Abstract
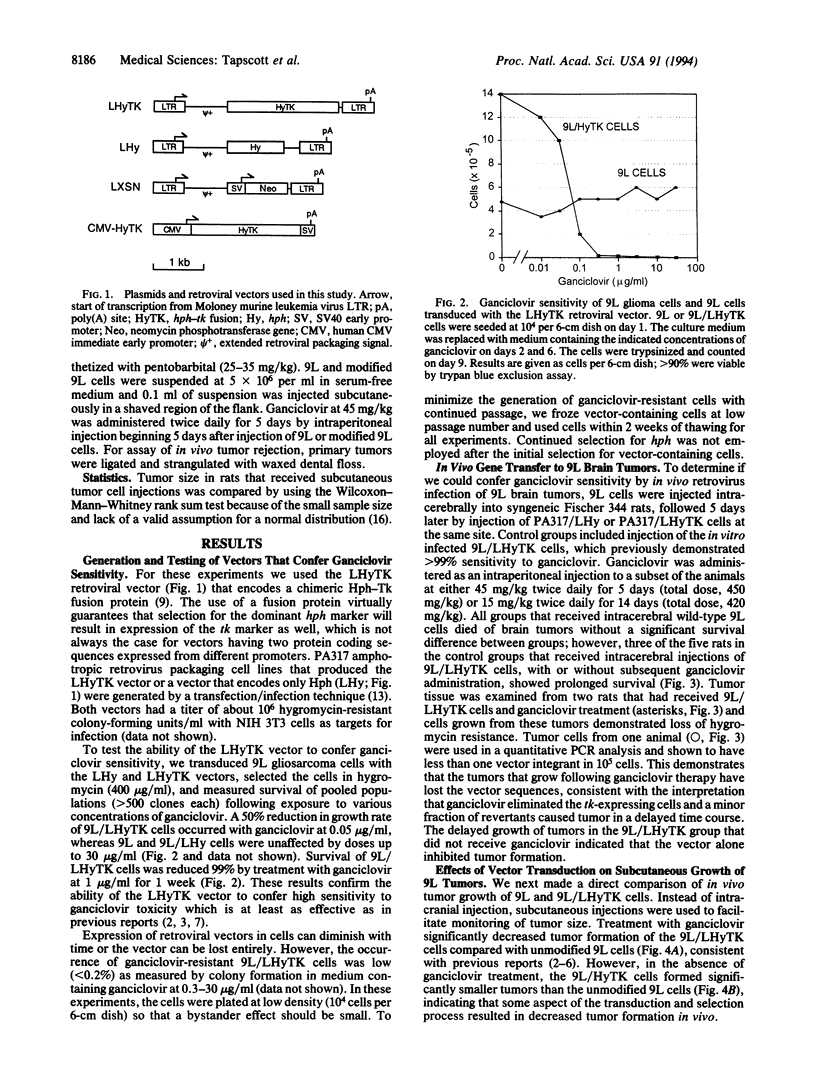
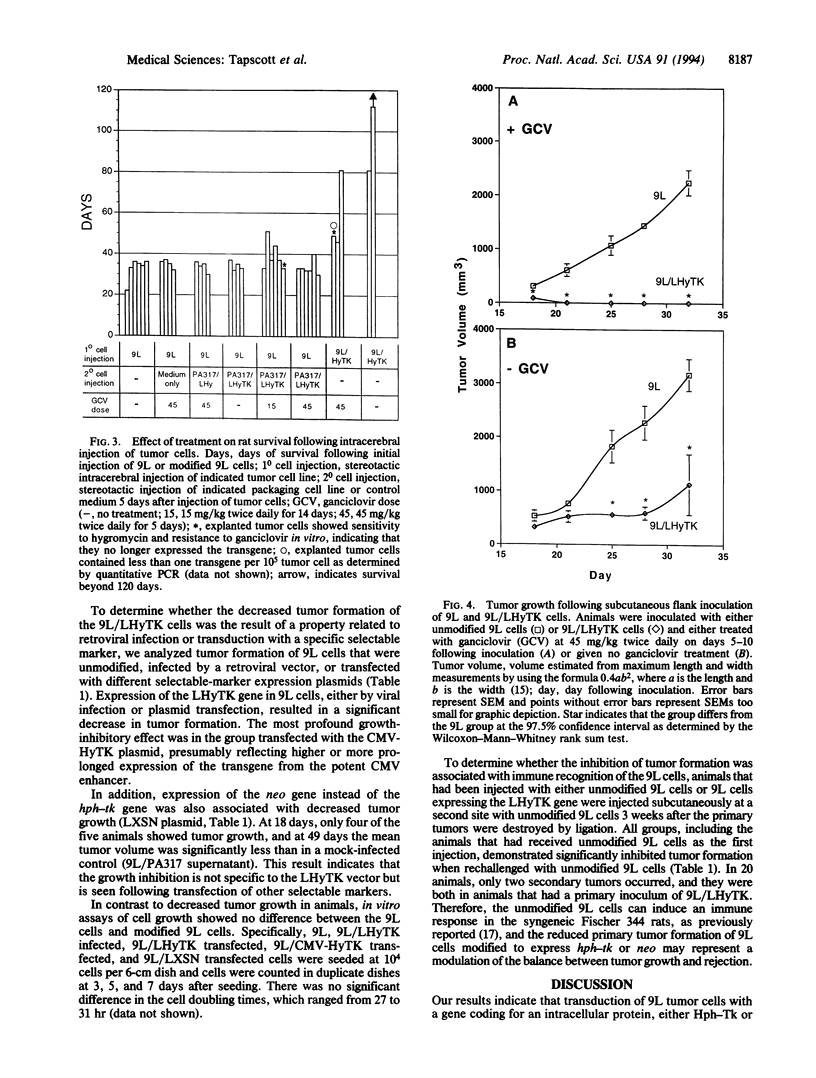
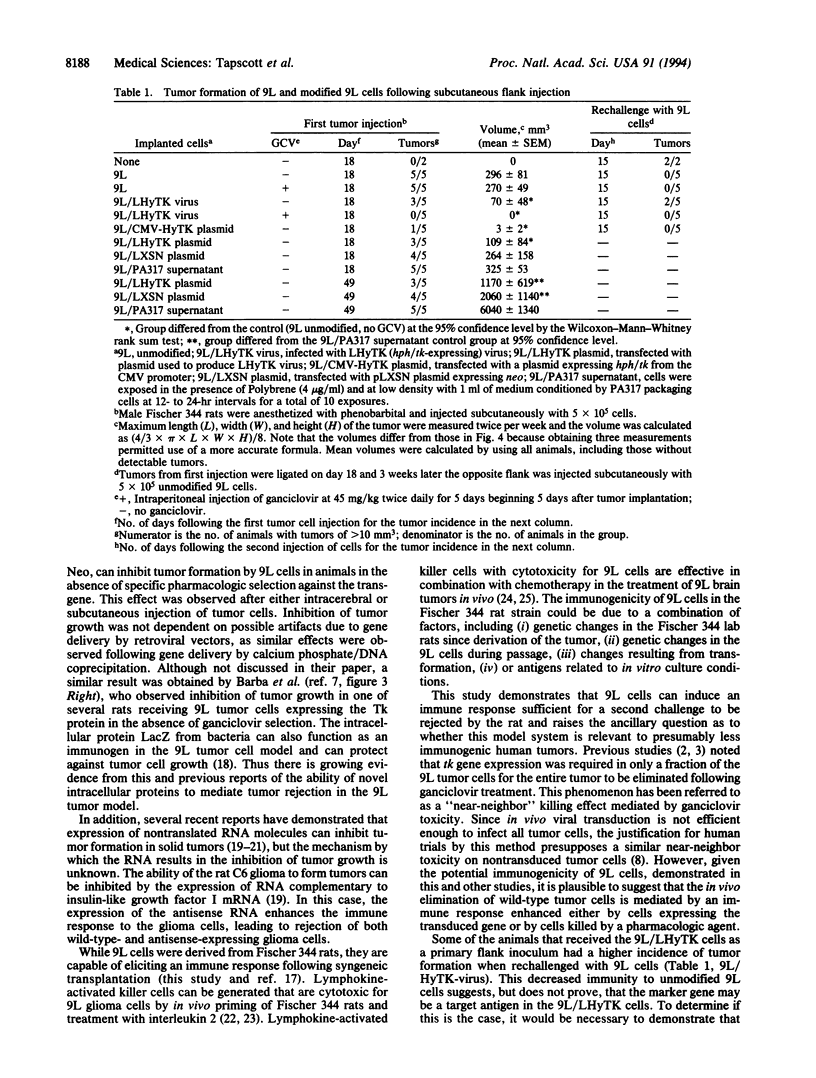
9L rat glioma cells have been used as a model for brain tumor therapies. It has been reported that in vivo infection of 9L cells with a replication-defective retrovirus expressing the herpes simplex thymidine kinase gene resulted in decreased tumor formation following treatment with the antiviral drug ganciclovir. In the study reported here, rats were injected either intracerebrally or subcutaneously with 9L glioma cells expressing a chimeric hygromycin phosphotransferase-thymidine kinase fusion protein or with unmodified 9L cells. Tumor formation was decreased in the rats receiving modified cells, even in the absence of treatment with ganciclovir. Suppression of tumor growth was also observed with cells modified to express the intracellular selectable marker neomycin phosphotransferase. These results indicate that an intracellular selectable marker, in the absence of pharmacologic selection, can inhibit tumor growth of 9L cells. The demonstration that intracellular marker genes can negatively influence the survival of transplanted cells has important implications for in vivo studies that use genetically modified cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attia M. A., Weiss D. W. Immunology of spontaneous mammary carcinomas in mice. V. Acquired tumor resistance and enhancement in strain A mice infected with mammary tumor virus. Cancer Res. 1966 Aug;26(8):1787–1800. [PubMed] [Google Scholar]
- Barba D., Hardin J., Ray J., Gage F. H. Thymidine kinase-mediated killing of rat brain tumors. J Neurosurg. 1993 Nov;79(5):729–735. doi: 10.3171/jns.1993.79.5.0729. [DOI] [PubMed] [Google Scholar]
- Barker M., Hoshino T., Gurcay O., Wilson C. B., Nielsen S. L., Downie R., Eliason J. Development of an animal brain tumor model and its response to therapy with 1,3-bis(2-chloroethyl)-1-nitrosourea. Cancer Res. 1973 May;33(5):976–986. [PubMed] [Google Scholar]
- Culver K. W., Ram Z., Wallbridge S., Ishii H., Oldfield E. H., Blaese R. M. In vivo gene transfer with retroviral vector-producer cells for treatment of experimental brain tumors. Science. 1992 Jun 12;256(5063):1550–1552. doi: 10.1126/science.1317968. [DOI] [PubMed] [Google Scholar]
- Ezzeddine Z. D., Martuza R. L., Platika D., Short M. P., Malick A., Choi B., Breakefield X. O. Selective killing of glioma cells in culture and in vivo by retrovirus transfer of the herpes simplex virus thymidine kinase gene. New Biol. 1991 Jun;3(6):608–614. [PubMed] [Google Scholar]
- Hao Y., Crenshaw T., Moulton T., Newcomb E., Tycko B. Tumour-suppressor activity of H19 RNA. Nature. 1993 Oct 21;365(6448):764–767. doi: 10.1038/365764a0. [DOI] [PubMed] [Google Scholar]
- Holladay F. P., Lopez G., De M., Morantz R. A., Wood G. W. Generation of cytotoxic immune responses against a rat glioma by in vivo priming and secondary in vitro stimulation with tumor cells. Neurosurgery. 1992 Apr;30(4):499–505. doi: 10.1227/00006123-199204000-00005. [DOI] [PubMed] [Google Scholar]
- Jacobs S. K., Melin G., Holcomb B., Parham C. W., Kornblith P. L., Grimm E. A. Lymphokine activated killer (LAK) cell-mediated lysis of murine glioma: trypsin-chymotrypsin-sensitive glioma protein is responsible for tumor-selective recognition by LAK cells. Brain Res. 1986 May 7;372(2):386–389. doi: 10.1016/0006-8993(86)91150-9. [DOI] [PubMed] [Google Scholar]
- Jacobs S. K., Wilson D. J., Melin G., Parham C. W., Holcomb B., Kornblith P. L., Grimm E. A. Interleukin-2 and lymphokine activated killer (LAK) cells in the treatment of malignant glioma: clinical and experimental studies. Neurol Res. 1986 Jun;8(2):81–87. doi: 10.1080/01616412.1986.11739735. [DOI] [PubMed] [Google Scholar]
- Kruse C. A., Mitchell D. H., Kleinschmidt-DeMasters B. K., Bellgrau D., Eule J. M., Parra J. R., Kong Q., Lillehei K. O. Systemic chemotherapy combined with local adoptive immunotherapy cures rats bearing 9L gliosarcoma. J Neurooncol. 1993 Feb;15(2):97–112. doi: 10.1007/BF01053931. [DOI] [PubMed] [Google Scholar]
- Lampson L. A., Lampson M. A., Dunne A. D. Exploiting the lacZ reporter gene for quantitative analysis of disseminated tumor growth within the brain: use of the lacZ gene product as a tumor antigen, for evaluation of antigenic modulation, and to facilitate image analysis of tumor growth in situ. Cancer Res. 1993 Jan 1;53(1):176–182. [PubMed] [Google Scholar]
- Lupton S. D., Brunton L. L., Kalberg V. A., Overell R. W. Dominant positive and negative selection using a hygromycin phosphotransferase-thymidine kinase fusion gene. Mol Cell Biol. 1991 Jun;11(6):3374–3378. doi: 10.1128/mcb.11.6.3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahaley M. S., Jr, Mettlin C., Natarajan N., Laws E. R., Jr, Peace B. B. National survey of patterns of care for brain-tumor patients. J Neurosurg. 1989 Dec;71(6):826–836. doi: 10.3171/jns.1989.71.6.0826. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Buttimore C. Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production. Mol Cell Biol. 1986 Aug;6(8):2895–2902. doi: 10.1128/mcb.6.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Miller D. G., Garcia J. V., Lynch C. M. Use of retroviral vectors for gene transfer and expression. Methods Enzymol. 1993;217:581–599. doi: 10.1016/0076-6879(93)17090-r. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Rosman G. J. Improved retroviral vectors for gene transfer and expression. Biotechniques. 1989 Oct;7(9):980-2, 984-6, 989-90. [PMC free article] [PubMed] [Google Scholar]
- Morantz R. A., Wood G. W., Foster M., Clark M., Gollahon K. Macrophages in experimental and human brain tumors. Part 1: Studies of the macrophage content of experimental rat brain tumors of varying immunogenicity. J Neurosurg. 1979 Mar;50(3):298–304. doi: 10.3171/jns.1979.50.3.0298. [DOI] [PubMed] [Google Scholar]
- Oldfield E. H., Ram Z., Culver K. W., Blaese R. M., DeVroom H. L., Anderson W. F. Gene therapy for the treatment of brain tumors using intra-tumoral transduction with the thymidine kinase gene and intravenous ganciclovir. Hum Gene Ther. 1993 Feb;4(1):39–69. doi: 10.1089/hum.1993.4.1-39. [DOI] [PubMed] [Google Scholar]
- Ram Z., Culver K. W., Walbridge S., Blaese R. M., Oldfield E. H. In situ retroviral-mediated gene transfer for the treatment of brain tumors in rats. Cancer Res. 1993 Jan 1;53(1):83–88. [PubMed] [Google Scholar]
- Ram Z., Culver K. W., Walbridge S., Frank J. A., Blaese R. M., Oldfield E. H. Toxicity studies of retroviral-mediated gene transfer for the treatment of brain tumors. J Neurosurg. 1993 Sep;79(3):400–407. doi: 10.3171/jns.1993.79.3.0400. [DOI] [PubMed] [Google Scholar]
- Rastinejad F., Conboy M. J., Rando T. A., Blau H. M. Tumor suppression by RNA from the 3' untranslated region of alpha-tropomyosin. Cell. 1993 Dec 17;75(6):1107–1117. doi: 10.1016/0092-8674(93)90320-p. [DOI] [PubMed] [Google Scholar]
- Short M. P., Choi B. C., Lee J. K., Malick A., Breakefield X. O., Martuza R. L. Gene delivery to glioma cells in rat brain by grafting of a retrovirus packaging cell line. J Neurosci Res. 1990 Nov;27(3):427–439. doi: 10.1002/jnr.490270322. [DOI] [PubMed] [Google Scholar]
- Steen R. G., Tamargo R. J., McGovern K. A., Rajan S. S., Brem H., Wehrle J. P., Glickson J. D. In vivo 31P nuclear magnetic resonance spectroscopy of subcutaneous 9L gliosarcoma: effects of tumor growth and treatment with 1,3-bis(2-chloroethyl)-1-nitrosourea on tumor bioenergetics and histology. Cancer Res. 1988 Feb 1;48(3):676–681. [PubMed] [Google Scholar]
- Trojan J., Johnson T. R., Rudin S. D., Ilan J., Tykocinski M. L., Ilan J. Treatment and prevention of rat glioblastoma by immunogenic C6 cells expressing antisense insulin-like growth factor I RNA. Science. 1993 Jan 1;259(5091):94–97. doi: 10.1126/science.8418502. [DOI] [PubMed] [Google Scholar]



