Abstract
With the hypothesis that vestibular sensitivity is regulated to deal with a range of environmental motion conditions, we explored the effects of passive whole-body motion on vestibular perceptual and balance responses. In 10 subjects, vestibular responses were measured before and after a period of imposed passive motion. Vestibulospinal balance reflexes during standing evoked by galvanic vestibular stimulation (GVS) were measured as shear reaction forces. Perceptual tests measured thresholds for detecting angular motion, perceptions of suprathreshold rotation and perceptions of GVS-evoked illusory rotation. The imposed conditioning motion was 10 min of stochastic yaw rotation (0.5–2.5 Hz ≤ 300 deg s−2) with subjects seated. This conditioning markedly reduced reflexive and perceptual responses. The medium latency galvanic reflex (300–350 ms) was halved in amplitude (48%; P = 0.011) but the short latency response was unaffected. Thresholds for detecting imposed rotation more than doubled (248%; P < 0.001) and remained elevated after 30 min. Over-estimation of whole-body rotation (30–180 deg every 5 s) before conditioning was significantly reduced (41.1 to 21.5%; P = 0.033). Conditioning reduced illusory vestibular sensations of rotation evoked by GVS (mean 113 deg for 10 s at 1 mA) by 44% (P < 0.01) and the effect persisted for at least 1 h (24% reduction; P < 0.05). We conclude that a system of vestibular sensory autoregulation exists and that this probably involves central and peripheral mechanisms, possibly through vestibular efferent regulation. We propose that failure of these regulatory mechanisms at different levels could lead to disorders of movement perception and balance control during standing.
Key points
Human activity exposes the vestibular organs to a wide dynamic range of motion.
We aimed to discover whether the CNS regulates sensitivity to vestibular afference during exposure to ambient motion.
Balance and perceptual responses to vestibular stimulation were measured before and after a 10 min period of imposed, moderate intensity, stochastic whole-body rotation.
After this conditioning, vestibular balance reflexes evoked by galvanic vestibular stimulation were halved in amplitude.
Conditioning doubled the thresholds for perceiving small rotations, and reduced perceptions of the amplitude of real rotations, and illusory rotation evoked by galvanic stimulation.
We conclude that the CNS auto-regulates sensitivity to vestibular sensory afference and that this probably involves central and peripheral mechanisms, as might arise from vestibular efferent regulation.
Failure of these regulatory mechanisms at different levels could lead to disorders of movement perception and balance control during standing.
Introduction
The vestibular system, alone and in combination with other sensory systems, detects head movement and contributes to sensations of whole-body movement, orientation and balance. The paired vestibular organs comprise the semicircular canals and the otolith organs (sacculus, utriculus), which are sensitive to angular and linear motion, respectively. Hair cells, the basic sensory units of these organs, transduce the gravito-inertial forces associated with movement into nerve impulses that provide an awareness of head alignment and movement and drive motor reflexes to maintain balance.
Consider a boat or bus trip. In modern life we are commonly exposed to such environments in which we are moved and jostled. For some animals, particularly the avian and aquatic, strong passive motion must be common. A highly sensitive vestibular system seems beneficial in a stationary environment, but excessive sensory inflow in a moving environment could be detrimental. Peripherally, sensory transduction could be overwhelmed, adversely affecting the perceptual and motor responses. Centrally, excessive stimulation in the vestibular sensory channel could mask behaviourally important signals in other sensory channels.
The vestibular system has central adaptive mechanisms that reduce the response to a sustained directional stimulus over time (Guedry & Lauver, 1961; St George et al. 2011) but the opposite after-effect on cessation of this stimulus indicates an increased sensitivity to a stimulus in the reverse direction. Such a mechanism is unsuitable to regulate the sensory inflow and consequences of rocking or random motion that has no net direction. However, a range of neural processes have been identified that could serve this function.
The vestibular afferent signal generated by constant velocity rotation rapidly returns to baseline (time constant ∼6 s) through the physical properties of the semicircular canals. Within the vestibular nuclei and brainstem a velocity storage integrator prolongs this afferent signal with resultant longer lasting (time constant ∼16 s) ocular and perceptual responses (Robinson, 1986; Vibert et al. 1997). Through vestibulocerebellar regulation, repeated passive movement reduces the velocity storage time constant and attenuates the vestibulo-ocular response to movement (Cohen et al. 1992). Vestibular efferent neurons make pre- and post-synaptic connections with hair cells and primary afferents (Boyle et al. 2009) and could also play a role in altering vestibular sensitivity to prolonged random motion. Well known but not specific to the vestibular system are non-associative learning processes of habituation and sensitisation involving synaptic depression at central interneurons. These processes can either reduce or enhance the subjective experience to repeated stimuli (Thompson & Spencer, 1966; Groves & Thompson, 1970).
Although not yet clearly implicated in the pathophysiological aetiology of any specific clinical disorder, failure of any of these regulatory processes could result in disorders of motion perception and balance control. Mal de debarquement syndrome is an uncommon clinical disorder in which sufferers experience rocking or swaying sensations of self-motion after an event of passive motion, classically a boat trip (Cha et al. 2008). Its pathophysiology is uncertain but is commonly believed to be a central disorder as patients do not experience rotatory vertigo and standard vestibular tests are essentially normal. A working hypothesis of the aetiology of this disorder is an inability to regulate and attenuate vestibular activity in response to a changing motion environment.
Prompted by these observations, the present study of normal subjects was designed to determine whether exposure to a period of vestibular (semi-circular canal) stimulation alters vestibular perceptual responses and balance reflexes. A key element of the study is the use of galvanic vestibular stimulation (GVS) to assess vestibular responses. Natural activation of the vestibular system requires a real movement of the head in space and inevitably affects other sensory systems. GVS overcomes this problem by creating a signal of virtual head motion without a real movement (Fitzpatrick & Day, 2004). A small, percutaneous current delivered behind the ears modulates vestibular afferent firing, increasing it on the cathodal side and decreasing it on the anodal side (Goldberg et al. 1982), which evokes characteristic motor and illusory responses (Fitzpatrick et al. 1994a, 1999). The signal evoked from the semi-circular canals dominates human GVS responses (Fitzpatrick et al. 2002; Schneider et al. 2002; Wardman et al. 2003; Cathers et al. 2005) and is interpreted by the CNS as earth-horizontal rotation when the head is flexed with Reid's stereotaxic plane inclined at ∼72 deg (Fitzpatrick & Day, 2004; Day & Fitzpatrick, 2004).
In this study we explore the effects of passive whole-body motion on human vestibular sensibility with a null hypothesis that it would not affect the perceptual or reflexive balance responses to a vestibular stimulus. The results show that a brief 10 min period of random rotatory motion markedly reduces both perceptual and balance responses. We conclude that a powerful system of vestibular sensory autoregulation exists and that this probably involves central mechanisms. We propose that failure of these regulatory mechanisms could lead to disorders of movement perception and balance control during standing.
Methods
Ten subjects (23–59 years, four female) were recruited from staff and students at the University of New South Wales to participate in this study. The tests were approved by the Human Research Ethics Committee of the University of New South Wales and were conducted in accord with the Declaration of Helsinki. Subjects provided written informed consent before participating. No subject had a history of neurological or vestibular disease. Seven subjects were naïve to the purpose and methods of the study and three had participated in previous studies that used GVS to study motion perception or balance reflexes.
The study was designed to examine the effects of a conditioning period of whole-body motion on vestibular balance reflexes and on vestibular perception of angular motion. Four vestibular assessments were made before and after a period of motion conditioning (Fig.1).
Figure 1.
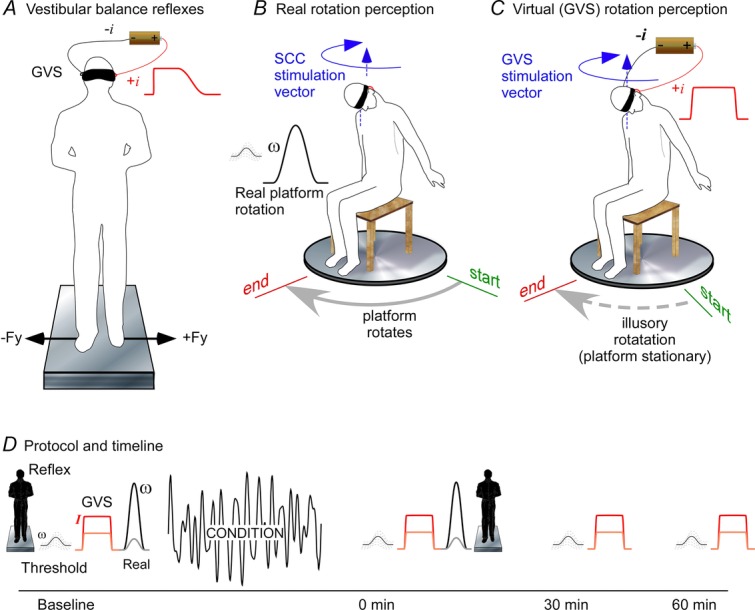
Method
A, vestibular balance reflexes. The blindfolded subject stood on a force plate while galvanic stimuli having a step profile were delivered. Medio-lateral shear forces were recorded. B, real rotation. Subjects sat blindfolded on a motorized rotating platform with the head aligned closely with the rotation axis and tilted forward to face the feet. This set-up was used to assess rotation detection thresholds and perceptions of rotation (illustrated as ‘start’ and ‘end’), and to apply the stochastic motion conditioning. C, virtual (GVS) rotation. The blindfolded subject sat in the platform chair with head tilted forward. The platform motor was engaged to create the small ‘noise’ motion that existed during real rotation but without any net rotation. Bipolar GVS was delivered and subjects reported the perception of rotation by pointing to the ‘start’ position. D, experimental protocol. Standing vestibulospinal reflex, detection threshold, GVS and real perceptions were obtained before administering 10 min of passive stochastic rotation, and then immediately after in the same order. Detection threshold and GVS perceptions were obtained 30 and 60 min after conditioning.
Vestibular assessments
Vestibular balance reflexes
Ag–AgCl electrodes (3 cm2) were attached bilaterally over the mastoid processes. Subjects stood barefoot and blindfolded with feet together on a force plate (Kistler 9286B Zürich) with an instruction to stand still (Fig.1A). Bipolar GVS was applied bilaterally through a computer-controlled high compliance current source. Twenty step stimuli of 1 mA intensity and 2 s duration, 10 of each polarity, were delivered in randomised order at 5–10 s intervals. Reactive lateral shear forces were recorded from the force plate to measure the evoked reflex responses.
Rotation detection thresholds
Subjects sat in a chair on a servo-controlled motorised platform that rotated about a vertical axis (whole-body yaw), positioned so that the head was in the axis of rotation. Throughout, the subject had his or her head flexed, facing the ground between the feet (Fig.1B). The subject wore ear defenders and was blindfolded and the room was dimly lit. The feet were placed on a block of foam to exclude any small tactile cues that might come from the platform. In individual trials, the platform was rotated between 1 and 15 deg over 5 s with a sine-square velocity profile and the subject was instructed to report the direction of any detected movement. A wrong direction or no response within 3 s after the end of the movement was recorded as not detected. When movements were detected correctly the next test rotation was reduced and when not detected the rotation was increased. In these stimuli, angular displacement (θ), velocity (ω) and acceleration (α) all co-vary such that: ωpeak = θ/2.5 deg s−1, and αpeak = θ/4 deg s−2.
Perception of real rotation
Subjects sat on the motorised platform, blindfolded and with ear defenders (Fig.1B). Larger rotations of θ = 30, 60, 90, 120 and 180 deg with a sine-square velocity profile were delivered, with ωpeak and αpeak co-varying as above. Rotations were both clockwise and anticlockwise, in randomised order, and superimposed on a small background stochastic motion (2–6 Hz, zero mean, ω < 0.1 deg s−1). After each rotation, subjects reported its direction and displacement by pointing to the perceived start position, which the experimenter measured (5 deg resolution) with a protractor scale on the platform perimeter. The room was silenced and instructions were always given from directly behind the subject.
Perception of virtual rotation
GVS was used to create an illusory horizontal rotation (Fig.1C). Subjects sat on the platform with the head flexed and facing between the feet. In this posture, GVS creates a vestibular afferent signal that is interpreted as whole-body yaw (Day & Fitzpatrick, 2005). Bipolar GVS was delivered as 10 s step stimuli of either 0.5 or 1.0 mA intensity (six trials, three of each polarity). They were told that they would be rotated with the same instructions as for the real rotation (above) and during application of the galvanic stimulus, the platform was driven with the same small stochastic motion (2–6 Hz, zero mean, ω < 1 deg s−1) that was superimposed on the real rotations (above) to encourage a sense that the platform could be moving. They were never informed that they had not been physically rotated. As above, subjects reported direction and displacement by pointing to the start position.
Protocol and conditioning
Figure1D shows the study timeline. The four vestibular assessments were made before conditioning in the order described above (1, 2, 3, 4). Immediately after conditioning, the assessments were repeated beginning with the perceptual assessments (2, 3, 4, 1). The entire assessment protocol typically took ∼25 min. Detection thresholds and virtual rotation (2, 4) perception were assessed 30 and 60 min after conditioning. This sequence was chosen to avoid interference between tests. The thresholds need to be done before exposure to the larger movements, and the virtual motion before exposure to the real motion.
Conditioning was 10 min of stochastic rotation (0.5–2.5 Hz, –40 dB per decade roll-off) with a peak velocity of ∼100 deg s−1 and a peak acceleration of ∼300 deg s−2. Subjects were seated blindfolded and wearing ear defenders on a platform in the same posture and head inclination as during the vestibular assessments (2, 3, 4). Subjects leaned with the forehead resting on the hands to lessen head-on-neck motion.
This head position of forward tilt brought Reid's plane and the horizontal canals close to vertical so that the vertical (anterior, posterior) semicircular canals are stimulated by the rotation but not the horizontal canals. The semicircular canals were activated in the same way during both the conditioning and the vestibular assessments (below). This position was used as GVS evokes a sensation of whole-body yaw rotation with this head alignment, thus allowing the effects of conditioning on a specific vestibular sensation to be assessed.
A control experiment was performed in five subjects who sat still (without the 10 min of passive motion) during the conditioning phase with all else the same.
Measurements and analysis
To measure the vestibular reflex responses, lateral shear forces were recorded from the force plate at 1 kHz. Anode-left and anode-right trials were normalised to the anodal direction and pooled for within-subject averaging. From these, the peak shear force of the short latency response (at ∼120 ms) and the medium latency response (300–350 ms) were identified for each subject. Pre- and post-conditioning responses were compared using a paired t test.
Detection thresholds were determined by fitting a cumulative Gaussian psychometric function to individual responses (0 = wrong, 1 = correct) and identifying the rotation amplitude estimated to produce 50% correct responses (P50, with its SEM). Repeated-measures ANOVA with Dunnett's post hoc test was used to identify significant effects of motion conditioning on detection thresholds (four times; Fig.1A), on perceptions of virtual (GVS) rotation (four times, with stimulus intensity as a factor) and on perceptions of real rotation (four times, with rotation angle as a factor). Significance was set at Pα = 0.05.
Results
Vestibular balance reflexes
Reflexive force responses evoked by electrical stimulation of vestibular afferents were recorded before and after conditioning. Both showed typical biphasic shear reaction force responses (Fig.2). The short latency response (∼120 ms) was unaffected by motion conditioning (t18 = 0.44, P = 0.66) whereas the medium latency response (300–350 ms) was halved in amplitude (–6.64 to –3.18; t18 = 2.86, P = 0.011). The control condition in which the motion conditioning was replaced by sitting still had no effect on responses (black curves, Fig.2).
Figure 2.
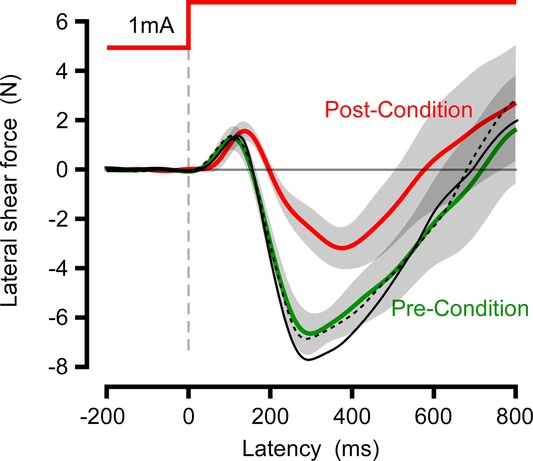
Galvanic reflexes
Subject mean (n = 10) lateral shear force reactions to a bipolar 1 mA stimulus, before (green) and after (red) motion conditioning. Trials are normalized for anode–cathode direction with force in the direction of the anodal electrode plotted as positive values. Black curves are the mean (n = 5) of a control experiment before (broken) and after (unbroken) a conditioning period of sitting stationary.
Rotation detection thresholds
Subjects could detect the direction of whole-body rotation of a few degrees (threshold P50 = 3.9 deg, SD 1.5 deg) when delivered as a sine-square function over 5 s (Fig.3). For this threshold movement, peak angular velocity was 1.6 deg s−1, and peak angular acceleration was 1.0 deg s−2. As thresholds had to be established rapidly with a limited number of presentations (due to time constraints imposed by the overall protocol), the confidence intervals for individual estimates were relatively wide compared with customary psychophysical estimates (mean 95% CI = ± 0.22%). There was a significant main effect of conditioning (pre-, post-) on threshold (F3,39 = 24.0, P < 0.001). Immediately after motion conditioning detection thresholds more than doubled (subject mean 248%, SD 31%). At 30 min after conditioning, thresholds were still elevated significantly (151%, 19%) but at 60 min the increase was no longer significant (141%, 23%).
Figure 3.
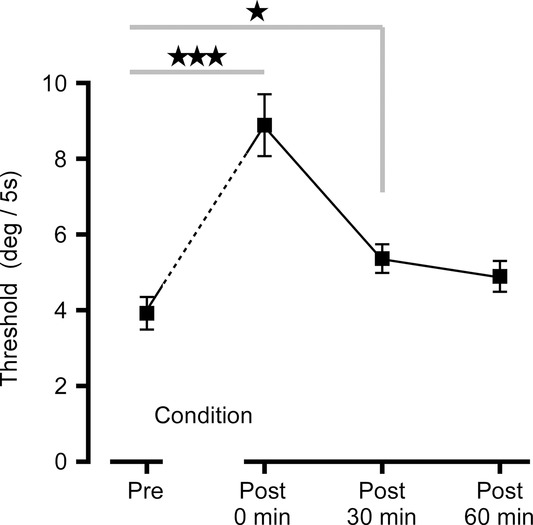
Thresholds for motion detection
Group mean ± SEM rotation at the detection threshold before conditioning (3.88 deg), immediately after (8.88 deg), and at 30 min (5.33 deg) and 60 min (4.88 deg) after conditioning. ***P < 0.001, *P < 0.05 by Dunnett's test.
Perception of real rotation
Subjects reported their perceived rotation by pointing to their start positions for each rotation. In Fig.4, clockwise and anticlockwise results are pooled as there was no difference in their reported errors (F1,189 = 0.15, P = 0.70). For each movement, perceived rotation error was calculated as a proportion of the actual rotation. Before conditioning subjects overestimated the real rotation by a mean of 41.1% (range 33.3–48.8%) but after motion conditioning this was reduced to a 21.5% (13.0–30.0%) overestimation (F1,189 = 6.4, P = 0.033), which represents a 16.1% mean reduction in the perception of the imposed movement. There was a statistically significant effect of the test displacement angle (F1,187 = 9.5, P < 0.001) with greater proportional errors with the smaller displacements (30 and 60 deg).
Figure 4.
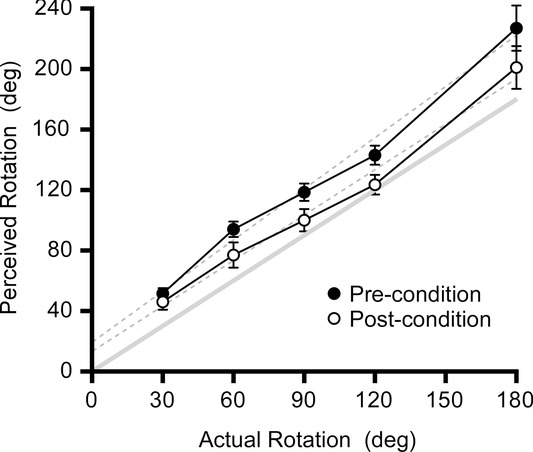
Perception of real rotation
Group mean (n = 10) ± SEM perceptions of rotation after different whole-body rotations, before and after motion conditioning. Broken lines are regressions through the raw data. The solid line is the line of equality. Motion conditioning reduced perceived rotation although it remained greater than actual motion.
Perception of virtual rotation (GVS)
All subjects reported strong sensations of illusory motion when the galvanic stimulus was applied in the absence of real rotation (Fig.5). For a 1 mA stimulus delivered for 10 s, the mean reported rotation was 113 deg (range 53−205 deg). No difference was found between anode-left and anode-right trials and data are pooled after normalizing to the anodal direction. Reported displacements were on average 54% greater for 1.0 mA than for 0.5 mA stimulus intensity (F1,79 = 22.5, P = 0.001). After motion conditioning, perceived rotations to the same stimuli were reduced by 44% overall and at 1 h was still reduced (24% reduction) to pre-conditioning levels (P <0.01 and P < 0.05, respectively, by repeated-measures ANOVA and Dunnett's test).
Figure 5.
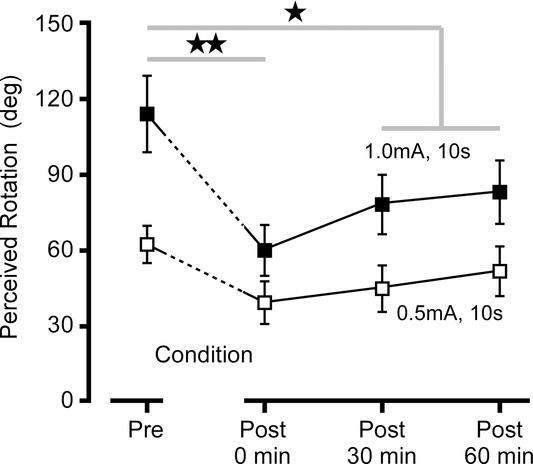
Perception of virtual rotation
Group mean (n = 10) ± SEM perceptions of rotation after 10 s exposure to GVS (0.5 and 1.0 mA). Motion conditioning resulted in an immediate reduction in perceived rotation that only partly recovered over 1 h. Before and after conditioning. **P < 0.01, *P < 0.05 by Dunnett's post hoc test.
Discussion
The results of this study show that even a relatively brief exposure to passive motion significantly attenuates vestibular signals used for perception and reflexive balance control. The passive movement imposed during conditioning was vigorous but not extreme and in the physiological range with peak angular velocity (∼100 deg s−1) and acceleration (∼300 deg s−2) typical of running or jumping activities such as playing basketball (Ng et al., 2006). The duration, however, was relatively brief – just 10 min. The doubling of detection thresholds, halving of the motion sensitivity (gain) to a pure vestibular stimulus and halving of the force developed by automatic balance reflexes indicate that this is a potent regulatory mechanism.
One possible outcome we anticipated was worsened perceptual performance after motion exposure, particularly elevated detection thresholds, through non-specific masking or decreased attentiveness. However, the automatic balance reflexes evoked by GVS have latency to force production of < 100 ms (Fitzpatrick et al. 1994a) and are thus too rapid to be based on a response driven by perception (St George et al. 2007). The main vestibular projection to the leg muscles is from the lateral vestibular nucleus via the ipsilateral lateral vestibulospinal tract (Wilson & Peterson, 1981), which, with the reticulospinal tract, is thought to mediate the GVS balance reflex (Britton et al. 1993; Watson & Colebatch, 1997, 1998). Thus, the large reduction in the size of the automatic balance reflex evoked by GVS shows vestibular specificity and indicates that the regulation is not purely a consequence of attention, volition or perception. Indeed, perceptual responses after conditioning showed increasing sensitivity at 30 and 60 min when attention would probably be declining.
Despite the change in vestibular reflex gain, subjects had no overt problem with balance. Comprehensive posturographic measures were not made after conditioning because of the time constraints of the study, but subjects had no difficulty standing blindfolded for the duration of the GVS reflex testing, their sway during the pre-stimulus epochs was unchanged and none reported disturbed balance. It is possible that challenging balance tasks that depend more on vestibular afference would have demonstrated abnormalities. During quiet standing on a stable base, vestibular signals do not contribute much to the detection or reflex control of body sway (Fitzpatrick & McCloskey, 1994; Fitzpatrick et al. 1994) with vestibulospinal GVS reflexes becoming many times larger when standing on unstable supports (Fitzpatrick et al. 1994a). With vestibular thesholds for detecting motion increased by conditioning to above their already high levels (i.e. further reduced sensitivity), it is not surprising that we did not observe balance problems after conditioning. One could speculate that an enhanced vestibular sensitivity to detecting motion (i.e. opposite to the findings of the current study) could bring it into the range of normal body sway during standing and thereby create disordered perception of sway such as exists in conditions such as mal de debarquement syndrome.
Vestibular stimulation
The natural adequate vestibular stimulus of head motion in space inevitably affects other sensory systems. Important for this study, mechanoreceptors throughout the body will be stimulated by real motion. GVS overcomes this problem by creating a pure signal of ‘virtual’ head motion without a real movement (Fitzpatrick & Day, 2004). The small, percutaneous current that is delivered behind the ears modulates vestibular afferent firing, increasing it on the cathodal side and decreasing it on the anodal side (Goldberg et al. 1982), which creates characteristic motor responses during standing and locomotion and illusory motion when stationary (Fitzpatrick et al. 1994a, 1999). The human GVS (bipolar) response is dominated by a net signal from the semicircular canals of head rotation towards the cathodal side about a sagittal axis inclined ∼20 deg down anteriorly (Wardman et al. 2003; Fitzpatrick & Day, 2004; Cathers et al. 2005; Day & Fitzpatrick, 2004). Thus, in this study we positioned the head facing down by ∼70 deg with this GVS axis vertical so that the evoked vestibular afference represented horizontal rotation – equivalent to the real rotation of the plate (Fig.1C). For consistency and to control potential confounding influences (e.g. neck proprioception, attention), we positioned the head at this same angle during the tests using real rotation studies (perception and threshold; Fig.1B). This meant that the conditioning had to be done with the head in identical orientation to ensure that the movements of testing and conditioning activated the same canals (anterior and posterior bilaterally). It is accepted that the relatively vigorous conditioning rotation will have resulted in a greater degree of stimulus contamination (e.g. neck proprioception) than for the specific vestibular assessments and thus stimulus matching is imperfect. It has yet to be determined whether there is canal specificity to this desensitization process or whether motion in any plane desensitizes all canal afference. Similarly it is possible that the same profile of linear motion driving utricular and saccular afferents might influence canal sensitivity, and vice versa.
Differences between the rotation-evoked and galvanic-evoked signals should be considered. Because it is a pure vestibular signal, the overall pattern of sensory inflow across modalities will be different. For virtual motion resulting from GVS, the vestibular organs signal movement but somatic inflow does not signal the contact and inertial forces that would have produced an equivalent real motion. All subjects could reliably detect both the suprathreshold virtual and real motions and a direction error was never reported. As seen in the error bars (Figs4 and 5) variability of the reported perception is greater with the virtual than with the real rotations (coefficients of variation ≈0.4 and 0.2, respectively). Probable reasons for this include the sensory conflict and a greater variability in the stimulus delivered at the hair cells. The absence of this coherent signal of contact force signal should, if anything, diminish the sensation of movement. As this potential effect would result in a greater attenuation of the larger pre-conditioning responses, the influence of motion conditioning on vestibular responses would be underestimated. Alternatively, could we have desensitized the somatic sensory pathways that signal contact force through the conditioning process? This would lead to an opposite bias with the vestibular effect overestimated. Overall the consistency between all measures of the response to real and galvanic stimulation indicates that these potential biasing effects are small and the finding of response attenuation is genuine.
Mechanisms
Vestibular signals of rotation are transformed by several mechanisms. Peripherally, the canal cupula mechanics form a high pass filter that integrates the angular acceleration with an ∼6 s time constant (Steinhausen, 1933; Goldberg & Fernandez, 1971; Rabbitt et al. 1994; Dai et al. 1999; Dickman & Angelaki, 2004), thus tuning the response to detect transitory changes in head motion. In the vestibular nucleus complex, a velocity storage integrator ‘lengthens’ the afferent signal to a time constant of ∼16 s (Robinson, 1986; Vibert et al. 1997) making ocular and perceptual responses last longer. With sustained angular acceleration and cupula deflection, perceived rotation and compensatory eye movements slowly decay over minutes and after-rotations are experienced in the opposite direction (Guedry & Lauver, 1961) showing a long-term vestibular adaptation. GVS generates a similar motion perception (St George et al. 2011), indicating that the afferent signal it evokes is interpreted by the brain as angular acceleration.
Unlike somatic mechanoreceptors that are dormant without an adequate stimulus and desensitize with sustained stimulation, hair cells have a high resting firing rate that is modulated up and down by motion (Goldberg & Fernandez, 1971). Cyclical or random motion of the type we delivered does not produce sustained stimulation, making hair cell desensitization of this type an unlikely candidate to explain these observations. For the same reason, velocity storage and long-term filtering mechanisms are unlikely have this effect on perceptual and reflex responses. However, it is possible that some of the fundamental synaptic activity on which these processes are based could contribute to the effects we have observed.
The vestibular receptors, like other sensory systems (visual, auditory, proprioceptive), receive efferent innervation that modulates afferent flow. Efferent neurons in the reticular formation receive dendritic input from the vestibular nuclei and other sensory centres and make pre- and post-synaptic connections with the hair cells and primary afferent (Boyle et al. 2009). In the presence of continuous sound, feedback through the auditory efferent system modulates and tunes incoming signals and produces a long-lasting inhibition of cochlear afferents so that a larger sound stimulus is required to evoke a response (Vlajkovic et al. 2006). The function of the vestibular efferent system is less well understood but electrophysiological studies have shown that efferent activity, driven in large part by afferent feedback, can increase or decrease the responsiveness of vestibular afferents to motion stimulation (Goldberg & Fernandez, 1980; Rossi et al. 1980; Holt et al. 2006). This suggests that the vestibular system, through efferent control on its sensors and afferents, can autoregulate its own afferent inflow, perhaps to keep it within a functional dynamic range and sensitivity for the prevailing conditions (Boyle et al. 2009). Recent work in alpha9 knockout mice that lack a vestibular efferent system reveals a markedly diminished adaptation to imposed head rotation. Such a crucial role of vestibular efferents in vestibular adaptation strengthens the argument for efferent involvement in the phenomenon we describe.
In contrast to the medium latency balance reflex, the short latency reflex was unaffected by motion conditioning. Short latency responses are less dependent on postural set (Britton et al. 1993; Fitzpatrick et al. 1994a) and dissociation between short latency and medium latency responses has been noted in a range of experimental contexts (Fitzpatrick et al. 1994a; Watson & Colebatch, 1997; Watson & Colebatch, 1998). Indeed, a more recent study showed that the short latency reflex does not respond to head alignment like the medium latency reflex and as expected from a vestibular reflex (Mian et al. 2010), leading the authors to speculate on a non-vestibular origin. If, however, we accept its vestibular origin, the absence of modulation in the present study argues that not all vestibular pathways are modified equally by passive motion. Other fast and ‘hard-wired’ vestibular reflexes, such as short latency vestibulo-collic reflexes (Colebatch et al. 1994) and the vestibulo-ocular reflex, might also prove relatively immune to motion conditioning. If this proves to be the case it would argue against an exclusive or predominant role of the efferent system on receptors and primary afferents and suggest a more nuanced alteration of central vestibular pathways.
Active, reactive and passive motion
Although the intensity of our imposed conditioning movement might correspond to common activities such as running, the nature of the movement should be considered. Being supported, our subjects had little or no need to react and maintain balance. Had they stood or otherwise had to control themselves in space, detecting the imposed motion would take on a different functional significance and the outcomes might have been different. While canal afferents themselves appear to respond similarly to active and passive head movement (Cullen & Minor, 2002), within the vestibular nuclei are neurons that receive semicircular canal afferents but respond less to active than to passive head movement (McCrea et al. 1999; Roy & Cullen, 2001). Thus, active head movement might also give different outcomes from the passive motion used here. These considerations are relevant to potential clinical correlates of the changes shown here, such as mal de debarquement syndrome (Cha et al. 2008) and motion sickness (Money, 1972), where the nature of motion and subject intention are recognized determinants of syndrome and symptom development.
Clinical implications
By exposure to passive motion we have demonstrated for the first time a powerful regulatory process that attenuates vestibular sensitivity. Altered activity in the neural pathways subserving this regulation could form a pathophysiological basis for overly sensitized or desensitized vestibular balance and perceptual responses. During quiet standing on a stable support, vestibular signals do not contribute to the sensation of body sway or the reflex control of body sway as vestibular sensitivity is weaker than other sensory modalities (Fitzpatrick & McCloskey, 1994; Fitzpatrick et al. 1994b). Failure of the regulatory process described here could increase vestibular sensitivity and create an entirely different experience during standing, with increased vestibular signals reaching perception. Thus, the perception of rocking, swaying and bobbing during standing described by patients with mal de debarquement syndrome, despite an absence of outward signs, could be a manifestation of a failure of this regulatory process.
Typically after acute loss of vestibular function, there is a fairly rapid recovery of vertiginous symptoms and objective deficits of balance and gaze stability. A range of compensatory behaviours can contribute to functional recovery but adaptation and compensation in central vestibular pathways are probably the most significant. The regulatory process identified here, which has the potential to modulate strongly the sensitivity and dynamic range of the vestibular response in the short term, is likely to contribute to early compensation and drive longer term plastic changes. Regulating exposure to passive motion, restricting or increasing it, could modify these compensatory processes and is clearly relevant to patient management.
Conclusions
This study has provided an initial description of a powerful short-term regulation of vestibular sensitivity by passive whole-body motion in normal subjects. It could serve to maintain vestibular sensitivity and dynamic range, keeping it aligned with other sensory modalities and with ambient sensorimotor conditions.
Acknowledgments
This study was supported by the National Health and Medical Research Council of Australia. We are grateful to Dr Frida Emilson, Sahlgrenska Academy, University of Gothenburg, for her valuable contribution to conducting these studies.
Glossary
- GVS
galvanic vestibular stimulation
Additional information
Competing interests
None of the authors has any conflicts of interest.
Contributions
R.F. and S.W. conceived and conducted the study, and wrote the paper. Both authors approved the final manuscript.
Funding
This study was supported by the National Health and Medical Research Council of Australia.
References
- Ackerley R. Barnes GR. The interaction of visual, vestibular and extra-retinal mechanisms in the control of head and gaze during head-free pursuit. J Physiol. 2011;589:1627–1642. doi: 10.1113/jphysiol.2010.199471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baloh RW, Honrubia V. Kerber KA. Baloh and Honrubia's Clinical Neurophysiology of the Vestibular System, 4th edn. Oxford University Press; 2010. New York. [Google Scholar]
- Boyle R, Rabbitt RD. Highstein SM. Efferent control of hair cell and afferent responses in the semicircular canals. J Neurophysiol. 2009;102:1513–1525. doi: 10.1152/jn.91367.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton TC, Day BL, Brown P, Rothwell JC, Thompson PD. Marsden CD. Postural electromyographic responses in the arm and leg following galvanic vestibular stimulation in man. Exp Brain Res. 1993;94:143–151. doi: 10.1007/BF00230477. [DOI] [PubMed] [Google Scholar]
- Cathers I, Day BL. Fitzpatrick RC. Otolith and canal reflexes in human standing. J Physiol. 2005;563:229–234. doi: 10.1113/jphysiol.2004.079525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha YH, Brodsky J, Ishiyama G, Sabatti C. Baloh RW. Clinical features and associated syndromes of mal de debarquement. J Neurol. 2008;255:1038–1044. doi: 10.1007/s00415-008-0837-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen H, Cohen B, Raphan T. Waespe W. Habituation and adaptation of the vestibuloocular reflex: a model of differential control by the vestibulocerebellum. Exp Brain Res. 1992;90:526–538. doi: 10.1007/BF00230935. [DOI] [PubMed] [Google Scholar]
- Colebatch JG, Halmagyi GM. Skuse NF. Myogenic potentials generated by a click-evoked vestibulocollic reflex. J Neurol Neurosurg Psych. 1994;57:190–197. doi: 10.1136/jnnp.57.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen KE. Minor LB. Semicircular canal afferents similarly encode active and passive head-on-body rotations: implications for the role of vestibular efference. J Neurosci. 2002;22:RC226. doi: 10.1523/JNEUROSCI.22-11-j0002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen KE. Roy JE. Signal processing in the vestibular system during active versus passive head movements. J Neurophysiol. 2004;91:1919–1933. doi: 10.1152/jn.00988.2003. [DOI] [PubMed] [Google Scholar]
- Cullen KE, Roy JE. Sylvestre PA. Signal processing by vestibular nuclei neurons is dependent on the current behavioral goal. Ann N Y Acad Sci. 2001;942:345–363. doi: 10.1111/j.1749-6632.2001.tb03759.x. [DOI] [PubMed] [Google Scholar]
- Dai M, Klein A, Cohen B. Raphan T. Model-based study of the human cupular time constant. J Vestib Res. 1999;9:293–301. [PubMed] [Google Scholar]
- Day BL. Fitzpatrick RC. Virtual head rotation reveals a process of route reconstruction from human vestibular signals. J Physiol. 2005;567:591–597. doi: 10.1113/jphysiol.2005.092544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickman JD. Angelaki DE. Dynamics of vestibular neurons during rotational motion in alert rhesus monkeys. Exp Brain Res. 2004;155:91–101. doi: 10.1007/s00221-003-1692-1. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick R, Burke D. Gandevia SC. a Task-dependent reflex responses and movement illusions evoked by galvanic vestibular stimulation in standing humans. J Physiol. 1994;478:363–372. doi: 10.1113/jphysiol.1994.sp020257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick R. McCloskey DI. Proprioceptive, visual and vestibular thresholds for the perception of sway during standing in humans. J Physiol. 1994;478:173–186. doi: 10.1113/jphysiol.1994.sp020240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick R, Rogers DK. McCloskey DI. b Stable human standing with lower-limb muscle afferents providing the only sensory input. J Physiol. 1994;480:395–403. doi: 10.1113/jphysiol.1994.sp020369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick RC. Day BL. Probing the human vestibular system with galvanic stimulation. J Appl Physiol. 2004;96:2301–2316. doi: 10.1152/japplphysiol.00008.2004. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick RC, Marsden J, Lord SR. Day BL. Galvanic vestibular stimulation evokes sensations of body rotation. Neuroreport. 2002;13:2379–2383. doi: 10.1097/00001756-200212200-00001. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick RC, Wardman DL. Taylor JL. Effects of galvanic vestibular stimulation during human walking. J Physiol. 1999;517:931–939. doi: 10.1111/j.1469-7793.1999.0931s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JM. Fernandez C. Physiology of peripheral neurons innervating semicircular canals of the squirrel monkey. I. Resting discharge and response to constant angular accelerations. J Neurophysiol. 1971;34:635–660. doi: 10.1152/jn.1971.34.4.635. [DOI] [PubMed] [Google Scholar]
- Goldberg JM. Fernandez C. Efferent vestibular system in the squirrel monkey: anatomical location and influence on afferent activity. J Neurophysiol. 1980;43:986–1025. doi: 10.1152/jn.1980.43.4.986. [DOI] [PubMed] [Google Scholar]
- Goldberg JM, Fernandez C. Smith CE. Responses of vestibular-nerve afferents in the squirrel monkey to externally applied galvanic currents. Brain Res. 1982;252:156–160. doi: 10.1016/0006-8993(82)90990-8. [DOI] [PubMed] [Google Scholar]
- Groves PM. Thompson RF. Habituation: a dual-process theory. Psychol Rev. 1970;77:419–450. doi: 10.1037/h0029810. [DOI] [PubMed] [Google Scholar]
- Guedry F. Lauver L. Vestibular reactions during prolonged constant angular acceleration. J Appl Physiol. 1961;16:215–220. [Google Scholar]
- Holt JC, Lysakowski A. Goldberg JM. Mechanisms of efferent-mediated responses in the turtle posterior crista. J Neurosci. 2006;26:13180–13193. doi: 10.1523/JNEUROSCI.3539-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hübner PP, Khan SI. Migliaccio AA The role of vestibular efferents in vestibulo-ocular reflex gain adaptation: a study in α9-nachr deficient miceJ Vest Res. 2014. p. 58. XXVIII Meeting of the Bárány Society, Buenos Aires, Argentina.
- McCrea RA, Gdowski GT, Boyle R. Belton T. Firing behavior of vestibular neurons during active and passive head movements: vestibulo-spinal and other non-eye-movement related neurons. J Neurophysiol. 1999;82:416–428. doi: 10.1152/jn.1999.82.1.416. [DOI] [PubMed] [Google Scholar]
- Mian OS, Dakin CJ, Blouin JS, Fitzpatrick RC. Day BL. Lack of otolith involvement in balance responses evoked by mastoid electrical stimulation. J Physiol. 2010;588:4441–4451. doi: 10.1113/jphysiol.2010.195222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Money KE. Motion sickness. Physiol Rev. 1972;50:1–39. doi: 10.1152/physrev.1970.50.1.1. [DOI] [PubMed] [Google Scholar]
- Rabbitt RD, Boyle R. Highstein SM. Sensory transduction of head velocity and acceleration in the toadfish horizontal semicircular canal. J Neurophysiol. 1994;72:1041–1048. doi: 10.1152/jn.1994.72.2.1041. [DOI] [PubMed] [Google Scholar]
- Robinson DA. The systems approach to the oculomotor system. Vis Res. 1986;26:91–99. doi: 10.1016/0042-6989(86)90073-8. [DOI] [PubMed] [Google Scholar]
- Rossi ML, Prigioni I, Valli P. Casella C. Activation of the efferent system in the isolated frog labyrinth: effects on the afferent EPSPs and spike discharge recorded from single fibers on the posterior nerve. Brain Res. 1980;185:125–137. doi: 10.1016/0006-8993(80)90677-0. [DOI] [PubMed] [Google Scholar]
- Roy JE. Cullen KE. Selective processing of vestibular reafference during self-generated head motion. J Neurosci. 2001;21:2131–2142. doi: 10.1523/JNEUROSCI.21-06-02131.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider E, Glasauer S. Dieterich M. Comparison of human ocular torsion patterns during natural and galvanic vestibular stimulation. J Neurophysiol. 2002;87:2064–2073. doi: 10.1152/jn.00558.2001. [DOI] [PubMed] [Google Scholar]
- St George RJ, Day BL. Fitzpatrick RC. Adaptation of vestibular signals for self-motion perception. J Physiol. 2011;589:843–853. doi: 10.1113/jphysiol.2010.197053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St George RJ, Fitzpatrick RC, Rogers MW. Lord SR. Choice stepping response and transfer times: effects of age, fall risk, and secondary tasks. J Gerontol A, Biol Sci Med Sci. 2007;62:537–542. doi: 10.1093/gerona/62.5.537. [DOI] [PubMed] [Google Scholar]
- Steinhausen W. Uber die beobachtung der cupula in den bogengangsampullen des labyrinths des lebenden hechts. Pflugers Arch Ges Physiol. 1933;232:500–512. [Google Scholar]
- Thopmson RF. Spencer WA. Habituation: a model phenomenon for the study of neuronal substrates of behavior. Psychol Rev. 1966;73:16–43. doi: 10.1037/h0022681. [DOI] [PubMed] [Google Scholar]
- Vibert N, De Waele C, Serafin M, Babalian A, Muhlethaler M. Vidal PP. The vestibular system as a model of sensorimotor transformations. A combined in vivo and in vitro approach to study the cellular mechanisms of gaze and posture stabilization in mammals. Prog Neurobiol. 1997;51:243–286. doi: 10.1016/s0301-0082(96)00057-3. [DOI] [PubMed] [Google Scholar]
- Vlajkovic SM, Vinayagamoorthy A, Thorne PR, Robson SC, Wang CJH. Housley GD. Noise-induced up-regulation of NTPDase3 expression in the rat cochlea: implications for auditory transmission and cochlear protection. Brain Res. 2006;1104:55–63. doi: 10.1016/j.brainres.2006.05.094. [DOI] [PubMed] [Google Scholar]
- Wardman DL, Day BL. Fitzpatrick RC. Position and velocity responses to galvanic vestibular stimulation during standing. J Physiol. 2003;549:293–299. doi: 10.1113/jphysiol.2002.030767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson SR. Colebatch JG. EMG responses in the soleus muscles evoked by unipolar galvanic vestibular stimulation. Electroenceph Clin Neurophysiol. 1997;105:476–483. doi: 10.1016/s0924-980x(97)00044-1. [DOI] [PubMed] [Google Scholar]
- Watson SR. Colebatch JG. Vestibular-evoked electromyographic responses in soleus: a comparison between click and galvanic stimulation. Exp Brain Res. 1998;119:504–510. doi: 10.1007/s002210050366. [DOI] [PubMed] [Google Scholar]
- Wilson VJ. Peterson BW. Vestibulospinal and reticulospinal systems. In: Brooks VB, editor; Handbook of Physiology, Sect. I. Bethesda, MD: American Physiological Society; 1981. pp 667–702. [Google Scholar]


