Abstract
The major link between the visual and motor systems is via the dorsal stream pathways from visual to parietal and frontal areas of the cortex. Although the pathway appears to be indirect, there is evidence that visual input can reach the motor cortex at relatively short latency. To shed some light on its neural basis, we studied the visuomotor interaction using paired transcranial magnetic stimulation (TMS). Motor-evoked potentials (MEPs) were recorded from the right first dorsal interosseous in sixteen healthy volunteers. A conditioning stimulus (CS) was applied over the phosphene hotspot of the visual cortex, followed by a test stimulus over the left primary motor cortex (M1) with a random interstimulus interval (ISI) in range 12–40 ms. The effects of paired stimulation were retested during visual and auditory reaction-time tasks (RT). Finally, we measured the effects of a CS on short-interval intracortical inhibition (SICI). At rest, a CS over the occiput significantly (P < 0.001) suppressed test MEPs with an ISI in the range 18–40 ms. In the visual RT, inhibition with an ISI of 40 ms (but not 18 ms) was replaced by a time-specific facilitation (P < 0.001), whereas, in the auditory RT, the CS no longer had any effect on MEPs. Finally, an occipital CS facilitated SICI with an ISI of 40 ms (P < 0.01). We conclude that it is possible to study separate functional connections from visual to motor cortices using paired-TMS with an ISI in the range 18–40 ms. The connections are inhibitory at rest and possibly mediated by inhibitory interneurones in the motor cortex. The effect with an ISI of 40 ms reverses into facilitation during a visuomotor RT but not an audiomotor RT. This suggests that it plays a role in visuomotor integration.
Key points
We studied the interaction between the primary visual cortex and the primary motor cortex using paired transcranial magnetic stimulation (TMS) with an interstimulus interval (ISI) in the range 12–40 ms.
The connection is inhibitory at rest and possibly mediated by inhibitory interneurones in the motor cortex.
The effect with an ISI of 40 ms reverses into facilitation during a visuomotor (but not audiomotor) reaction task. By contrast, there is no change in inhibition with an ISI of 18 ms, suggesting that separate pathways can be probed at different ISIs.
We conclude that a physiologically relevant occipito-motor connection can be activated by means of TMS. It may contribute to visuomotor integration, as well as being involved in certain types of visual epilepsy.
Introduction
Corticospinal excitability is modulated by a variety of sensory inputs, including auditory (Furubayashi et al. 2000), somatosensory (Tokimura et al. 2000), visual (Cantello et al. 2000) and even gustatory (Mistry et al. 2006) inputs. This probably contributes to the sensorimotor integration underlying hand/limb movements (Goodale, 2011). In particular, somatosensory input has often been given special prominence, in view of its direct and short latency inputs. A large proportion of motor cortex neurones recorded in non-human primates respond at short latency to somatosensory inputs (Cheney & Fetz, 1984), and such responses are probably involved in long-latency transcortical stretch and cutaneous reflexes in humans (Macefield et al. 1996). By contrast, visual inputs are classically viewed as relatively indirect and weak, with only ∼3% neurones in the primate motor cortex responding to visual stimulation (Lamarre et al. 1983). However, later studies found visually responsive neurones in many areas of the cerebral cortex not directly involved in vision (i.e. premotor cortex, supplementary motor area, prefrontal cortex, frontal ocular fields) (Fadiga et al. 2000). How these areas are involved in visuomotor integration is still largely unknown.
In humans, there have been relatively few direct investigations of the effects of visual input on the primary motor cortex (M1), although those that have been carried out suggest that moderately strong effects can be observed at a relatively short latency. The earliest studies were conducted in patients with photic reflex myoclonus in whom flashes of light can evoke a generalized myoclonic jerk (Shibasaki & Neshige, 1987; Artieda & Obeso, 1993). In a series of investigations on six patients, Artieda & Obeso (1993) suggested that visual input was reaching the motor cortex rapidly from primary visual areas because transcranial magnetic stimulation (TMS) over the occiput during 1 Hz flash stimuli (to increase visuomotor excitability) provoked a muscle twitch some 7 ms later than direct TMS over M1. A later study by Cantello et al. (2000) in healthy volunteers followed up on these observations by using single pulses of TMS to assess the excitability of the motor cortex after a light flash. They found that excitability was reduced some 55–70 ms after the flash and noted that the response to a flash reaches the visual cortex at ∼40 ms, so that, if a cortico-cortical pathway was involved from the primary visual cortex (V1) to M1, the transit time would be of the order of 15 ms, at least in normal subjects. These effects might be interpreted as the physiological counterpart of a pathological visuomotor connectivity seen earlier in patients with photic reflex myoclonus (Artieda & Obeso, 1993). Rapid access of visual input to motor areas of the cortex is also evident from reaction time (RT) studies (Thut et al. 2000; Makin et al. 2009) and many event-related potential studies (Saron et al. 2001; Foxe & Simpson, 2002; Ledberg et al. 2007). Yet the precise neural basis of these phenomena is still largely obscure. Intuitively, the primary visual area would be the first cortical relay of the circuit and M1 would represent the final output.
The present study aimed to devise a method for examining the visuomotor interaction in healthy participants. We used a ‘twin coil’ TMS approach to test whether a conditioning pulse over the occiput influences the amplitude of the muscle twitches evoked from a later TMS pulse applied over M1. Connectivity was tested at rest, as well as during the warning period, prior to a simple visual RT task, aiming to examine whether it showed any task-related changes in excitability.
Methods
Subjects
A total of 16 healthy volunteers (eight women; aged 21–51 years) were recruited. One subject was excluded because he reported no phosphenes. All of the remaining 15 subjects participated in Experiment 1; 10 of these then participated in Experiments 3–5 (i.e. the same individuals in all three experiments). All subjects were right-handed based on the Edinburgh Handedness Inventory and provided their written informed consent. Experiments were approved by the Ethical Committee of University College London and were performed in accordance with the Declaration of Helsinki.
TMS
For paired-TMS, we used two high-power Magstim 200 machines (Magstim, Whitland, UK). The magnetic stimulus had an almost monophasic pulse configuration, with a rise time of ∼100 μs, decaying back to zero over ∼0.8 μs. The stimulators were connected to a figure-of-eight coil (outer winding diameter of 70 mm).
Test stimuli
Motor-evoked potentials (MEPs) were recorded from the first dorsal interosseous (FDI) muscles using 9 mm diameter Ag-AgCl surface-cup electrodes, in a typical belly-tendon montage. Responses were amplified by a Digitimer D360 device (Digitimer, Welwyn Garden City, UK). Filters were 20 Hz – 3 kHz, and the sampling rate was 10 kHz. The signal was then recorded by a PC using Signal, version 4.08 (Cambridge Electronic Devices, Cambridge, UK). The test coil was placed tangentially to the scalp at a 45 deg angle to the mid-line, to induce a posterior–anterior current flow across the central sulcus. The hand motor area of the left M1 was defined as the point where stimulation consistently evoked the largest MEP. We defined the resting motor threshold (RMT) as the lowest intensity that evoked five small responses (∼50 μV) in the relaxed FDI muscle in a series of 10 stimuli (Rossini et al. 1994). The intensity of the test stimulus (TS) was finally adjusted to evoke an MEP of ∼1 mV peak-to-peak amplitude in the relaxed right FDI.
Experiment 1
Paired-TMS stimulation was conducted as follows. The TS alone and CS plus TS were randomly intermixed at each ISI. Fifteen responses were collected for TS and 12 responses for CS plus TS. There was a 5 s (±20%) intertrial interval. For each trial, we measured the average peak-to-peak MEP amplitude. The conditioned MEP was expressed as a percentage of the unconditioned MEP size. The centre of the conditioning coil was placed over the phosphene hot spot. This was located and the phosphene threshold (PT) determined according to the method of Stewart et al. (2001). Subjects wore a blindfold and a cap when they were seated in a comfortable chair in a dimly lit room. Three points were marked over the occipital mid-line, 2, 3 and 4 cm above the inion. The coil handle pointed upwards and was parallel to the subject's spine. The coil centre was first positioned 2 cm above the inion, then moved anteriorly across the marks, to determine the best site to elicit phosphenes (hot spot). Stimuli were initially applied at 60% of the stimulator output and at a maximum frequency of 0.2 Hz. The subject was asked about the presence of phosphenes immediately after each pulse. If a phosphene was reported five or more times out of 10, the pulse intensity was reduced by steps of 5%, and then stimuli were repeated another 10 times. This protocol progressed until no phosphene was reported. The minimum intensity at which the subject perceived a phosphene five times out of 10 was the PT. If the initial intensity of 60% was ineffective, it was increased by steps of 5% maximum power, until phosphenes appeared. If the subject still failed to perceive a phosphene on the mid-line, the coil was shifted to a lateral position and the procedure was repeated at this location. One subject was excluded because he reported no phosphenes. The intensity of the CS was adjusted to be 80% PT or 90% PT. ISIs were 12, 15, 18, 21, 24, 27, 30, 35 and 40 ms. There were two sessions: one with eyes open and another with eyes closed.
Experiment 2
From Experiment 1, eight subjects were selected because they showed the strongest inhibition with an ISI of 18 and 40 ms. We then studied the effects of changing the CS site, in a setting otherwise identical to Experiment 1. There were two sessions: conditioning stimuli with an intensity of 80% PT were applied to the phosphene hot spot or to a site 3 cm lateral to Pz (according to the International 10–20 system) on the right side. The subjects’ eyes were open.
Experiment 3
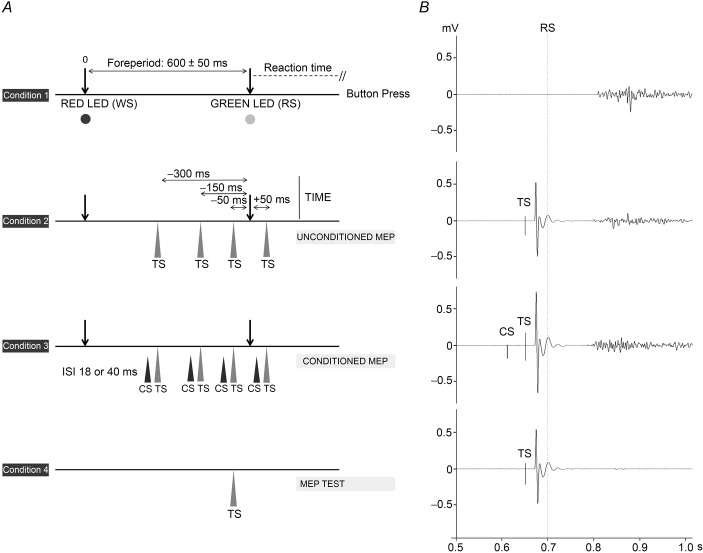
The protocol described in Experment 1 was then repeated during a visuomotor RT task. We hypothesized that a physiologically relevant connectivity would show time-specific changes in such a context. We used a task similar to that of Touge et al. (1998). Subjects sat relaxed in a chair with their right forearm lying comfortably on a pillow and their right hand on a button box. Eyes were open. A surface EMG was recorded from the FDI, abductor pollicis brevis and abductor digiti minimi muscles. We ensured that there was no EMG activity at baseline. A black screen was placed in front of the subjects at a distance of 50 cm, which carried two light-emitting diodes (LEDs) separated by 1.5 cm. The red LED was the warning signal (WS) and the green LED was the response signal (RS). Subjects were instructed to use the WS to prepare for the upcoming response and to contract their right FDI muscle as quickly as possible and press a button with their right index finger as soon as they saw the RS. Each trial began with a WS followed by a RS given randomly 600 ± 50 ms later. The intertrial interval was 5 s (±20%). There were two randomized sessions separated by at least 1 week. In each session, we measured the effects of the CS on TS when subjects were at rest, outside of the RT task. The CS was 90% PT. ISIs of 18 and 40 ms (i.e. the most effective in previous experiments) were randomly intermingled. Subjects also performed four blocks of the RT task. Each block had four conditions that were randomized within the block. Condition 1: subjects received a WS, followed 600 ± 50 ms later by a RS, to which they had to react as quickly as possible. Condition 2: a TS alone given at −300, −150, −50 or +50 ms relative to RS (depending on the block; see below). Condition 3: the same as condition 2, except that the TS was preceded by a CS with an ISI of either 18 or 40 ms (depending on the block; see below). Condition 4: a TS alone was given in the intertrial interval (Fig.1). Thirty trials were recorded for each condition for a total of 120 trials. In one of the experimental sessions, the four trial blocks were: (1) TS at −300 ms, CS 18 ms before test; (2) TS at −150 ms, CS at 18 ms; (3) TS at −300 ms, CS at 40 ms before test; and (4) TS at −150 ms, CS at 40 ms. The other experimental session comprised TS at −50 ms and +50 ms. Before each session, at least 50 practice trials were given.
Figure 1.
Schematic representation of Experiment 3 with examples of recorded traces
A, the setting of Experiment 3. In condition 1, subjects received a WS, followed 600 ± 50 ms later by a RS, after which they had to react as fast as possible. In condition 2, subjects received a TS alone given at one of four different ‘times’ (−300, −150, −50 or +50 ms). Condition 3 was the same in condition 2, although the TS was preceded by a CS with an ISI of either 18 or 40 ms. In condition 4, a TS alone was given in the intertrial interval. B, typical example of changes in the MEP (grand average of the recorded trials) during the RT task. In this particular subject, a clear MEP increase can be seen 50 ms before the RS with an ISI of 40 ms (condition 3).
The responses to each single trial were stored on a PC and analysed offline at the end of the experiment. Rejection criteria were: (1) baseline EMG levels ≥ 50 μV; (2) RT < 100 ms and > 1000 ms; and (3) failure to react. Later, we analysed the root mean square (RMS) values of the baseline EMG in the 100 ms before the TMS pulses in each trial to ensure the task-specific conditioned MEP data were not contaminated by background EMG activity.
Experiment 4
We tested the paired-TMS protocol during an auditory RT task. The subjects, settings and conditions were the same as in Experiment 3. First, we measured the effects of the CS on TS with an ISI of 40 ms when the subjects were at rest outside of the RT task. There followed two sessions: in one, we used an auditory RT task, where the first tone (500 Hz, 50 ms) was the WS and the second tone (1000 Hz, 50 ms) was the RS; in the other, we retested the visual RT task. We also restricted our timings to a TS at −50 ms (i.e. just prior to the RS) using an ISI between CS and TS of 40 ms because these parameters had produced large effects in Experiment 3.
Experiment 5
This experiment investigated the effects of a CS over the visual cortex on short interval intracortical inhibition (SICI) in the left M1 (Kujirai et al. 1993). We used three high-power Magstim 200 machines. The first conditioning stimulus (CS1) was delivered with an intensity of 90% PT over the phosphene hot spot and the second one (CS2) over the left M1. Finally, the TS was applied over the left M1 with an intensity that elicited a MEP of ∼1 mV. The intensity of CS2 was set to the relatively low value of 70% active motor threshold (AMT) to avoid floor effects on the percentage SICI. AMT was defined as the lowest intensity that evoked five small responses (∼100 μV) in a series of ten stimuli when the subject made a 10% of the maximum voluntary contraction of the right FDI. The ISIs between CS1 and CS2 were 18 and 40 ms, whereas the ISI between CS2 and TS was 2.2 ms. A randomized conditioning test design was used. First, we tested the effects on the test MEP (MEP1) of giving CS1 alone (with an ISI of 40 ms, CS140ms; or an ISI of 18 ms, CS118ms) or CS2 alone (MEP2). Then, the intensity of the TS was re-adjusted so that, when CS118ms + TS or CS140ms + TS were applied, the combined effect would elicit a MEP of ∼1 mV (MEP31mV). Finally, two conditions were randomly intermingled: CS1(18 ms or 40 ms) + TS (MEP31mV) and CS1(18 ms or 40 ms) + CS2 + TS (MEP4). Fifteen trials were recorded for each condition. The ratio of MEP4/MEP31mV was the amount of SICI in the presence of CS1(18 ms or 40 ms), whereas the ratio MEP2/MEP1 was the baseline SICI.
Statistical analysis
All data are expressed as the mean ± SEM. Student's paired t tests (two-tailed) were used to compare mean RMT with eyes open and closed obtained from all the participants. Spearman's rho was applied to study the correlation between motor and PT. In general, the effects of the CS on MEP amplitude were analysed with separate one-way ANOVAs for any given stimulation intensity and eyes state, with ‘ISI’ (TS alone, CS plus TS at various ISIs) as the main factor. A significant main effect in these ANOVAs was followed by post hoc tests with Bonferroni corrections. Based on the conditions of the various experiments, we performed preliminary two- or three-way repeated measures ANOVAs that accounted for the various factors to be analysed. Supplementary ANOVAs or repeated measures ANOVAs were finally carried out, as dictated by the specific experiment, to assess the effects of additional confounders; for example, in Experiment 3, a two-way repeated measures ANOVA explored the ‘time’ (Fig.1) × ‘ISI’ interactions. Mauchley's test was used to examine for sphericity. The Greenhouse-Geisser correction was used for non-spherical data. Occasionally, two-tailed Student's paired t tests were used (Experiment 5). P < 0.05 was considered statistically significant. Data were analysed using SPSS, version 19.0 (SPSS Inc.).
Results
Baseline physiological data are shown in Table1. No differences were found between each experimental session. All subjects completed the experiments without complications.
Table 1.
Physiological data (mean ± SEM)
| RMT (%) | PT (%) | UC MEP (mV) | ||
|---|---|---|---|---|
| Experiment 1 (n = 15) | ||||
| EO | 80% PT | 41.4 ± 1.9 | 62.8 ± 2.6 | 1.07 ± 0.08 |
| 90% PT | 1.13 ± 0.09 | |||
| EC | 80% PT | 40.6 ± 1.9 | 0.98 ± 0.08 | |
| 90% PT | 0.96 ± 0.05 | |||
| Experiment 2 (n = 8) | ||||
| CS over control site | 36.3 ± 1.6 | 65.7 ± 2.5 | 1.11 ± 0.14 | |
| CS over phosphene hotspot | 1.06 ± 0.05 | |||
| Experiment 3 (n = 10) | ||||
| TS at rest | 39.1 ± 1.6 | 62.3 ± 2.7 | 1.09 ± 0.07 | |
| TS −300 ms | 1.15 ± 0.09 | |||
| TS −150 ms | 1.04 ± 0.07 | |||
| TS at rest | 40.8 ± 2.1 | 65.2 ± 2.3 | 1.13 ± 0.10 | |
| TS −50 ms | 1.10 ± 0.12 | |||
| TS +50 ms | 1.17 ± 0.06 | |||
| Experiment 4 (n = 10) | ||||
| TS at rest | 39.8 ± 1.5 | 63.5 ± 2.1 | 1.11 ± 0.06 | |
| Auditory task | 0.98 ± 0.08 | |||
| Visual task | 1.08 ± 0.05 | |||
| Experiment 5 (n = 10) | AMT (%) | |||
| TS | 35.8 ± 1.4 | 66.5 ± 3.4 | 1.15 ± 0.08 | |
| ISI 18 ms | 1.14 ± 0.08 | |||
| ISI 40 ms | 1.01 ± 0.06 | |||
EO, eyes open; EC, eyes closed; RMS, root mean square; UC, unconditioned.
Mean RMT with eyes open was 41.4% (range 30–52%), which is the same as with eyes closed (40.6%; range 30–53%) (Student's t = 0.50, P = 0.63). The phosphene hot spot was located in the mid-line in all subjects: it was 3 cm above the inion in 10 of 15 subjects, 2 cm in four of 15 subjects and 4 cm in one subject (Fig.2). Phosphenes were reported across both sides of the visual field. Mean PT was 62.8% (range 40–76%). Motor and PTs did not correlate (Spearman's rho = −0.15, P = 0.62 with eyes open; rho = 0.07, P = 0.82 with eyes closed).
Figure 2.
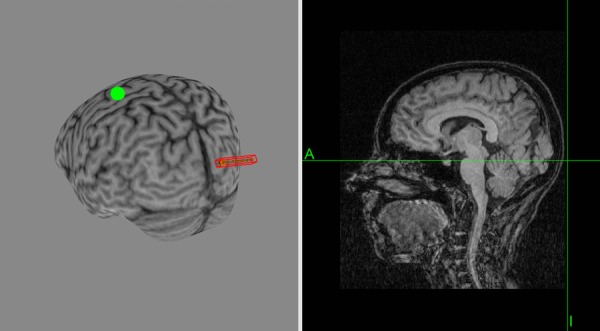
MRI reconstruction of a single subject
The red mark indicates the orientation of the magnetic field at the phosphene hot spot (striate cortex). The anterior green dot is at the hand area of the left motor cortex.
Experiment 1: conditioning MEPs with stimuli over the phosphene hot spot at rest
In this experiment, the CS was placed over the phosphene hot spot. The effect of two different intensities of CS was measured on MEPs evoked from the left M1 with eyes open or closed throughout the testing (Fig.3A–D). A preliminary three-way repeated measures ANOVA showed a significant main effect of ‘ISI’ (F5,67 = 10.93, P < 0.001) but no effect of ‘eye state’ (F1,14 = 1.50, P = 0.24) or ‘intensity’ (F1,14 = 0.32, P = 0.58) and no significant interactions (P > 0.05). Thus, the time course of MEP suppression was the same at each intensity of CS and was unaffected by eye closure. The ISIs in each state where post hoc testing revealed significant (P < 0.05) effects compared to control (Fig.3A–D) are also indicated. Because ISIs of 18 and 40 ms were effective in all states, these two intervals were then used in Experiments 2–5.
Figure 3.
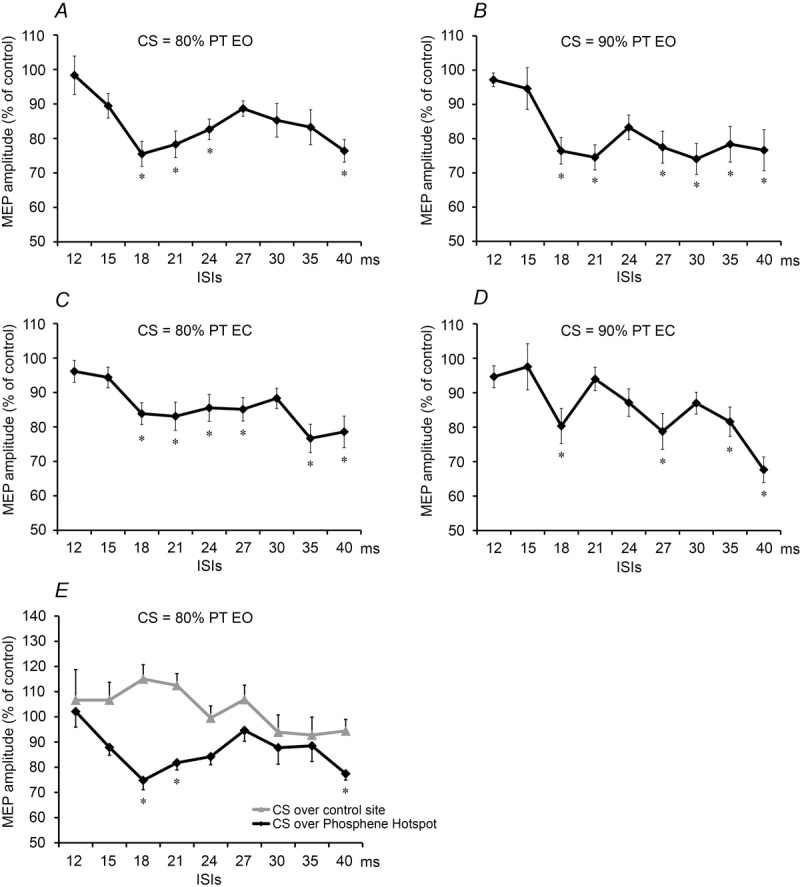
The effects of paired-TMS in Experiment 1 and 2
A –D, effects of a CS applied over the phosphene hot spot at different intensities (80% or 90% PT) and eye states (eyes open or closed) on the test MEPs with subjects at rest. Amplitude of MEPs (mV) is normalized and expressed as a percentage of control. Errors bars indicate the SEM. Asterisks indicate P < 0.05 on post hoc analysis. E, effects of changing the location of the CS (90% of the PT) on the test MEPs with subjects at rest. Grey line: CS applied to a scalp site 3 cm lateral to Pz on the right side. Black line: CS applied to the phosphene hot spot. Amplitude of MEPs (mV) is normalized and expressed as a percentage of control. Errors bars indicate the SEM. Asterisks indicate P < 0.05 on post hoc analysis.
Experiment 2: changing the site of the CS
To confirm that the effect of the CS was spatially specific, we compared the effect of conditioning over the phosphene hot spot with conditioning over a point 3 cm lateral to Pz. Figure3E shows that stimulation over the parietal site at this intensity had no effect, whereas there was clear MEP suppression if the CS was over V1. A two-way repeated measures ANOVA showed a significant main effect of ‘stimulation site’ (F1,7 = 37.52, P < 0.001), as well as a significant interaction between ‘stimulation site’ and ‘ISI’ (F8,56 = 2.475, P = 0.02), indicating that the time course of the effect on MEPs differed between sites. Follow-up one-way ANOVAs revealed a significant main effect of ISI (F9,63 = 4.73, P < 0.001) at the phosphene hot spot but no effect of ISI over the parietal site (F9,63 = 1.65, P = 0.12). On post hoc analysis, the size of the MEP conditioned from V1 was significantly reduced with ISIs of 18 ms (P = 0.001), 21 ms (P = 0.014) and 40 ms (P = 0.002). No subject reported phosphenes after the control (parietal) stimulus.
Experiment 3: visuomotor functional connectivity during a visual RT task
We next tested whether the effect of the CS varied during the course of a warned simple visual RT task. MEPs were conditioned by stimulation over the phosphene hot spot during the warning interval prior to the onset of the RS and 50 ms after the RS prior to onset of movement. The effects were compared with those seen at complete rest outside the reaction task. MEPs to the M1 stimulus given alone were the same at rest at all intervals tested during the task (one-way repeated measures ANOVA, first session of task: F3,27 = 0.62, P = 0.61; second session: F3,27 = 0.24, P = 0.87).
Figure4A plots the size of the conditioned MEP as a percent of the test MEP alone for the two ISIs between CS and TS (18 and 40 ms). There are five bars for each ISI corresponding to suppression at rest and at −300, −150, −50 and +50 (with respect to the time of the RS) during the reaction task. The percentage suppression of MEP with an ISI of 18 ms was unchanged during the task, whereas suppression with an ISI of 40 ms gradually shifted to facilitation around the time of the RS.
Figure 4.

The effects of paired-TMS in Experiment 3,4 and 5
A, effects of the CS (phosphene hot spot) on the test MEP amplitude at rest and at different times during the behavioural task (−300, −150, −50 and +50 ms). Left: ISI 18 ms. Right: ISI 40 ms. Amplitude of MEPs (mV) is normalized and expressed as a percentage of control. Errors bars indicate the SEM. B, effects of the CS (phosphene hot spot) with an ISI of 40 ms on the MEP amplitude at rest and during a visual and an auditory reaction task 50 ms before the RS. Amplitude of MEPs (mV) is normalized and expressed as a percentage of control. Errors bars indicate the SEM. C, comparison of the effects on SICI of conditioning stimuli applied over the visual cortex with an ISI of 18 and 40 ms. Errors bars indicate the SEM.
This was confirmed by a two-way repeated measures ANOVA showing a significant main effect of ‘time’ (F4,36 = 39.64, P < 0.001), ‘ISI’ (F1,9 = 25.40, P = 0.001) and a significant ‘time’ × ‘ISI’ interaction (F2,18 = 12.20, P < 0.001). Follow-up one-way ANOVAs showed no effect of ‘time’ with an ISI of 18 ms (F3,25 = 0.44, P = 0.73) and no effects of ‘background EMG’ both on the unconditioned (F1,25 = 0.017, P = 0.90) and conditioned MEPs (F1,25 = 0.007, P = 0.93) (Table2). By contrast, there was a significant effect with an ISI of 40 ms (F3,25 = 9.44, P < 0.001) and no effects of ‘background EMG’ on the unconditioned (F1,25 = 0.28, P = 0.60) and conditioned trials (F1,25 = 0.32, P = 0.574) (Table2). Post hoc analysis showed that the conditioned MEP was significantly larger at 300 ms (P = 0.034), 150 ms and 50 ms before and after the RS (P < 0.001).
Table 2.
EMG levels (100 ms before the TMS pulse) in the visual RT task (mean ± SD)
| EMG levels (RMS, mV) | ||||
|---|---|---|---|---|
| RT conditions | ||||
| Experiment 3 (n = 10) | –300 ms | –150 ms | –50 ms | +50 ms |
| ISI 18 ms | ||||
| Pre-test MEP | 0.0327 ± 0.0015 | 0.0326 ± 0.0016 | 0.0239 ± 0.0129 | 0.0237 ± 0.0130 |
| Pre-UC MEP | 0.0327 ± 0.0015 | 0.0327 ± 0.0016 | 0.0238 ± 0.0129 | 0.0238 ± 0.0128 |
| Pre-C MEP | 0.0328 ± 0.0017 | 0.0327 ± 0.0016 | 0.0238 ± 0.0129 | 0.0237 ± 0.0130 |
| ISI 40 ms | ||||
| Pre-test MEP | 0.0326 ± 0.0015 | 0.0327 ± 0.0016 | 0.0236 ± 0.0129 | 0.0238 ± 0.0129 |
| Pre-UC MEP | 0.0327 ± 0.0015 | 0.0327 ± 0.0016 | 0.0237 ± 0.0130 | 0.0237 ± 0.0130 |
| Pre-C MEP | 0.0327 ± 0.0016 | 0.0327 ± 0.0016 | 0.0236 ± 0.0130 | 0.0237 ± 0.0129 |
RMS, root mean square; UC, unconditioned; C, conditioned.
Experiment 4: visuomotor functional connectivity during an auditory RT task
In the visual task, the CS (ISI of 40 ms) facilitated the conditioned MEP 50 ms prior to the RS. In the same subjects, we compared this with the effect when using the same timing in an auditory reaction task. The unconditioned MEP at rest was the same as during the visual and auditory task (50 ms before the RS) (F2,18 = 1.20, P = 0.323). Figure4B shows that the CS suppressed the MEP when subjects were tested at rest. However, during performance of the auditory task (−50 ms), there was no longer any effect of the CS on the TS, whereas, in the visual task, it was facilitated. A one-way repeated measures ANOVA on the data confirmed that the effect of the CS differed between the three conditions (F2,18 = 49.26, P < 0.001). Follow-up analysis showed that, although there was a significant difference between the effect at rest and at the −50 ms time points in both tasks (visual, P < 0.001; auditory, P < 0.001), the effect was larger in the visual task compared to the auditory task (P < 0.001).
Experiment 5: effects on SICI
A CS over the phosphene hot spot increased the amount of SICI compared to baseline (baseline SICI: 77.5%; SICI in the presence of CS: 56%) (Student's t = 6.86, P < 0.001) with an ISI of 40 ms but not 18 ms (Student's t = 0.254, P = 0.80) (Fig.4C). As a result of intensity re-adjustment, the MEP31mV size was 1.01 ± 0.1 mV (ISI 40 ms) and 1.14 ± 0.1 (ISI 18 ms) and was not statistically different from the MEP1 (1.15 ± 0.1 mV) (F2,18 = 1.11, P = 0.35).
Discussion
The present data show that TMS over the occipital region affects excitability of M1 when tested 18–40 ms later. Because the TMS coil was located over the optimal point to elicit stationary phosphenes (Afra et al. 1998; Stewart et al. 2001; Franca et al. 2006) and an intensity below PT was used, we suggest that the effect depends on activation of V1. We assumed that both hemispheres were activated because the coil position was on the mid-line in all of the subjects and phosphenes were reported across both sides of the visual field. The effect was present at both 80% and 90% PT but was not significantly influenced by whether the eyes were open or closed. It was not caused by the auditory click made by the coil when discharged (Furubayashi et al. 2000) because it was no longer present when the site of stimulation was moved 3 cm lateral to Pz.
Our results confirm the evidence suggesting that activity in visual cortex can modulate corticospinal excitability at short latency in subjects at rest. One of the limits of the previous approaches is that they used natural visual stimuli and there is some uncertainty about the precise time at which these arrive in the visual cortex. Most studies indicate that the first occipital visual evoked potentials begin at ∼35–40 ms (ffytche et al. 1995), whereas intracranial electrodes recorded a latency of ∼31–33 ms (Ducati et al. 1988). Using these values, the earliest TMS effect with an ISI of 18 ms is compatible with the data on flash evoked suppression of MEPs noted at 55–70 ms after a flash (Cantello et al. 2000; Makin et al. 2009) but later than the very rapid (7 ms) visuomotor connectivity described in photic reflex myoclonus (Nakashima et al. 1985; Shibasaki & Neshige, 1987; Artieda & Obeso, 1993; Kanouchi et al. 1997). The shorter occipito-motor conduction time in the patients might well be explained by a pathological exaggeration of the normal physiological mechanism, resulting in a shorter latency response and a shift from inhibition to excitation of the motor cortex. A similar connection might explain the spread of the epileptic discharge from the hyperexcitable visual cortex to the motor cortex in photosensitive idiopathic epilepsies (Strigaro et al. 2012; Strigaro et al. 2013).
The later phase of interaction with an ISI of 40 ms is compatible with the earliest signs of visual effect on motor cortex excitability described in a number of behavioural studies (e.g. 70 ms; Makin et al. 2009). Longer latency visuomotor effects have also been described by Suppa et al. (2015) who showed that it was possible to induce long-term potentiation and depression-like plasticity in the primary motor cortex in healthy humans after repetitive pairing of a patterned visual stimulus and a TMS stimulus at specific time intervals around the latency of the P100 evoked potential. These varied between 40 and 140 ms after the individual P100 latency (i.e. between 140 and 240 ms after onset of the visual stimulus) (Suppa et al. 2015) and are therefore longer than the ISIs considered in the present study.
Apart from estimates of transit time, our data do not provide any information about the possible anatomical pathways that might mediate these functional effects. Connections in the dorsal visual stream via parietal and premotor cortex could provide one route. In addition, diffusion tensor imaging techniques (Catani et al. 2002) and anatomical dissection studies (Martino et al. 2010; Sarubbo et al. 2013) have demonstrated the existence in humans of the inferior fronto-occipital fascicle, a long associative bundle connecting the occipital cortex and other posterior areas to the frontal lobe (Martino et al. 2010). Although often seen as playing a role in transmitting information from frontal cortex to the occiput for the purposes of ‘top down’ control, the inferior fronto-occipital fascicle might also contain a direct efferent pathway from the occipital cortex, which can rapidly transmit visual information to the frontal regions (Martino et al. 2010).
Most long range cortico-cortical connections are considered to be excitatory, as in the transcallosal pathway (Asanuma & Okuda, 1962; Ferbert et al. 1992). We obtained an overall inhibitory effect in the present study, which is compatible with the idea that these excitatory projections synapse onto inhibitory interneurones in M1 that suppress corticospinal excitability. This is supported by our findings that a CS over the visual cortex increased SICI in the left M1, at least with an ISI of 40 ms (and not 18 ms). SICI is considered to test a GABAAergic form of intracortical inhibition in the motor cortex (Ziemann et al. 1996). Because SICI is made more effective by stimulation over the visual cortex, this suggests that occipital input has access to inhibitory circuits in M1 and that this may contribute to MEP suppression. Visuomotor suppression at 18 ms presumably does not depend on activity in the same set of interneurones because it has no effect on SICI. However, there are a number of possibilities that can be tested with TMS methods, including a GABABergic system (tested with the long interval intracortical inhibition paradigm) (Valls-Sole et al. 1992; Werhahn et al. 1999) and a further pathway modulated by cholinergic input (tested with short afferent inhibition) (Tokimura et al. 2000). Further work could distinguish between these possibilities. At present, we conclude that the two phases of inhibition are caused by activity in two separate pathways.
To assess the potential physiological role of this visuomotor pathway, we examined connectivity during a visual RT task using ISIs of 18 and 40 ms because they produced the most consistent inhibitory effects. The task had no effect on MEP suppression with an ISI of 18 ms at any of the time points studied during the task. This was not true with an ISI of 40 ms. The inhibitory effect at rest (MEP reduced by 30–40%) gradually reversed into facilitation during movement preparation. Facilitation appeared to begin ∼150 ms prior to the RS and was very clear at +50 ms (MEP increased by 40–50%). This contrasts with the results obtained in an equivalent auditory reaction task. The usual visuomotor suppression observed at rest was absent 50 ms prior to the RS, although there was no clear facilitation of the MEP as in the visual task. We suggest that rapid visuomotor connectivity is suppressed during an auditory task but becomes facilitatory during a visual task, perhaps improving access of visual input to motor areas. It is unclear why connectivity with an ISI of 18 ms was unaffected in the visual reaction task. Nevertheless, the finding does confirm the conclusion that these two effects are mediated by quite separate pathways.
During the RT tasks, we saw no significant changes in the unconditioned MEP at the time intervals studied. In previous studies, the MEP has been suppressed in the interval between the WS and RS (Hasbroucq et al. 1997; Touge et al. 1998; Davranche et al. 2007). However, suppression is best observed when the WS–RS interval is constant and subjects can anticipate precisely when the RS is about to be delivered (Touge et al. 1998). In the present task, the timing of the RS was not predictable because it was randomized to occur 550–650 ms after the WS. MEPs also are known to increase after the RS prior to onset of EMG activity. However, the effect usually starts more than 50 ms after the RS, which was beyond the time range investigated in the present study.
There was one slightly unexpected feature of the present results: the excitability of the occipito-motor connection was the same when it was tested with the eyes open or closed. Previous work had shown that transient removal of vision increases the amplitude of early components of the flash-evoked EEG potential (Cantello et al. 2011) and we had initially anticipated that it might also increase the size of any effects we observed. However, the amplitude of the visually-evoked potentials may well be influenced by subcortical rather than cortical changes. For example, eye closure produces effects on retinal sensitivity that could affect the flash-evoked input without affecting the excitability of V1 to TMS. We propose that, although ambient light levels may affect the excitability of inputs to the visual cortex, they do not influence the excitability of the output elements activated by TMS. One study noted that blindfolding increases the excitability of M1, as tested by its effect on the amplitude of TMS-evoked muscle twitches (Leon-Sarmiento et al. 2005). The effect was larger after 30 min of blindfolding compared to immediately after eye closure. In the present study, the eyes were only closed for a short period and we did not detect any change of RMT or baseline MEPs between open and closed eyes. We are less certain why the responses to conditioning stimuli of 80% and 90% PT were similar. It is possible that this was the result of a lack of statistical power, given the tendency for more inhibition to occur at 90% PT regardless of whether the eyes were open or closed.
Conclusions
Our findings support the existence of physiologically relevant occipito-motor connections, which can be activated by means of TMS. They may contribute to the rapid integration of visual input into motor tasks, as well as being involved in the spread of a seizure from visual to motor areas in certain types of visual epilepsy.
Glossary
- AMT
active motor threshold
- CS
conditioning stimulus
- FDI
first dorsal interosseous
- ISI
interstimulus interval
- LED
light-emitting diode
- M1
primary motor cortex
- MEP
motor-evoked potential
- PT
phosphene threshold
- RMT
resting motor threshold
- RS
response signal
- SICI
short-interval intracortical inhibition
- TMS
transcranial magnetic stimulation
- TS
test stimulus
- V1
primary visual cortex
- WS
warning signal
Additional information
Competing interests
The authors declare that they have no competing interests.
Author contributions
GS, JCR, DR and RC conceived and designed the experiments. GS, JCC, LM and MD collected the data. All authors participated in the analysis and interpretation of the data and in the drafting of the manuscript. All authors approved the final version of the manuscript submitted for publication.
Funding
GS is funded by the Fondazione Veronesi Young Investigator Programme 2013.
References
- Afra J, Mascia A, Gerard P, Maertens de Noordhout A. Schoenen J. Interictal cortical excitability in migraine: a study using transcranial magnetic stimulation of motor and visual cortices. Ann Neurol. 1998;44:209–215. doi: 10.1002/ana.410440211. [DOI] [PubMed] [Google Scholar]
- Artieda J. Obeso JA. The pathophysiology and pharmacology of photic cortical reflex myoclonus. Ann Neurol. 1993;34:175–184. doi: 10.1002/ana.410340213. [DOI] [PubMed] [Google Scholar]
- Asanuma H. Okuda O. Effects of transcallosal volleys on pyramidal tract cell activity of cat. J Neurophysiol. 1962;25:198–208. doi: 10.1152/jn.1962.25.2.198. [DOI] [PubMed] [Google Scholar]
- Cantello R, Civardi C, Cavalli A, Varrasi C. Vicentini R. Effects of a photic input on the human cortico-motoneuron connection. Clin Neurophysiol. 2000;111:1981–1989. doi: 10.1016/s1388-2457(00)00431-4. [DOI] [PubMed] [Google Scholar]
- Cantello R, Strigaro G, Prandi P, Varrasi C, Mula M. Monaco F. Paired-pulse flash-visual evoked potentials: new methods revive an old test. Clin Neurophysiol. 2011;122:1622–1628. doi: 10.1016/j.clinph.2011.01.008. [DOI] [PubMed] [Google Scholar]
- Catani M, Howard RJ, Pajevic S. Jones DK. Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage. 2002;17:77–94. doi: 10.1006/nimg.2002.1136. [DOI] [PubMed] [Google Scholar]
- Cheney PD. Fetz EE. Corticomotoneuronal cells contribute to long-latency stretch reflexes in the rhesus monkey. J Physiol. 1984;349:249–272. doi: 10.1113/jphysiol.1984.sp015155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davranche K, Tandonnet C, Burle B, Meynier C, Vidal F. Hasbroucq T. The dual nature of time preparation: neural activation and suppression revealed by transcranial magnetic stimulation of the motor cortex. Eur J Neurosci. 2007;25:3766–3774. doi: 10.1111/j.1460-9568.2007.05588.x. [DOI] [PubMed] [Google Scholar]
- Ducati A, Fava E. Motti ED. Neuronal generators of the visual evoked potentials: intracerebral recording in awake humans. Electroencephalogr Clin Neurophysiol. 1988;71:89–99. doi: 10.1016/0168-5597(88)90010-x. [DOI] [PubMed] [Google Scholar]
- Fadiga L, Fogassi L, Gallese V. Rizzolatti G. Visuomotor neurons: ambiguity of the discharge or ‘motor’ perception? Int J Psychophysiol. 2000;35:165–177. doi: 10.1016/s0167-8760(99)00051-3. [DOI] [PubMed] [Google Scholar]
- Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG. Marsden CD. Interhemispheric inhibition of the human motor cortex. J Physiol. 1992;453:525–546. doi: 10.1113/jphysiol.1992.sp019243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ffytche DH, Guy CN. Zeki S. The parallel visual motion inputs into areas V1 and V5 of human cerebral cortex. Brain. 1995;118:1375–1394. doi: 10.1093/brain/118.6.1375. [DOI] [PubMed] [Google Scholar]
- Foxe JJ. Simpson GV. Flow of activation from V1 to frontal cortex in humans. A framework for defining "early" visual processing. Exp Brain Res. 2002;142:139–150. doi: 10.1007/s00221-001-0906-7. [DOI] [PubMed] [Google Scholar]
- Franca M, Koch G, Mochizuki H, Huang YZ. Rothwell JC. Effects of theta burst stimulation protocols on phosphene threshold. Clin Neurophysiol. 2006;117:1808–1813. doi: 10.1016/j.clinph.2006.03.019. [DOI] [PubMed] [Google Scholar]
- Furubayashi T, Ugawa Y, Terao Y, Hanajima R, Sakai K, Machii K, Mochizuki H, Shiio Y, Uesugi H, Enomoto H. Kanazawa I. The human hand motor area is transiently suppressed by an unexpected auditory stimulus. Clin Neurophysiol. 2000;111:178–183. doi: 10.1016/s1388-2457(99)00200-x. [DOI] [PubMed] [Google Scholar]
- Goodale MA( Transforming vision into action. Vision Res. 2011;51:1567–1587. doi: 10.1016/j.visres.2010.07.027. [DOI] [PubMed] [Google Scholar]
- Hasbroucq T, Kaneko H, Akamatsu M. Possamai CA. Preparatory inhibition of cortico-spinal excitability: a transcranial magnetic stimulation study in man. Brain Res Cogn Brain Res. 1997;5:185–192. doi: 10.1016/s0926-6410(96)00069-9. [DOI] [PubMed] [Google Scholar]
- Kanouchi T, Yokota T, Kamata T, Ishii K. Senda M. Central pathway of photic reflex myoclonus. J Neurol Neurosurg Psychiatry. 1997;62:414–417. doi: 10.1136/jnnp.62.4.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P. Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamarre Y, Busby L. Spidalieri G. Fast ballistic arm movements triggered by visual, auditory, and somesthetic stimuli in the monkey. I. Activity of precentral cortical neurons. J Neurophysiol. 1983;50:1343–1358. doi: 10.1152/jn.1983.50.6.1343. [DOI] [PubMed] [Google Scholar]
- Ledberg A, Bressler SL, Ding M, Coppola R. Nakamura R. Large-scale visuomotor integration in the cerebral cortex. Cereb Cortex. 2007;17:44–62. doi: 10.1093/cercor/bhj123. [DOI] [PubMed] [Google Scholar]
- Leon-Sarmiento FE, Bara-Jimenez W. Wassermann EM. Visual deprivation effects on human motor cortex excitability. Neurosci Lett. 2005;389:17–20. doi: 10.1016/j.neulet.2005.06.061. [DOI] [PubMed] [Google Scholar]
- Macefield VG, Rothwell JC. Day BL. The contribution of transcortical pathways to long-latency stretch and tactile reflexes in human hand muscles. Exp Brain Res. 1996;108:147–154. doi: 10.1007/BF00242912. [DOI] [PubMed] [Google Scholar]
- Makin TR, Holmes NP, Brozzoli C, Rossetti Y. Farne A. Coding of visual space during motor preparation: Approaching objects rapidly modulate corticospinal excitability in hand-centered coordinates. J Neurosci. 2009;29:11841–11851. doi: 10.1523/JNEUROSCI.2955-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino J, Brogna C, Robles SG, Vergani F. Duffau H. Anatomic dissection of the inferior fronto-occipital fasciculus revisited in the lights of brain stimulation data. Cortex. 2010;46:691–699. doi: 10.1016/j.cortex.2009.07.015. [DOI] [PubMed] [Google Scholar]
- Mistry S, Rothwell JC, Thompson DG. Hamdy S. Modulation of human cortical swallowing motor pathways after pleasant and aversive taste stimuli. Am J Physiol Gastrointest Liver Physiol. 2006;291:G666–G671. doi: 10.1152/ajpgi.00573.2005. [DOI] [PubMed] [Google Scholar]
- Nakashima K, Araga S. Takahashi K. Electrophysiological studies of myoclonic jerks provoked by photic stimulation. Acta Neurol Scand. 1985;71:401–407. doi: 10.1111/j.1600-0404.1985.tb03220.x. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, Dimitrijevic MR, Hallett M, Katayama Y, Lucking CH, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol. 1994;91:79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Saron CD, Schroeder CE, Foxe JJ. Vaughan HG., Jr Visual activation of frontal cortex: segregation from occipital activity. Brain Res Cogn Brain Res. 2001;12:75–88. doi: 10.1016/s0926-6410(01)00036-2. [DOI] [PubMed] [Google Scholar]
- Sarubbo S, De Benedictis A, Maldonado IL, Basso G. Duffau H. Frontal terminations for the inferior fronto-occipital fascicle: anatomical dissection, DTI study and functional considerations on a multi-component bundle. Brain Struct Funct. 2013;218:21–37. doi: 10.1007/s00429-011-0372-3. [DOI] [PubMed] [Google Scholar]
- Shibasaki H. Neshige R. Photic cortical reflex myoclonus. Ann Neurol. 1987;22:252–257. doi: 10.1002/ana.410220210. [DOI] [PubMed] [Google Scholar]
- Stewart LM, Walsh V. Rothwell JC. Motor and phosphene thresholds: a transcranial magnetic stimulation correlation study. Neuropsychologia. 2001;39:415–419. doi: 10.1016/s0028-3932(00)00130-5. [DOI] [PubMed] [Google Scholar]
- Strigaro G, Prandi P, Varrasi C, Magistrelli L, Falletta L. Cantello R. Intermittent photic stimulation affects motor cortex excitability in photosensitive idiopathic generalized epilepsy. Epilepsy Res. 2013;104:78–83. doi: 10.1016/j.eplepsyres.2012.08.009. [DOI] [PubMed] [Google Scholar]
- Strigaro G, Prandi P, Varrasi C, Monaco F. Cantello R. Defective visual inhibition in photosensitive idiopathic generalized epilepsy. Epilepsia. 2012;53:695–704. doi: 10.1111/j.1528-1167.2012.03411.x. [DOI] [PubMed] [Google Scholar]
- Suppa A, Li Voti P, Rocchi L, Papazachariadis O. Berardelli A. Early visuomotor integration processes induce LTP/LTD-like plasticity in the human motor cortex. Cereb Cortex. 2015;25:703–712. doi: 10.1093/cercor/bht264. [DOI] [PubMed] [Google Scholar]
- Thut G, Hauert CA, Blanke O, Morand S, Seeck M, Gonzalez SL, Grave de Peralta R, Spinelli L, Khateb A, Landis T. Michel CM. Visually induced activity in human frontal motor areas during simple visuomotor performance. Neuroreport. 2000;11:2843–2848. doi: 10.1097/00001756-200009110-00004. [DOI] [PubMed] [Google Scholar]
- Tokimura H, Di Lazzaro V, Tokimura Y, Oliviero A, Profice P, Insola A, Mazzone P, Tonali P. Rothwell JC. Short latency inhibition of human hand motor cortex by somatosensory input from the hand. J Physiol. 2000;523:503–513. doi: 10.1111/j.1469-7793.2000.t01-1-00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touge T, Taylor JL. Rothwell JC. Reduced excitability of the cortico-spinal system during the warning period of a reaction time task. Electroencephalogr Clin Neurophysiol. 1998;109:489–495. doi: 10.1016/s0924-980x(98)00050-2. [DOI] [PubMed] [Google Scholar]
- Valls-Sole J, Pascual-Leone A, Wassermann EM. Hallett M. Human motor evoked responses to paired transcranial magnetic stimuli. Electroencephalogr Clin Neurophysiol. 1992;85:355–364. doi: 10.1016/0168-5597(92)90048-g. [DOI] [PubMed] [Google Scholar]
- Werhahn KJ, Kunesch E, Noachtar S, Benecke R. Classen J. Differential effects on motorcortical inhibition induced by blockade of GABA uptake in humans. J Physiol. 1999;517(Pt 2):591–597. doi: 10.1111/j.1469-7793.1999.0591t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Lonnecker S, Steinhoff BJ. Paulus W. Effects of antiepileptic drugs on motor cortex excitability in humans: a transcranial magnetic stimulation study. Ann Neurol. 1996;40:367–378. doi: 10.1002/ana.410400306. [DOI] [PubMed] [Google Scholar]