Abstract
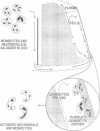
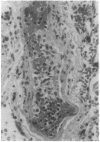
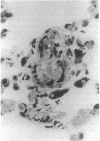
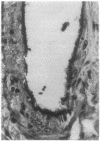
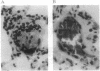
We have identified a leukocyte activation syndrome that is occasionally associated with the transfusion of intraoperatively recovered erythrocytes. This syndrome appears to result from intravascular damage caused by leukocytes activated during the erythrocyte salvage process. We hypothesize that this syndrome is part of a larger disease grouping: disseminated intravascular inflammation (DII). DII is the analog of the coagulation disorder disseminated intravascular coagulation. In disseminated intravascular coagulation, the organ damage results from uncontrolled activation of the clotting pathway; in DII the damage is caused by leukocytes that have become activated by direct contact with bacteria or in rare instances--such as erythrocyte salvage--in the absence of bacteria and bacterial products. Recent studies of the hazards associated with intraoperative blood salvage indicate that activation of leukocytes can be achieved by exposure to activated platelets alone. If such activated leukocytes are reinfused along with the washed erythrocytes, widespread organ damage may result. The lung is the organ most severely affected by activated leukocytes. Adult respiratory distress syndrome is one outcome. It is likely that DII is a presently unrecognized pathophysiological process that complicates a variety of primary disease states and increases their lethality.
Full text
PDF

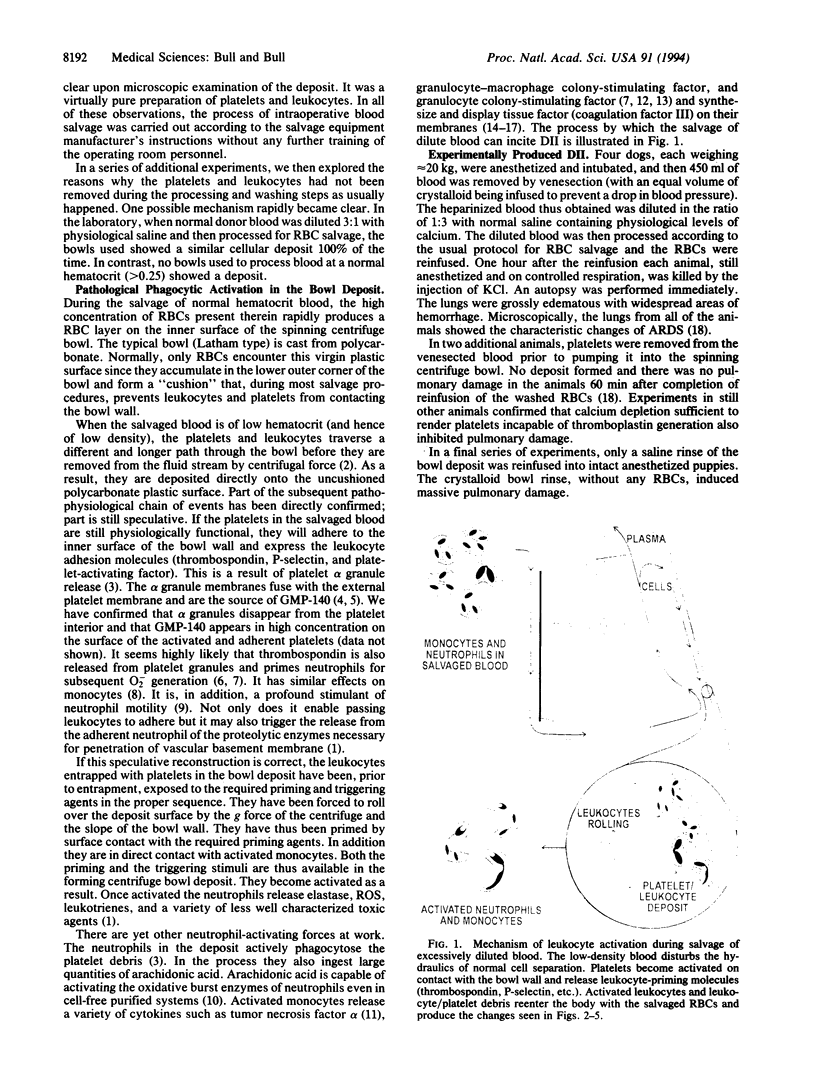
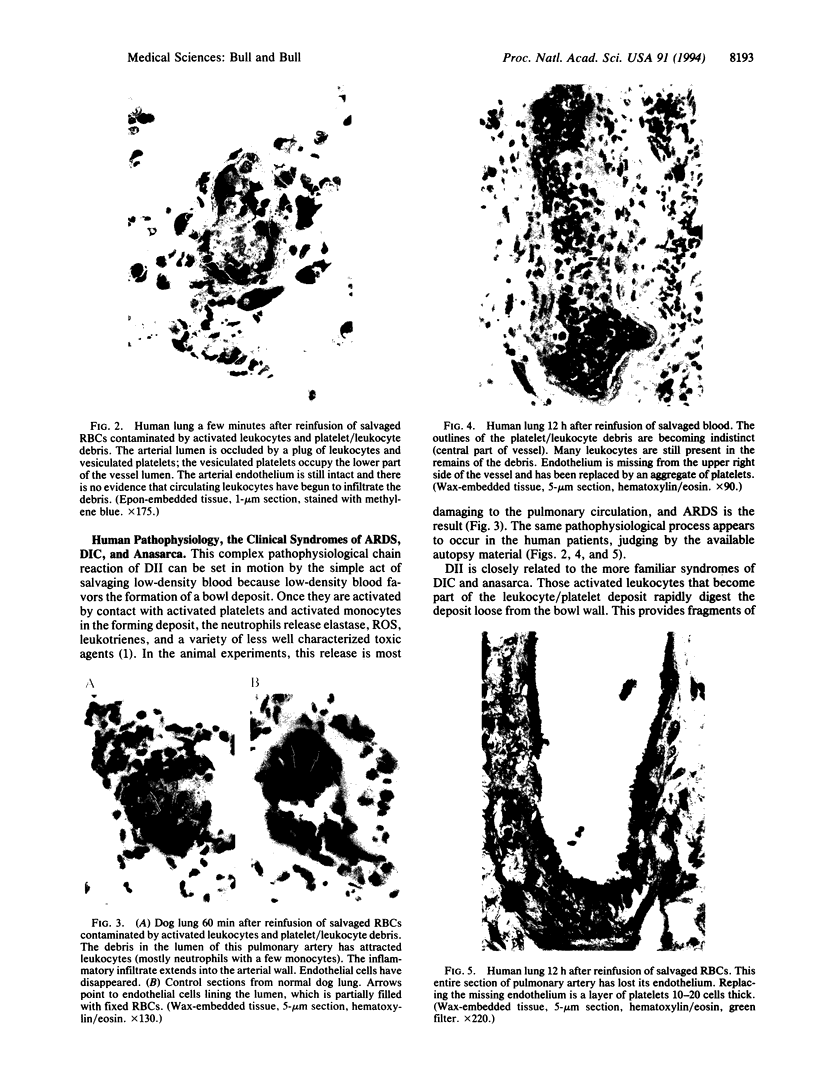

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boxer L. A., Axtell R., Suchard S. The role of the neutrophil in inflammatory diseases of the lung. Blood Cells. 1990;16(1):25–42. [PubMed] [Google Scholar]
- Bull B. S., Bull M. H. The salvaged blood syndrome: a sequel to mechanochemical activation of platelets and leukocytes? Blood Cells. 1990;16(1):5–23. [PubMed] [Google Scholar]
- Bull M. H., Bull B. S., Van Arsdell G. S., Smith L. L. Clinical implications of procoagulant and leukoattractant formation during intraoperative blood salvage. Arch Surg. 1988 Sep;123(9):1073–1078. doi: 10.1001/archsurg.1988.01400330049007. [DOI] [PubMed] [Google Scholar]
- Dean R. T., Prydz H. Inflammatory particles stimulate thromboplastin production by human monocytes. Thromb Res. 1983 May 15;30(4):357–367. doi: 10.1016/0049-3848(83)90227-x. [DOI] [PubMed] [Google Scholar]
- Edgington T. S. Recognition coupled responses of the monocyte: activation of coagulation pathways. Nouv Rev Fr Hematol. 1983;25(1):1–6. [PubMed] [Google Scholar]
- Frazier W. A. Thrombospondin: a modular adhesive glycoprotein of platelets and nucleated cells. J Cell Biol. 1987 Aug;105(2):625–632. doi: 10.1083/jcb.105.2.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan J. M. Leukocyte-endothelial interactions. Blood. 1985 Mar;65(3):513–525. [PubMed] [Google Scholar]
- Lyberg T. Clinical significance of increased thromboplastin activity on the monocyte surface--a brief review. Haemostasis. 1984;14(5):430–439. doi: 10.1159/000215101. [DOI] [PubMed] [Google Scholar]
- Mansfield P. J., Boxer L. A., Suchard S. J. Thrombospondin stimulates motility of human neutrophils. J Cell Biol. 1990 Dec;111(6 Pt 2):3077–3086. doi: 10.1083/jcb.111.6.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPhail L. C., Shirley P. S., Clayton C. C., Snyderman R. Activation of the respiratory burst enzyme from human neutrophils in a cell-free system. Evidence for a soluble cofactor. J Clin Invest. 1985 May;75(5):1735–1739. doi: 10.1172/JCI111884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F. Neutrophil activation on biological surfaces. Massive secretion of hydrogen peroxide in response to products of macrophages and lymphocytes. J Clin Invest. 1987 Dec;80(6):1550–1560. doi: 10.1172/JCI113241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F. Respiratory burst in adherent human neutrophils: triggering by colony-stimulating factors CSF-GM and CSF-G. Blood. 1989 Jan;73(1):301–306. [PubMed] [Google Scholar]
- Nathan C., Srimal S., Farber C., Sanchez E., Kabbash L., Asch A., Gailit J., Wright S. D. Cytokine-induced respiratory burst of human neutrophils: dependence on extracellular matrix proteins and CD11/CD18 integrins. J Cell Biol. 1989 Sep;109(3):1341–1349. doi: 10.1083/jcb.109.3.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterud B., Bjørklid E. The production and availability of tissue thromboplastin in cellular populations of whole blood exposed to various concentrations of endotoxin. An assay for detection of endotoxin. Scand J Haematol. 1982 Aug;29(2):175–184. doi: 10.1111/j.1600-0609.1982.tb00580.x. [DOI] [PubMed] [Google Scholar]
- Schüepp B. J., Jungi T. W. Thrombospondin-exposed human monocytes display augmented luminol-enhanced chemiluminescence upon receptor triggering. Biochem Biophys Res Commun. 1991 Jun 28;177(3):1087–1094. doi: 10.1016/0006-291x(91)90650-v. [DOI] [PubMed] [Google Scholar]