Abstract
Background
In recent years, a high prevalence of vitamin D deficiency amongst pregnant women and newborns has been observed throughout several regions of the world, especially in the presence of preeclampsia (PE) or obesity (OB). The aim of this study was to investigate whether nonobese and obese preeclamptic pregnant women and their newborns have low 25(OH)D compared with nonobese and obese nonpreeclamptic pregnant women; and to verify whether the maternal level of this vitamin correlates with the newborns’ level.
Methods
This is a cross-sectional study conducted with 179 pregnant women recruited immediately before delivery, divided into four groups: PE+/OB−; PE+/OB+; PE−/OB+; and PE−/OB−, with gestational age ≥ 34 weeks. Maternal peripheral blood and newborns umbilical cord blood were collected and 25(OH)D levels were measured by chemiluminescence (LIAISON®).
Results
Infants born to preeclamptic mothers had a lower median 25(OH)D level than those born to nonpreeclamptic mothers (p < 0.01). Obese pregnant women and their newborns had higher frequencies of 25(OH)D deficiency, but the difference with respect to nonobese pregnant women and their newborns was not significant. The vitamin D status of preeclamptic obese women was not worse than that of their nonobese counterparts. Newborns and maternal 25(OH)D levels were significantly correlated (p = 0.01). Obesity weakened this correlation.
Conclusions
Preeclamptic women and their newborns presented higher frequencies of 25(OH)D deficiency, but 25(OH)D levels were not significantly influenced by obesity. Obese pregnant women transferred less 25(OH)D to their fetuses.
Keywords: Vitamin D, Vitamin D deficiency, Preeclampsia, Obesity, Pregnancy, Newborn
Background
Vitamin D deficiency during pregnancy is common [1, 2] and concerning because of its association with many conditions, such as preeclampsia (PE), obesity, and gestational diabetes, as well as its medium- and long-term repercussions for newborns [1, 3, 4].
Classically, emphasis rests on the importance of vitamin D to bone metabolism [5], but increasingly greater biological and clinical roles have been attributed to its active form 1,25(OH)2days in the last years [6]. There has been a paradigm shift such that, today, 1, 25(OH)2days is considered a hormone that acts on specific receptors found in at least 36 different types of cells [6].
The participation of 1, 25(OH)2days in immune system regulation has been demonstrated [7, 8], so its deficiency is now considered a risk factor for inflammatory diseases, such as PE [8–11]. Prepregnancy obesity is also associated with higher susceptibility to PE [12]. Additionally, studies have shown an inverse relationship between BMI (Body Mass Index) and vitamin D status [13–15]. Hence, low vitamin D in obese pregnant women [16] can contribute to the onset of PE, which is complex and multifactorial [17].
Since fetal vitamin D status depends on maternal levels of this vitamin [1, 18], all situations that cause vitamin D deficiency during pregnancy imply in low vitamin D in the newborn. Bone (craniotabes, low bone mineral content, and rickets) [19] and nonbone repercussions (greater risk of respiratory infections, asthma, and diabetes type 1) [20] have been associated with vitamin D deficiency at birth.
Hence, the objective of this study is to investigate whether nonobese and obese preeclamptic pregnant women and their newborns have low 25(OH)D compared with nonobese and obese nonpreeclamptic pregnant women; and to verify whether the maternal level of this vitamin correlates with the newborns’ level.
Methods
Study design and subjects
This cross-sectional study was conducted from November 2012 to March 2013 in the maternity ward of the Hospital Barão de Lucena in the city of Recife, Northeast Brazil. The study was approved by the Human Research Ethics Committee of the Health Sciences Center of the Federal University of Pernambuco and followed the principles of the Declaration of Helsinki. Pregnant women were recruited immediately before delivery after signing the informed consent form. They were divided into four groups: nonobese preeclamptic (PE+/OB−), obese preeclamptic (PE+/OB+); obese nonpreeclamptic (PE−/OB+); and nonobese nonpreeclamptic women (PE−/OB−).
All subjects had singleton pregnancies with gestational age of at least 34 weeks. They were aged 16 to 40 years and attended the first prenatal care visit before gestational week 16. The exclusion criteria were hypertension before gestational week 20, history of chronic hypertension, gestational diabetes, diabetes mellitus, liver or kidney disease, carrying an infection, or taking anticonvulsants.
Twenty-two of the 201 initially recruited mother-newborn dyads were excluded because of hemolysis or insufficient blood sample (n = 16), or inappropriate storage or transportation (n = 6), therefore, the study sample comprised 179 mother-newborn dyads.
Study variables
Gestational age was determined by the date of the last menstrual period. PE was defined as the presence of hypertension (systolic blood pressure level of 140 mmHg or higher or a diastolic blood pressure level of ≥ 90 mmHg) after the twentieth week of gestation in previously normotensive women, plus the presence of proteinuria (≥1+ on dipstick in single urine sample), according to criteria adopted by the American College of Obstetricians and Gynecologists (ACOG) [21]. Maternal nutritional status was assessed by means of BMI obtained in the first prenatal consultation before the 16th gestational week. Women were defined as obese or not according to the classification developed by Atallah et al. [22] and adopted by the Ministry of Health of Brazil, which classifies pregnant women according to the appropriateness of BMI in relation to gestational age. No measurements were undertaken to assess women adiposity.
Vitamin D status was classified according to the cutoff points proposed by Holick et al. [23] for adults (pregnant women), and the same values were used for the newborns [24]: ≥ 30 ng/mL is considered enough to promote bone and no bone health, 20 to 29.9 ng/mL indicates insufficiency and <20 ng/mL deficiency.
A single researcher used a form to interview the participants after delivery and collect information on socioeconomic and demographic factors, clinical pregnancy data, amount of exposure to sunlight, frequency of sunscreen use, newborns weight and length. Newborns nutritional status was classified according to the appropriateness of weight in relation to gestational age, using the fetal growth curve of Lubchenco et al. [25].
Laboratory analyses
Peripheral and umbilical cord blood samples were collected from the mothers and newborns, respectively, using a Vacuette® tube, which contains clot activator and serum separator gel. The tubes were immediately centrifuged and refrigerated to 2-8 °C until the vitamin D level was determined, no later than 72 h after blood collection. Vitamin D level was determined by chemiluminescence using the commercial assay LIAISON 25 OH Vitamin D Total (310600)®, from DiaSorin Inc. (USA), which has an analytical measurement range of 4.0 to 150.0 ng/mL. All samples were analyzed by a single technician using the same equipment throughout the study period in a reference laboratory. The inter-assay coefficient of variation varied from 6.5 % to 8.1 % during this period, in accordance with the manufacturer’s standards.
Statistical power and analyses
A retrospective statistical power calculation from the frequencies of vitamin D deficiency observed in PE+/OB− and PE+/OB+ groups in relation to the PE−/OB− with a significance level of 5 % showed a sample power around 95 %.
Normality of continuous variables was checked using a histogram. Those with symmetric distribution were expressed as means and standard deviation and the asymmetric ones as medians and inter quartile range (IQR). The associations between categorical variables were tested by the chi-square test. For continuous variables one way analysis of variance (ANOVA) and the Kruskal-Wallis test were employed as appropriate. Pearson correlation coefficient tested the association of two continuous variables. The Statistical Package for the Social Science (SPSS), version 13 was used for the statistical analysis. The significance level was set at 5% for all tests.
Results
Table 1 shows the socioeconomic and clinical characteristics of the pregnant women and their newborns. Around 63 % (58/92) of the preelamptic women were primigravida as opposed to 31 % (27/87) of the nonpreeclamptic women, and nonobese pregnant women were younger than the obese. There was a greater percentage of small-for-gestational-age (SGA) newborns in the group PE+/OB− and a higher frequency of large-for-gestational-age (LGA) newborns in the obese groups (PE+/OB+ and PE−/OB+).
Table 1.
Maternal and newborn characteristicsa according to maternal nutritional status and occurrence of preeclampsia
| Variables | PE +/OB − | PE +/OB+ | PE −/OB+ | PE −/OB− | P-value |
|---|---|---|---|---|---|
| (n = 48) | (n = 44) | (n = 40) | (n = 47) | ||
| Maternal age (years) | 21.0(19.0-27.0) | 26.5(21.5-31.5) | 27.0(22.0-33.0) | 24.0(19.0-29.0) | 0.003* |
| Maternal BMI (kg/m2) | 23.7(21.2-25.7) | 32.9(31.5-36.8) | 32.8(31.6-35.9) | 24.2(21.3-26.3) | <0.001* |
| Number of pregnancies | |||||
| 1 | 70.8% (34) | 54.5% (24) | 27.5% (11) | 34.0% (16) | <0.001** |
| ≥2 | 29.2% (14) | 45.5% (20) | 72.5% (29) | 66.0% (31) | |
| Gestational age (weeks) | |||||
| <37 | 25.0% (12) | 18.2% (08) | 12.5% (05) | 14.9% (07) | 0.43** |
| ≥37 | 75.0% (36) | 81.8% (36) | 87.5% (35) | 85.1% (40) | |
| Skin color | |||||
| White | 16.7% (08) | 25.0% (11) | 17.5% (07) | 14.9% (07) | 0.62** |
| Other | 83.3% (40) | 75.0% (33) | 82.5% (33) | 85.1% (40) | |
| Per capita family income (in Reais) | 312(208–500) | 339(211–450) | 252.00(169–336) | 282.70(200–400) | 0.09* |
| Literacy | |||||
| Illiterate | 2.1% (01) | 4.5% (02) | 10.0% (04) | 10.6% (05) | 0.28** |
| Literate | 97.9% (47) | 95.5% (42) | 90.0% (36) | 89.4% (42) | |
| Exposure to sunlight (minutes/day) | 20.0(10.0-32.5) | 20.0(15.0-35.0) | 32.5(15.0-50.0) | 20.0(10.0-30.0) | 0.16* |
| Sunscreen | |||||
| Yes | 10.4% (05) | 09.1% (04) | 07.5% (03) | 08.5% (04) | 0.97** |
| No | 89.6% (43) | 90.9%(40) | 92.5% (37) | 91.5% (43) | |
| Infant sex | |||||
| Female | 45.8% (22) | 59.1% (26) | 55.0% (22) | 53.2% (25) | 0.63** |
| Male | 54.2% (26) | 40.9% (18) | 45.0% (18) | 46.8% (22) | |
| Birth weight (g) | 2782.3 ± 750.9 | 3272.6 ± 648.6 | 3326.7 ± 589.3 | 3221.2 ± 544.3 | <0.001*** |
| Birth length (cm) | 46.5(44.5-48.0) | 48.0(47.0-49.0) | 49.0(47.0-50.0) | 48.0(46.0-49.0) | 0.006* |
| Nutritional status at birth | |||||
| AGA | 58.3% (28) | 70.5% (31) | 75.0% (30) | 83.0% (39) | <0.001** |
| SGA | 33.3% (16) | 0% (0) | 05.0% (02) | 04.3% (02) | |
| LGA | 08.4% (04) | 29.5% (13) | 20.0% (08) | 12.7% (06) | |
aValues are shown as mean ± SD, median (inter quartile range) or %. NB: newborn; AGA: appropriate for gestational age; SGA: small for gestational age; LGA: large for gestational age; OB: obesity; PE: preeclampsia
1.0 Real = US$ 2.0
*Kruskal-Wallis test; **Chi-square test; ***Analysis of variance
Preeclamptic women and their newborns had a higher frequency of 25(OH)D deficiency than nonpreeclamptic women. The differences of vitamin D status between PE+/OB− and PE−/OB− pregnant women was significant (p = 0.0006) as well as between their newborns (p = 0.006). The same was observed among the groups of PE+/OB+ and PE−/OB− (p = 0.002) and between their newborns (p = 0.03). Women and newborns in the group PE−/OB+ had a higher frequency of 25(OH)D deficiency than those in the group PE−/OB−, but the difference was not significant (p = 0.10 e 0.16, respectively). The vitamin D status of the mother-newborn dyads in group PE+/OB+ was not worse than that of mother-newborn dyads in group PE+/OB− (p = 0.62 e 0.72, respectively). (Table 2).
Table 2.
Distribution of the 25(OH)D status among pregnant women and newborns
| 25(OH)D | PE +/OB− | PE +/ OB+ | PE −/ OB+ | PE −/OB− | P-value* |
|---|---|---|---|---|---|
| (n = 48) | (n = 44) | (n = 40) | (n = 47) | ||
| Pregnant women | |||||
| Adequate | 08.3 %(04) | 04.6 %(02) | 20.0 %(08) | 17.0 %(08) | 0.002 |
| Insufficient | 39.6 %(19) | 47.7 %(21) | 47.5 %(19) | 68.1 %(32) | |
| Deficient | 52.1 %(25) | 47.7 %(21) | 32.5 %(13) | 14.9 %(07) | |
| Newborns | |||||
| Adequate | 33.3 %(16) | 36.4 %(16) | 52.5 %(21) | 57.4 %(27) | 0.06 |
| Insufficient | 52.1 %(25) | 54.5 %(24) | 40.0 %(16) | 42.6 %(20) | |
| Deficient | 14.6 %(07) | 09.1 %(04) | 07.5 %(03) | 0 % (0) | |
PE: preeclampsia; OB: obesity; *Chi-square test
Preeclamptic women and newborns had a lower median vitamin D level than the nonpreeclamptic women and newborns (Fig. 1). The median differences of vitamin D between PE+/OB− and PE−/OB− groups were significant among pregnant women (p = 0.0002) and among the neonates (p = 0.007). The same was observed between the PE+/OB+ and PE−/OB− groups (p = 0.0003 for pregnant women and p = 0.002 for newborns). However, when analyzing the median differences between PE−/OB+ and PE−/OB− groups we found no statistical significance (p = 0.22 among pregnant women and p = 0.10 among neonates). The median 25(OH)D level of all newborns was roughly one-third higher than that of all mothers (Fig. 1).
Fig. 1.
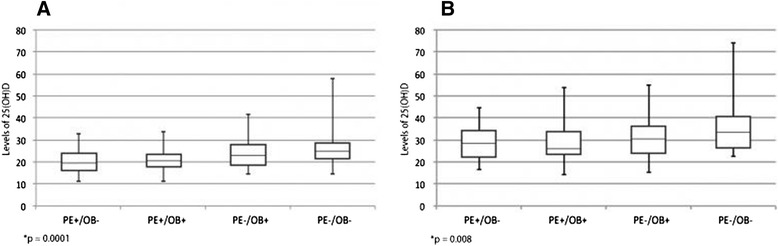
Median, inter quartile range and minimum and maximum values of 25(OH)D in pregnant women (A) and newborns – umbilical cord blood (B). PE: preeclampsia; OB: obesity. *Kruskal-Wallis test
Fig. 2 shows that the 25(OH)D levels of nonobese women and their newborns were strongly and positively correlated; the 25(OH)D levels in obese women and their newborns were also positively correlated, but not as much. The correlations in all groups were significant (p = 0.01).
Fig. 2.
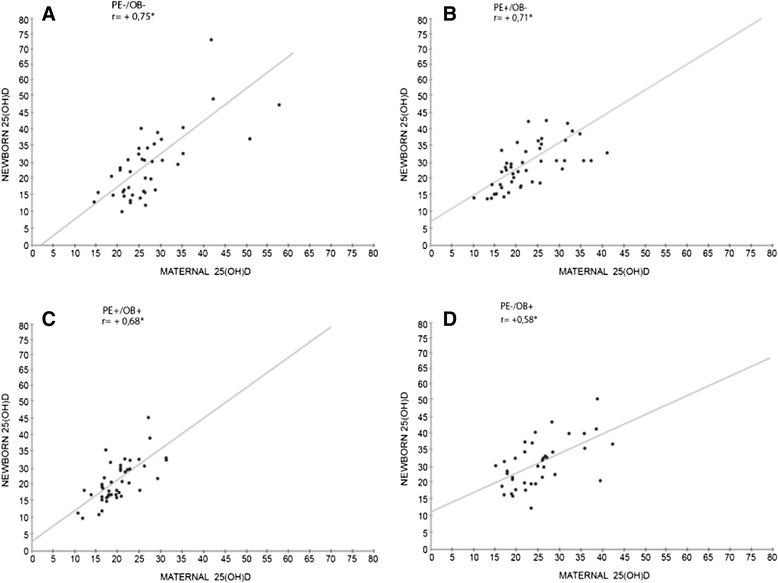
Correlations between maternal and newborn (umbilical cord blood) levels of 25(OH)D. *p = 0.01
Discussion
Preeclampsia was associated with a lower median vitamin D and a higher frequency of vitamin D deficiency in pregnant women and their newborns. Other studies have also reported worse vitamin D status in preeclamptic women and their newborns [9–11, 26]. Robinson et al. [11] and Baker et al. [10] found 25(OH)D deficiency percentages of 54% and 26%, respectively, in severely preeclamptic American women, and both percentages significantly exceeded those in nonpreeclamptic women. These studies used the same vitamin D status cutoff points as the present study. In another case–control study, Bodnar et al. [9] found a higher percentage of vitamin D deficiency in American women who developed PE than in controls (p = 0.08). Likewise, infants born to preeclamptic mothers had significant worse 25(OH)D status than those born to control mothers.
Studies have postulated that maternal vitamin D status affects the risk of preeclampsia [27]. This condition can be partly explained by the manner in which the trophoblast is implanted in the endometrium: an exacerbated maternal immune response (increase of proinflammatory cytokines) allows only superficial implantation, leading to lower maternal tolerance of the fetus [17, 28]. The immunomodulating properties of the active form of vitamin D may be essential during trophoblast implantation, so vitamin D deficiency at the beginning of pregnancy could cause an unbalance between pro- and anti-inflammatory cytokines, constituting a risk factor for PE [29].
Baker et al. [10] and Bodnar et al. [9] collected blood from pregnant women in the first half of pregnancy and found that low 25(OH)D levels precede clinical PE presentation. Thus, higher causal inference may be speculated, and perhaps low vitamin D levels at the beginning of pregnancy may have had some effect on the complex PE pathophysiology.
The study results show greater vitamin D deficiency in obese women, but the difference was not significant. Bodnar et al. [16] also found a higher percentage of mother-NB dyads with 25(OH)D levels below 20 ng/mL when prepregnancy BMI ≥ 30 kg/m2 (p < 0.05). Low vitamin D levels in obese individuals may stem from this vitamin’s fat-soluble nature: as the percentage of body fat increases, serum 25(OH)D level decreases [13].
Surprisingly, the group PE+/OB+ did not have worse 25(OH)D status than the group PE+/OB−. Studies testing serum levels of 25(OH)D in obese preeclamptic women were not found, so comparisons were not possible. Since the present study is observational, selection bias is possible. Most obese participants had BMI from 30 to 35 (grade I obesity), so the results could have been different if there were more women with obesity grades II and III in the group PE+/OB+. Additionally, the classification of obesity according to BMI only, without analysis of body composition, could have resulted in the selection of pregnant women with a smaller percentage of body fat, implying in smaller sequestration of circulating 25(OH)D [15].
The newborns vitamin D medians were higher than those of the mothers in all groups. In a literature review, Dror & Alen [1] found reports of 25(OH)D levels in cord blood being as much as 108% higher than those of the mothers. The different methods used for measuring serum vitamin D may partly justify the differences. In line with other studies [30, 31], the study newborns levels of vitamin D were positively correlated with those of their mothers during pregnancy, showing that fetuses are dependent on maternal serum 25(OH)D levels. However, according to the coefficients of determination, there was less maternofetal transfer of 25(OH)D in the groups of pregnant women with higher BMI. In a recent study, Josefson et al. [32] also found that obese mothers transferred less 25(OH)D to their fetuses than nonobese mothers. Although this fact awaits an explanation, some hypotheses with a nonlinear explanation have been offered, based on the physiological aspects of placental vitamin D transportation and the pathophysiology of obesity.
The mechanism by which vitamin D crosses the placenta is not yet clear. Studies from the 1980s [33] and recent literature reviews [34] report that the main mechanism is passive diffusion, as occurs with sex steroid hormones. The free serum 25(OH)D fraction may reflect the amount available for cells according to the free hormone theory [35]. Therefore, only free 25(OH)D would cross the cellular lipid bilayer because of its solubility in fat. Karlsson et al. [36] found that obese women of childbearing age had higher circulating DBP levels than normal weight women, resulting in a smaller percentage of free 25(OH)D. If higher levels of circulating DBP also occur in obese pregnant women, and placental transportation of 25(OH)D is passive, smaller maternofetal transfer of vitamin D is to be expected.
On the other hand, the placenta is a complex organ, and different clinical contexts may change its structure. The first months of pregnancy should promote greater placental expression of the gene CYP27B1, which codifies the enzyme 1α-hydroxylase, necessary for the conversion of calcidiol into calcitriol; and simultaneously silence the gene CYP24A1, responsible for the catabolism of 25(OH)D and 1,25(OH)2days [29]. In vitro studies have shown that tumor necrosis factor-alpha (TNF-α) increased CYP24A1 expression in trophoblast cultures [27]. In obese women, the placenta is inflamed, containing high levels of pro-inflammatory cytokines [37]. Therefore, the inflamed placenta of obese women may be an epigenetic factor that increases placental expression of the gene CYP24A1, resulting in lower 25(OH)D levels in the maternofetal interface and consequently, smaller amounts of 25(OH)D transferred to the fetus.
We believe that future studies, especially in vivo, can elucidate the mechanism by which vitamin D is transported across the placenta of obese and nonobese women with and without complications, such as preeclampsia, and thereby contribute to a better understanding of newborns’ vitamin D status.
The study results must be analyzed in light of its limitations. Measuring 25(OH)D right before delivery reflects its status only for the past two to three weeks [38]. Moreover, any biomarker has limitations, either because of physiological factors or the methodological difficulties associated with its measurement [35]. The main factors capable of affecting 25(OH)D levels are: higher physiological requirements, body fat, hemodilution and ageing effects, affinity of vitamin D for its target tissues, and cellular absorption efficiency, among others [35]. Although 25(OH)D is the most widely used marker by clinicians and researchers, it only indicates vitamin D supply, so it may not be the best biomarker of biological function [35]. Finally, not having used the gold standard, high-performance liquid chromatography (HPLC), to determine the sample’s vitamin D levels may have returned less accurate 25(OH)D measurements.
It should be emphasized that the present study was conducted close to the equator, at a latitude of 9° south, where UVB radiation is abundant throughout the four seasons of the year. This fact alone controlled this geographic confounder present in studies conducted at high latitudes.
Conclusion
The study found a smaller median and consequently, worse vitamin D status in mother-newborns dyads with preeclampsia or gestational obesity. Since obesity became one of the greatest global epidemics at the dawn of this century, besides the high prevalence of preeclampsia in pregnancies, we believe that new studies should investigate whether maternal supplementation with higher doses of vitamin D can improve the 25(OH)D status of newborns.
Acknowledgements
We thank National Council for Research and Development (CNPq) for sponsoring the study (grant number 473147/2012-2) and providing Research Grant for Giselia Alves and Marilia Lima. The authors also thank the manager of the Clinical Pathology and Pathological Anatomy Laboratory of Hospital Barão de Lucena, Mrs. Ana Maria de Lima Barros.
Abbreviations
- 1,25(OH)2days
1,25-dihydroxyvitamin D
- 25(OH)D
25-hydroxyvitamin D
- ACOG
American College of Obstetricians and Gynecologists
- ANOVA
Analysis of variance
- BMI
Body mass index
- DBP
Vitamin D-binding protein
- HPLC
High-performance liquid chromatography
- IOM
Institute of Medicine
- IQR
Inter quartile range
- LGA
Large-for-gestational-age
- OB
Obesity
- PE
Preeclampsia
- RDA
Recommended dietary allowance
- SGA
Small-for-gestational-age
- SPSS
Statistical package for the social science
- TNF-α
Tumor necrosis factor-alpha
- USA
United States of America
- UVB
Ultraviolet B.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
HRP conceived and designed the study, conducted data collection, analyzed data and drafted the manuscript; MCL analyzed and interpreted data, provided advice and critically revised the manuscript; KGB conceived and designed the study, conducted data collection, provided advice and critically revised the manuscript; MMCA conceived and designed the study, analyzed and interpreted data, provided advice and critically revised the manuscript; GAPS conceived and designed the study, interpreted data, provided advice and critically revised the manuscript. All authors approved the final version of the manuscript.
Contributor Information
Homero Rabelo Pena, Email: homero.rabelo@terra.com.br.
Marilia Carvalho de Lima, Email: mlima@ufpe.br.
Katia Galeão Brandt, Email: katiabrandt@uol.com.br.
Margarida Maria Castro de Antunes, Email: margarida.mmcastro@gmail.com.
Giselia Alves Pontes da Silva, Email: giseliaalves@gmail.com.
References
- 1.Dror DK, Allen L. Vitamin D inadequacy in pregnancy: biology, outcomes, and interventions. Nutr Rev. 2010;68:465–77. doi: 10.1111/j.1753-4887.2010.00306.x. [DOI] [PubMed] [Google Scholar]
- 2.Bener A, Al-Hamaq AO, Saleh NM. Association between vitamin D insufficiency and adverse pregnancy outcome: global comparisons. Int J Womens Health. 2013;5:523–31. doi: 10.2147/IJWH.S51403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaludjerovic J, Vieth R. Relationship between vitamin D during perinatal development and health. J Midwifery Womens Health. 2010;55:550–60. doi: 10.1016/j.jmwh.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 4.Principi N, Bianchini S, Baggi E, Esposito S. Implications of maternal vitamin D deficiency for the fetus, the neonate and the young infant. Eur J Nutr. 2013;52:859–67. doi: 10.1007/s00394-012-0476-4. [DOI] [PubMed] [Google Scholar]
- 5.Christakos S, Ajibade DV, Puneet Dhawan P, Fechner AJ, Mady LJ. Vitamin D: Metabolism. Endocrinol Metab Clin North Am. 2010;39:243–53. doi: 10.1016/j.ecl.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Norman AW. From vitamin D to hormone D: fundamentals of the vitamin D endocrine system essential for good health. Am J Clin Nutr. 2008;88(Suppl 2):491–9. doi: 10.1093/ajcn/88.2.491S. [DOI] [PubMed] [Google Scholar]
- 7.Jeffery LE, Burke F, Mura M, Zhenget Y, Qureshi OS, Hewison M, et al. 1,25 dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FOXP3. J Immunol. 2009;183:5458–67. doi: 10.4049/jimmunol.0803217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shin JS, Choi MY, Longtine MS, Nelson DM. Vitamin D effects on pregnancy and the placenta. Placenta. 2010;31:1027–34. doi: 10.1016/j.placenta.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bodnar LM, Catov JM, Simhan HN, Holick MF, Powers RW, Roberts JM. Maternal Vitamin D deficiency increases the risk of preeclampsia. J Clin Endocrinol Metab. 2007;92:3517–22. doi: 10.1210/jc.2007-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baker AM, Haeri S, Camargo CA, Jr, Espinola JA, Stuebe AM. A nested case–control study of midgestation vitamin D deficiency and risk of severe preeclampsia. J Clin Endocrinol Metab. 2010;95:5105–9. doi: 10.1210/jc.2010-0996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robinson CJ, Alanis MC, Wagner CL, Hollis BW, Johnson DD. Plasma 25-OH-Vitamin D levels in early onset severe preeclampsia. Am J Obstet Gynecol. 2010;203:366.e1–366.e6. doi: 10.1016/j.ajog.2010.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365(9461):785–99. doi: 10.1016/S0140-6736(05)71003-5. [DOI] [PubMed] [Google Scholar]
- 13.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72:690–3. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 14.Young KA, Engelman CD, Langefeld CD, Hairston KG, Haffner SM, Bryer-Ash M, et al. Association of plasma vitamin D levels with adiposity in Hispanic and African Americans. J Clin Endocrinol Metab. 2009;94:3306–13. doi: 10.1210/jc.2009-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Earthman CP, Beckman LM, Masodkar K, Sibley SD. The link between obesity and low circulating 25-hydroxyvitamin D concentrations: considerations and implications. Int J Obes. 2012;36:387–96. doi: 10.1038/ijo.2011.119. [DOI] [PubMed] [Google Scholar]
- 16.Bodnar LM, Catov JM, Roberts JM, Simhan HN. Prepregnancy obesity predicts poor vitamin D status in mothers and their neonates. J Nutr. 2007;137:2437–42. doi: 10.1093/jn/137.11.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pennington KA, Schlitt JM, Jackson DL, Schulz LC, Schust DL. Preeclampsia: multiple approaches for a multifactorial disease. Dis Model Mech. 2012;5:9–18. doi: 10.1242/dmm.008516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pearce SH, Cheetham TD. Diagnosis and management of vitamin D deficiency. BMJ. 2010;340:142–7. doi: 10.1136/bmj.b5664. [DOI] [PubMed] [Google Scholar]
- 19.Viljakainen HT, Saarnio E, Hytinantti T, Miettinen M, Surcel H, Mäkitie O, et al. Maternal vitamin D status determines bone variables in the newborn. J Clin Endocrinol Metab. 2010;95:1749–57. doi: 10.1210/jc.2009-1391. [DOI] [PubMed] [Google Scholar]
- 20.Camargo CA, Ingham T, Wickens K, Thadhani R, Silvers KM, Epton MJ, et al. Cord-blood 25-hydroxyvitamin D levels and risk of respiratory infection, wheezing, and asthma. Pediatrics. 2011;127:e180–7. doi: 10.1542/peds.2010-0442. [DOI] [PubMed] [Google Scholar]
- 21.American College of Obstetricians and Gynecologists Practice Bulletin No. 33: Diagnosis and management of preeclampsia and eclampsia. Obstet Gynecol. 2002;99:159–167. doi: 10.1016/S0029-7844(01)01747-1. [DOI] [PubMed] [Google Scholar]
- 22.Atalah ES, Castillo CL, Castro RS, Aldea AP. Propuesta de un nuevo estándar de evaluación nutricional en embarazadas. Rev Med Chile. 1997;125:1429–36. [PubMed] [Google Scholar]
- 23.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–30. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 24.Holick MF. Resurrection of vitamin D deficiency and rickets. J Clin Invest. 2006;116:2062–72. doi: 10.1172/JCI29449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lubchenco LO, Hansman C, Dressler M, Boyd E. Intrauterine growth as estimated from liveborn birth-weight data at 24 to 42 weeks of gestation. Pediatrics. 1963;32:793–800. [PubMed] [Google Scholar]
- 26.Aghajafari F, Nagulesapillai T, Ronksley PE, Tough SC, O'Beirne M, Rabi DM. Association between maternal serum 25-hydroxyvitamin D level and pregnancy and neonatal outcomes: systematic review and meta-analysis of observational studies. BMJ. 2013;346:f1169. doi: 10.1136/bmj.f1169. [DOI] [PubMed] [Google Scholar]
- 27.Díaz L, Noyola-Martínez N, Barrera D, Hernández G, Avila E, Halhali A, et al. Calcitriol inhibits TNF-α-induced inflammatory cytokines in human trophoblasts. J Reprod Immunol. 2009;81:17–24. doi: 10.1016/j.jri.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 28.Borzychowski AM, Sargent IL, Redman CWG. Inflammation and pre-eclampsia. Semin Fetal Neonatal Med. 2006;11:309–16. doi: 10.1016/j.siny.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 29.Liu NQ, Hewison M. Vitamin D, the placenta and pregnancy. Arch Biochem Biophys. 2012;523:37–47. doi: 10.1016/j.abb.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 30.Halicioglu O, Aksitc S, Kocc F, Akmana SA, Albudaka E, Yapraka I, et al. Vitamin D deficiency in pregnant women and their neonates in spring time in western Turkey. Paediatr Perinat Epidemiol. 2011;26:53–60. doi: 10.1111/j.1365-3016.2011.01238.x. [DOI] [PubMed] [Google Scholar]
- 31.Molla AM, Badawi MA, Hammoud MS, Shukkur M, Thalib L, Eliwa MS. Vitamin D status of mothers and their neonates in Kuwait. Pediatr Int. 2005;47:649–52. doi: 10.1111/j.1442-200x.2005.02141.x. [DOI] [PubMed] [Google Scholar]
- 32.Josefson JL, Feinglass J, Rademaker AW, Metzger BE, Zeiss DM, Price HE, et al. Maternal Obesity and Vitamin D Sufficiency Are Associated with Cord Blood Vitamin D Insufficiency. J Clin Endocrinol Metab. 2013;98:114–9. doi: 10.1210/jc.2012-2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gertner JM, Glassman MS, Coustan DR, Goodman DBP. Fetomaternal vitamin D relationships at term. J Pediatr. 1980;97:637–40. doi: 10.1016/S0022-3476(80)80029-1. [DOI] [PubMed] [Google Scholar]
- 34.Salle BL, Delvin EE, Lapillonne A, Bishop NJ, Glorieux FH. Perinatal metabolism of vitamin D. Am J Clin Nutr. 2000;71(Suppl):1317–24. doi: 10.1093/ajcn/71.5.1317s. [DOI] [PubMed] [Google Scholar]
- 35.Prentice A, Goldberg GR, Schoenmakers I. Vitamin D across the lifecycle: physiology and biomarkers. Am J Clin Nutr. 2008;88(Suppl 5):500–6. doi: 10.1093/ajcn/88.2.500S. [DOI] [PubMed] [Google Scholar]
- 36.Karlsson T, Osmancevic A, Jansson N, Hulthén L, Holmäng A, Larsson I. Increased vitamin D-binding protein and decreased free 25(OH)D in obese women of reproductive age. Eur J Nutr. 2014;53:259–67. doi: 10.1007/s00394-013-0524-8. [DOI] [PubMed] [Google Scholar]
- 37.Roberts KA, Riley SC, Reynolds RM, Barr S, Evans M, Statham A, et al. Placental structure and inflammation in pregnancies associated with obesity. Placenta. 2011;32:247–54. doi: 10.1016/j.placenta.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 38.Tsiaras WG, Weinstock MA. Factors influencing vitamin D status. Acta Derm Venereol. 2011;91:115–24. doi: 10.2340/00015555-0980. [DOI] [PubMed] [Google Scholar]


