Abstract
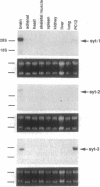
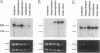
The synaptotagmins are integral membrane proteins of synaptic vesicles thought to serve as Ca2+ sensors in the process of vesicular trafficking and exocytosis. Results from antibody microinjection and gene-disruption experiments have led to a controversy over whether synaptotagmins are essential for neurotransmission. However, the studies casting doubt on the role of synaptotagmins have assumed that no further isoforms of these molecules exist. Here we report the isolation of a third member of the synaptotagmin family (Syt3) from mouse brain. Although retaining the characteristic five-domain structure of the other synaptotagmins, SYT3 is considerably more divergent at the level of amino acid sequence. In the most highly conserved C2 domain, the mammalian synaptotagmins, SYT1 and SYT2, share 88% sequence identity, whereas SYT3 has only approximately 45% identity to either. Overall, SYT3 has the greatest sequence identity with rat SYT2 and marine ray p65A (both 37%), although homology to all of the known synaptotagmins is > 30%. However, SYT3 is most like p65C when comparing domain structure. Syt3 is expressed in many regions of the nervous system but is undetectable in extraneural tissues. The three murine synaptotagmins have differential expression patterns in the brain. Furthermore, in PC12 cells, Syt3 is coexpressed with Syt1 and is more abundant than the latter. This result suggests that individual neurons may have specific combinations of synaptotagmins that could provide for diversity in vesicular release.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett M. K., Calakos N., Scheller R. H. Syntaxin: a synaptic protein implicated in docking of synaptic vesicles at presynaptic active zones. Science. 1992 Jul 10;257(5067):255–259. doi: 10.1126/science.1321498. [DOI] [PubMed] [Google Scholar]
- Bommert K., Charlton M. P., DeBello W. M., Chin G. J., Betz H., Augustine G. J. Inhibition of neurotransmitter release by C2-domain peptides implicates synaptotagmin in exocytosis. Nature. 1993 May 13;363(6425):163–165. doi: 10.1038/363163a0. [DOI] [PubMed] [Google Scholar]
- Brose N., Petrenko A. G., Südhof T. C., Jahn R. Synaptotagmin: a calcium sensor on the synaptic vesicle surface. Science. 1992 May 15;256(5059):1021–1025. doi: 10.1126/science.1589771. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clark J. D., Lin L. L., Kriz R. W., Ramesha C. S., Sultzman L. A., Lin A. Y., Milona N., Knopf J. L. A novel arachidonic acid-selective cytosolic PLA2 contains a Ca(2+)-dependent translocation domain with homology to PKC and GAP. Cell. 1991 Jun 14;65(6):1043–1051. doi: 10.1016/0092-8674(91)90556-e. [DOI] [PubMed] [Google Scholar]
- DeBello W. M., Betz H., Augustine G. J. Synaptotagmin and neurotransmitter release. Cell. 1993 Sep 24;74(6):947–950. doi: 10.1016/0092-8674(93)90716-4. [DOI] [PubMed] [Google Scholar]
- Elferink L. A., Peterson M. R., Scheller R. H. A role for synaptotagmin (p65) in regulated exocytosis. Cell. 1993 Jan 15;72(1):153–159. doi: 10.1016/0092-8674(93)90059-y. [DOI] [PubMed] [Google Scholar]
- Geppert M., Archer B. T., 3rd, Südhof T. C. Synaptotagmin II. A novel differentially distributed form of synaptotagmin. J Biol Chem. 1991 Jul 25;266(21):13548–13552. [PubMed] [Google Scholar]
- Gribskov M., Burgess R. R. Sigma factors from E. coli, B. subtilis, phage SP01, and phage T4 are homologous proteins. Nucleic Acids Res. 1986 Aug 26;14(16):6745–6763. doi: 10.1093/nar/14.16.6745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata Y., Davletov B., Petrenko A. G., Jahn R., Südhof T. C. Interaction of synaptotagmin with the cytoplasmic domains of neurexins. Neuron. 1993 Feb;10(2):307–315. doi: 10.1016/0896-6273(93)90320-q. [DOI] [PubMed] [Google Scholar]
- Leveque C., Hoshino T., David P., Shoji-Kasai Y., Leys K., Omori A., Lang B., el Far O., Sato K., Martin-Moutot N. The synaptic vesicle protein synaptotagmin associates with calcium channels and is a putative Lambert-Eaton myasthenic syndrome antigen. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3625–3629. doi: 10.1073/pnas.89.8.3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littleton J. T., Stern M., Schulze K., Perin M., Bellen H. J. Mutational analysis of Drosophila synaptotagmin demonstrates its essential role in Ca(2+)-activated neurotransmitter release. Cell. 1993 Sep 24;74(6):1125–1134. doi: 10.1016/0092-8674(93)90733-7. [DOI] [PubMed] [Google Scholar]
- Matthew W. D., Tsavaler L., Reichardt L. F. Identification of a synaptic vesicle-specific membrane protein with a wide distribution in neuronal and neurosecretory tissue. J Cell Biol. 1981 Oct;91(1):257–269. doi: 10.1083/jcb.91.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuta M., Inagaki N., Nemoto Y., Matsukura S., Takahashi M., Seino S. Synaptotagmin III is a novel isoform of rat synaptotagmin expressed in endocrine and neuronal cells. J Biol Chem. 1994 Apr 22;269(16):11675–11678. [PubMed] [Google Scholar]
- Morgan J. I., Cohen D. R., Hempstead J. L., Curran T. Mapping patterns of c-fos expression in the central nervous system after seizure. Science. 1987 Jul 10;237(4811):192–197. doi: 10.1126/science.3037702. [DOI] [PubMed] [Google Scholar]
- Nonet M. L., Grundahl K., Meyer B. J., Rand J. B. Synaptic function is impaired but not eliminated in C. elegans mutants lacking synaptotagmin. Cell. 1993 Jul 2;73(7):1291–1305. doi: 10.1016/0092-8674(93)90357-v. [DOI] [PubMed] [Google Scholar]
- Perin M. S., Brose N., Jahn R., Südhof T. C. Domain structure of synaptotagmin (p65) J Biol Chem. 1991 Jan 5;266(1):623–629. [PubMed] [Google Scholar]
- Perin M. S., Fried V. A., Mignery G. A., Jahn R., Südhof T. C. Phospholipid binding by a synaptic vesicle protein homologous to the regulatory region of protein kinase C. Nature. 1990 May 17;345(6272):260–263. doi: 10.1038/345260a0. [DOI] [PubMed] [Google Scholar]
- Perin M. S., Johnston P. A., Ozcelik T., Jahn R., Francke U., Südhof T. C. Structural and functional conservation of synaptotagmin (p65) in Drosophila and humans. J Biol Chem. 1991 Jan 5;266(1):615–622. [PubMed] [Google Scholar]
- Petrenko A. G., Perin M. S., Davletov B. A., Ushkaryov Y. A., Geppert M., Südhof T. C. Binding of synaptotagmin to the alpha-latrotoxin receptor implicates both in synaptic vesicle exocytosis. Nature. 1991 Sep 5;353(6339):65–68. doi: 10.1038/353065a0. [DOI] [PubMed] [Google Scholar]
- Shirataki H., Kaibuchi K., Sakoda T., Kishida S., Yamaguchi T., Wada K., Miyazaki M., Takai Y. Rabphilin-3A, a putative target protein for smg p25A/rab3A p25 small GTP-binding protein related to synaptotagmin. Mol Cell Biol. 1993 Apr;13(4):2061–2068. doi: 10.1128/mcb.13.4.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens C. F. Quantal release of neurotransmitter and long-term potentiation. Cell. 1993 Jan;72 (Suppl):55–63. doi: 10.1016/s0092-8674(05)80028-5. [DOI] [PubMed] [Google Scholar]
- Söllner T., Whiteheart S. W., Brunner M., Erdjument-Bromage H., Geromanos S., Tempst P., Rothman J. E. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993 Mar 25;362(6418):318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- Wendland B., Miller K. G., Schilling J., Scheller R. H. Differential expression of the p65 gene family. Neuron. 1991 Jun;6(6):993–1007. doi: 10.1016/0896-6273(91)90239-v. [DOI] [PMC free article] [PubMed] [Google Scholar]