Abstract
Diffuse pleural thickening (DPT) is a well-recognized consequence of asbestos exposure and often follows a benign asbestos-related pleural effusion. At our tertiary center, in the North West of England where the prevalence of asbestos-related pleural disease is high, we have encountered a series of patients that have had led us to consider a new hypothesis in DPT. We postulate that non-asbestos-related pleural effusions, particularly transudative pleural effusions, caused by heart failure can trigger the development of DPT. We present one such case, discuss the limitations of our temporal observations, and invite further discussions from readers.
Keywords: Asbestos, Asbestos-related pleural disease, Diffuse pleural thickening, Benign asbestos-related pleural effusion
INTRODUCTION
Pleural fibrosis leading to diffuse pleural thickening (DPT) is a well-recognized consequence of asbestos exposure. Most cases of asbestos-related DPT are thought to follow benign asbestos pleural effusions (BAPE), suggesting a common inflammatory pathway.1,2 A BAPE is defined as exudative pleural effusion in an asbestos exposed patient in whom all other causes have been excluded. Other causes of a highly viscous exudative pleural effusion (empyema and hemothorax) can also lead to localized pleural thickening and are not related to asbestos exposure. There are specific radiological findings on computed tomography (CT) that are characteristic of asbestos-induced DPT, which include rounded atelectasis and linear parenchymal bands.3 Pleural plaques are also frequently present, confirming asbestos exposure.
To our knowledge there have been no reports observing the development of DPT in asbestos exposed patients following pleural effusions not classified as BAPE, empyema, or hemothorax. In particular there have been no reports of DPT following transudative pleural effusions, such as those caused by heart failure. The University Hospital of South Manchester is a tertiary respiratory center in the North West of England; a region with a high prevalence of asbestos-related pleural disease. We have encountered a series of patients in whom we have observed this phenomenon and wonder if this may represent a novel pathway in the pathogenesis of asbestos-related pleural disease. One such case is presented below.
CASE REPORT
Patient X presented with progressive breathlessness and intermittent palpitations over a period of several weeks. The patient had previously worked as a joiner cutting asbestos sheets for roofing on a daily basis for 5 years and was an ex-smoker with 65-packs per year history. Past medical history was confined to peripheral vascular disease. Physical examination revealed an irregular pulse with tachycardia, a raised jugular venous pressure, an ejection systolic murmur radiating to the carotid area, and bilateral pitting edema in the lower legs. A 12-lead electrocardiogram (ECG) confirmed atrial fibrillation with left ventricular hypertrophy by voltage criteria and a chest X-ray (CXR) showed a small right pleural effusion. A CXR 12 months prior to this presentation was normal (Fig. 1). Given the unilateral appearance of the pleural disease on CXR, a respiratory opinion was sought. Thoracic ultrasound revealed bilateral, anechoic, and non-septated pleural effusions, larger on the right side. Analysis of pleural fluid aspirated from the right-sided effusion revealed a transudate (fluid protein, 28 g/l; serum protein, 71 g/l; fluid LDH, 165 IU/l; serum LDH, 339 IU/l; upper limit of serum LDH reference range, 264 IU/l). No organisms were observed on Gram stain or culture and occasional lymphocytes and mesothelial cells were seen on cytology but no malignant cells. Echocardiography revealed bi-atrial enlargement, severe aortic stenosis (valve area 0.6 cm2), and moderate left ventricular impairment. The diagnosis made was decompensated heart failure precipitated by the onset of atrial fibrillation on the background of aortic stenosis. The immediate management centered on appropriate cardiac interventions including rate-control with beta-blockers, diuresis, anti-coagulation, and a cardiac surgery assessment in preparation for aortic valve replacement.
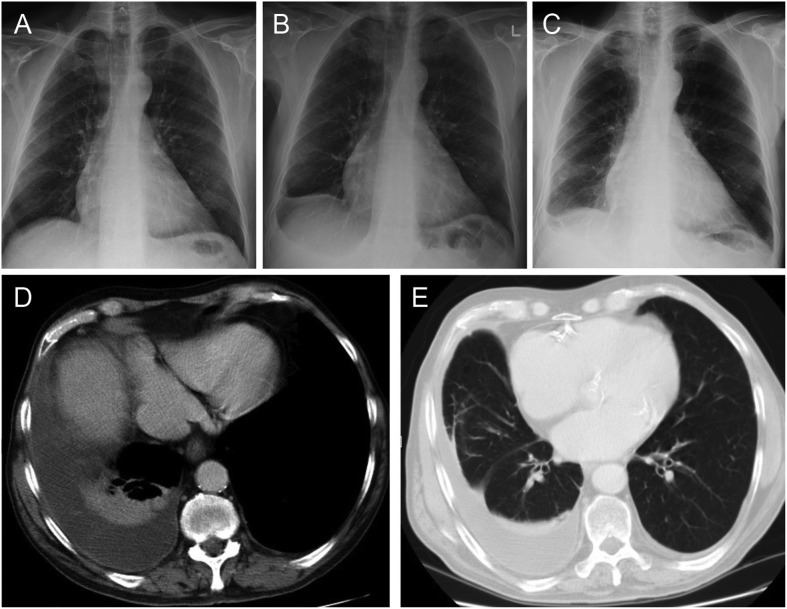
Figure 1.
Case demonstrating the development of right sided diffuse pleural thickening (DPT) following bilateral transudative pleural effusions secondary to heart failure. (A) Baseline chest X-ray (CXR) with no evidence of pre-existing pleural disease. (B) Presentation of CXR demonstrating a right-sided pleural effusion (subsequently shown to be bilateral on thoracic ultrasound). (C) Chest X-ray 6 months after presentation showing right sided pleural thickening with associated loss of volume in the right hemithorax. (D) Computed tomography (CT) thorax demonstrating smooth pleural thickening, a small pleural effusion, and rounded atelectasis in the adjacent lung parenchyma. (E) Computed tomography thorax demonstrating linear parenchymal bands.
Surprisingly, over the next 6 months, the patient developed unilateral right-sided smooth pleural thickening with additional features of rounded atelectasis and linear bands on CT consistent with asbestos-related DPT (Fig. 1). Radiological surveillance was undertaken to ensure stability prior to cardiac surgery. The right-sided pleural disease remained stable with no radiological concern for malignancy and no evidence of left sided pleural disease. Aortic valve replacement was undertaken following this period of radiological stability (approximately 12 months). Unfortunately, the patient developed widespread small bowel ischemia in the post-operative period and died.
DISCUSSION
This case, in conjunction with others we have encountered, has led us to postulate on the possibility of a novel hypothesis in asbestos-related DPT. Could a pleural effusion of any etiology, including heart failure, trigger the onset of DPT in asbestos exposed individuals? Could it be that transudative effusions are not simple and non-inflammatory effusions? Perhaps the cytokine and growth stimulant release seen in exudative effusions such as parapneumonic effusions can also occur in transudative processes. Perhaps the pleura of asbestos exposed individuals are ‘primed’ in a pro-inflammatory state following exposure and if this were true, could any pleural insult potentially trigger a cascade of cytokine activation leading to inflammation and subsequently fibrosis? Answers to these questions are obviously not demonstrable at this stage and we emphasize that these are purely speculative hypotheses. Our observations in this case and others are temporal and do not provide any evidence on causation. With specific reference to the case presented here, it is not possible to know if the DPT would have developed in the absence of the cardiac disease. It is also not possible to know that the effusion was not a BAPE unrelated to the cardiac disease, simply coinciding with it. The CXR 12 months prior to presentation showing no evidence of pleural disease, the transudative biochemistry coupled with the clinical scenario, and the immediate development of DPT following the onset of symptomatic heart failure, however, are the reasons for considering this new hypothesis.
There are a number of limitations to this hypothesis. If pleural effusions secondary to heart failure can trigger DPT in asbestos exposed patients, then we would expect a sizeable cohort of patients in the western world with such phenomena, given the prevalence of heart failure effusions. Perhaps DPT following transudative pleural effusions only occurs in patients with a genetic susceptibility to asbestos as has been suggested in mesothelioma.4 This is in line with the observation that patients with similar exposure to asbestos may develop different disease entities related to that exposure. This could explain why such a hypothesis has not been previously proposed. Furthermore, while DPT is commonly thought to follow a BAPE, DPT can occur without a preceding effusion and an effusion is not a pre-requisite for the development of DPT.
In summary, this case along with others has prompted us to consider a novel hypothesis in the pathogenesis of DPT in asbestos exposure. Although this hypothesis is based on an observation of a temporal relationship we believe it warrants further discussion. We welcome the thoughts of the respiratory and occupational community on whether similar observations have been made elsewhere and suggestions of the biological pathways that may be involved.
DISCLAIMER STATEMENTS
Contributors The authors are the sole contributors.
Funding None
Conflicts of interest None
Ethics approval Not required
REFERENCES
- 1.Cookson WO, De Klerk NH, Musk AW, Glancy JJ, Armstrong BK, Hobbs MS. Benign and malignant pleural effusions in former Wittenoom crocidolite millers and miners. Aust N Z J Med. 1985;15:731–7. [PubMed] [Google Scholar]
- 2.Huggins JT, Sahn SA. Causes and management of pleural fibrosis. Respirology. 2004;9:441–7. doi: 10.1111/j.1440-1843.2004.00630.x. [DOI] [PubMed] [Google Scholar]
- 3.Roach HD, Davies GJ, Attanoos R, Crane M, Adams H, Phillips S. Asbestos: when the dust settles an imaging review of asbestos-related disease. Radiographics. 2002;22:S167–84. doi: 10.1148/radiographics.22.suppl_1.g02oc10s167. [DOI] [PubMed] [Google Scholar]
- 4.Testa JR, Cheung M, Pei J, Below JE, Tan Y, Sementino E, et al. Germline BAP1 mutations predispose to malignant mesothelioma. Nat Genet. 2011;43:1022–5. doi: 10.1038/ng.912. [DOI] [PMC free article] [PubMed] [Google Scholar]