Abstract
Background
Neutralizing antibodies (NAb) to interferon-beta (IFN-β) are associated with reduced bioactivity and efficacy of IFN-β in multiple sclerosis (MS). The myxovirus resistance protein A (MxA) gene expression is one of the most appropriate markers of biological activity of exogenous IFN-β. We hypothesized that therapeutic plasma exchange (TPE) can restore the ability of IFN-β to induce the MxA mRNA expression and that maintenance plasmapheresis can sustain the bioavailability of IFN-β.
Material/Methods
Eligible patients underwent 4 primary separate plasma exchange sessions. After the induction TPE sessions, they were transferred to maintenance plasmapheresis. Bioactivity of IFN-β was expressed as in vivo MxA mRNA induction in whole blood using RT-qPCR.
Results
Six patients with low IFN-β bioavailability detected by the MxA mRNA response were included. Four patients became biological responders after induction plasmapheresis. In 2 patients an increase of MxA mRNA expression was found, but the values persisted below the cut-off and the patients remained as “poor biological responders”. The effect of maintenance plasmapheresis was transient: MxA mRNA expression values reverted to the baseline levels after 1–2 months.
Conclusions
Therapeutic plasma exchange is able to restore the bioavailability of IFN-β in the majority of studied patients, but the effect of TPE on the IFN-β bioavailability was transient.
MeSH Keywords: Antibodies, Neutralizing; Interferon-beta; Multiple Sclerosis; Plasma Exchange
Background
Multiple sclerosis (MS) is generally considered a chronic inflammatory demyelinating disease of the central nervous system, thought to be triggered by an autoimmune response to myelin. The exact cause of MS is unknown. However, it is believed that some factors, including genetics, environment, and childhood infection, may play a role. Even vascular etiologies (chronic cerebrospinal venous insufficiency) due to the periventricular predilection of the lesions have been considered [1–4].
Nowadays there are a number of disease modifying treatment options, which have been shown to slow the progression of MS in significant proportion of patients. Interferon beta (IFN-β) preparations are the most widely used initial therapies prescribed for patients with relapsing-remitting MS (RRMS) [5–8]. However, IFN-β preparations, like other protein-based biopharmaceuticals produced by recombinant gene technologies, are potentially immunogenic [9]. It is known that IFN-β preparations are associated with the development of 2 different classes of antibodies against IFN-β: binding antibodies (BAbs) and neutralizing antibodies (NAbs). BAbs bind to the IFN-β molecule and may or may not interfere with its functions. NAbs interfere with functions of the IFN-β molecule in vitro, most likely by preventing binding of IFN-β to the IFN receptor on cells used in the assay, thereby inhibiting the functional activation of the receptor. BAbs can be demonstrated in the majority of patients treated with an IFN-β preparation, but only a small proportion of patients develop NAbs [10,11].
Persistent and high-titer NAbs reduce or eliminate the biological activity of IFN-β therapies for MS and are associated with reduced efficacy. The majority of studies greater than 2 years in duration reported a higher attack rate, disease activity measured on brain MRI, and disability progression on EDSS in NAb-positive compared to NAb-negative patients [11–15].
Low concentrations of NAbs can be detected in vitro with sensitive assay after 6 months, whereas clinically relevant NAbs usually develop between 9 and 18 months after start of IFN-β therapy. It is clear that if NAbs have not developed by this time, they are unlikely to develop in the future [10]. The frequency of developing NAb and BAb to IFN-βeta varied according to the IFN-βeta given. Specifically, the NAb seroconversion frequency was significantly higher in patients treated with IFN-β-1b (up to 30% and more) than in patients treated with both preparations of recombinant IFN-β-1a (approx. 6–7%) [16].
The most commonly used method for measuring of BAbs is ELISA [10,17], particularly capture ELISA, which correlates better with NAb titers than does direct ELISA [18]. In general, ELISA titers correlate only weakly with NAb titers, but BAb-negative samples measured by ELISA reliably predict NAb-negativity [19]. A number of methods have been developed for the measurement of NAbs and all are based on measuring the in vivo responses of IFN-β-sensitive human cell lines to the application of IFN-β. Binding of IFN-β to the IFN receptor complex on the cells leads to a change in the expression levels of many genes that have antiviral, antiproliferative, and immunological properties. In the presence of Nabs, these changes are inhibited [17]. The CPE assay is considered the gold standard method for measuring NAbs. However, the assay is prone to variation, and is very time-consuming and non-specific [10,17]. For this reason, CPE assay has been modified by measuring the amount of MxA protein or MxA mRNA produced following stimulation with IFN-β. Stimulation with IFN-β leads to a dose-dependent increase in MxA protein and MxA mRNA [17]. Measuring MxA production has the advantage of being faster and more reliable; however, its relatively high cost may limit its adoption as a routine assay in clinical laboratories [17,20]. Other method for measuring of NAbs is the assay based on the in vivo induction of MxA. Maximal MxA mRNA concentrations are achieved about 12 h after the dose of IFN-β is given. Results are reliable and reproducible; however, costs are relatively high [17,21–23]. As described earlier in NAb-positive patients, especially those with high titers, the MxA response decreases to baseline levels, indicating that NAbs abrogate the bioactivity of IFN-β [24], meaning that MxA mRNA levels inversely correlate with NAb titers [20,25–27].
There is no consensus on preventing development of Nabs or management of NAb-positive patients except for switching them to non-interferon therapy. When NAbs develops, it is difficult to revert patients to a NAb-negative state. Monthly pulses of intravenous methylprednisolone may reduce the risk of developing NAbs during the first year of treatment, but possibly this is only of clinical importance in cases without high-titer Nabs and only in those cases when methylprednisolone is given together with IFN-β from the beginning of the treatment [28,29]. However, in established NAb-positive MS patients who continue IFN-β therapy, treatment with monthly cycles of high-dose methylprednisolone does not restore IFN-β biologic response [29–31] nor did a combination of azathioprine or oral low-dose methotrexate and monthly methylprednisolone cycles [31,32].
Plasmapheresis might be considered as possible procedures to diminish NAbs generation and to restore the abolished bioavailability of IFN-β, because it effectively removes autoantibodies from the plasma [10,33,34]. However it is still unknown whether TPE can promote recovery of IFN-β bioavailability.
The objectives of the study were
To assess the effect of TPE on the ability of IFN-β to induce the MxA mRNA;
To evaluate the possibility of sustaining recovered IFN-β bioavailability by maintenance plasmapheresis, if the induction TPE restores the activity of IFN-β; and
To assess when the markers of IFN-β bioavailability return to baseline levels after TPE use.
Material and methods
Study design
An open-label, single-center proof of concept study was initiated in 2013 at the Neurology and Neurosurgery Clinic, Faculty of Medicine at Vilnius University, Vilnius University Hospital Santariskiu Clinics, Department of Neurology. The study protocol was approved by the Vilnius Regional Bioethics Committee (Trial registration number 158200-13-644-191) and written informed consent was signed by each subject.
Study population
The participants were MS patients treated at Vilnius University Hospital Santariskiu Clinics. We included 6 patients in the study and the study was ended earlier than initially planned because the results were so evident that it was considered unethical to continue the study.
The trial population consisted of subjects with RRMS according to the following inclusion and exclusion criteria.
Inclusion criteria
Willing and able to sign written informed consent;
Diagnosis of RRMS according to the revised McDonald criteria (2010 Revisions);
Subjects aged 18–55 years (inclusive) at the time of informed consent signing;
EDSS score of 0.0 up to and including 5.5;
Neurologically stable with no evidence of relapse and steroid-free for at least 3 months preceding the enrolment;
Treatment with high-doses of IFN-β. All participants were treated with IFN-β-1b (Betaferon) 250 μg every other day subcutaneously for more than 18 months;
No IFN-β bioactivity detected by means of in vivo MxA mRNA response 9–12 h after IFN-β injection at least 3 months preceding the enrolment and at the screening. High BAbs titers, measured by ELISA method, were found before MxA mRNA assay.
Exclusion criteria
Any life-threatening, medically unstable, or otherwise clinically significant condition or findings other than MS, in particular neoplastic disease, seizure disorders, or psychiatric diseases;
Medical conditions or laboratory abnormalities that might be negatively influenced by plasma exchange sessions e.g., patients with hypocalcemia, immune compromise, or malignancy);
Severe allergic/anaphylactic reactions, in particular if a history of prior reaction to blood products existed, or any known drug hypersensitivity;
Hemodynamically unstable patients; or
Patients with limited vascular access.
The mean age of these patients at the screening onset was 43.7 years (range, 36–54 years). Four patients were women (66.7%) and 2 patients were men (33.3%). The average treatment duration of immunomodulatory therapy prior to the screening was 4.5 (±1.8) years. The mean disability score on the EDSS was 3.7 (±0.9) (Table 1). All patients were clinically stable during the study.
Table 1.
Demographic characteristics of study subjects.
| Patient # | Age, year | Gender | MS duration, year | EDSS | IM treatment duration, year |
|---|---|---|---|---|---|
| 1 | 40 | M | 6 | 3.5 | 3 |
| 2 | 40 | F | 15 | 5.0 | 3 |
| 3 | 43 | F | 7 | 3.5 | 3 |
| 4 | 36 | F | 9 | 2.5 | 5 |
| 5 | 49 | M | 16 | 3.0 | 7 |
| 6 | 54 | F | 28 | 4.5 | 6 |
The procedure
Six patients met the inclusion criteria and were included over a 4-month recruitment period. Patients underwent 4 separate plasma exchange sessions of 2.0–2.5 plasma volumes over a 5–8 day span. After induction plasmapheresis, in order to sustain the bioavailability of IFN-β, the patients were transferred to the maintenance plasmapheresis – 3 sessions were performed in total, with 1 plasma exchange session per month. Donor plasma was used for plasma replacement. All participants received IFN-β-1b (Betaferon) 250 μg subcutaneously every other day during the entire study. One patient after the first incomplete plasma exchange session (1100 ml plasma volume was replaced) was switched to the centrifugal plasmapheresis due to adverse reactions to donor plasma; the patient underwent 6 centrifugal plasmapheresis sessions of 310–380 ml volumes every day (Table 2). Premedication with clemastine IM and dexamethasone IV before TPE was given to avoid hypersensitivity reactions and calcium gluconate was infused IV during the TPE to avoid hypocalcemic symptoms.
Table 2.
Study procedures and assessments.
| Screening | Induction TPE | Maintenance TPE | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Day/month | 0 | 1D | 2D | 3D | 4D | 5D | 1M | 2M | 3M | 4M |
| Informed consent | x | |||||||||
| In/Exclusion criteria | x | |||||||||
| Physical examination* | x | x | x | x | x | x | x | x | x | x |
| Neurological examination** | x | x | x | x | x | x | ||||
| IFN-β BAbs*** | x | x | x | x | ||||||
| Lab values# | x | x | x | x | ||||||
| IFN-β bioavailability## | x | x | x | x | x | x | ||||
| TPE | x | x | x | x | x | x | x | |||
| (S)AEs assessment and reporting | x | Whenever (serious) adverse events occurs | ||||||||
ABP, HR, Resp, body temperature, ECG;
Neurological examination, EDSS;
BAbs measured with ELISA before MxA expression;
Hb, WBC, PLT, electrolytes, TP, coagulation (APTT, PA, fibrinogen);
MxA mRNA expression (before IFN-β injection and 12 hours after injection). INF-β – interferon beta; BAbs – binding antibodies; MxA – myxovirus resistance protein A; mRNA – messenger RNA; TPE – therapeutic plasma exchange.
IFN-β bioactivity measurement
Blood from MS patients was drawn into PAXgene Blood RNA Tube (PreAnalytiX GmbH, Hombrechtikon, Switzerland) before and 12 hours after injection of INF-β. Total RNA was extracted using Purlink FFPE Total RNA Isolaton Kit (Life Technologies, Carlsbad CA, USA) according to the manufacturer‘s protocol. Total RNA was subsequently reverse-transcribed to cDNA using RevertAid™ M-MULV reverse transcriptase and random hexamers (Thermo Fisher Scientific, Vilnius, Lithuania). Previously published sequences for MxA and GAPDH primers and probes were used in duplex qPCR reactions [35]. qPCR reactions were performed in a 20-μl reaction volume containing Maxima® Probe qPCR Master Mix supplemented with Uracil-DNA glycosylase (Thermo Fisher Scientific, Vilnius, Lithuania), 300-nmol/l final concentration of each primer, 200 nmol/l of each probe, and 2-μl cDNA solution. qPCR was performed on a RotorGene 6000 analyzer (Qiagen, Hilden, Germany) using the following cycling conditions: 2 min at 50°C followed by 5 min at 95°C and 45 amplification cycles at 95°C for 20 s, 60°C for 15 s, and 72°C for 20 s. Relative quantification of MxA expression was calculated by the ΔΔCq method. Peripheral blood cDNA from a pool of healthy donors was used as a calibrator in ΔΔCq calculations and was run in parallel with patient samples. This calibrator was assigned the normalization ratio 1. MS patient samples before and after INF-β injection were always analyzed in the same qPCR run. Two cut-off values for INF-β bioactivity were determined based on MxA expression results of 30 untreated MS patients, as described previously [36].
The definition of biological responders, poor biological responders, and biological non-responders
Biological responders, poor biological responders, and biological non-responders were defined based on the absolute values of their MxA expression/induction indicators regarding 2 cut-off values established before the study. Biological responders were defined as the patients whose MxA expression values were above both threshold levels. In case only 1 threshold was reached, a patient was assigned to the group of poor biological responder. If neither of the 2 thresholds was reached, a patient was defined as a biological non-responder. MxA mRNA expression cut-off of <0.586 before INF-β injection and MxA mRNA expression cut-off of <3.84 after INF-β injection were considered as negative.
Results
TPE effect on the bioavailability of IFN-β
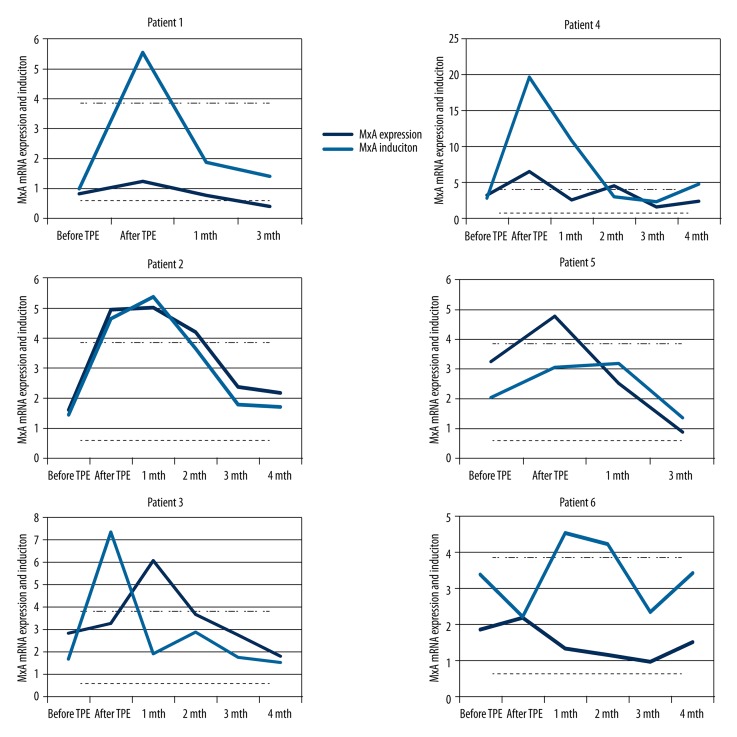
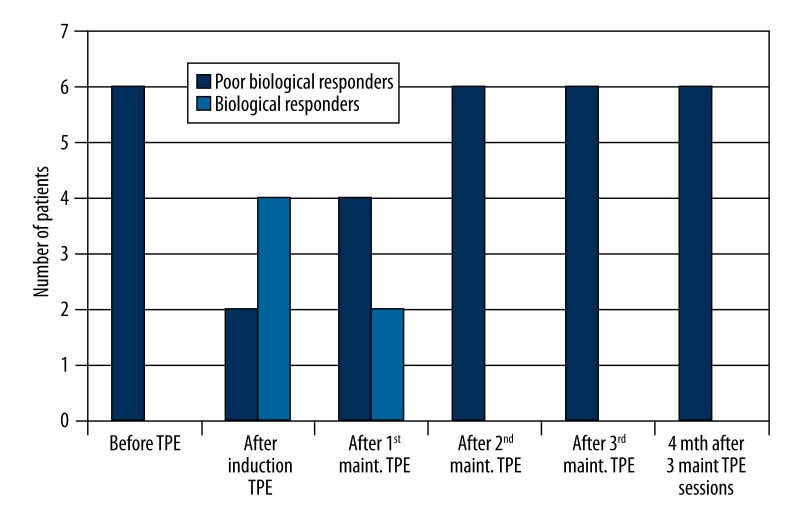
A sharp increase of MxA mRNA expression and induction was found in 4 patients after induction plasmapheresis (in 1 case after centrifugal plasmapheresis); the patients regained an in vivo MxA response to IFN-β therapy and became “biological responders” (Figure 1). In 2 patients, an increase of MxA mRNA expression or induction was found but the values persisted below the expression and induction cut-off and the patients remained as “poor biological responders”. In the patients who became “biological responders”, MxA mRNA expression values after the maintenance plasmapheresis sessions reverted to the baseline levels after 1 or 2 months (Figure 2), in 2 patients after the first maintenance plasmapheresis and in the other 2 patients after the second session.
Figure 1.
MxA mRNA expression and MxA mRNA induction levels before and after four sessions of induction TPE and after maintenance TPE each subsequent month in six MS patients separately. MxA mRNA expression cut-off is shown as a dashed line, MxA mRNA induction cut-off – as a dot-dashed line. MxA – myxovirus resistance protein A; mRNA – messenger RNA; TPE – therapeutic plasma exchange.
Figure 2.
The proportion of “biological” and “poor biological” responders before and after therapeutic plasma exchange sessions. TPE – therapeutic plasma exchange; maint – maintenance.
The effect of TPE on the BAbs
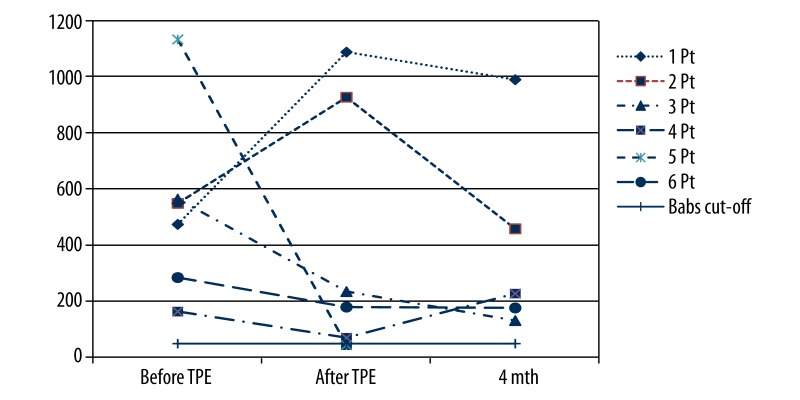
The mean BAbs titer in all patients before TPE was 528 BTU (±335.0 BTU). In 4 patients, a decrease of BAbs titer was found after induction TPE; a decrease by 403.8 BTU on average was found. In the first and in the second patient, an increase of BAbs titer by 610 and 377 BTU, respectively, was found. In contrast to increased BAbs titers, these 2 patients regained an in vivo MxA response to IFN-β therapy after the initial course of plasmapheresis. The mean titer of BAbs in all patients after induction plasmapheresis was 423.8 BTU (±459.5 BTU) and 396.8 (±354.0 BTU) in 5 patients after the maintenance plasmapheresis (Figure 3).
Figure 3.
BAbs titers before and after induction and maintenance TPE in six MS patients separately. TPE – therapeutic plasma exchange; Pt – patient; BAbs – binding antibodies.
Safety and tolerability of the TPE
One of the 6 patients after the first incomplete plasma exchange session was switched to the centrifugal plasmapheresis due to the excessive itchy rashes. The rashes persisted after the additional treatment with dexamethasone and clemastine. One patient during the first and the second sessions of induction plasmapheresis had urticaria, which regressed completely after the treatment with antihistamines. One patient after the induction plasmapheresis sessions underwent only 2 procedures of maintenance plasmapheresis; the third session was canceled due to abdominal pain. The pain appeared 3 weeks later after the last (second) maintenance plasmapheresis, and appendicitis was diagnosed. After the surgery, plasma exchange was not performed because the patient refused to continue to participate in the study.
Discussion
Most RRMS patients treated with IFN-β preparations do not develop persistent and high-titer NAbs associated with reduced measures of radiographic and clinical efficacy. For those that do, however, NAbs is a significant clinical problem, especially if other therapies have already been used, are not tolerated, or are not available. There is no consensus on prevention of NAb development or management of NAb-positive MS patients except for switching them to non-interferon therapy. However, NAb reversion cannot be accelerated by the concomitant treatment with corticosteroids or by the combined treatment with azathioprine or oral low-dose methotrexate and corticosteroids [29,31,32].
Because TPE is effectively used to remove serum proteins from the circulation, we decided in the present study to perform plasma exchange sessions to restore and then sustain the recovered bioavailability of IFN-β. All the patients in this study had been treated with high-dose IFN-β for more than 18 months and did not have an in vivo MxA response to IFN-β. We used the in vivo response to IFN-β for IFN-β bioavailability because MxA mRNA levels inversely correlate with NAb titers. Furthermore, several studies have shown that high titers of NAbs invariably are associated with decreased IFN-β bioactivity but low NAbs titers are correlated poorly with expression of IFN-β induced genes or gene products [37–39].
The present data support the hypothesis that induction TPE may promote the recovery of in vivo biological response to IFN-β, because 4 patients became “biological responders” after induction plasmapheresis. It should be noted that 2 patients who did not regain the ability of IFN-β to induce the MxA mRNA were the oldest (54 and 49 years old) and had with the longest treatment duration (6 and 7 years, respectively). In contrast, the mean age of all others was 39.8 years and mean treatment duration was 3.5 years (Table 1). However, in other studies, which assessed the effect of immunosuppression on NAbs, no data about the impact of immunosuppression in the different age groups or in the groups with different treatment duration were evaluated.
Unfortunately, the effect of maintenance plasmapheresis on the IFN-β bioavailability was transient – even during the maintenance plasmapheresis sessions when one TPE session was performed per months, the biological activity of IFN-β returned to baseline levels after 1 or 2 months.
On the contrary, the effect of TPE on BAbs was different from the effect on the MxA mRNA expression values. Whereas BAbs levels markedly increased after TPE (Figure 3) in patients with restored IFN-β bioavailability, they did not change in patients in who low availability of IFN-β remained (Figure 1).
In chronic diseases, TPE use is more problematic than in acute diseases due to the phenomenon of rebound antibody production. Therefore, TPE alone is not effective for persistent reduction of NAbs and sustaining of IFN-β bioavailability. Antibodies are effectively removed after plasmapheresis in various autoimmune diseases (e.g., myasthenia gravis and chronic inflammatory demyelinating polyneuropathy), but the additional immunosuppressive therapy after plasmapheresis courses is required for long-term management to prevent the re-synthesis of antibodies. It is possible that the same combination of plasmapheresis and immunosuppression must be used in NAb-positive patients to achieve and maintain adequate bioavailability of IFN-β.
The results of the present study demonstrate that there is still no optimal methodology to restore or improve the markers of IFN-β bioactivity in abolished IFN-β ability or to prevent the development of NAbs in IFN-β-treated RRMS patients. Consequently, to tailor the best treatment for MS patients and to prevent the development of NAbs, the neurologist must carefully consider the results of clinical trials and the particular individual clinical and prognostic characteristics of each patient, including susceptibility to NAbs and clinical effects of therapy.
Conclusions
TPE can restore the bioavailability of IFN-β in most patients, especially younger patients and patients with shorter immunomodulatory treatment duration.
The applied schedule of maintenance TPE is insufficient to sustain the recovered IFN-β bioavailability.
The markers of IFN-β bioavailability return to the baseline levels during 1 to 2 months when 1 TPE session is performed per month.
Acknowledgements
The authors thank Dr. Antanas Griskevicius and Dr. Judita Audzijoniene for their support performing the plasmapheresis sessions. They also thank all participants for their efforts in the study.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Ethics approval
The study protocol was approved by the Vilnius Regional Bioethics Committee (Trial registration number 158200-13-644-191) and written informed consent was signed by all participants.
Source of support: Departmental sources
References
- 1.Bohlega S. Epidemiology of MS. Mult Scler Relat Disord. 2014;3(6):766–67. [Google Scholar]
- 2.Ramagopalan SV, Guimond C, Dyment DA, et al. Early life child exposure and the risk of multiple sclerosis: a population based study. J Neurol Sci. 2011;307(1–2):162–63. doi: 10.1016/j.jns.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 3.Djelilovic-Vranic J, Alajbegovic A, Tiric-Campara M, et al. Stress as provoking factor for the first and repeated multiple sclerosis seizures. Mater Sociomed. 2012;24(3):142–47. doi: 10.5455/msm.2012.24.142-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jandaghi AB, Amanian D, Roudbari SA, et al. Evaluation of hemodynamic properties of cerebral venous drainage in patients with multiple sclerosis: a case-control study. Pol J Radiol. 2014;79:323–27. doi: 10.12659/PJR.890690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.IFN beta Multiple Sclerosis Study Group. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis: clinical results of a multi-center randomized, double-blind, placebo controlled trial. Neurology. 1993;43:655–61. doi: 10.1212/wnl.43.4.655. [DOI] [PubMed] [Google Scholar]
- 6.The IFNb Multiple Sclerosis Study Group and the University of British Columbia MS/MRI Analysis Group. Interferon beta-1b in the treatment of multiple sclerosis: final outcome of the randomized controlled trial. Neurology. 1995;45:1277–85. [PubMed] [Google Scholar]
- 7.The PRISMS (Prevention of Relapses and Disability by Interferona-β-1a Subcutaneously in Multiple Sclerosis) Study Group, the University of British Columbia MS/MRI Analysis Group. PRISMS-4: Long-term efficacy of interferon-beta-1a in relapsing MS. Neurology. 2001;56:1628–36. doi: 10.1212/wnl.56.12.1628. [DOI] [PubMed] [Google Scholar]
- 8.Jacobs LD, Beck RW, Simon JH, et al. Intramuscular Interferon Beta-1a therapy initiated during a first demyelinating event in multiple sclerosis. N Engl J Med. 2000;343:898–904. doi: 10.1056/NEJM200009283431301. [DOI] [PubMed] [Google Scholar]
- 9.Schellekens H. Bioequivalence and the immunogenicity of biopharmaceuticals. Nat Rev Drug Discov. 2002;1(6):457–62. doi: 10.1038/nrd818. [DOI] [PubMed] [Google Scholar]
- 10.Sorensen PS. Neutralizing antibodies against interferon-beta. Ther Adv Neurol Disord. 2008;1(2):62–78. doi: 10.1177/1756285608095144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sorensen PS, Ross C, Clemmesen KM, et al. Danish Multiple Sclerosis Study Group. Clinical importance of neutralising antibodies against interferon beta in patients with relapsing-remitting multiple sclerosis. Lancet. 2003;362(9391):1184–91. doi: 10.1016/S0140-6736(03)14541-2. [DOI] [PubMed] [Google Scholar]
- 12.Polman C, Kappos L, White R, et al. European Study Group in Interferon Beta-1b in Secondary Progressive MS. Neutralizing antibodies during treatment of secondary progressive MS with interferon beta-1b. Neurology. 2003;60(1):37–43. doi: 10.1212/wnl.60.1.37. [DOI] [PubMed] [Google Scholar]
- 13.Goodin DS, Hartung HP, O’Connor P, et al. Neutralizing antibodies to interferon beta-1b multiple sclerosis: a clinico-radiographic paradox in the BEYOND trial. Mult Scler. 2012;18(2):181–95. doi: 10.1177/1352458511418629. [DOI] [PubMed] [Google Scholar]
- 14.Francis GS, Rice GP, Alsop JC PRISMS Study Group. Interferon beta-1a in MS: results following development of neutralizing antibodies in PRISMS. Neurology. 2005;65(1):48–55. doi: 10.1212/01.wnl.0000171748.48188.5b. [DOI] [PubMed] [Google Scholar]
- 15.Kappos L, Clanet M, Sandberg-Wollheim M, et al. European Interferon Beta-1a IM Dose-Comparison Study Investigators. Neutralizing antibodies and efficacy of interferon beta-1a: a 4-year controlled study. Neurology. 2005;65(1):40–47. doi: 10.1212/01.wnl.0000171747.59767.5c. [DOI] [PubMed] [Google Scholar]
- 16.Scagnolari C, Bellomi F, Turriziani O, et al. Neutralizing and binding antibodies to IFN-beta: relative frequency in relapsing-remitting multiple sclerosis patients treated with different IFN-beta preparations. J Interferon Cytokine Res. 2002;22(2):207–13. doi: 10.1089/107999002753536176. [DOI] [PubMed] [Google Scholar]
- 17.Farrell RA, Marta M, Gaeguta AJ, et al. Development of resistance to biologic therapies with reference to IFN-β. Rheumatology (Oxford) 2012;51(4):590–99. doi: 10.1093/rheumatology/ker445. [DOI] [PubMed] [Google Scholar]
- 18.Gneiss C, Brugger M, Millonig A, et al. Comparative study of four different assays for the detection of binding antibodies against interferon-beta. Mult Scler. 2008;14:830–36. doi: 10.1177/1352458508089228. [DOI] [PubMed] [Google Scholar]
- 19.Gilli F, Marnetto F, Caldano M, et al. Anti-interferon-β neutralising activity is not entirely mediated by antibodies. J Neuroimmunol. 2007;192:198–205. doi: 10.1016/j.jneuroim.2007.09.025. [DOI] [PubMed] [Google Scholar]
- 20.Bertolotto A, Sala A, Caldano M, et al. Development and validation of a real time PCR-based bioassay for quantification of neutralizing antibodies against human interferon-beta. J Immunol Methods. 2007;321:19–31. doi: 10.1016/j.jim.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 21.Malucchi S, Gilli F, Caldano M, et al. One-year evaluation of factors affecting the biological activity of interferon beta in multiple sclerosis patients. J Neurol. 2011;258:895–903. doi: 10.1007/s00415-010-5844-5. [DOI] [PubMed] [Google Scholar]
- 22.Hesse D, Sellebjerg F, Sorensen PS. Absence of MxA induction by interferon beta in patients with MS reflects complete loss of bioactivity. Neurology. 2009;73:372–77. doi: 10.1212/WNL.0b013e3181b04c98. [DOI] [PubMed] [Google Scholar]
- 23.Bertolotto A, Gilli F, Sala A, et al. Persistent neutralizing antibodies abolish the interferon beta bioavailability in MS patients. Neurology. 2003;60:634–39. doi: 10.1212/01.wnl.0000046662.03894.c5. [DOI] [PubMed] [Google Scholar]
- 24.Pachner AR, Warth JD, Pace A, et al. INSIGHT investigators. Effect of neutralizing antibodies on biomarker responses to interferon beta: the INSIGHT study. Neurology. 2009;73(18):1493–500. doi: 10.1212/WNL.0b013e3181bf98db. [DOI] [PubMed] [Google Scholar]
- 25.Santos R, Weinstock-Guttman B, Tamaño-Blanco M, et al. Dynamics of interferon-beta modulated mRNA biomarkers in multiple sclerosis patients with anti-interferon-beta neutralizing antibodies. J Neuroimmunol. 2006;176(1–2):125–33. doi: 10.1016/j.jneuroim.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 26.Pachner AR, Dail D, Pak E, et al. The importance of measuring IFNbeta bioactivity: monitoring in MS patients and the effect of anti-IFNbeta antibodies. J Neuroimmunol. 2005;166(1–2):180–88. doi: 10.1016/j.jneuroim.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 27.Van der Voort LF, Kok A, Visser A, et al. Interferon-beta bioactivity measurement in multiple sclerosis: feasibility for routine clinical practice. Mult Scler. 2009;15(2):212–18. doi: 10.1177/1352458508096877. [DOI] [PubMed] [Google Scholar]
- 28.Pozzilli C, Antonini G, Bagnato F, et al. Monthly corticosteroids decrease neutralizing antibodies to IFNbeta1 b: a randomized trial in multiple sclerosis. J Neurol. 2002;249(1):50–56. doi: 10.1007/pl00007847. [DOI] [PubMed] [Google Scholar]
- 29.Zarkou S, Carter JL, Wellik KE, et al. Are corticosteroids efficacious for preventing or treating neutralizing antibodies in multiple sclerosis patients treated with beta-interferons? A critically appraised topic. Neurologist. 2010;16(3):212–14. doi: 10.1097/NRL.0b013e3181de4924. [DOI] [PubMed] [Google Scholar]
- 30.Hesse D, Frederiksen JL, Koch-Henriksen N, et al. Methylprednisolone does not restore biological response in multiple sclerosis patients with neutralizing antibodies against interferon-β. Eur J Neurol. 2009;16(1):43–47. doi: 10.1111/j.1468-1331.2008.02336.x. [DOI] [PubMed] [Google Scholar]
- 31.Cohen JA, Imrey PB, Calabresi PA, et al. Results of the Avonex Combination Trial (ACT) in relapsing-remitting MS. Neurology. 2009;72(6):535–41. doi: 10.1212/01.wnl.0000341934.12142.74. [DOI] [PubMed] [Google Scholar]
- 32.Ravnborg M, Bendtzen K, Christensen O, et al. Treatment with azathioprine and cyclic methylprednisolone has little or no effect on bioactivity in anti-interferon beta antibody-positive patients with multiple sclerosis. Mult Scler. 2009;15(3):323–28. doi: 10.1177/1352458508099476. [DOI] [PubMed] [Google Scholar]
- 33.Winters JL. Plasma exchange: concepts, mechanisms, and an overview of the American Society for Apheresis guidelines. Hematology Am Soc Hematol Educ Program. 2012;2012:7–12. doi: 10.1182/asheducation-2012.1.7. [DOI] [PubMed] [Google Scholar]
- 34.Rudick RA, Goodkin DE. Multiple sclerosis therapeutics. Martin Dunitz; London: 1999. [Google Scholar]
- 35.Pachner AR, Narayan K, Pak E. Multiplex analysis of expression of three IFNbeta-induced genes in antibody-positive MS patients. Neurology. 2006;66(3):444–46. doi: 10.1212/01.wnl.0000196467.71646.72. [DOI] [PubMed] [Google Scholar]
- 36.Gilli F, Marnetto F, Caldano M, et al. Biological responsiveness to first injections of interferon-beta in patients with multiple sclerosis. J Neuroimmunol. 2005;158(1–2):195–203. doi: 10.1016/j.jneuroim.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 37.Lam R, Farrell R, Aziz T, et al. Validating parameters of a luciferase reporter gene assay to measure neutralizing antibodies to IFNbeta in multiple sclerosis patients. J Immunol Methods. 2008;336:113–18. doi: 10.1016/j.jim.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 38.Pachner A, Narayan K, Price N, et al. MxA gene expression analysis as an interferon-beta bioactivity measurement in patients with multiple sclerosis and the identification of antibody-mediated decreased bioactivity. Mol Diagn. 2003;7(1):17–25. doi: 10.1007/BF03260016. [DOI] [PubMed] [Google Scholar]
- 39.Bertolotto A, Gilli F, Sala A, et al. Evaluation of bioavailability of three types of IFNbeta in multiple sclerosis patients by a new quantitative-competitive-PCR method for MxA quantification. J Immunol Methods. 2001;256(1–2):141–52. doi: 10.1016/s0022-1759(01)00434-3. [DOI] [PubMed] [Google Scholar]