFP)-tagged BIN2 in N. benthamiana. We found that BIN2-ECFP inhibited BES1-L-mCherry- and BES1-LΔ43-100-mCherry-induced BES1-S/BZR1 nuclear accumulation, but did not affect their nuclear accumulation mediated by BES1-L1-120-mCherry (Figures 5C and 5D). The inhibitory effect of bin2-1-ECFP on the BES1-S and BZR1 nuclear accumulation promoted by BES1-L was similar to that of BIN2 (Supplemental Figures 7A and 7B). Furthermore, BES1-L1-120-mCherry overexpression in A. thaliana led to dwarfism (Supplemental Figures 6B and 6C), suggesting that BES1-L1-120 has a dominant-negative effect likely by forming nonfunctional homodimer or heterodimers with BES1 and BZR1.
BES1-L Contributes to the Nuclear Localization of BES1-S in A. thaliana
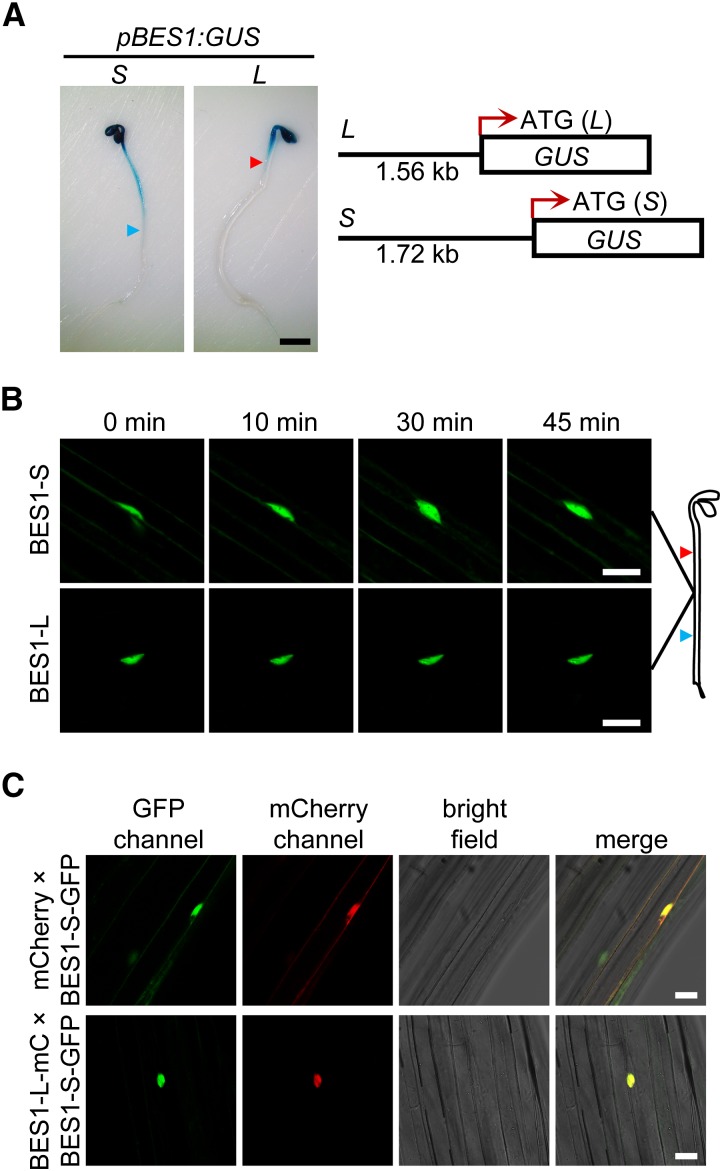
Since the subcellular localization of BES1-S/BZR1 is differentially regulated by BRs in hypocotyls in the dark and light (Wang et al., 2002; Yin et al., 2002; Zhao et al., 2002; Vert and Chory, 2006), we then checked the expression patterns of BES1-S and BES1-L in the dark-grown seedlings using their GUS reporters (Figure 6A). BES1-S was widely expressed in the entire hypocotyl, while BES1-L was only expressed in the upper part of the hypocotyl (Figure 6A), raising the possibility that the reduced BES1-L expression in the middle region of the hypocotyl may cause the cytoplasmic distribution of BES1-S and BZR1. Consistent with a previous report (Yin et al., 2002), the BES1-S-GFP was partially distributed in the cytoplasm, and eBL treatment promoted its nuclear localization in the middle of dark-grown hypocotyls (Figure 6B). In contrast, BES1-L-GFP was always localized in the nucleus (Figure 6B). Furthermore, we crossed the P35S:mCherry and P35S:BES1-L-mCherry plants with the BES1-S-GFP plants. In the F1, coexpression of BES1-L-mCherry promoted the nuclear localization of BES1-S-GFP in the middle of dark-grown hypocotyls, which was consistent with the observations in N. benthamiana pavement cells (Figures 5A and 6C). These data indicate that BES1-L contributes to the nuclear localization of BES1-S in A. thaliana and may explain the discrepancy of the BES1-S localization in different tissues and under different growth conditions.
Figure 6.
The Expression and Localization of BES1-S and BES1-L in Middle Hypocotyl Cells under Darkness.
(A) GUS staining of two different pBES1:GUS reporter lines. About 1.56-kb fragment upstream from ATG of BES1-L was labeled as L; the same site to ATG of BES1-S labeled as S. The 4-d-old seedlings (T2) grown under dark were used. The blue and red arrowheads refer to the approximate expression boundaries of pBES1(S):GUS and pBES1(L):GUS, respectively. Bar = 1 mm.
(B) The subcellular localization of BES1-S-GFP and BES1-L-GFP in Col-0 before and after 1 μM eBL treatment for indicated time. Bars = 25 μm.
(C) The localization of BES1-S-GFP with mCherry or BES1-L-mCherry coexpression. The 4-d-old dark-grown F1 plants of P35S:mCherry or P35S:BES1-L-mCherry crossed with BES1-S-GFP were used. Bars = 25 μm.
Differential Transcriptional Regulation of BES1-S and BES1-L
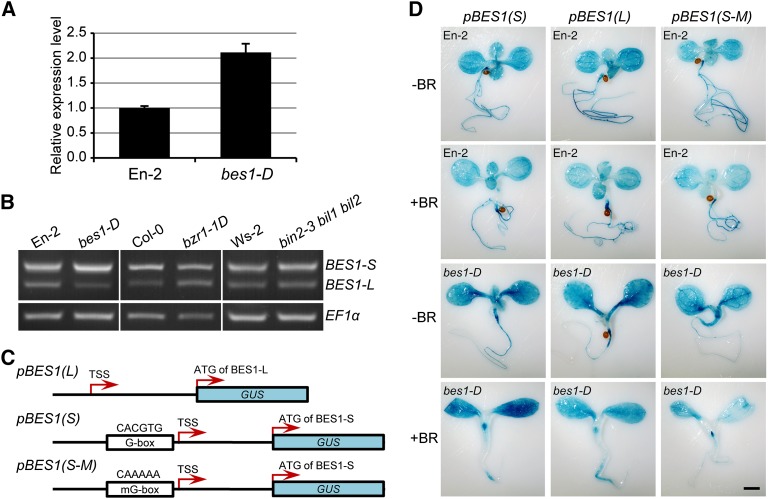
It was reported that BES1 is a target gene of BES1/BZR1, and its expression is positively regulated by itself (Sun et al., 2010; Yu et al., 2011). We observed that BES1 expression was largely enhanced in the bes1-D mutant (Figure 7A). RT-PCR and qRT-PCR further showed that BES1-S expression was increased, while the BES1-L expression was reduced, in bes1-D (Figure 7B; Supplemental Figure 2E). However, in the bzr1-1D and the GSK3 triple knockout mutant, bin2-3 bil1 bil2, the ratio of BES1-S/BES1-L expression was not remarkably altered (Figure 7B; Supplemental Figure 2E), suggesting that the BES1-S expression is enhanced by BES1 itself. In the wild-type and bes1-D seedlings, BR treatment greatly enhanced the BES1-S expression, but reduced the BES1-L expression, which was especially apparent in the root (Figures 7C and 7D). Genomic DNA sequence analysis indicated that a conserved extra G-box is present in the promoter of BES1-S versus the 5′-UTR of BES1-L (Figure 7C; Supplemental Figure 8C). We mutated this G-box to generate the GUS reporter line pBES1(S-M). Histochemical analysis showed that the GUS signal in plants harboring the pBES1(S-M) reporter was similar to that in plants harboring pBES1(L) (Figure 7D), indicating the G-box plays an important role in positively regulating BES1-S expression.
Figure 7.
Differential Regulation of BES1-S and BES1-L Expression.
(A) Relative expression level of BES1 determined by qRT-PCR in the wild type (En-2) and bes1-D backgrounds. Error bars indicate sd. Three technical replicates were conducted.
(B) The expression of BES1-S and BES1-L in bes1-D, bzr1-1D, bin2-3 bil1 bil2, and wild-type controls determined by RT-PCR. EF1α was used as a control.
(C) Schematic representation of the different BES1 promoter-driven GUS reporters.
(D) GUS staining of pBES1(S):GUS, pBES1(L):GUS, and pBES1(S-M):GUS transgenic plants in En-2 and bes1-D backgrounds. Seedlings were grown on half-strength MS without or with 100 nM eBL for 7 d. Bar = 1 mm.
BES1-L Is a More Recently Evolved Isoform in A. thaliana
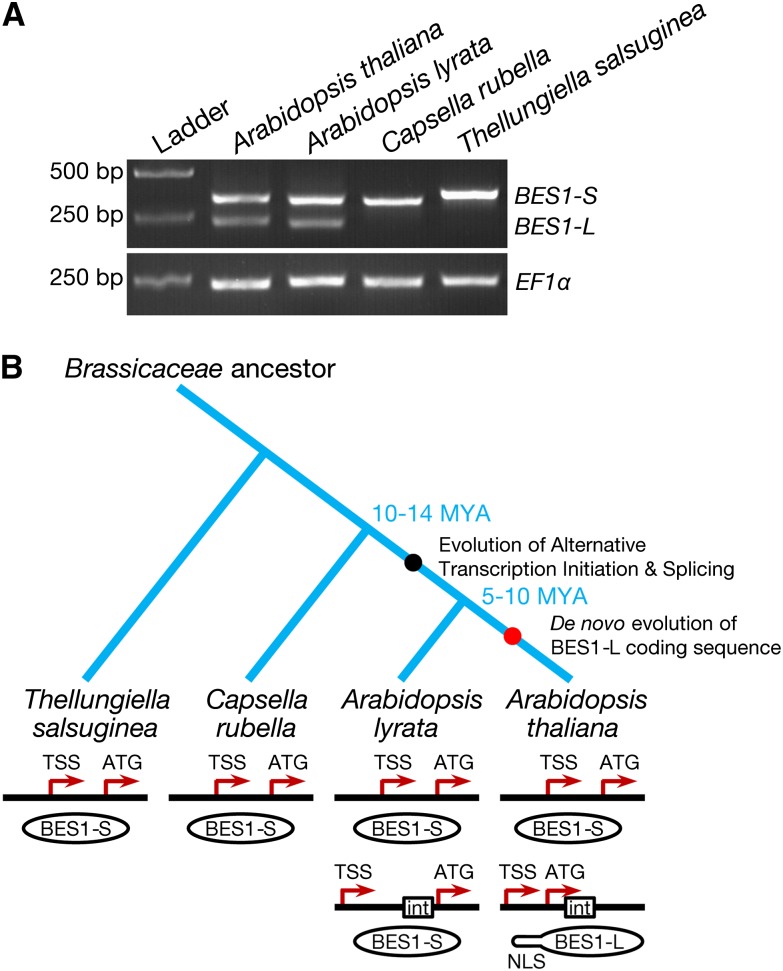
Transcription factors play important roles in organism evolution (Chen and Rajewsky, 2007). To avoid the deleterious effects of mutation, gene duplication events occur to allow one copy of a gene to maintain its function, while another copy may diverge to acquire new functions (Sayou et al., 2014). BES1 and BZR1 belong to a small gene family encoding plant-specific transcription factors, and they may have originated from a common ancestor and then diverged (Yin et al., 2005; Lachowiec et al., 2013). A previous examination of the dN/dS ratio, which is used to infer the direction and magnitude of the effect of natural selection on protein-coding genes, revealed that compared with BZR1, BES1 has been undergoing relaxed purifying selection, suggesting that BES1 may have a more rapid evolution rate. Furthermore, the molecular chaperone heat shock protein 90 acts as a capacitor to tolerate changes in its client BES1, allowing BES1 to evolve rapidly (Lachowiec et al., 2013). To evaluate the evolutionary novelty of BES1-L, we constructed a phylogenetic tree of BES1 proteins from multiple flowering plant species. We found that BES1-S is conserved in all flowering plant species including monocots and dicots, and the most recent common ancestor of living angiosperms Amborella trichopoda (Amborella Genome Project, 2013) (Supplemental Figure 8A). These results indicate that BES1-S is the ancient isoform. A BLASTp search with the 22-amino acid sequence of the BES1-L did not retrieve any sequences with high similarity (Supplemental Figure 8B), suggesting that this small region may have specifically evolved de novo by noncoding sequence exonization. We further investigated the occurrence of BES1-L in the A. thaliana-related Brassicaceae species. Interestingly, BES1-L transcript is not present in Capsella rubella or Thellungiella salsuginea, but it exists in the closer relative Arabidopsis lyrata (Figure 8A; Supplemental Figure 2F). However, sequence analysis revealed that the BES1-L-like transcript of A. lyrata may not encode a BES1-L protein because a premature stop codon makes Al-BES1-L encode a short peptide or Al-BES1-S (Supplemental Figures 8C and 8D). Moreover, alignment of this possible short peptide sequence with the N-terminal sequence of At-BES1-L revealed that two key positively charged Lys and Arg residues were replaced by two negatively charged Glu residues (Supplemental Figure 8D), likely resulting in a loss of NLS function. These data suggest that BES1-L is a more recently evolved isoform with a function specific to A. thaliana.
Figure 8.
The Evolution of BES1-L.
(A) RT-PCR gel electrophoretic analysis of BES1 transcripts in several Brassicaceae species. The A. thaliana ecotype Col-0, A. lyrata subsp lyrata ecotype MN47, C. rubella ecotype MTE, and T. salsuginea ecotype Shandong were used.
(B) A proposed model illustrating the two-step evolution of BES1-L in A. thaliana. First, in the Arabidopsis genus, a new TSS arises, leading to ATI and sequential AS. Then, in A. thaliana, BES1-L de novo encodes a novel BES1-L protein with an additional NLS. “int” stands for intron.
DISCUSSION
BES1-L Is an Important Isoform of BES1 in the BR Signaling Pathway
Since BES1 was first cloned from A. thaliana, one of the most well-studied model plants, the short isoform BES1-S has been widely used to study BR signaling. Here, we provided at least several lines of evidence to support the notion that the recently evolved isoform BES1-L plays a more important role in BR signaling, plant growth, and development than BES1-S in A. thaliana. First, overexpression of BES1-L but not BES1-S led to obvious phenotypes and rescued bin2-1+/−, implying that BES1-L may possess stronger functions. Second, nuclear localization is important for the function of a transcription factor. The unique NLS of BES1-L determines its constitutive nuclear localization in both N. benthamiana and A. thaliana. In addition, BES1-L can enhance the nuclear accumulation of BES1-S and BZR1 through dimerization. Apparently, the enhanced nuclear localization of BES1-L and its homologs accounts for its strong positive role in BR signaling. Third, BES1-L is widely expressed in various tissues, and BES1-L protein is almost identical to BES1-S. Domains including DNA binding domain, BIN2 phosphorylation domain, the PEST motif, and the C-terminal domain are the same except for the additional NLS at N terminus (Supplemental Figures 1D and 1E). Therefore, BES1-L may also be regulated by BIN2, 14-3-3 proteins, PP2A, or other interacting proteins in a similar manner to that of BES1-S. To test whether BES1-L is retained in the cytosol by 14-3-3 proteins, we coexpressed BES1-L-mCherry or mCherry with 14-3-3κ-yellow fluorescent protein (YFP) in A. thaliana. Interestingly, 14-3-3κ-YFP did not retain BES1-L-mCherry in the cytoplasm (Supplemental Figure 9). Thus, we proposed a working model of BES1-L in A. thaliana: After BES1-L and BES1-S are translated in the cytosol, BES1-L can translocate into the nucleus more strongly than BES1-S. The movement of BES1-L promotes the nuclear accumulation of BES1-S or other proteins via their dimerization, which can be negatively regulated by BIN2 phosphorylation (Supplemental Figure 10). BES1 was reported to regulate many physiological processes, such as male fertility, chloroplast function, shoot branching, and cellulose synthesis (Ye et al., 2010; Xie et al., 2011; Yu et al., 2011; Wang et al., 2013). In the future, BES1-L should be considered when studying the function of BES1 in A. thaliana.
The Mechanism of BES1-L Appearance
AS, ATI, and alternative transcription termination (ATT) are several mechanisms by which multiple transcripts are produced from a single gene. In the AS process, pairs of splice sites are differentially selected to generate multiple mRNA variants from a precursor (pre-) mRNA. In the ATI and ATT processes, the transcription sites are alternately initiated or terminated, resulting in alternate 5′- and 3′-UTRs in the transcripts (Shabalina et al., 2010). In mammals, ATI and ATT contribute to the diversity of human and mouse transcriptome more than AS, largely expanding the transcriptome and proteome diversity (Shabalina et al., 2010). However, very few examples of ATI and ATT are reported in plants. One example is that two variants of Glutathione S-Transferase F8 generated by ATI have differentially tissue-specific and stress-responsive expression patterns and different subcellular localizations in A. thaliana (Thatcher et al., 2007). Another example is the isoforms of protease Lon1 that arise from ATI, which are localized and function in the chloroplasts and mitochondria, respectively (Daras et al., 2014). For the BES1 locus, the BES1-L transcript initiates from an alternative TSS, and then the pre-mRNA undergoes alternative splicing to encode an in-frame BES1 protein with an extra NLS (Supplemental Figure 10).
The Mechanism of Homo- or Heterodimerization of BES1
Although BES1 and/or BZR1 were reported to interact with multiple bHLH transcription factors, such as BIM1-3 and phytochrome-interacting factor 4 (Yin et al., 2005; Wang et al., 2009; Oh et al., 2012), their working mechanisms are still not fully understood. Our secondary structure prediction indicates that the DNA binding domain of BES1 contains a bHLH motif, classifying BES1 as a bHLH transcription factor. The HLH domain mediates dimerization with high affinity, and the basic region usually recognizes and binds to major groove of DNA double helix (Ma et al., 1994). The basic region of BES1 was previously predicted to be a NLS (Yin et al., 2002). The basic regions of some bHLH transcription factors overlap with and function as NLS, such as SPATULA (Groszmann et al., 2008). Therefore, it is reasonable to speculate that the HLH domain of BES1 dimerizes with that of other proteins, including BES1, BZR1, and BIM1-3. Interestingly, many bHLH transcription factors are involved in the BR signaling pathway (Li, 2010). Our data demonstrated that the bHLH motif of BES1 mediates the dimerization with itself or its homologs. Thus, BES1-L participates in the nuclear localization of BES1-S and BZR1. It should be noted that except for the bHLH domain, some other domains may also mediate the dimerization of BES1. Because BES1-L is expressed in most tissues of A. thaliana, BES1-S and BZR1 are observed solely in the nucleus of A. thaliana roots, while they are localized in both the nucleus and cytoplasm in N. benthamiana, which lacks a BES1-L isoform (Figures 4A to 4C; Supplemental Figure 8A) (Zhao et al., 2002; Vert and Chory, 2006; Gampala et al., 2007). Furthermore, since BES1-L expression is low in hypocotyls of dark-grown A. thaliana seedlings, a certain amount of cytosolic BES1 and BZR1 can be observed, and BRs can promote their nuclear accumulation (Figures 6A to 6C) (Wang et al., 2002; Yin et al., 2002).
The Two-Step Evolution of BES1-L
Considering the unique existence of BES1-L in A. thaliana, we propose a two-step evolutionary model of BES1-L (Figure 8B). The first step is the appearance of a new TSS of the BES1 locus. The ancestor of the BES1 locus produced the BES1-S-type transcript, starting from a TSS behind the G-box. In the Arabidopsis genus of the Brassicaceae family, another TSS emerged and resulted in ATI to produce a longer transcript, which undergoes alternative splicing to encode a short peptide or BES1-S. The second step is the birth of BES1-L protein. Among the ancestors of A. thaliana, certain mutations led to a novel BES1 with a brand new NLS in the N terminus. This BES1-L can strongly promote BR signaling and other aspects of plant development, such as flowering time, by enhancing the nuclear localization of itself, BES1-S, and many other interacting proteins. The diversification of C. rubella and A. thaliana occurred ∼10 to 14 million years ago (MYA), and that of A. lyrata and A. thaliana occurred ∼5 to 10 MYA (Wright et al., 2002; Koch and Kiefer, 2005). Therefore, it is likely that ATI occurred in the ancestral Arabidopsis 10 to 14 MYA, and BES1-L appeared 5 to 10 MYA, which was likely accompanied by the origin and expansion of A. thaliana (Figure 8B).
Because the BES1-L overexpression lines displayed larger plants with accelerated flowering, and the ecotype Oy-0 lacking BES1-L showed smaller plants with delayed flowering (Figures 2A to 2C and 3A to 3C), it is likely that the occurrence of BES1-L shortened the lifecycle of A. thaliana and strengthened its fitness and competitiveness in the diversification and expansion from its ancestral home in central Asia to Europe, most of Asia, Australia, Africa, and the Americas (Al-Shehbaz and O'Kane, 2002; Hoffmann, 2002). More evidence, such as a fitness test in the field, is necessary to test this hypothesis.
METHODS
Plasmid Construction
To construct plasmids for transgenic plant production, the coding sequences of BES1-L, BES1-S, and BZR1 were inserted into the binary vector pCAMBIA2302. BES1-L, BES1-L1-120, and BES1-LΔ43-100 were cloned into pCAMBIA1300-mCherry. BIN2, bin2-1 (BIN2E263K), and BIN2K69R were cloned into pCAMBIA1300-mCherry or pCAMBIA1300-ECFP; the ECFP was mutated (Y145A and H148D, and T65S) from msf-CyPet (a derived form of CFP optimized for Förster resonance energy transfer with YPet for a longer lifetime and brightness improvement) (Goedhart et al., 2012). These binary vectors consist of a 5′ cauliflower mosaic virus 35S promoter and a 3′ GFP/mCherry/ECFP coding sequence. The promoters used for the GUS reporters and gBES1 expression are ∼1.56 and ∼1.72 kb from the start codons of BES1-L and BES1-S, respectively (using the same forward primer). Site-directed mutagenesis was conducted via overlapping PCR. All promoters were inserted into pCAMBIA-1300-221 containing a GUS coding sequence. For CRISPR/Cas9 construction, the guide sequence of a previously described vector pMD18 (Mao et al., 2013) was replaced by a sequence targeting BES1 or BES1-L using the overlapping PCR method and then digested by HindIII/KpnI and ligated to pCAMBIA1300. The gBES1/gBES1-ΔG fragments were inserted into pCAMBIA1302 (GFP-tagged) via the homologous recombination method (GBClonart). The restriction sites used in cloning are labeled in the primer names. The primers are listed in Supplemental Data Set 1.
Plant Materials and Growth Conditions
The Agrobacterium tumefaciens-mediated floral dip method (Clough and Bent, 1998) was used to generate transgenic plants. Agrobacterium strain GV3101 was transformed with binary plasmids and grown in Luria-Bertani media with appropriate antibiotics at 28°C. The bacteria was resuspended to OD600 ∼1.0 in transformation buffer (50 g sucrose, 2.2 g Murashige and Skoog [MS] powder, 200 μL Silwet-77, and 10 μL 6-BA for 1 liter, pH 5.8 to 6.0). Seeds were screened on hygromycin-containing half-strength MS agar plates (35 mg/L) and then transferred to soil. Several T2 or T3 plant lines or T1 plants were used for phenotypic analysis and other experiments. The Arabidopsis thaliana ecotype Po-0 and Oy-0 were obtained from the ABRC (stock numbers CS28648 and CS28591, respectively). Plants were grown at 23°C/21°C (day/night) under long-day conditions (16-h-light/8-h-dark cycle). Seeds of Arabidopsis lyrata, Capsella rubella, and Thellungiella salsuginea were vernalized at 4°C for 3 d and then grown in growth chambers. The seedlings were used for RT-PCR analysis. Nicotiana benthamiana plants were grown at 30°C under long-day conditions.
Hormone and Abiotic Stress Treatments
Nine-day-old Col-0 seedlings were soaked in half-strength MS liquid medium with or without 5 μM eBL or 50 μM ABA for 1 h. For long-term treatments, the Col-0 seedlings were grown on half-strength MS medium with 100 μM GA3, 50 μM IAA, 100 or 200 mM mannitol, or 50 mM sodium chloride (NaCl) for 9 d. Nine-day-old Col-0 seedlings grown on half-strength MS plates were incubated at 38°C or 4°C for 1 h, and plants grown under 23°C were used as a control. For the long-term BR treatment, plants were grown on half-strength MS plates with 100 nM eBL for 9 d. The treated seedlings were frozen in liquid nitrogen and preserved at −80°C.
GUS Staining Assay
Histochemical analysis of GUS expression was performed as described (Wang and Chory, 2006; Cheng et al., 2014). Briefly, transgenic plants containing the pBES1(S):GUS, pBES1(L):GUS, and pBES1(S-M):GUS reporters were grown on the half-strength MS plates with or without 100 nM eBL. Different tissues and organs from adult plant grown on soil were used. The samples were soaked in 90% acetone for 30 min for fixation and then washed and stained in X-Gluc solution (100 mM NaH2PO4, pH7.2, 2 mM K-ferrocyanide, 2 mM K-ferricyanide, 2 mM X-Gluc, and 0.1% Triton X-100) at 37°C in the dark for 8 to 12 h (overnight). Samples were rinsed sequentially with 70% (v/v) and 100% (v/v) ethanol to remove the chlorophyll. Digital images were taken with a Leica MZ FLIII stereomicroscope.
Quantitative and Standard RT-PCR
Total RNA was extracted using a Tiangen RNApre Plant Kit (Tiangen), and the first-strand cDNA was synthesized using a Takara PrimeScript First-strand cDNA synthesis kit (TaKaRa). For qRT-PCR, cDNAs were combined with SYBR master mix for PCR (Invitrogen/Bio-Rad). A U-box gene (At5g15400) or ACT7 (At5g09810) was used to normalize the data. qRT-PCR was performed in triplicate with an Eppendorf Cycler (Eppendorf) or Bio-Rad C1000 Cycler (Bio-Rad). Data were collected and analyzed with Eppendorf or Bio-Rad real-time PCR detection system and software. For RT-PCR, an Elongation Factor 1α (EF1α; At5g60390) gene was used to normalize the data. The number of PCR cycles for EF1α and BES1 was 24 and 36, respectively. PCR products were loaded onto a 2% agarose TAE gel and stained with ethidium bromide. The primers are listed in Supplemental Data Set 2.
Confocal Microscopy
For observation of the subcellular localization in N. benthamiana pavement cells, the indicated vectors were transformed into Agrobacterium strain GV3101 (Sparkes et al., 2006). Cells were cultured and resuspended to OD600∼2.0 in buffer (10 mM MES, 10 mM MgCl2, and 200 μM acetosyringone, pH 5.7) and infiltrated into young leaves. After 36 to 48 h, the fluorescent signals were analyzed. The root tips or indicated tissues of the 4-d-old transgenic seedlings of A. thaliana were used to observe subcellular localization. For eBL treatment, the middle hypocotyl of 4-d-old dark grown seedling was soaked in 1 μM eBL (100 mM stock in DMSO) on slides for the indicated time. Fluorescent signals were observed with a Leica SP8 confocal microscope.
Protein Gel Blotting
For detecting the protein levels in plants, the plant leaves were ground to a fine powder in liquid nitrogen. Total protein was extracted with 2× extraction buffer (100 mM Tris-HCl, pH 7.4, 100 mM NaCl, 5 mM EDTA, 1 mM PMSF, 5 mM DTT, 0.5% SDS, and 10 mM β-mercaptoethanol) or 5% SDS, separated with a 10% SDS-PAGE gel, and transferred to a nitrocellulose membrane. The membrane was blocked and incubated with monoclonal anti-mCherry (1/5000) or polyclonal anti-GFP (1/1000) and anti-BES1 (1/1000) antibodies, and goat anti-mouse/rabbit horseradish peroxidase-conjugated secondary antibodies. For in vitro pull-down assays, the MBP-BES1 and YFP-BES1-His proteins were purified via affinity chromatography. The MBP-BES1 protein was incubated with Amylose Resin (New England Biolabs) and then the YFP-BES1-His proteins was incubated with the resin, washed five times, and boiled. The signals were detected by anti-His antibody and visualized with x-ray film (Kodak) or the ChemiScope Mini CCD system (Clinx).
Bioinformatics and Phylogenetic Analysis
The secondary structure prediction was performed by PSIPRED server v3.3 (http://bioinf.cs.ucl.ac.uk/psipred/) (McGuffin et al., 2000). The NLS prediction was conducted by cNLS mapper (http://nls-mapper.iab.keio.ac.jp/cgi-bin/NLS_Mapper_form.cgi) (Kosugi et al., 2009), ELM tools (http://elm.eu.org) (Dinkel et al., 2012), or seqNLS (http://mleg.cse.sc.edu/seqNLS) (Lin and Hu, 2013). Protein domain prediction was done by SMART (http://smart.embl-heidelberg.de/) (Letunic et al., 2012). BLASTp search was conducted with a cutoff E value of 10 using the tool provided by the NCBI online (http://blast.ncbi.nlm.nih.gov/Blast.cgi). The BES1 protein sequences from various flowering plant species used for phylogenetic analysis are described in the Accession Numbers section and listed in Supplemental Data Set 2. The multiple sequence alignment was performed by ClustalW implemented in MEGA5 using default parameters with protein sequence and manually adjusted. The aligned protein sequences are listed in Supplemental Data Set 2. In our phylogenetic analysis, we selected BES1 as the outgroup from the basal angiosperm, Amborella trichopoda, which is the sister lineage to the ancestry of monocots and dicots in the phylogeny of angiosperms (Amborella Genome Project, 2013). The neighbor-joining tree with Dayhoff model was constructed using MEGA5 software (version 5.10) (Tamura et al., 2011). Bootstrap analysis was performed with 1000 replicates. The bootstrap values are shown at the nodes.
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative database and the GenBank/EMBL libraries under the following accession numbers: BES1-L (AT1G19350.3, NM_202134); BES1-S (AT1G19350.1, NM_101792); BZR1 (AT1G75080, NM_202416); BIN2 (AT4G18710, BT026031); 14-3-3κ (AT5G65430, NP_851274); U-box (AT5G15400, NM_121544); EF1α (AT5G60390, AK318784); and ACT7 (AT5G09810, U27811). The BES1 sequences of the following species can be found in the GenBank/EMBL libraries under the following accession numbers: A. lyrata, XM_002890284; C. rubella, XM_006305091; Thellungiella halophila, XM_006416450; Solanum tuberosum, XM_006346868; Populus trichocarpa, XM_002301181; Glycine max, XM_006601248; Oryza sativa Japonica, NM_001066635; Zea mays, XM_008655060; A. trichopoda, XM_006833009. The N. benthamiana BES1 sequence is from a released draft genome sequence by Boyce Thompson Institute (http://bti.cornell.edu/nicotiana-benthamiana/) (Bombarely et al., 2012) as NbS00054694g0005.1.
Supplemental Data
Supplemental Figure 1. Sequence Analysis of BES1-L.
Supplemental Figure 2. Expression of BES1-L and BES1-S Detected by Quantitative PCR.
Supplemental Figure 3. Phenotypes of BES1-L Overexpression Lines in the bin2-1+/−, det2-1, and Col-0 Backgrounds.
Supplemental Figure 4. CRISPR/Cas9 Engineering of BES1 Allele Mutants.
Supplemental Figure 5. Pull-Down Assay of MBP-BES1 with YFP-BES1-His.
Supplemental Figure 6. Functional Analysis of the bHLH Domain of BES1.
Supplemental Figure 7. Effect of bin2-1 on BES1-L Promoted Nuclear Localization of BES1-S and BZR1.
Supplemental Figure 8. Phylogenetic and Evolutionary Analysis of BES1.
Supplemental Figure 9. Effect of 14-3-3κ-YFP on the Subcellular Localization of BES1-L-mCherry.
Supplemental Figure 10. Proposed Working Model of BES1-L in Arabidopsis thaliana.
Supplemental Data Set 1. Primer Sequences Used in This Study.
Supplemental Data Set 2. BES1 Protein Sequences Used in the Phylogenetic Analysis.
Supplementary Material
Acknowledgments
We thank Hongya Gu (Peking University) for providing A. lyrata seedlings and seeds, Yalong Guo (Institute of Botany, Chinese Academy of Sciences [CAS]) for C. rubella seeds, Qi Xie (Institute of Genetics and Development, CAS) for T. salsuginea seeds, Yanhai Yin (Iowa State University) for the BES1-S-GFP overexpression seeds, Jianming Li (University of Michigan) for bzr1-1D mutant seeds, and Zhimin Zheng and Jian-Kang Zhu (Shanghai Center for Plant Stress Biology, CAS) for CRISPR/Cas9 systems. This work was supported by Grants 91317302, 31271300, and 31430046 of the National Natural Science Foundation of China (to X.W.), by Grant 2012CB114300 of the National Basic Research Program of China (to X.W.), and by a startup fund of Huazhong Agricultural University (to X.W.).
AUTHOR CONTRIBUTIONS
J.J., C.Z., and X.W. designed the research. J.J. and C.Z. performed research. J.J., C.Z., and X.W. analyzed data. J.J, C.Z., and X.W. wrote the article.
Glossary
- BR
brassinosteroid
- NLS
nuclear localization signal
- bHLH
basic helix-loop-helix
- ATI
alternative transcription initiation
- TSS
transcription start site
- AS
alternative splicing
- Col-0
Columbia-0
- UTR
untranslated region
- qRT-PCR
quantitative RT-PCR
- eBL
epibrassinolide
- ABA
abscisic acid
- IAA
indole-3-acetic acid
- Po-0
Poppelsdorf-0
- Oy-0
Oystese-0
- ATT
alternative transcription termination
- MYA
million years ago
- MS
Murashige and Skoog
- GA3
gibberellin
- En-2
Enkheim-2
References
- Al-Shehbaz I.A., O’Kane S.L. Jr. (2002). Taxonomy and phylogeny of Arabidopsis (brassicaceae). The Arabidopsis Book 1: e0001, doi/10.1199/tab.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amborella Genome Project (2013). The Amborella genome and the evolution of flowering plants. Science 342: 1241089. [DOI] [PubMed] [Google Scholar]
- Bombarely A., Rosli H.G., Vrebalov J., Moffett P., Mueller L.A., Martin G.B. (2012). A draft genome sequence of Nicotiana benthamiana to enhance molecular plant-microbe biology research. Mol. Plant Microbe Interact. 25: 1523–1530. [DOI] [PubMed] [Google Scholar]
- Cao J., et al. (2011). Whole-genome sequencing of multiple Arabidopsis thaliana populations. Nat. Genet. 43: 956–963. [DOI] [PubMed] [Google Scholar]
- Chen K., Rajewsky N. (2007). The evolution of gene regulation by transcription factors and microRNAs. Nat. Rev. Genet. 8: 93–103. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Zhu W., Chen Y., Ito S., Asami T., Wang X. (2014). Brassinosteroids control root epidermal cell fate via direct regulation of a MYB-bHLH-WD40 complex by GSK3-like kinases. eLife 3: e2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough S.J., Bent A.F. (1998). Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16: 735–743. [DOI] [PubMed] [Google Scholar]
- Clouse S.D., Sasse J.M. (1998). BRASSINOSTEROIDS: essential regulators of plant growth and development. Annu. Rev. Plant Physiol. Plant Mol. Biol. 49: 427–451. [DOI] [PubMed] [Google Scholar]
- Cong L., Ran F.A., Cox D., Lin S., Barretto R., Habib N., Hsu P.D., Wu X., Jiang W., Marraffini L.A., Zhang F. (2013). Multiplex genome engineering using CRISPR/Cas systems. Science 339: 819–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daras G., Rigas S., Tsitsekian D., Zur H., Tuller T., Hatzopoulos P. (2014). Alternative transcription initiation and the AUG context configuration control dual-organellar targeting and functional competence of Arabidopsis Lon1 protease. Mol. Plant 7: 989–1005. [DOI] [PubMed] [Google Scholar]
- Dinkel H., et al. (2012). ELM—the database of eukaryotic linear motifs. Nucleic Acids Res. 40: D242–D251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gampala S.S., et al. (2007). An essential role for 14-3-3 proteins in brassinosteroid signal transduction in Arabidopsis. Dev. Cell 13: 177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan X., et al. (2011). Multiple reference genomes and transcriptomes for Arabidopsis thaliana. Nature 477: 419–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedhart J., von Stetten D., Noirclerc-Savoye M., Lelimousin M., Joosen L., Hink M.A., van Weeren L., Gadella T.W. Jr., Royant A. (2012). Structure-guided evolution of cyan fluorescent proteins towards a quantum yield of 93%. Nat. Commun. 3: 751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groszmann M., Paicu T., Smyth D.R. (2008). Functional domains of SPATULA, a bHLH transcription factor involved in carpel and fruit development in Arabidopsis. Plant J. 55: 40–52. [DOI] [PubMed] [Google Scholar]
- He J.X., Gendron J.M., Yang Y., Li J., Wang Z.Y. (2002). The GSK3-like kinase BIN2 phosphorylates and destabilizes BZR1, a positive regulator of the brassinosteroid signaling pathway in Arabidopsis. Proc. Natl. Acad. Sci. USA 99: 10185–10190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J.X., Gendron J.M., Sun Y., Gampala S.S., Gendron N., Sun C.Q., Wang Z.Y. (2005). BZR1 is a transcriptional repressor with dual roles in brassinosteroid homeostasis and growth responses. Science 307: 1634–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M.H. (2002). Biogeography of Arabidopsis thaliana (L.) Heynh. (Brassicaceae). J. Biogeogr. 29: 125–134. [Google Scholar]
- Jiang J., Zhang C., Wang X. (2013). Ligand perception, activation, and early signaling of plant steroid receptor brassinosteroid insensitive 1. J. Integr. Plant Biol. 55: 1198–1211. [DOI] [PubMed] [Google Scholar]
- Kim T.W., Guan S., Burlingame A.L., Wang Z.Y. (2011). The CDG1 kinase mediates brassinosteroid signal transduction from BRI1 receptor kinase to BSU1 phosphatase and GSK3-like kinase BIN2. Mol. Cell 43: 561–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T.W., Guan S., Sun Y., Deng Z., Tang W., Shang J.X., Sun Y., Burlingame A.L., Wang Z.Y. (2009). Brassinosteroid signal transduction from cell-surface receptor kinases to nuclear transcription factors. Nat. Cell Biol. 11: 1254–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M.A., Kiefer M. (2005). Genome evolution among cruciferous plants: a lecture from the comparison of the genetic maps of three diploid species—Capsella rubella, Arabidopsis lyrata subsp. petraea, and A. thaliana. Am. J. Bot. 92: 761–767. [DOI] [PubMed] [Google Scholar]
- Kosugi S., Hasebe M., Tomita M., Yanagawa H. (2009). Systematic identification of cell cycle-dependent yeast nucleocytoplasmic shuttling proteins by prediction of composite motifs. Proc. Natl. Acad. Sci. USA 106: 10171–10176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachowiec J., Lemus T., Thomas J.H., Murphy P.J., Nemhauser J.L., Queitsch C. (2013). The protein chaperone HSP90 can facilitate the divergence of gene duplicates. Genetics 193: 1269–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamesch P., et al. (2012). The Arabidopsis Information Resource (TAIR): improved gene annotation and new tools. Nucleic Acids Res. 40: D1202–D1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letunic I., Doerks T., Bork P. (2012). SMART 7: recent updates to the protein domain annotation resource. Nucleic Acids Res. 40: D302–D305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. (2010). Regulation of the nuclear activities of brassinosteroid signaling. Curr. Opin. Plant Biol. 13: 540–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Chory J. (1997). A putative leucine-rich repeat receptor kinase involved in brassinosteroid signal transduction. Cell 90: 929–938. [DOI] [PubMed] [Google Scholar]
- Li J., Nam K.H. (2002). Regulation of brassinosteroid signaling by a GSK3/SHAGGY-like kinase. Science 295: 1299–1301. [DOI] [PubMed] [Google Scholar]
- Li J., Wen J., Lease K.A., Doke J.T., Tax F.E., Walker J.C. (2002). BAK1, an Arabidopsis LRR receptor-like protein kinase, interacts with BRI1 and modulates brassinosteroid signaling. Cell 110: 213–222. [DOI] [PubMed] [Google Scholar]
- Li L., Ye H., Guo H., Yin Y. (2010). Arabidopsis IWS1 interacts with transcription factor BES1 and is involved in plant steroid hormone brassinosteroid regulated gene expression. Proc. Natl. Acad. Sci. USA 107: 3918–3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Yu X., Thompson A., Guo M., Yoshida S., Asami T., Chory J., Yin Y. (2009). Arabidopsis MYB30 is a direct target of BES1 and cooperates with BES1 to regulate brassinosteroid-induced gene expression. Plant J. 58: 275–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J.R., Hu J. (2013). SeqNLS: nuclear localization signal prediction based on frequent pattern mining and linear motif scoring. PLoS ONE 8: e76864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Li X., Li K., Liu H., Lin C. (2013). Multiple bHLH proteins form heterodimers to mediate CRY2-dependent regulation of flowering-time in Arabidopsis. PLoS Genet. 9: e1003861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma P.C., Rould M.A., Weintraub H., Pabo C.O. (1994). Crystal structure of MyoD bHLH domain-DNA complex: perspectives on DNA recognition and implications for transcriptional activation. Cell 77: 451–459. [DOI] [PubMed] [Google Scholar]
- Mao Y., Zhang H., Xu N., Zhang B., Gou F., Zhu J.K. (2013). Application of the CRISPR-Cas system for efficient genome engineering in plants. Mol. Plant 6: 2008–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuffin L.J., Bryson K., Jones D.T. (2000). The PSIPRED protein structure prediction server. Bioinformatics 16: 404–405. [DOI] [PubMed] [Google Scholar]
- Nam K.H., Li J. (2002). BRI1/BAK1, a receptor kinase pair mediating brassinosteroid signaling. Cell 110: 203–212. [DOI] [PubMed] [Google Scholar]
- Oh E., Zhu J.Y., Wang Z.Y. (2012). Interaction between BZR1 and PIF4 integrates brassinosteroid and environmental responses. Nat. Cell Biol. 14: 802–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu H., Cho H., Kim K., Hwang I. (2010). Phosphorylation dependent nucleocytoplasmic shuttling of BES1 is a key regulatory event in brassinosteroid signaling. Mol. Cells 29: 283–290. [DOI] [PubMed] [Google Scholar]
- Ryu H., Cho H., Bae W., Hwang I. (2014). Control of early seedling development by BES1/TPL/HDA19-mediated epigenetic regulation of ABI3. Nat. Commun. 5: 4138. [DOI] [PubMed] [Google Scholar]
- Ryu H., Kim K., Cho H., Park J., Choe S., Hwang I. (2007). Nucleocytoplasmic shuttling of BZR1 mediated by phosphorylation is essential in Arabidopsis brassinosteroid signaling. Plant Cell 19: 2749–2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago J., Henzler C., Hothorn M. (2013). Molecular mechanism for plant steroid receptor activation by somatic embryogenesis co-receptor kinases. Science 341: 889–892. [DOI] [PubMed] [Google Scholar]
- Sayou C., et al. (2014). A promiscuous intermediate underlies the evolution of LEAFY DNA binding specificity. Science 343: 645–648. [DOI] [PubMed] [Google Scholar]
- Shabalina S.A., Spiridonov A.N., Spiridonov N.A., Koonin E.V. (2010). Connections between alternative transcription and alternative splicing in mammals. Genome Biol. Evol. 2: 791–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparkes I.A., Runions J., Kearns A., Hawes C. (2006). Rapid, transient expression of fluorescent fusion proteins in tobacco plants and generation of stably transformed plants. Nat. Protoc. 1: 2019–2025. [DOI] [PubMed] [Google Scholar]
- Sun Y., Han Z., Tang J., Hu Z., Chai C., Zhou B., Chai J. (2013). Structure reveals that BAK1 as a co-receptor recognizes the BRI1-bound brassinolide. Cell Res. 23: 1326–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., et al. (2010). Integration of brassinosteroid signal transduction with the transcription network for plant growth regulation in Arabidopsis. Dev. Cell 19: 765–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K., Peterson D., Peterson N., Stecher G., Nei M., Kumar S. (2011). MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28: 2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W., et al. (2011). PP2A activates brassinosteroid-responsive gene expression and plant growth by dephosphorylating BZR1. Nat. Cell Biol. 13: 124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thatcher L.F., Carrie C., Andersson C.R., Sivasithamparam K., Whelan J., Singh K.B. (2007). Differential gene expression and subcellular targeting of Arabidopsis glutathione S-transferase F8 is achieved through alternative transcription start sites. J. Biol. Chem. 282: 28915–28928. [DOI] [PubMed] [Google Scholar]
- Toledo-Ortiz G., Huq E., Quail P.H. (2003). The Arabidopsis basic/helix-loop-helix transcription factor family. Plant Cell 15: 1749–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vert G., Chory J. (2006). Downstream nuclear events in brassinosteroid signalling. Nature 441: 96–100. [DOI] [PubMed] [Google Scholar]
- Wang H., Zhu Y., Fujioka S., Asami T., Li J., Li J. (2009). Regulation of Arabidopsis brassinosteroid signaling by atypical basic helix-loop-helix proteins. Plant Cell 21: 3781–3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Jiang J., Wang J., Chen L., Fan S.L., Wu J.W., Wang X., Wang Z.X. (2014). Structural insights into the negative regulation of BRI1 signaling by BRI1-interacting protein BKI1. Cell Res. 24: 1328–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Chory J. (2006). Brassinosteroids regulate dissociation of BKI1, a negative regulator of BRI1 signaling, from the plasma membrane. Science 313: 1118–1122. [DOI] [PubMed] [Google Scholar]
- Wang X., Li X., Meisenhelder J., Hunter T., Yoshida S., Asami T., Chory J. (2005). Autoregulation and homodimerization are involved in the activation of the plant steroid receptor BRI1. Dev. Cell 8: 855–865. [DOI] [PubMed] [Google Scholar]
- Wang X., Chen J., Xie Z., Liu S., Nolan T., Ye H., Zhang M., Guo H., Schnable P.S., Li Z., Yin Y. (2014). Histone lysine methyltransferase SDG8 is involved in brassinosteroid-regulated gene expression in Arabidopsis thaliana. Mol. Plant 7: 1303–1315. [DOI] [PubMed] [Google Scholar]
- Wang Y., Sun S., Zhu W., Jia K., Yang H., Wang X. (2013). Strigolactone/MAX2-induced degradation of brassinosteroid transcriptional effector BES1 regulates shoot branching. Dev. Cell 27: 681–688. [DOI] [PubMed] [Google Scholar]
- Wang Z.Y., Nakano T., Gendron J., He J., Chen M., Vafeados D., Yang Y., Fujioka S., Yoshida S., Asami T., Chory J. (2002). Nuclear-localized BZR1 mediates brassinosteroid-induced growth and feedback suppression of brassinosteroid biosynthesis. Dev. Cell 2: 505–513. [DOI] [PubMed] [Google Scholar]
- Wright S.I., Lauga B., Charlesworth D. (2002). Rates and patterns of molecular evolution in inbred and outbred Arabidopsis. Mol. Biol. Evol. 19: 1407–1420. [DOI] [PubMed] [Google Scholar]
- Xie L., Yang C., Wang X. (2011). Brassinosteroids can regulate cellulose biosynthesis by controlling the expression of CESA genes in Arabidopsis. J. Exp. Bot. 62: 4495–4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C.J., Zhang C., Lu Y.N., Jin J.Q., Wang X.L. (2011). The mechanisms of brassinosteroids’ action: from signal transduction to plant development. Mol. Plant 4: 588–600. [DOI] [PubMed] [Google Scholar]
- Ye H., Li L., Guo H., Yin Y. (2012). MYBL2 is a substrate of GSK3-like kinase BIN2 and acts as a corepressor of BES1 in brassinosteroid signaling pathway in Arabidopsis. Proc. Natl. Acad. Sci. USA 109: 20142–20147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q., Zhu W., Li L., Zhang S., Yin Y., Ma H., Wang X. (2010). Brassinosteroids control male fertility by regulating the expression of key genes involved in Arabidopsis anther and pollen development. Proc. Natl. Acad. Sci. USA 107: 6100–6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y., Vafeados D., Tao Y., Yoshida S., Asami T., Chory J. (2005). A new class of transcription factors mediates brassinosteroid-regulated gene expression in Arabidopsis. Cell 120: 249–259. [DOI] [PubMed] [Google Scholar]
- Yin Y., Wang Z.Y., Mora-Garcia S., Li J., Yoshida S., Asami T., Chory J. (2002). BES1 accumulates in the nucleus in response to brassinosteroids to regulate gene expression and promote stem elongation. Cell 109: 181–191. [DOI] [PubMed] [Google Scholar]
- Yu X., Li L., Li L., Guo M., Chory J., Yin Y. (2008). Modulation of brassinosteroid-regulated gene expression by Jumonji domain-containing proteins ELF6 and REF6 in Arabidopsis. Proc. Natl. Acad. Sci. USA 105: 7618–7623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., Li L., Zola J., Aluru M., Ye H., Foudree A., Guo H., Anderson S., Aluru S., Liu P., Rodermel S., Yin Y. (2011). A brassinosteroid transcriptional network revealed by genome-wide identification of BESI target genes in Arabidopsis thaliana. Plant J. 65: 634–646. [DOI] [PubMed] [Google Scholar]
- Zhang D., Ye H., Guo H., Johnson A., Zhang M., Lin H., Yin Y. (2014). Transcription factor HAT1 is phosphorylated by BIN2 kinase and mediates brassinosteroid repressed gene expression in Arabidopsis. Plant J. 77: 59–70. [DOI] [PubMed] [Google Scholar]
- Zhao J., Peng P., Schmitz R.J., Decker A.D., Tax F.E., Li J. (2002). Two putative BIN2 substrates are nuclear components of brassinosteroid signaling. Plant Physiol. 130: 1221–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.