Abstract
Endocrine disrupting chemicals (EDC) abound in the environment since many compounds are released from chemical, agricultural, pharmaceutical, and consumer product industries. Many of the EDCs such as Bisphenol A (BPA) have estrogenic activity or interfere with endogenous sex hormones. Experimental studies have reported a positive correlation of BPA with reproductive toxicity, altered growth, and immune dysregulation. Although the precise relevance of these studies to the environmental levels is unclear, nevertheless, their potential health implications remain a concern. One possible mechanism by which BPA can alter genes is by regulating epigenetics, including microRNA, alteration of methylation, and histone acetylation. There is now wealth of information on BPA effects on non-lymphoid cells and by comparison, paucity of data on effects of BPA on the immune system. In this mini review, we will highlight the BPA regulation of estrogen receptor-mediated immune cell functions and in different inflammatory conditions. In addition, BPA-mediated epigenetic regulation of non-lymphoid cells is emphasized. We recognize that most of these studies are on non-lymphoid cells, and given that BPA also affects the immune system, it is plausible that BPA could have similar epigenetic regulation in immune cells. It is hoped that this review will stimulate studies in this area to ascertain whether or not BPA epigenetically regulates the cells of the immune system.
Keywords: bisphenol A, EDC, immune, epigenetics, estrogenic
Introduction
Exposure to environmental chemicals is suspected in increase in the incidence of allergies and autoimmune diseases (1, 2). Among these compounds that are released in the environment, a group of compounds that alter the endocrine functions of body are termed as endocrine disrupting chemicals (EDCs). EDCs can interfere with synthesis, transport, function and activity, or elimination of natural hormones such as estrogen. This mini-review is focused on Bisphenol A (BPA; 2,2-bis (4-hydroxyphenyl) propane), a ubiquitous EDC, known to possess both agonistic and antagonistic estrogen action. It interferes with estrogen-regulated endocrine and physiological functions (3, 4). There is now growing evidence that BPA can alter epigenetics in various non-lymphoid cells. There is paucity of similar data on epigenetic regulation of BPA on the cells of the immune system. Given that BPA can modulate the immune system, it is plausible that the findings of BPA regulation of epigenetics in non-lymphoid cells may also apply to the immune system.
Bisphenol A, a xenoestrogen, is found in a variety of daily consumer products such as polycarbonate plastics, food can liners, epoxy resin, and flame retardant (5, 6). Nearly eight billion pounds of BPA is produced/year and more than 100 t is released in atmosphere (5). In 2003–2004 National Health and Nutrition Examination Survey, around 92.6% of 2517 subjects had detectable levels of BPA in their urine (7). In addition, BPA is also detected in sera, amniotic fluid, placenta, umbilical cord blood, ovarian follicular fluid, and colostrum (8–10). Although BPA interacts with both estrogen receptors (ERα and ERβ), BPA has 10 times higher affinity for ERβ (11, 12). Interestingly, BPA estrogenic metabolite, 4-Methyl-2,4-bis(4-hydroxyphenyl)pent-1-ene is more potent (∼500 fold) (13, 14). BPA exposure in different experimentally and naturally exposed populations during early developmental stages has been associated with reproductive abnormalities such as infertility in both males and females, altered male sexual function, spermatogenesis, endometrial disorders, polycystic ovary syndrome, interference of embryonic development programs, sex differentiation of the brain and behavior, metabolic disorders, and immune responsiveness (15–22).
BPA a Potent Immunomodulator
It is well established that estrogen is a natural target of the immune system since both ERα and ERβ are present on cells of the immune system (23–25). Extensive studies have documented the immunomodulatory role of estrogen (23, 26–31). Increasing evidence suggests that BPA also modulate immune pathways, which may contribute to the development of inflammatory conditions and autoimmune diseases (1, 2, 32). BPA exposure modulates estrogen-associated immune signaling, molecular mimicry (33), disruption of cytochrome p450 enzyme (34), alteration of immune signaling in cells of innate and adaptive immune system, cytokine polarization to Th1 and Th2 (35), inhibition of Tregs (36), dysregulation of immunoglobulin (37), and hyperprolactinemia reviewed in detail previously (38, 39).
BPA binds to ER to modulate immune cell signaling and function
Bisphenol A binds and stimulates ERα and ERβ transcriptional activity at concentrations of 100–1000 nM (12). However, BPA potency is 10–1000-fold less than other EDCs such as diethylstilbestrol (DES) and ethinyl estradiol (40). BPA treatment had opposing effects on ERα expression with decreased expression in males and increased expression in females F(0) and F(1) offspring rats (41). In a proteome study, apo-AI, DPPIII, and VAT were identified as protein biomarkers for BPA-induced endocrine disruption in spleen and thymus from mice prenatally exposed to BPA (42). Interestingly, female and young offsprings were more susceptible to alterations in these proteins suggesting gender and age interplay in BPA (42). Furthermore, there was decreased IL-2, IL-12, IFNγ, and TNFα expression in the spleen of BPA-treated rats when compared to control rats (41).
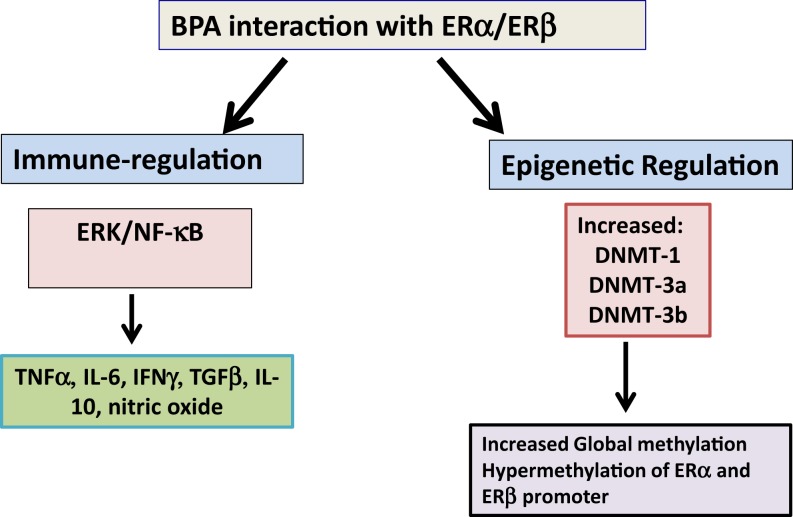
It has been recently shown that treatment of BV2 murine microglial cell line, THP1 macrophage and primary human macrophages with BPA increased TNFα and IL-6 but decreased IL-10 and TGFβ (Figure 1) (43, 44). This was mediated through ERα/β and extracellular-regulated protein kinases (ERK)/nuclear factor κB (NF-κB) signal cascade (43, 44). Interestingly, treatment of LPS-activated RAW 264.7 cells and murine macrophages with BPA decreased nitric oxide production (45–47). These effects are potentially mediated through BPA-ER mediated downregulation of NF-κB transactivation (48). However, BPA-exposed zebra fish embryos have increased expression of iNOS, IFNγ, IL-1β, IL-10, TNFα, CC-chemokine, and CXCL-clc. In addition, BPA altered expression of members of Toll-like receptors (TLRs) signaling pathway TLR3, TRIF, MyD88, IRAK4, and TRAF6 (49). The above studies show that the pathophysiological outcome of experimental exposure to BPA varies with routes, concentration and dose, cell culture systems, and organisms. Since, the majority of these studies are performed on experimental animals or are cell-culture based studies, it is likely that there is some heterogeneity between the effects of BPA in the different experimental designs and organisms studied.
Figure 1.
Bisphenol A interaction with estrogen receptors. Bisphenol A (BPA) interacts with estrogen receptor (ER)-α and β to regulate different proinflammatory cytokines such as TNFα, IL-6, and IFNγ and anti-inflammatory cytokines TGFβ and IL-10. In addition, it upregulates DNA methyl transferase enzymes (DNMTs) to epigenetically regulate gene expression. Abbreviation: IL: interleukin; ERK: extracellular signal-regulated kinases.
BPA effects on allergic and autoimmune conditions
Different studies have reported BPA altered Th1, Th2, and Tregs profile. Majority of these studies have demonstrated augmentation of Th1 type response in BPA-exposed subjects (35, 50–52). Offsprings of C57B6/129svj mice exposed to BPA during gestation have altered cytokine profile with increased Th1 profile and skewing toward Th17 responses (53). Maternal exposure to environmentally relevant BPA dose results in decreased innate immune responses in influenza A-infected adult offspring (54). Perinatal exposure to low doses of BPA from 15th day of pregnancy to pups weaning age, increased anti-ovalbumin (OVA) IgG titers in OVA-tolerized rats accompanied with increased activation of T cells and IFNγ secretion. Oral OVA challenge in these mice increased colonic inflammation, neutrophil infiltration; IFNγ and decreased TGFβ suggesting perinatal exposure of BPA in low doses can make neonatal immune system susceptible to food intolerance (55). BPA exposure via in utero and through breast milk promotes development of allergic asthma in BALB/c mice pups sensitized to low dose of OVA (56). However, postnatal BPA exposure alone (through breast milk) was not sufficient to induce allergy in the mouse pups indicating the importance of identification of critical period of BPA exposure in studying different inflammatory conditions (56). In contrast, a recent study showed that BPA intake during pregnancy and breastfeeding or orally did not have significant effect in murine offsprings in experimental allergic asthma (57) and inflammatory bowel disease (IBD) (58).
Contrasting effect of perinatal exposure BPA in different mouse models of MS, experimental autoimmune encephalomyelitis (EAE) disease expression has also been recently reported (59, 60). In Theiler’s-virus induced demyelination, perinatal BPA exposure resulted in decreased anti-viral antibodies, increased onset of disease, and increased inflammation in spinal cord and digestive tract (60). Whereas, in EAE models, C57BL/6J mice (chronic progressive) and SJL/J mice (relapsing-remitting), prenatal BPA exposure did not have significant effect on EAE disease severity and progression (59). These reports suggest that biological effects of BPA exposure vary with age, gender, route and dose, and model of disease. These are important variables to consider when studying immunomodulatory role of BPA.
There was increased autoantibody production by B1 cells from BPA-treated BWF1 mice, a murine model of systemic lupus erythematous (SLE) (61). However, other studies have demonstrated that BPA-fed NZB X NZW F(1) mice had late onset of proteinuria and lowered IFNγ and IL-10 production suggesting protective effect of BPA on SLE (62). Trans-maternal BPA exposure increased diabetes type-1 development in the NOD mice offspring by increasing apoptotic cells and Tregs and decreasing resident macrophages in islets and by inducing systemic immune changes including altered cytokine production (63). Together these studies indicate the importance of critical windows of exposure since early exposure to BPA has modulatory effects on the immune system later in life.
BPA an Epigenetic Regulator
An important mechanism by which environmental agents can modify gene expression is through altering epigenetics, DNA methylation, histone modification, and microRNA (miRNA). In this regard, several recent studies have confirmed epigenetic regulation by BPA, although most of these studies have focused on epigenetic regulation in non-lymphoid tissues.
BPA and DNA methylation
Long-lasting effects of perinatal and trans-generational BPA exposure suggest the potential disruption of epigenetic programing of gene expression critical in development, cancer, behavioral, ovarian, and other reproductive functions (64–67). Studies have demonstrated that BPA exposure results in alteration in DNA methylation and expression of specific genes (68). Whether these BPA-mediated alteration in methylation are related to its estrogenic activity remains still unclear. However, a recent study has demonstrated that BPA regulation of methylation was mediated through BPA-ERα regulation of DNA methyltransferase (DNMT)-1 and DNMT-3a expression (Figure 1) (64). BPA exposure of neonatal male rats increased DNMT-3a and -3b expression and also increased methylation at promoter region of ERα and ERβ in testis (69). Different independent groups have also reported BPA-mediated alteration in DNMTs and methyl-CpG binding protein 2 (MECP2) levels and in genome-wide methylation level (70–73). Exposure of BPA promotes global and cytochrome P450 aromatase (cyp19a1a gene-specific) methylation in gonads of adult rare minnow Gobiocypris rarus (74). This was also associated with alteration of DNMT mRNA levels. In ovaries, the methylation levels at four CpGs at the 5′ flanking region of cyp19a1a varied with the time of BPA exposure; suppression by 7 days and augmentation by 35 days of BPA exposure (43).
In a recent cross-sectional epidemiological study of pre-pubescent girls in Egypt, it was found that BPA exposure resulted in alteration of methylation profiles. Higher urinary BPA levels were associated with hypomethylation of CpG islands on the X-chromosome and lowered methylation of genes involved in immune function, transport activity, metabolism, and caspase activity (75). Further, it has been found that there is hypomethylation of long interspersed nucleotide elements (LINE 1) in sperms of men exposed to BPA when compared to control group indicating potential of BPA in epigenetic reprograming (76). Non-monotonic dose-dependent effects of DNA methylation patterns were observed in mouse liver following perinatal BPA exposure (77). There was enrichment of regions of altered methylation (RAMs) within CpG island shores (77).
Bisphenol A exposure of F(0) pregnant rats during gestation and lactation, resulted in decreased global DNA methylation in F(1) offspring sperms. In addition, glucokinase (Gck) promoter was completely methylated in F(2) offspring hepatic tissue. There were five unmethylated sites in control offspring indicating maternal BPA exposure can have multigenerational effects on glucose metabolism (78). BPA- and DES-induced antisense transcript, long non-coding RNA HOTAIR in breast cancer cells and in mammary gland of rats, which was mediated by ER-ERE pathway and by chromatin modification (histone methylation and acetylation) (79). BPA increases the expression of Enhancer of Zeste Homolog 2 (EZH2), a histone methyl transferase, in breast cancer cell line (79, 80). Furthermore, EZH2-regulated histone H3 trimethylation was also increased in MCF-7 cell line and in mammary glands of mice exposed to BPA in utero (80). In utero BPA exposure decreased the expression of phase I and II xenobiotic metabolizing enzyme (XME) genes. This was associated with increased site-specific methylation at COMT and increased average methylation at SULT2A1 promoters (81).
BPA Epigenetic Modulation of Gamete, Embryo and Placenta
Epigenetic trans-generational inheritance (ETI) or Germline transmission of epigenetic information between generation is modulated by different environmental stimuli including BPA. These epimutations are changes in methylation and histone modification in germ line and are passed on to subsequent generations (82). Imprinted genes are regulated by differential DNA methylation. Maternal BPA exposure during late stages of oocyte development and early embryonic stages significantly reduced genome-wide methylation levels in placenta and altered methylation of differentially methylated regions (DMRs) such as Snrpn imprinting control region (ICR) and Igf2 DMR1 (83). Low-dose BPA exposure during follicle culture from preantral to antral stage resulted in significant increase in allele methylation errors in DMRs of maternally imprinted genes (Snrpn, Igf2r, and Mest) and decreased in histone H3K9 trimethylation and interkinetochore distance and epigenetic changes in germinal vesicle and metaphase II oocyte, which potentially contribute to chromosome congression failure, meiotic errors, and overall health of offspring (84). BPA exposure of neonatal male rats (F0) resulted in significant hypomethylation at the H19 ICR in sperms of F(0) and in resorbed embryo (F1) (85). In addition, there were decreased Igf2 and H19 mRNA levels in BPA resorbed embryo (F1) compared to viable control embryo. Murine N2A cells exposed to BPA had modest decrease in global DNA methylation accompanied with increased adipocyte differentiation (86). Together, these studies indicate that BPA exposure epigenetically modulates gametes, embryo and placenta, which potentially results in defects in fetal and postnatal development.
Bisphenol A decreased methylation upstream of Agouti gene in viable yellow agouti [A(vy)] mice (87, 88). In BPA-exposed human mammary epithelial cells (HMEC), there was hypermethylation of genes related to the development of most or all tumor types indicating modulatory effect of BPA on HMEC proliferation and senescence (89). In utero BPA exposure decreased methylation at Hoxa10 promoter, resulting in increased ERα binding to Hoxa10 ERE thereby increasing ERE-driven Hoxa10 expression (90). These epigenetic modifications result in alteration in ERE sensitivity of different genes and could possibly be a general mechanism of BPA-mediated gene expression.
BPA and MicroRNAs
Different studies have demonstrated that BPA exposure results in aberrant miRNA expression profile. These miRNAs are believed to target gonadal differentiation, folliculogenesis, and insulin homeostasis (91, 92). Estrogen, BPA, and DDT similarly altered the expression of multiple miRNAs including miR-21 in ER(+) and hormone sensitive MCF-7 breast cancer cell line (93). Placental cell line exposed to BPA had significantly increased miR-146a levels (94). BPA upregulated the expression of oncogenic miR-19a and miR-19b accompanied with the downregulation of miR-19-related downstream proteins such as PTEN, p-AKT, p-MDM2, p53, and proliferating cell nuclear antigen (78). Interestingly, curcumin, which is clinically used for cancer treatment modulated miR-19/PTEN/AKT/p53 axis to protect against BPA-associated breast cancer promotion (95). BPA treatment decreased miR-134 and upregulated the expression of miR-134 targets including pluripotency markers (Oct4, Sox2, and Nanog) in embryonic stem cells (ESC) and embryoid bodies (EB) (96).
BPA and histone acetylation
Long-term BPA exposure increased N-methyl-d-aspartic acid (NMDA) receptor levels and enhanced the expression and function of histone deacetylase 2 (HDAC2) in hippocampus of adult mice (97). Prenatal exposure of Wistar-Furth rats to BPA resulted in increased pro-activation histone H3K4 trimethylation at the promoter region of alpha lactalbumin gene at postnatal day 4 (PND4) and increased alpha lactalbumin mRNA expression in mammary gland (98). Interestingly, majority of differences in gene expression in BPA vs. vehicle-treated group were evident at later stage of life (PND50). These results indicate that fetal BPA exposure can modify postnatal and adult mammary gland epigenome and gene expression, which may contribute to development of pre-neoplastic and neoplastic lesions in adult rat mammary gland (98). To date, there is no information on BPA effects on methylation, miRNAs, and histone acetylation in specific immune subsets.
Conclusion
Bisphenol A, a model EDC has been shown to modulate not only reproductive and non-lymphoid systems, but it can also affect the immune system. One potential mechanism by which EDC can physiologically affect tissue functioning is by epigenetically regulating ER-targeted genes. While there are only limited studies on epigenetic regulation of BPA on the immune system, studies on many non-lymphoid tissues clearly demonstrate that BPA can alter methylation, histone modification, and miRNAs. Together by integrating current knowledge of trans-generational effect of EDCs on developmental biology, immune function, and epigenetic regulation, it is imperative to design thorough systematic and comprehensive studies to further define the dose, route, and critical windows of exposure of these EDCs and their effects on different chronic inflammatory conditions. A better understanding of BPA regulation of epigenetic mechanism will add to our current understanding about estrogens and their potential contribution in etiopathogenesis in immune-altered states including autoimmune diseases. By extrapolation, it is likely that other EDCs may also epigenetically regulate immune cells, which may have implications in immune conditions including response to infectious agents or susceptibility to autoimmune diseases. There is a distinct gap in this area that warrants studies. These studies will help in establishing safe levels of EDCs in environment especially during the vulnerable fetal life, during which critical immunological education occurs.
Author Contributions
DK and SAA designed the work, drafted and revised the work and finally approved the version to be published and agree to be accountable for all aspects of the work.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbrevations
AKT, protein kinase B (PKB); COMT, catechol-O-methyltransferase; CXCL, chemokine (C-X-C motif) ligand; DPPIII, dipeptidyl-peptidase 3; ERE, estrogen response element; Gata, GATA family transcription factor; IFN, interferon; Igf2r, insulin-like growth factor 2 receptor; IL, interleukin; iNOS, inducible nitric oxide synthase; IRAK, interleukin-1 receptor-associated kinase; Mest, mesoderm-specific transcript homolog protein; MyD88, myeloid differentiation primary response gene 88; Oct4, octamer-binding transcription factor 4; PTEN, phosphatase and tensin homolog; Slc22a18, solute carrier family 22, member 18; Snrpn, small nuclear ribonucleoprotein particles; Sox, SRY (sex determining region Y)-box; SULT2A1, sulfotransferase family, cytosolic, 2A; TNF, tumor necrosis factor; TRAF, TNF receptor associated factor; TRIF, TIR-domain-containing adapter-inducing interferon-β.
References
- 1.Lang IA, Galloway TS, Scarlett A, Henley WE, Depledge M, Wallace RB, et al. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA (2008) 300(11):1303–10. 10.1001/jama.300.11.1303 [DOI] [PubMed] [Google Scholar]
- 2.Melzer D, Rice NE, Lewis C, Henley WE, Galloway TS. Association of urinary bisphenol A concentration with heart disease: evidence from NHANES 2003/06. PLoS One (2010) 5(1):e8673. 10.1371/journal.pone.0008673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wetherill YB, Akingbemi BT, Kanno J, McLachlan JA, Nadal A, Sonnenschein C, et al. In vitro molecular mechanisms of bisphenol A action. Reprod Toxicol (2007) 24(2):178–98. 10.1016/j.reprotox.2007.05.010 [DOI] [PubMed] [Google Scholar]
- 4.Cao J, Rebuli ME, Rogers J, Todd KL, Leyrer SM, Ferguson SA, et al. Prenatal bisphenol A exposure alters sex-specific estrogen receptor expression in the neonatal rat hypothalamus and amygdala. Toxicol Sci (2013) 133(1):157–73. 10.1093/toxsci/kft035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. Human exposure to bisphenol A (BPA). Reprod Toxicol (2007) 24(2):139–77. 10.1016/j.reprotox.2007.07.010 [DOI] [PubMed] [Google Scholar]
- 6.Rubin BS, Soto AM. Bisphenol A: perinatal exposure and body weight. Mol Cell Endocrinol (2009) 304(1–2):55–62. 10.1016/j.mce.2009.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003-2004. Environ Health Perspect (2008) 116(1):39–44. 10.1289/ehp.10753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ikezuki Y, Tsutsumi O, Takai Y, Kamei Y, Taketani Y. Determination of bisphenol A concentrations in human biological fluids reveals significant early prenatal exposure. Hum Reprod (2002) 17(11):2839–41. 10.1093/humrep/17.11.2839 [DOI] [PubMed] [Google Scholar]
- 9.Kuruto-Niwa R, Tateoka Y, Usuki Y, Nozawa R. Measurement of bisphenol A concentrations in human colostrum. Chemosphere (2007) 66(6):1160–4. 10.1016/j.chemosphere.2006.06.073 [DOI] [PubMed] [Google Scholar]
- 10.Cobellis L, Colacurci N, Trabucco E, Carpentiero C, Grumetto L. Measurement of bisphenol A and bisphenol B levels in human blood sera from healthy and endometriotic women. Biomed Chromatogr (2009) 23(11):1186–90. 10.1002/bmc.1241 [DOI] [PubMed] [Google Scholar]
- 11.Gould JC, Leonard LS, Maness SC, Wagner BL, Conner K, Zacharewski T, et al. Bisphenol A interacts with the estrogen receptor alpha in a distinct manner from estradiol. Mol Cell Endocrinol (1998) 142(1–2):203–14. 10.1016/S0303-7207(98)00084-7 [DOI] [PubMed] [Google Scholar]
- 12.Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology (1998) 139(10):4252–63. 10.1210/endo.139.10.6216 [DOI] [PubMed] [Google Scholar]
- 13.Okuda K, Takiguchi M, Yoshihara S. In vivo estrogenic potential of 4-methyl-2,4-bis(4-hydroxyphenyl)pent-1-ene, an active metabolite of bisphenol A, in uterus of ovariectomized rat. Toxicol Lett (2010) 197(1):7–11. 10.1016/j.toxlet.2010.04.017 [DOI] [PubMed] [Google Scholar]
- 14.Cipelli R, Harries L, Okuda K, Yoshihara S, Melzer D, Galloway T. Bisphenol A modulates the metabolic regulator oestrogen-related receptor-alpha in T-cells. Reproduction (2014) 147(4):419–26. 10.1530/REP-13-0423 [DOI] [PubMed] [Google Scholar]
- 15.Yin DQ, Hu SQ, Gu Y, Wei L, Liu SS, Zhang AQ. Immunotoxicity of bisphenol A to Carassius auratus lymphocytes and macrophages following in vitro exposure. J Environ Sci (China) (2007) 19(2):232–7. 10.1016/S1001-0742(07)60038-2 [DOI] [PubMed] [Google Scholar]
- 16.Minier C, Forget-Leray J, Bjornstad A, Camus L. Multixenobiotic resistance, acetyl-choline esterase activity and total oxyradical scavenging capacity of the arctic spider crab, Hyas araneus, following exposure to bisphenol A, tetra bromo diphenyl ether and diallyl phthalate. Mar Pollut Bull (2008) 56(8):1410–5. 10.1016/j.marpolbul.2008.05.005 [DOI] [PubMed] [Google Scholar]
- 17.Kim K, Son TG, Park HR, Kim SJ, Kim HS, Kim HS, et al. Potencies of bisphenol A on the neuronal differentiation and hippocampal neurogenesis. J Toxicol Environ Health A (2009) 72(21–22):1343–51. 10.1080/15287390903212501 [DOI] [PubMed] [Google Scholar]
- 18.Clayton EM, Todd M, Dowd JB, Aiello AE. The impact of bisphenol A and triclosan on immune parameters in the U.S. population, NHANES 2003-2006. Environ Health Perspect (2011) 119(3):390–6. 10.1289/ehp.1002883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salian S, Doshi T, Vanage G. Perinatal exposure of rats to bisphenol A affects fertility of male offspring – an overview. Reprod Toxicol (2011) 31(3):359–62. 10.1016/j.reprotox.2010.10.008 [DOI] [PubMed] [Google Scholar]
- 20.Diamanti-Kandarakis E, Christakou C, Marinakis E. Phenotypes and enviromental factors: their influence in PCOS. Curr Pharm Des (2012) 18(3):270–82. 10.2174/138161212803307590 [DOI] [PubMed] [Google Scholar]
- 21.Keiter S, Baumann L, Farber H, Holbech H, Skutlarek D, Engwall M, et al. Long-term effects of a binary mixture of perfluorooctane sulfonate (PFOS) and bisphenol A (BPA) in zebrafish (Danio rerio). Aquat Toxicol (2012) 11(8–119):116–29. 10.1016/j.aquatox.2012.04.003 [DOI] [PubMed] [Google Scholar]
- 22.Kendziorski JA, Kendig EL, Gear RB, Belcher SM. Strain specific induction of pyometra and differences in immune responsiveness in mice exposed to 17alpha-ethinyl estradiol or the endocrine disrupting chemical bisphenol A. Reprod Toxicol (2012) 34(1):22–30. 10.1016/j.reprotox.2012.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ansar Ahmed S, Karpuzoglu E, Khan D. Effects of sex steroids on innate and adaptive immunity. In: Klein CA, editor. Sex Hormones and Immunity to Infection. Berlin: Springer; (2010). p. 19–51. [Google Scholar]
- 24.Khan D, Cowan C, Ansar Ahmed S. Estrogen and signaling in the cells of immune system. Adv Neuroimmune Biol (2012) 3(1):73–93. 10.3233/NIB-2012-012039 [DOI] [Google Scholar]
- 25.Kovats S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell Immunol (2015) 294(2):63–9. 10.1016/j.cellimm.2015.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mo R, Chen J, Grolleau-Julius A, Murphy HS, Richardson BC, Yung RL. Estrogen regulates CCR gene expression and function in T lymphocytes. J Immunol (2005) 174(10):6023–9. 10.4049/jimmunol.174.10.6023 [DOI] [PubMed] [Google Scholar]
- 27.Dai R, Phillips RA, Ahmed SA. Despite inhibition of nuclear localization of NF-kappa B p65, c-Rel, and RelB, 17-beta estradiol up-regulates NF-kappa B signaling in mouse splenocytes: the potential role of Bcl-3. J Immunol (2007) 179(3):1776–83. 10.4049/jimmunol.179.2.1068 [DOI] [PubMed] [Google Scholar]
- 28.Carreras E, Turner S, Paharkova-Vatchkova V, Mao A, Dascher C, Kovats S. Estradiol acts directly on bone marrow myeloid progenitors to differentially regulate GM-CSF or Flt3 ligand-mediated dendritic cell differentiation. J Immunol (2008) 180(2):727–38. 10.4049/jimmunol.180.2.727 [DOI] [PubMed] [Google Scholar]
- 29.Subramanian S, Yates M, Vandenbark AA, Offner H. Oestrogen-mediated protection of experimental autoimmune encephalomyelitis in the absence of Foxp3+ regulatory T cells implicates compensatory pathways including regulatory B cells. Immunology (2011) 132(3):340–7. 10.1111/j.1365-2567.2010.03380.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moulton VR, Holcomb DR, Zajdel MC, Tsokos GC. Estrogen upregulates cyclic AMP response element modulator alpha expression and downregulates interleukin-2 production by human T lymphocytes. Mol Med (2012) 18:370–8. 10.2119/molmed.2011.00506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khan D, Dai R, Ansar Ahmed S. Sex differences and estrogen regulation of miRNAs in lupus, a prototypical autoimmune disease. Cell Immunol (2015) 294(2):70–9. [DOI] [PubMed] [Google Scholar]
- 32.Rubin BS. Bisphenol A: an endocrine disruptor with widespread exposure and multiple effects. J Steroid Biochem Mol Biol (2011) 127(1–2):27–34. 10.1016/j.jsbmb.2011.05.002 [DOI] [PubMed] [Google Scholar]
- 33.Moriyama K, Tagami T, Akamizu T, Usui T, Saijo M, Kanamoto N, et al. Thyroid hormone action is disrupted by bisphenol A as an antagonist. J Clin Endocrinol Metab (2002) 87(11):5185–90. 10.1210/jc.2002-020209 [DOI] [PubMed] [Google Scholar]
- 34.Hassan ZK, Elobeid MA, Virk P, Omer SA, ElAmin M, Daghestani MH, et al. Bisphenol A induces hepatotoxicity through oxidative stress in rat model. Oxid Med Cell Longev (2012) 2012:6. 10.1155/2012/194829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Youn JY, Park HY, Lee JW, Jung IO, Choi KH, Kim K, et al. Evaluation of the immune response following exposure of mice to bisphenol A: induction of Th1 cytokine and prolactin by BPA exposure in the mouse spleen cells. Arch Pharm Res (2002) 25(6):946–53. 10.1007/BF02977018 [DOI] [PubMed] [Google Scholar]
- 36.Yan H, Takamoto M, Sugane K. Exposure to bisphenol A prenatally or in adulthood promotes T(H)2 cytokine production associated with reduction of CD4CD25 regulatory T cells. Environ Health Perspect (2008) 116(4):514–9. 10.1289/ehp.10829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee MH, Chung SW, Kang BY, Park J, Lee CH, Hwang SY, et al. Enhanced interleukin-4 production in CD4+ T cells and elevated immunoglobulin E levels in antigen-primed mice by bisphenol A and nonylphenol, endocrine disruptors: involvement of nuclear factor-AT and Ca2+. Immunology (2003) 109(1):76–86. 10.1046/j.1365-2567.2003.01646.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rogers JA, Metz L, Yong VW. Review: endocrine disrupting chemicals and immune responses: a focus on bisphenol-A and its potential mechanisms. Mol Immunol (2013) 53(4):421–30. 10.1016/j.molimm.2012.09.013 [DOI] [PubMed] [Google Scholar]
- 39.Kharrazian D. The potential roles of bisphenol A (BPA) pathogenesis in autoimmunity. Autoimmune Dis (2014) 2014:743616. 10.1155/2014/743616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Richter CA, Birnbaum LS, Farabollini F, Newbold RR, Rubin BS, Talsness CE, et al. In vivo effects of bisphenol A in laboratory rodent studies. Reprod Toxicol (2007) 24(2):199–224. 10.1016/j.reprotox.2007.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miao S, Gao Z, Kou Z, Xu G, Su C, Liu N. Influence of bisphenol A on developing rat estrogen receptors and some cytokines in rats: a two-generational study. J Toxicol Environ Health A (2008) 71(15):1000–8. 10.1080/15287390801907467 [DOI] [PubMed] [Google Scholar]
- 42.Yang M, Lee HS, Pyo MY. Proteomic biomarkers for prenatal bisphenol A-exposure in mouse immune organs. Environ Mol Mutagen (2008) 49(5):368–73. 10.1002/em.20394 [DOI] [PubMed] [Google Scholar]
- 43.Liu Y, Mei C, Liu H, Wang H, Zeng G, Lin J, et al. Modulation of cytokine expression in human macrophages by endocrine-disrupting chemical bisphenol-A. Biochem Biophys Res Commun (2014) 451(4):592–8. 10.1016/j.bbrc.2014.08.031 [DOI] [PubMed] [Google Scholar]
- 44.Zhu J, Jiang L, Liu Y, Qian W, Liu J, Zhou J, et al. MAPK and NF-kappaB pathways are involved in bisphenol A-induced TNF-alpha and IL-6 production in BV2 microglial cells. Inflammation (2014) 38(2):637–48. 10.1007/s10753-014-9971-5 [DOI] [PubMed] [Google Scholar]
- 45.Byun JA, Heo Y, Kim YO, Pyo MY. Bisphenol A-induced downregulation of murine macrophage activities in vitro and ex vivo. Environ Toxicol Pharmacol (2005) 19(1):19–24. 10.1016/j.etap.2004.02.006 [DOI] [PubMed] [Google Scholar]
- 46.Yoshitake J, Kato K, Yoshioka D, Sueishi Y, Sawa T, Akaike T, et al. Suppression of NO production and 8-nitroguanosine formation by phenol-containing endocrine-disrupting chemicals in LPS-stimulated macrophages: involvement of estrogen receptor-dependent or -independent pathways. Nitric Oxide (2008) 18(3):223–8. 10.1016/j.niox.2008.01.003 [DOI] [PubMed] [Google Scholar]
- 47.Kim KH, Yeon SM, Kim HG, Choi HS, Kang H, Park HD, et al. Diverse influences of androgen-disrupting chemicals on immune responses mounted by macrophages. Inflammation (2014) 37(3):649–56. 10.1007/s10753-013-9781-1 [DOI] [PubMed] [Google Scholar]
- 48.Kim JY, Jeong HG. Down-regulation of inducible nitric oxide synthase and tumor necrosis factor-alpha expression by bisphenol A via nuclear factor-kappaB inactivation in macrophages. Cancer Lett (2003) 196(1):69–76. 10.1016/S0304-3835(03)00219-2 [DOI] [PubMed] [Google Scholar]
- 49.Xu H, Yang M, Qiu W, Pan C, Wu M. The impact of endocrine-disrupting chemicals on oxidative stress and innate immune response in zebrafish embryos. Environ Toxicol Chem (2013) 32(8):1793–9. 10.1002/etc.2245 [DOI] [PubMed] [Google Scholar]
- 50.Yoshino S, Yamaki K, Li X, Sai T, Yanagisawa R, Takano H, et al. Prenatal exposure to bisphenol A up-regulates immune responses, including T helper 1 and T helper 2 responses, in mice. Immunology (2004) 112(3):489–95. 10.1111/j.1365-2567.2004.01900.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alizadeh M, Ota F, Kassu A. Immune response to ovalbumin following bisphenol A administration in mice fed with a low level of dietary protein. J Microbiol Immunol Infect (2007) 40(4):364–70. [PubMed] [Google Scholar]
- 52.Goto M, Takano-Ishikawa Y, Ono H, Yoshida M, Yamaki K, Shinmoto H. Orally administered bisphenol A disturbed antigen specific immunoresponses in the naive condition. Biosci Biotechnol Biochem (2007) 71(9):2136–43. 10.1271/bbb.70004 [DOI] [PubMed] [Google Scholar]
- 53.Holladay SD, Xiao S, Diao H, Barber J, Nagy T, Ye X, et al. Perinatal bisphenol A exposure in C57B6/129svj male mice: potential altered cytokine/chemokine production in adulthood. Int J Environ Res Public Health (2010) 7(7):2845–52. 10.3390/ijerph7072845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roy A, Bauer SM, Lawrence BP. Developmental exposure to bisphenol A modulates innate but not adaptive immune responses to influenza A virus infection. PLoS One (2012) 7(6):e38448. 10.1371/journal.pone.0038448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Menard S, Guzylack-Piriou L, Leveque M, Braniste V, Lencina C, Naturel M, et al. Food intolerance at adulthood after perinatal exposure to the endocrine disruptor bisphenol A. FASEB J (2014) 28(11):4893–900. 10.1096/fj.14-255380 [DOI] [PubMed] [Google Scholar]
- 56.Nakajima Y, Goldblum RM, Midoro-Horiuti T. Fetal exposure to bisphenol A as a risk factor for the development of childhood asthma: an animal model study. Environ Health (2012) 11:8. 10.1186/1476-069X-11-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Petzold S, Averbeck M, Simon JC, Lehmann I, Polte T. Lifetime-dependent effects of bisphenol A on asthma development in an experimental mouse model. PLoS One (2014) 9(6):e100468. 10.1371/journal.pone.0100468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roy A, Gaylo A, Cao W, Saubermann LJ, Lawrence BP. Neither direct nor developmental exposure to bisphenol A alters the severity of experimental inflammatory colitis in mice. J Immunotoxicol (2013) 10(4):334–40. 10.3109/1547691X.2012.747231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Krementsov DN, Katchy A, Case LK, Carr FE, Davis B, Williams C, et al. Studies in experimental autoimmune encephalomyelitis do not support developmental bisphenol A exposure as an environmental factor in increasing multiple sclerosis risk. Toxicol Sci (2013) 135(1):91–102. 10.1093/toxsci/kft141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brinkmeyer-Langford C, Rodrigues A, Kochan KJ, Haney R, Rassu F, Steelman AJ, et al. Consequences of perinatal bisphenol A exposure in a mouse model of multiple sclerosis. Autoimmunity (2014) 47(1):57–66. 10.3109/08916934.2013.832220 [DOI] [PubMed] [Google Scholar]
- 61.Yurino H, Ishikawa S, Sato T, Akadegawa K, Ito T, Ueha S, et al. Endocrine disruptors (environmental estrogens) enhance autoantibody production by B1 cells. Toxicol Sci (2004) 81(1):139–47. 10.1093/toxsci/kfh179 [DOI] [PubMed] [Google Scholar]
- 62.Sawai C, Anderson K, Walser-Kuntz D. Effect of bisphenol A on murine immune function: modulation of interferon-gamma, IgG2a, and disease symptoms in NZB X NZW F1 mice. Environ Health Perspect (2003) 111(16):1883–7. 10.1289/ehp.6359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bodin J, Bolling AK, Becher R, Kuper F, Lovik M, Nygaard UC. Transmaternal bisphenol A exposure accelerates diabetes type 1 development in NOD mice. Toxicol Sci (2014) 137(2):311–23. 10.1093/toxsci/kft242 [DOI] [PubMed] [Google Scholar]
- 64.Kundakovic M, Gudsnuk K, Franks B, Madrid J, Miller RL, Perera FP, et al. Sex-specific epigenetic disruption and behavioral changes following low-dose in utero bisphenol A exposure. Proc Natl Acad Sci U S A (2013) 110(24):9956–61. 10.1073/pnas.1214056110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cruz G, Foster W, Paredes A, Yi KD, Uzumcu M. Long-term effects of early-life exposure to environmental oestrogens on ovarian function: role of epigenetics. J Neuroendocrinol (2014) 26(9):613–24. 10.1111/jne.12181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mileva G, Baker SL, Konkle AT, Bielajew C. Bisphenol-A: epigenetic reprogramming and effects on reproduction and behavior. Int J Environ Res Public Health (2014) 11(7):7537–61. 10.3390/ijerph110707537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ferreira LL, Couto R, Oliveira PJ. Bisphenol A as epigenetic modulator: setting the stage for carcinogenesis? Eur J Clin Invest (2015) 45(Suppl 1):32–6. 10.1111/eci.12362 [DOI] [PubMed] [Google Scholar]
- 68.Kundakovic M, Champagne FA. Epigenetic perspective on the developmental effects of bisphenol A. Brain Behav Immun (2011) 25(6):1084–93. 10.1016/j.bbi.2011.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Doshi T, Mehta SS, Dighe V, Balasinor N, Vanage G. Hypermethylation of estrogen receptor promoter region in adult testis of rats exposed neonatally to bisphenol A. Toxicology (2011) 289(2–3):74–82. 10.1016/j.tox.2011.07.011 [DOI] [PubMed] [Google Scholar]
- 70.Doshi T, D’Souza C, Dighe V, Vanage G. Effect of neonatal exposure on male rats to bisphenol A on the expression of DNA methylation machinery in the postimplantation embryo. J Biochem Mol Toxicol (2012) 26(9):337–43. 10.1002/jbt.21425 [DOI] [PubMed] [Google Scholar]
- 71.Patel BB, Raad M, Sebag AI, Chalifour LE. Lifelong exposure to bisphenol A alters cardiac structure/function, protein expression, and DNA methylation in adult mice. Toxicol Sci (2013) 133(1):174–85. 10.1093/toxsci/kft026 [DOI] [PubMed] [Google Scholar]
- 72.Warita K, Mitsuhashi T, Ohta K, Suzuki S, Hoshi N, Miki T, et al. Gene expression of epigenetic regulatory factors related to primary silencing mechanism is less susceptible to lower doses of bisphenol A in embryonic hypothalamic cells. J Toxicol Sci (2013) 38(2):285–9. 10.2131/jts.38.285 [DOI] [PubMed] [Google Scholar]
- 73.Yeo M, Berglund K, Hanna M, Guo JU, Kittur J, Torres MD, et al. Bisphenol A delays the perinatal chloride shift in cortical neurons by epigenetic effects on the Kcc2 promoter. Proc Natl Acad Sci U S A (2013) 110(11):4315–20. 10.1073/pnas.1300959110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu Y, Yuan C, Chen S, Zheng Y, Zhang Y, Gao J, et al. Global and cyp19a1a gene specific DNA methylation in gonads of adult rare minnow Gobiocypris rarus under bisphenol A exposure. Aquat Toxicol (2014) 156:10–6. 10.1016/j.aquatox.2014.07.017 [DOI] [PubMed] [Google Scholar]
- 75.Kim JH, Rozek LS, Soliman AS, Sartor MA, Hablas A, Seifeldin IA, et al. Bisphenol A-associated epigenomic changes in prepubescent girls: a cross-sectional study in Gharbiah, Egypt. Environ Health (2013) 12:33. 10.1186/1476-069X-12-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Miao M, Zhou X, Li Y, Zhang O, Zhou Z, Li T, et al. LINE-1 hypomethylation in spermatozoa is associated with bisphenol A exposure. Andrology (2014) 2(1):138–44. 10.1111/j.2047-2927.2013.00166.x [DOI] [PubMed] [Google Scholar]
- 77.Kim JH, Sartor MA, Rozek LS, Faulk C, Anderson OS, Jones TR, et al. Perinatal bisphenol A exposure promotes dose-dependent alterations of the mouse methylome. BMC Genomics (2014) 15:30. 10.1186/1471-2164-15-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li G, Chang H, Xia W, Mao Z, Li Y, Xu S. F0 maternal BPA exposure induced glucose intolerance of F2 generation through DNA methylation change in Gck. Toxicol Lett (2014) 228(3):192–9. 10.1016/j.toxlet.2014.04.012 [DOI] [PubMed] [Google Scholar]
- 79.Bhan A, Hussain I, Ansari KI, Bobzean SA, Perrotti LI, Mandal SS. Bisphenol-A and diethylstilbestrol exposure induces the expression of breast cancer associated long noncoding RNA HOTAIR in vitro and in vivo. J Steroid Biochem Mol Biol (2014) 141:160–70. 10.1016/j.jsbmb.2014.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Doherty LF, Bromer JG, Zhou Y, Aldad TS, Taylor HS. In utero exposure to diethylstilbestrol (DES) or bisphenol-A (BPA) increases EZH2 expression in the mammary gland: an epigenetic mechanism linking endocrine disruptors to breast cancer. Horm Cancer (2010) 1(3):146–55. 10.1007/s12672-010-0015-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nahar MS, Liao C, Kannan K, Harris C, Dolinoy DC. Inutero bisphenol A concentration, metabolism, and global DNA methylation across matched placenta, kidney, and liver in the human fetus. Chemosphere (2015) 124:54–60. 10.1016/j.chemosphere.2014.10.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Manikkam M, Tracey R, Guerrero-Bosagna C, Skinner MK. Plastics derived endocrine disruptors (BPA, DEHP and DBP) induce epigenetic transgenerational inheritance of obesity, reproductive disease and sperm epimutations. PLoS One (2013) 8(1):e55387. 10.1371/journal.pone.0055387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Susiarjo M, Sasson I, Mesaros C, Bartolomei MS. Bisphenol A exposure disrupts genomic imprinting in the mouse. PLoS Genet (2013) 9(4):e1003401. 10.1371/journal.pgen.1003401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Trapphoff T, Heiligentag M, El Hajj N, Haaf T, Eichenlaub-Ritter U. Chronic exposure to a low concentration of bisphenol A during follicle culture affects the epigenetic status of germinal vesicles and metaphase II oocytes. Fertil Steril (2013) 100(6)1758–67.e1. 10.1016/j.fertnstert.2013.08.021 [DOI] [PubMed] [Google Scholar]
- 85.Doshi T, D’Souza C, Vanage G. Aberrant DNA methylation at Igf2-H19 imprinting control region in spermatozoa upon neonatal exposure to bisphenol A and its association with post implantation loss. Mol Biol Rep (2013) 40(8):4747–57. 10.1007/s11033-013-2571-x [DOI] [PubMed] [Google Scholar]
- 86.Bastos Sales L, Kamstra JH, Cenijn PH, van Rijt LS, Hamers T, Legler J. Effects of endocrine disrupting chemicals on in vitro global DNA methylation and adipocyte differentiation. Toxicol In vitro (2013) 27(6):1634–43. 10.1016/j.tiv.2013.04.005 [DOI] [PubMed] [Google Scholar]
- 87.Weinhouse C, Anderson OS, Jones TR, Kim J, Liberman SA, Nahar MS, et al. An expression microarray approach for the identification of metastable epialleles in the mouse genome. Epigenetics (2011) 6(9):1105–13. 10.4161/epi.6.9.17103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Anderson OS, Nahar MS, Faulk C, Jones TR, Liao C, Kannan K, et al. Epigenetic responses following maternal dietary exposure to physiologically relevant levels of bisphenol A. Environ Mol Mutagen (2012) 53(5):334–42. 10.1002/em.21692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Qin XY, Fukuda T, Yang L, Zaha H, Akanuma H, Zeng Q, et al. Effects of bisphenol A exposure on the proliferation and senescence of normal human mammary epithelial cells. Cancer Biol Ther (2012) 13(5):296–306. 10.4161/cbt.18942 [DOI] [PubMed] [Google Scholar]
- 90.Bromer JG, Zhou Y, Taylor MB, Doherty L, Taylor HS. Bisphenol-A exposure in utero leads to epigenetic alterations in the developmental programming of uterine estrogen response. FASEB J (2010) 24(7):2273–80. 10.1096/fj.09-140533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Veiga-Lopez A, Luense LJ, Christenson LK, Padmanabhan V. Developmental programming: gestational bisphenol-A treatment alters trajectory of fetal ovarian gene expression. Endocrinology (2013) 154(5):1873–84. 10.1210/en.2012-2129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kovanecz I, Gelfand R, Masouminia M, Gharib S, Segura D, Vernet D, et al. Oral bisphenol A (BPA) given to rats at moderate doses is associated with erectile dysfunction, cavernosal lipofibrosis and alterations of global gene transcription. Int J Impot Res (2014) 26(2):67–75. 10.1038/ijir.2013.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tilghman SL, Bratton MR, Segar HC, Martin EC, Rhodes LV, Li M, et al. Endocrine disruptor regulation of microRNA expression in breast carcinoma cells. PLoS One (2012) 7(3):e32754. 10.1371/journal.pone.0032754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Avissar-Whiting M, Veiga KR, Uhl KM, Maccani MA, Gagne LA, Moen EL, et al. Bisphenol A exposure leads to specific microRNA alterations in placental cells. Reprod Toxicol (2010) 29(4):401–6. 10.1016/j.reprotox.2010.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li X, Xie W, Xie C, Huang C, Zhu J, Liang Z, et al. Curcumin modulates miR-19/PTEN/AKT/p53 axis to suppress bisphenol A-induced MCF-7 breast cancer cell proliferation. Phytother Res (2014) 28(10):1553–60. 10.1002/ptr.5167 [DOI] [PubMed] [Google Scholar]
- 96.Chen X, Xu B, Han X, Mao Z, Talbot P, Chen M, et al. Effect of bisphenol A on pluripotency of mouse embryonic stem cells and differentiation capacity in mouse embryoid bodies. Toxicol In vitro (2013) 27(8):2249–55. 10.1016/j.tiv.2013.09.018 [DOI] [PubMed] [Google Scholar]
- 97.Zhang Q, Xu X, Li T, Lu Y, Ruan Q, Lu Y, et al. Exposure to bisphenol-A affects fear memory and histone acetylation of the hippocampus in adult mice. Horm Behav (2014) 65(2):106–13. 10.1016/j.yhbeh.2013.12.004 [DOI] [PubMed] [Google Scholar]
- 98.Dhimolea E, Wadia PR, Murray TJ, Settles ML, Treitman JD, Sonnenschein C, et al. Prenatal exposure to BPA alters the epigenome of the rat mammary gland and increases the propensity to neoplastic development. PLoS One (2014) 9(7):e99800. 10.1371/journal.pone.0099800 [DOI] [PMC free article] [PubMed] [Google Scholar]